Abstract
Migraine significantly impacts quality of life, with eptinezumab emerging as a promising calcitonin gene-related peptide-targeting therapy. Real-world data and clinical trials are crucial for evaluating its safety and effectiveness comprehensively. This study evaluated its safety using a dual approach: pharmacovigilance analysis of the FDA’s Adverse Event Reporting System (FAERS) database (2020–2024) and a systematic review with meta-analysis of clinical trials. FAERS data identified 5,306 adverse event (AE) reports, with “drug ineffective” (ROR = 6.71) and “migraine” (ROR = 67.45) as the strongest signals. Serious adverse events (SAEs) included anaphylactic reactions (ROR = 4.19) and rare events like increased intracranial pressure. Most AEs occurred within the first treatment month. A meta-analysis of six trials (n = 3,148) found no increased overall AE risk versus placebo (RR = 1.02, 95% CI 0.95–1.10) but a higher SAE incidence (RR = 2.87, 95% CI 1.27–6.48). Upper respiratory infections were more frequent (RR = 1.49, P = 0.04), while dizziness, nausea, and fatigue showed no significant differences. Eptinezumab shows promise but warrants further research on safety in vulnerable populations and real-world settings.
Similar content being viewed by others
Introduction
Migraine is a common neurological disorder that significantly affects patients’ quality of life, with recent research exploring its interictal burden, patient-reported outcomes, and related measurement tools1,2. It affects emotional function, work productivity, and is associated with stigma-related disability3,4,5. Eptinezumab, a humanized monoclonal antibody targeting calcitonin gene-related peptide (CGRP), prevents both episodic and chronic migraines6,7. It became the first infusion therapy for migraine prevention by targeting the CGRP pathway, critical in migraine pathophysiology8. Research has demonstrated that it is effective in preventing episodic and chronic migraines, with effects noticed from day one after dosing9. Long-term safety and tolerability were assessed over two years, with doses every 12 weeks, showing mostly mild or moderate adverse events unrelated to the drug10,11. Eptinezumab has received approval for preventing migraines, showing positive efficacy and safety, but continuous evaluation of its safety profile is essential12,13.
Real-world safety assessments are vital in the post-marketing phase of drug development due to the limitations of pre-marketing clinical trials, which often involve a small, controlled patient group14. These trials may not fully reflect a drug’s safety in diverse populations with various health conditions15. Regulatory agencies such as the FDA and EMA are crucial in ensuring drug safety, utilizing systems like the FDA’s Adverse Event Reporting System (FAERS) to detect safety signals. They emphasize real-world data to supplement clinical trials, helping to detect rare adverse events, identify at-risk groups, and assess long-term safety, ultimately improving patient safety and guiding clinical decisions14,15.
There is a significant lack of thorough safety evaluations that combine information from the FAERS database and conventional clinical trials. Reports of adverse events associated with eptinezumab exist in individual studies, yet a combined analysis of spontaneous reports and systematic clinical trial reviews is absent16. This study seeks to address this gap by conducting a comprehensive safety analysis of eptinezumab, providing insights to improve clinical decision-making and patient care.
This study employs a dual-methodology approach that combines pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database with a systematic review and meta-analysis of clinical trial data, enabling a holistic evaluation of eptinezumab’s safety. Real-world safety assessments are crucial in the post-marketing phase of drug development because pre-marketing clinical trials often involve controlled patient populations that do not fully represent the diverse general population. By incorporating data from both the FAERS database and relevant real-world studies, this combined strategy aims to identify both common and rare adverse events, assess their clinical relevance, and provide a more comprehensive understanding of eptinezumab’s safety profile across different demographics and clinical settings. Through these analyses, the study seeks to contribute valuable insights to the existing literature on eptinezumab’s safety in migraine management.
Method and materials
Pharmacovigilance analysis of FAERS
Data sources, management, and study design
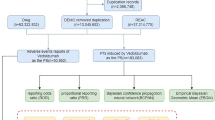
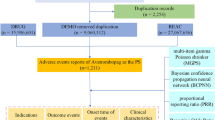
The study utilized data from the publicly accessible FAERS database, a spontaneous reporting system for submissions by consumers, physicians, and pharmacists. This open archive has garnered notable attention from clinicians for assessing drug safety and characterizing adverse events (AEs) of special interest after marketing. It is especially useful for identifying rare but severe adverse events that might not be detected or reported in clinical trials17. The FAERS data files are composed of seven databases, which include demographic and administrative information (DEMO), adverse drug reaction information (REAC), patient outcome information (OUTC), drug information (DRUG), drug therapy starts dates and end dates (THER), information on report sources (RPSR), and indications for use/diagnosis (INDI). We included data on eptinezumab to the fullest extent possible, encompassing its brand name "Vyepti," as well as the codes “ALD403” and "ALD-403," among others. Given that the database contains spontaneously reported data, there may be instances of non-standard spelling. To address this, we conducted a fuzzy search for drug names. We examined all adverse reaction reports associated with eptinezumab from Q2 2020 to Q3 2024. Reports were included only if eptinezumab was designated as the primary suspect drug (ROLE_COD = ‘PS’) in the Therapy (THER) file, following FDA-recommended role code standards18. The data management process included removing duplicate reports and standardizing adverse event terminology. FDA-recommended practices were followed when processing duplicates19.Based on the method recommended by the FDA for removing duplicate reports, this study selects the fields PRIMARYID, CASEID, and FDA DT from the DEMO table. The data is sorted in the order of CASEID, FDA DT, and PRIMARYID. For reports with the same CASEID, the one with the highest FDA DT value is retained; additionally, among reports with identical CASEID and FDA DT values, the one with the highest PRIMARYID value is preserved18,20,21,22. Adverse event terms were standardized using the Medical Dictionary for Regulatory Activities (MedDRA) dictionary, version 26.1, thereby enhancing the reliability of statistical analyses. The MedDRA hierarchy was used to identify the System Organ Class (SOC) corresponding to each PT. Figure S1 illustrates a comprehensive flowchart outlining the design of the pharmacovigilance analysis conducted in the research.
Statistical analysis
Descriptive statistics were used to analyze adverse event reports related to eptinezumab. The formulas used in this analysis are referenced from previously published pharmacovigilance studies, particularly the guidelines outlined in 'The REporting of A Disproportionality Analysis for DrUg Safety Signal Detection Using Individual Case Safety Reports in PharmacoVigilance (READUS-PV): Explanation and Elaboration,' specifically Item 7b19,23,24. Four disproportionality analysis methods were utilized to detect potential adverse reaction signals related to eptinezumab: reporting odds ratio (ROR)25, proportional reporting ratio (PRR)26, multi-item gamma Poisson shrinker (MGPS)27, and Bayesian confidence propagation neural network (BCPNN)28. The positive signals identified through signal detection were compared with the adverse events mentioned in the FDA-approved label to identify any unmentioned adverse events.
This study’s FAERS data analysis utilized a multimodal stepwise method, integrating various criteria for clinical prioritization of adverse events, a strategy recently suggested in pharmacovigilance17,29,30. A semi-quantitative score evaluating the clinical importance of adverse events that display statistically significant disproportionality. Supplementary Table S1 contains detailed two-by-two contingency tables. Supplementary Table S2 details the disproportionality calculations. A patient’s onset time of eptinezumab-related adverse events is defined as the time interval between adverse events that is reported in the Demographic (DEMO) file and treatment initiation that is reported in the THER file. The Weibull distribution modeled changes in adverse event incidence over time. Data analysis was performed using R software version 4.4.1, including data cleaning, statistical analysis, and result visualization. A detailed flowchart illustrating the entire pharmacovigilance analysis process, including data curation, is provided in Supplementary Figure S2 for visual reference.
Classifcation and prioritization of relevant disproportionality signals
Adverse events significantly associated in at least one disproportionality analysis were ranked using a semiquantitative score based on specific criteria (Table 1).
-
The reporting rate is defined as the proportion of specific AEs relative to all recorded AEs, expressed as the ratio of cases to non-cases. While utilizing conventional categories—very common (≥ 10%), common (1–10%), and uncommon (≤ 1%)—can facilitate the comparison with clinical trial data, we acknowledge the inherent limitations of the FAERS database. Specifically, the total number of patients prescribed eptinezumab or the total number of prescriptions is not available. However, as supported by previous research, categorizing AEs this way provides a pragmatic framework for evaluating the relative frequencies of AEs17. This method serves to highlight the clinical relevance of various adverse events and supports healthcare providers in prioritizing safety monitoring in a real-world context.
-
Signal stability refers to the consistency and robustness of disproportionality signals across various analyses. Maximum score was awarded to consistency that disproportionality signals in four or three out of four disproportionality analyses.
-
Reported case fatality rate: determined by the proportion of adverse event reports resulting in death: rates over 50% receive 2 points, rates between 25 and 50% receive 1 point, and rates below 25% receive 0 points.
-
Clinical relevance was assessed using the Important Medical Events (IMEs, version 26.0) and Designated Medical Events (DMEs) lists, which include serious and rare drug-related events, provided by the European Medical Agency17,29.
AEs were categorized as low, moderate, or high priority with scores of 0–2, 3–5, and 6–8, respectively.
Meta-analysis and systematic review of clinical trials
Search strategy and study selection
Research articles from the inception of the database until January 31, 2025, were retrieved using PubMed, Embase, Cochrane, Web of Science, OVID, and Scopus. The inclusion criteria for studies encompassed both randomized controlled trials (RCTs) and real-world studies (RWS) that utilized eptinezumab (including alternative names such as ALD403 and vyepti) as the experimental group. This broader study selection was made to better capture the safety profile of eptinezumab in a diverse patient population. Supplementary Table S3 contains a detailed list of search strategies. The articles were first evaluated by their title and abstract. Subsequently, two investigators (YS Chen and SQ Huang) examined the full texts of potentially relevant studies to assess their eligibility for inclusion. Any differences among the investigators were settled by reaching a consensus. Only English-language full-text articles were included.
The criteria for inclusion were as follows: 1. Studies that utilized eptinezumab (including ALD403 and Vyepti) as the experimental group in RCTs. 2. Studies that employed a placebo as the control group in RCTs. 3. Studies involving patients diagnosed with migraine or related migraine disorders. 4. Studies that reported AEs. 5. Both RCTs and real-world studies were considered. The criteria for exclusion were as follows: 1. Duplicate publications or studies. 2. Meta-analyses, reviews, case reports, conference abstracts, animal studies, and comments. 3. Studies that did not include eptinezumab treatment in the experimental group. 4. Studies lacking a placebo treatment in the control group. 5. Studies with flawed designs that did not meet rigorous methodological standards. The current meta-analysis has been registered with INPLASY (INPLASY202510104).
Data extraction and quality assessment
Data collection included the first author’s name, publication year, National Clinical Trial number, study design, RCT phase, overall duration, treatment arms, demographic details (age and gender), AEs, treatment-related adverse events (TEAEs), and serious adverse events (SAEs).Two authors assessed the methodological quality of the included studies using the Cochrane Collaboration’s tool for RCTs. In our systematic review, we included RCTs and assessed the risk of bias in these studies using the “Cochrane Collaboration’s Risk of Bias Tool”. This tool evaluates various domains of bias, including selection bias, performance bias, detection bias, attrition bias, and reporting bias, thus providing a comprehensive assessment of the methodological quality of each study.
The risk of bias for all studies was evaluated according to these criteria to ensure the reliability and validity of the results.
Statistical analysis
Dichotomous data were summarized using risk ratios (RR) with 95% confidence intervals (CI), and study heterogeneity was evaluated using the I2 statistic based on Cochran’s Q31. For analysis, fixed-effects models were applied when I2 was 50% or less, while random-effects models were used when I2 exceeded 50%. A P-value below 0.05 was deemed statistically significant. A sensitivity analysis was performed to verify the stability of the findings. Funnel plots were utilized for examining publication bias32. The meta-analysis was conducted using Review Manager 5.3 and Stata 16.1 (Stata Corporation, College Station, Texas, USA).
Results
Pharmacovigilance analysis of FAERS
Descriptive analysis
This investigation encompassed 5306 reports of adverse events in which eptinezumab was the suspected primary agent. The gender distribution revealed that 10.5% of the patients were male, while a significant majority of 62.6% were female, with 26.9% of the reports missing gender information. The majority of cases (59.8%) were in the 18–65 age group, followed by 10.0% in the 65–85 age group, while only 0.2% were under 18.Notably, 29.8% of the reports did not specify the age of the patients. The majority of the reports, 79.5%, were submitted by non-healthcare professionals, whereas healthcare professionals accounted for 20.0% of the submissions. Geographically, the United States was the predominant source of reports, contributing 93.7% of the total, followed by Canada at 4.1%, and France at 0.5%. Additional descriptive outcomes are detailed in Table 2.
Distribution of adverse events by SOC level
The analysis of AEs associated with eptinezumab in the FAERS database revealed significant signals in several SOCs (Supplementary Table S4).The category 'Nervous System Disorders’ demonstrated the highest association, with an ROR of 3.64 (95% CI 3.48–3.81), a PRR of 3.06, an EBGM of 3.06 (EBGM05 2.94), and an IC of 1.61 (IC025 1.55).Significant signals were also noted in 'General Disorders and Administration Site Conditions’ (ROR 2.25, IC 0.88) and 'Respiratory, Thoracic, and Mediastinal Disorders’ (ROR 2.07, IC 0.98).The study identifies significant SOCs associated with eptinezumab-related AEs, particularly in the nervous system and general disorders. The distribution of adverse events at the SOC level is visually represented in Fig. 1, providing a comprehensive overview of the data.
Clinical prioritization of relevant disproportionality signals
Supplementary Table S5 show the analysis of each evaluated PT. The analysis of AEs associated with eptinezumab at the PT level indicated events of moderate and low clinical significance, with no high-priority occurrences. Table 3, Supplementary Table S6 and Table S7 provide a summary of events with moderate clinical priority, along with their scores and statistical data. Among the most frequently reported AEs, drug ineffective had the highest number of cases (n = 1457), with a ROR of 6.71 (95% CI 6.35–7.09), PRR of 5.95, EBGM of 5.94 (EBGM05: 5.67), and IC of 2.57 (IC025: 2.49). Similarly, migraine (n = 1012) exhibited the strongest signal, with an ROR of 67.45 (95% CI 63.17–72.03), PRR of 61.34, EBGM of 59.41 (EBGM05: 56.24), and IC of 5.89 (IC025: 5.80). Other notable AEs included headache (n = 674, ROR: 7.21, IC: 2.77), therapeutic response shortened (n = 327, ROR: 36.41, IC: 5.12), and fatigue (n = 295, ROR: 2.11, IC: 1.06).
In subgroup analyses by sex, drug ineffective was the most reported AE in both males (n = 188, ROR: 9.72, IC: 3.05) and females (n = 833, ROR: 5.92, IC: 2.42). Migraine showed a particularly strong signal in males (n = 94, ROR: 143.74, IC: 7.01) and females (n = 737, ROR: 54.02, IC: 5.56). Other AEs, such as headache, therapeutic response shortened, and nausea, were also prominent in both sexes, with slight variations in reporting rates and signal strength.
Age subgroup analyses revealed that drug ineffective was the most frequently reported AE across all age groups, with the highest signal observed in patients aged 65–85 years (n = 197, ROR: 14.01, IC: 3.57). Migraine was also strongly associated with eptinezumab in patients aged 18–65 years (n = 719, ROR: 58.34, IC: 5.64) and 65–85 years (n = 94, ROR: 135.02, IC: 6.91). Other AEs, such as therapeutic response shortened, throat irritation, and nasal congestion, were more commonly reported in older age groups, with higher ROR and IC values compared to younger populations.
In terms of clinical relevance, DME and IME were also identified. For example, anaphylactic reaction (n = 39) had an ROR of 4.19 (95% CI 3.06–5.74), PRR of 4.18, EBGM of 4.17 (EBGM05: 3.21), and IC of 2.06 (IC025: 1.60). Rare but clinically significant events, such as intracranial pressure increased (n = 3, ROR: 3.47, IC: 1.79) and facial paralysis (n = 6, ROR: 3.25, IC: 1.70), were also observed, particularly in the older population.
Analysis of adverse event onset and weibull distribution
Among cases with complete temporal information, most adverse events occurred within 30 days following eptinezumab administration. Figure 2 illustrates the temporal distribution of these events. Figure S3 illustrates the cumulative incidence curve of adverse events. Weibull distribution analysis revealed an early failure mode. Table 4 outlines the parameters of the analysis.
Meta-analysis and systematic review of clinical trials
Study selection and study characteristics
A total of 1175 publications were identified through six databases, and 444 records remained after duplicates were removed. Following the screening of titles and abstracts, 93 studies were excluded as they were meta-analyses, reviews, comments, corrections, or involved animal research. Among the 351 full-text articles reviewed, 345 were excluded for reasons such as non-RCT designs, flawed methodologies, or inconsistent outcomes. Ultimately, six studies were included in the qualitative synthesis. The flowchat is showed in Figure S4.
The reviewed studies comprised six RCTs conducted from 2014 to 2022, with participant numbers ranging from 163 to 1,045.These multicenter trials were conducted at sites across the United States, Europe, Australia, New Zealand, and the Republic of Georgia. The trials assessed the efficacy and safety of varying doses of eptinezumab (10 mg, 30 mg, 100 mg, and 300 mg) against a placebo, with treatment durations spanning 12 to 72 weeks. Participants’ ages ranged from 35.7 to 44.9 years, and the proportion of female participants was consistently higher across all studies, with the eptinezumab groups including 403 males and 2,745 females in total. The studies were primarily Phase II, IIb, III, and IIIb trials, reflecting a progression from early-phase dose-finding studies to large-scale confirmatory trials. Participant characteristics are outlined in Table 5.
Risk bias analysis
The bias risk evaluation for the six studies indicated a generally low risk, with most areas demonstrating minimal bias. All studies exhibited a low risk of bias in both random sequence generation and allocation concealment, confirming strong randomization methods. All studies demonstrated low risk of performance bias due to effective blinding of participants and personnel. Blinding of outcome assessment consistently presented a low risk, thereby minimizing detection bias. Two studies, Ashina M (2020) and Dodick DW (2014), exhibited an unclear risk of bias regarding incomplete outcome data due to inadequate reporting on dropout rates and the management of missing data. Additionally, one study (Winner PK2021) had an unclear risk of bias in selective reporting, as some secondary outcomes mentioned in the protocol were not fully reported. Overall, the included studies were of high methodological quality, with only minor concerns in specific domains that were unlikely to significantly impact the reliability of the meta-analysis findings (Fig. 3). In our analysis of publication bias using Egger’s test for various AEs, we found the following: for AEs, the t-value was 1.44 (p = 0.223), indicating no significant bias; for SAEs, the t-value was 1.10 (p = 0.384), also suggesting no significant bias; and for TEAEs, the t-value was 0.72 (p = 0.526), indicating no evidence of publication bias. These results collectively confirm that there is no significant publication bias in the evaluated AEs, reinforcing the validity of our findings.
Safety outcomes and adverse events
AEs were reported in all six included studies. The eptinezumab group showed a total RR for AEs of 1.02 compared to the placebo group (95% CI 0.95–1.10, P = 0.54), indicating no significant differences, with low heterogeneity (I2 = 0%).TEAEs exhibited a comparable pattern, with a pooled RR of 1.02 (95% CI 0.95–1.10, P = 0.61), indicating no significant difference between groups (I2 = 0%).However, for SAEs , the pooled RR was 2.87 (95% CI 1.27–6.48, P = 0.01), indicating a significantly higher risk of SAEs in the eptinezumab group, though heterogeneity remained low (I2 = 0%) (Figs. 4, 5; Figure S5).
The forest plots of pooled risk ratio (RR) of safety outcomes of different categories: (A) Adverse events; (B) Treatment-related adverse events; (C) Serious adverse events. The black squares indicate the estimated RR for each randomized controlled trial (RCT), and the extending lines indicate the estimated 95% confidence interval (CI) of RR for each RCT. Weights are from a random-effects analysis. RR = Risk Ratio; CI = Confidence interval; RCT = Randomized controlled trial.
Subgroup analysis of various eptinezumab doses showed no significant difference in adverse events compared to the placebo across all dose levels. For the 1,000 mg dose (1 study), the RR was 1.08 (95% CI 0.82–1.43, P = 0.58), indicating no significant increase in AEs. The 300 mg dose, analyzed across four studies, demonstrated a relative risk (RR) of 1.05 (95% CI 0.97–1.15, P = 0.23) with no observed heterogeneity (I2 = 0%).Similarly, the 100 mg dose (5 studies) had an RR of 1.01 (95% CI 0.93–1.11, P = 0.75) and no heterogeneity (I2 = 0%).In two studies evaluating the 30 mg dose, the relative risk (RR) was 0.93 (95% CI 0.81–1.06, P = 0.26), indicating moderate heterogeneity (I2 = 35%).Finally, the 10 mg dose (1 study) showed an RR of 1.01 (95% CI 0.82–1.26, P = 0.91).The findings indicate that eptinezumab, at all tested doses, does not significantly elevate the risk of adverse events compared to placebo, with consistent results across most subgroups and minimal heterogeneity (Table 6).
The analysis of specific AEs associated with eptinezumab revealed varying results across different event types. The incidence of upper respiratory tract infections was notably higher in the eptinezumab group than in the placebo group, with a pooled relative risk of 1.49 (95% CI 1.02–2.18, P = 0.04) and no observed heterogeneity (I2 = 0%).Conversely, urinary tract infections showed no significant difference between groups (RR: 0.79, 95% CI 0.49–1.28, P = 0.34, I2 = 0%).Other AEs, such as dizziness (RR: 0.85, 95% CI 0.53–1.36, P = 0.50, I2 = 0%) and nausea/vomiting (RR: 0.92, 95% CI 0.61–1.38, P = 0.70, I2 = 0%), also demonstrated no significant differences. Fatigue showed a trend toward increased risk in the eptinezumab group (RR: 1.92, 95% CI 0.84–4.39, P = 0.12, I2 = 41%), though it was not statistically significant. Rare events, such as tooth abscess, dry mouth, and malaise, had high RRs but wide confidence intervals due to limited data, making their significance inconclusive. Overall, while most AEs showed no significant differences between eptinezumab and placebo, upper respiratory tract infections were notably more common in the eptinezumab group (Table 7).
Discussion
This study aimed to thoroughly analyze the safety of eptinezumab by examining AEs using FAERS data and conducting a systematic review and meta-analysis of clinical trials. This dual approach sought to improve comprehension of eptinezumab’s safety profile by detecting potential safety signals and contrasting adverse event incidence between the eptinezumab and placebo groups. The FAERS analysis revealed notable safety signals for eptinezumab, indicated by elevated ROR and PRR values for adverse events like ‘migraine recurrence’ and 'nervous system disorders,' with the latter exhibiting an ROR of 3.64 and a PRR of 3.06. These events were compared against the FDA-approved product level for eptinezumab, which outlines the expected adverse events based on clinical trial data. Adverse events showing significant increases in ROR and PRR values in comparison to the established safety profile in the monograph were categorized as potential safety signals, highlighting possibly unexpected effects associated with eptinezumab. These findings suggest potential central nervous system effects linked to eptinezumab, warranting further investigation. Similarly, the meta-analysis highlighted differences in adverse event incidence between eptinezumab and placebo groups. The overall risk ratio for adverse events was 1.02 (95% CI 0.95–1.10), indicating no significant difference. However, the pooled risk ratio for SAEs was significantly higher at 2.87 (95% CI 1.27–6.48), reflecting a statistically significant increased risk. This contrast between general and serious adverse events suggests varying clinical implications and highlights the need for thorough safety evaluation. Our results indicated that the increased risk of SAEs was consistent across different dosage levels. This consistency reinforces the premise that the heightened risks associated with eptinezumab are not dose-dependent, suggesting that all tested doses did not show a significant elevation in risk compared to placebo.
The FAERS analysis identified “drug ineffective” and “migraine” as the most commonly reported adverse events linked to eptinezumab, with “migraine” showing the highest signal strength. Other notable signals included “headache” and "therapeutic response shortened," which also demonstrated significant associations. However, it is important to interpret these findings cautiously. While the reports of ‘drug ineffective’ and ‘migraine’ are leading adverse events, they may reflect concerns about the drug’s efficacy rather than indicating toxicity. Distinguishing between efficacy complaints and safety signals is critical for guiding clinical decision-making and further research. Additionally, significant associations were observed with "increased intracranial pressure" and "upper respiratory infections." These findings suggest that the reported issues could indicate a broader immune response or central nervous system interactions influenced by CGRP modulation. The meta-analysis indicated no significant differences in overall adverse events or TEAEs between the eptinezumab and placebo groups. However, the eptinezumab group exhibited a notably higher risk of serious adverse events, particularly an increased incidence of upper respiratory tract infections.
Compared to prior studies, these results align with the established safety profile of eptinezumab, which has shown a low incidence of general adverse events in clinical trials9,10. However, it is essential to address the observed discrepancy between the meta-analysis and FAERS findings. While the meta-analysis indicated a significantly higher risk of serious adverse events (RR = 2.87), the FAERS results identified only moderate or low-priority signals with no high-priority events. This mismatch deserves further elaboration. The nature of SAEs reported in clinical trials may differ from those recorded in FAERS, with possible variations in patient demographics and reporting practices influencing these outcomes. However, the higher signal strength for ‘migraine’ and 'therapeutic response shortened’ in FAERS suggests that real-world data may capture patient-reported outcomes and dissatisfaction with treatment more effectively than controlled trials. Furthermore, the differences noted, such as the absence of high-priority events in FAERS, may indicate that the serious adverse events observed in trials, such as specific infections or neurological issues, are not being reported with the same frequency or severity in the FAERS database. This aspect highlights the necessity of recognizing the variability in reported SAEs and underscores the importance of understanding how trial conditions may not completely emulate real-world scenarios. This discrepancy underscores the importance of integrating real-world pharmacovigilance data with clinical trial findings to identify potential safety signals and better understand patient experiences8,15. These findings emphasize the need for continued monitoring of eptinezumab’s safety profile, particularly for rare but clinically significant events.
Both FAERS and the meta-analysis identified associations between eptinezumab and specific adverse events, such as “migraine” and "therapeutic response shortened," suggesting these may represent potential safety signals. However, discrepancies were observed. For instance, “drug ineffective” was frequently reported in FAERS but was not reflected in the meta-analysis, which showed no significant differences in overall AEs. This divergence may stem from reporting bias in FAERS, where subjective reports of dissatisfaction with treatment efficacy are more likely to be submitted15,25. Mechanistically, eptinezumab exerts its effects by targeting CGRP, a key mediator in migraine pathophysiology. Variability in individual responses to CGRP inhibition may explain reports of “drug ineffective” or "therapeutic response shortened." This variability arises from factors such as genetic differences, which influence patient responses to CGRP monoclonal antibody therapies. For example, genetic markers like those in the LRRC4C gene, identified in a Han Chinese population, underscore the role of genetic variability in treatment outcomes and highlight the potential of personalized approaches to enhance precision33. Individual variability in drug response extends beyond CGRP inhibitors to other therapeutic areas, such as chronic pain and diabetic kidney disease, where genetic makeup significantly impacts efficacy and safety. This has driven the adoption of personalized medicine to tailor treatments based on genetic profiles, improving outcomes and optimizing trial designs34,35. Additionally, the variability in drug response can also be influenced by compensatory mechanisms that the body activates in response to chronic drug administration. These mechanisms can lead to a partial or complete loss of drug effect over time, further complicating the management of chronic conditions. Understanding these mechanisms and developing strategies to overcome them is crucial for ensuring long-term, sustainable therapeutic benefits36. Furthermore, the strong signal for “migraine” in FAERS could reflect heightened patient sensitivity to migraine symptoms and a tendency to report these events more frequently8,16.
The analysis of FAERS data revealed gender and age-related differences in AE reporting for eptinezumab, with female patients reporting more AEs than males. While this observation may be partially attributable to the higher prevalence of migraine among women, other potential contributing factors include gender differences in healthcare-seeking behavior, reporting bias, and biological factors37,38,39,40,41,42.
Several studies highlight the complexity of interpreting these differences, emphasizing both biological and systemic contributors. Pharmacokinetic differences between sexes are significant; Zucker et al. demonstrated that sex-specific pharmacokinetics can predict AEs, suggesting that inherent biological differences in drug metabolism and distribution may predispose women to higher AEs rates43. This aligns with the broader understanding that sex-based physiological variations influence drug response and adverse outcomes. Additionally, reporting behaviors and healthcare system factors play a crucial role. Holm et al. found that age, sex, and the seriousness of AEs influence reporting patterns, implying that women may be more likely to report AEs, especially those perceived as serious44. However, D'Incau et al. observed no significant differences between men and women in ADR reports related to psychotropic drugs across multiple countries, indicating that the influence of sex on AEs reporting may vary depending on drug class and context45. Furthermore, Isbister and Strom discussed how reporting frequency and prescription patterns can skew perceived AEs rates, implying that higher reporting in women could partly reflect increased vigilance or differences in drug utilization46.These findings should be interpreted with consideration of the inherent limitations in spontaneous reporting systems. This gender disparity in migraine prevalence likely explains the higher AE reporting rate among women. However, the signal strength for “migraine” was stronger in males, possibly indicating a higher likelihood of reporting severe events in this group. This may indicate reporting bias, as men with migraines might be less inclined to seek treatment, leading to a higher likelihood of reporting severe or unresolved symptoms when they do seek help.
Older patients (65–85 years) also showed a stronger signal for "drug ineffective," suggesting reduced treatment efficacy or increased dissatisfaction with eptinezumab in this population47. While the diminished effectiveness of eptinezumab in older patients may be associated with age-related modifications in the CGRP pathway and changes in drug absorption, it is important to recognize that various physiological changes occurring with aging can significantly influence pharmacokinetics and pharmacodynamics. These factors can lead to variations in drug efficacy and safety profiles. However, the exact contributions of these changes and other potential influences on drug response cannot be fully elucidated from our database alone. For instance, the pharmacokinetics and pharmacodynamics of drugs can be altered due to age-related changes in body composition, organ function, and receptor sensitivity, which may affect the absorption, distribution, metabolism, and excretion of medications48. The CGRP pathway, integral to migraine pathophysiology, may alter with aging. This could potentially alter the effectiveness of CGRP-targeting therapies such as eptinezumab. Studies have shown that CGRP receptor blockers can effectively reduce the number of monthly migraine headache days, but their efficacy might vary across different patient populations, including older adults49. Furthermore, the efficacy of monoclonal antibodies against CGRP or its receptor, such as eptinezumab, has been evaluated in patients with prior preventive treatment failures, suggesting that individual patient factors, including age, could influence treatment outcomes50.Compared with FAERS, the meta-analysis found no significant differences in AEs or TEAEs based on gender or age. This discrepancy may be due to the controlled environment of clinical trials, which limits variability by excluding older or more complex patient groups, as opposed to the FAERS database, which reflects real-world use14.
The FAERS analysis identified certain rare but SAEs, such as increased intracranial pressure and facial paralysis, which were more commonly reported in older patients and showed higher signal strength. Similarly, the meta-analysis revealed a significantly increased overall risk of SAEs with eptinezumab, although specific event types were not detailed. These findings suggest a potential association between eptinezumab and an elevated risk of SAEs, particularly in vulnerable populations. The FAERS database provides specific details on event types, such as increased intracranial pressure, which aligns with the known physiological role of CGRP in maintaining intracranial pressure and neural function51. In contrast, the meta-analysis only reported overall SAE risk, likely due to the smaller sample size and controlled design of RCTs, which may limit the detection of rare events14. CGRP is essential for controlling intracranial pressure and ensuring neural homeostasis. It modulates neurogenic inflammation, influencing intracranial pressure dynamics and contributing to cerebral edema in acute CNS injuries like traumatic brain injury and stroke52. CGRP also plays a key role in migraine pathophysiology by promoting peripheral and central sensitization through the trigeminal nociceptive pathway, impacting both intracranial pressure and neural function53. CGRP affects central nervous system immune responses by modulating meningeal lymphatic vessels, essential for cerebrospinal fluid clearance and intracranial pressure regulation54. Its inhibition by eptinezumab could disrupt these processes, potentially leading to increased intracranial pressure or neurological dysfunction. This risk may be further exacerbated in older patients, whose blood–brain barrier function is often compromised, making them more susceptible to the effects of CGRP inhibition55. These findings highlight the necessity of combining real-world pharmacovigilance data with clinical trials to enhance understanding of SAE mechanisms and identify high-risk groups for focused monitoring and intervention. Additionally, it is crucial to clarify that serious adverse events, including fatalities, were evaluated separately in the trials included in the meta-analysis. In contrast, the FAERS analysis aggregated all reported adverse events without specifically categorizing outcomes related to death. This methodological difference may contribute to the discrepancy observed in the overall assessment of safety signals.
In a meta-analysis, even though the overall adverse event rate showed no significant difference, certain events, such as upper respiratory infections(URIs), occurred more frequently in the eptinezumab group. URIs might be associated with the immunosuppressive effects of calcitonin CGRP. CGRP has been shown to inhibit certain immune responses, including the proliferation of T cells and the activity of natural killer cells56,57. Additionally, CGRP’s role in modulating inflammation and immune responses suggests that it could influence the immune response during URI58,59. Therefore, CGRP’s immunosuppressive properties could potentially impact the immune system’s ability to respond to URI.
The concentration of early AEs observed in FAERS may also be influenced by real-world reporting behaviors, as patients are more likely to notice and report new symptoms shortly after starting a medication. The study highlights the critical need for close monitoring during the initial month of treatment to identify and address adverse reactions, ensuring patient safety and optimal treatment outcomes19. Conversely, the controlled design of RCTs in the meta-analysis may have obscured the temporal clustering of early events, as RCTs often focus on overall AE rates rather than their timing.
This study has certain limitations. First, the analysis of the FAERS database is inherently limited by underreporting, reporting bias, and incomplete patient information, which can obscure true incidence rates and hinder causal inference60. Furthermore, the significant number of cases with missing timing information for adverse events raises the concern that our findings may be biased, as the completeness of temporal data is essential for accurately assessing the occurrence of adverse events. In addition, the meta-analysis was based on a relatively small number of RCTs with strict inclusion criteria, which may not adequately represent the broader, more heterogeneous real-world population, thereby limiting the external validity of the findings. Discrepancies in adverse event reporting between the FAERS data and RCTs may also stem from differences in data collection methods and the controlled settings of clinical trials, which often exclude patients with multiple comorbidities or atypical presentations. Furthermore, the short follow-up periods in many RCTs might have precluded the detection of late-onset adverse events. Future research should aim to integrate prospective, real-world data sources, such as active surveillance registries, to overcome these limitations. Although we currently focus on analysis using SOCs, we recognize the significance of Standardized MedDRA Queries (SMQs) in drug safety signal detection. Therefore, in future research, we will consider incorporating SMQ-based signal detection as a complementary analytical approach to comprehensively evaluate adverse events associated with eptinezumab. Additionally, longer follow-up durations, improved standardization in adverse event reporting, and mechanistic studies investigating the underlying pathways of eptinezumab-related adverse events could enhance our understanding and management of these safety signals61.
Conclusion
Based on this study, the impact of eptinezumab on migraine management and its associated adverse events warrants further exploration. The research highlights demographic patterns in adverse event reporting, particularly across age groups and genders, consistent with prior findings on the predominance of women in migraine disorders. While a comprehensive understanding of eptinezumab-related adverse events has been achieved, further investigation is needed to clarify the underlying mechanisms. Future studies should integrate real-world data with clinical trial findings to better understand subgroup responses and optimize therapeutic strategies.
The trends in serious adverse events also call for greater attention to vulnerable populations, such as the elderly. Tailored treatment protocols that account for age-specific responses and monitoring strategies are essential. Additionally, the role of CGRP in mediating both efficacy and potential adverse outcomes deserves further study, particularly its involvement in neuroinflammation and migraine pathophysiology. These mechanisms may underlie early adverse events and highlight the need for careful patient monitoring during treatment.
In conclusion, this study emphasizes the need for ongoing research to address existing gaps and refine treatment approaches for migraine patients. Integrating insights from diverse data sources and focusing on vulnerable populations will be key to improving clinical outcomes and advancing the understanding of migraine management. Future investigations into CGRP’s physiological roles could contribute to more effective treatments while minimizing associated risks.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Vincent, M., Viktrup, L., Nicholson, R. A., Ossipov, M. H. & Vargas, B. B. The not so hidden impact of interictal burden in migraine: A narrative review. Front. Neurol. 13, 1032103 (2022).
Alpuente, A., Torres-Ferrus, M., Caronna, E. & Pozo-Rosich, P. The state of art on the use of patient reported outcomes in migraine. Curr. Opin. Neurol. 37, 271–282 (2024).
Lombard, L. et al. A global real-world assessment of the impact on health-related quality of life and work productivity of migraine in patients with insufficient versus good response to triptan medication. J. Headache Pain 21, 41 (2020).
Lipton, R. B. et al. Impact of monthly headache days on migraine-related quality of life: Results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study. Headache 63, 1448–1457 (2023).
Shapiro, R. E. et al. Migraine-related stigma and its relationship to disability, interictal burden, and quality of life: Results of the OVERCOME (US) study. Neurology 102, e208074 (2024).
Baker, B. et al. Population pharmacokinetic and exposure-response analysis of eptinezumab in the treatment of episodic and chronic migraine. Pharmacol. Res. Perspect 8, e00567 (2020).
Datta, A., Maryala, S. & John, R. A review of eptinezumab use in migraine. Cureus 13, e18032 (2021).
Scuteri, D., Corasaniti, M. T., Tonin, P. & Bagetta, G. Eptinezumab for the treatment of migraine. Drugs Today (Barcelona, Spain) 1998(55), 695–703 (2019).
Dodick, D. W. et al. Eptinezumab demonstrated efficacy in sustained prevention of episodic and chronic migraine beginning on day 1 after dosing. Headache 60, 2220–2231 (2020).
Kudrow, D. et al. Long-term safety and tolerability of eptinezumab in patients with chronic migraine: A 2-year, open-label, phase 3 trial. BMC Neurol. 21, 126 (2021).
Smith, T. R. et al. Safety and tolerability of eptinezumab in patients with migraine: A pooled analysis of 5 clinical trials. J. Headache Pain 22, 16 (2021).
Zhong, Y. et al. Efficacy and safety of eptinezumab for migraine: A systematic review and meta-analysis. J. Res. Med. Sci. 28, 82 (2023).
Pozo-Rosich, P. et al. Eptinezumab demonstrated efficacy regardless of prior preventive migraine treatment failure type: Post hoc analyses of the DELIVER study. Neurol. Ther. 13, 339–353 (2024).
Eichler, H. G. et al. Bridging the efficacy-effectiveness gap: A regulator’s perspective on addressing variability of drug response. Nat. Rev. Drug Discov. 10, 495–506 (2011).
Giezen, T. J., Mantel-Teeuwisse, A. K. & Leufkens, H. G. Pharmacovigilance of biopharmaceuticals: Challenges remain. Drug Saf. 32, 811–817 (2009).
Winner, P. K. et al. Effects of intravenous eptinezumab vs placebo on headache pain and most bothersome symptom when initiated during a migraine attack: A randomized clinical trial. JAMA 325, 2348–2356 (2021).
Cecco, S. et al. Emerging toxicities of antibody-drug conjugates for breast cancer: Clinical prioritization of adverse events from the FDA adverse event reporting system. Target. Oncol. 19, 435–445 (2024).
Lin, H. et al. Post-market safety profile of cefiderocol: A real-world pharmacovigilance exploratory analysis based on U.S. FDA adverse event reporting system (FAERS). BMC Pharmacol. Toxicol. 26, 58 (2025).
Zhao, K., Zhao, Y., Xiao, S. & Tu, C. Assessing the real-world safety of tralokinumab for atopic dermatitis: Insights from a comprehensive analysis of FAERS data. Front. Pharmacol. 15, 1458438 (2024).
Liu, Y., Chen, C., Rong, C., He, X. & Chen, L. Anaplastic lymphoma kinase tyrosine kinase inhibitor-associated cardiotoxicity: A recent five-year pharmacovigilance study. Front. Pharmacol. 13, 858279 (2022).
Yin, Y., Shu, Y., Zhu, J., Li, F. & Li, J. A real-world pharmacovigilance study of FDA Adverse Event Reporting System (FAERS) events for osimertinib. Sci. Rep. 12, 19555 (2022).
Zhang, Y. et al. Adverse event signal mining and severe adverse event influencing factor analysis of Lumateperone based on FAERS database. Front. Pharmacol. 15, 1472648 (2024).
Fusaroli, M. et al. The reporting of a disproportionality analysis for drug safety signal detection using individual case safety reports in pharmacovigilance (READUS-PV): Explanation and elaboration. Drug Saf. 47, 585–599 (2024).
Zhou, C. et al. Psychiatric disorders associated with immune checkpoint inhibitors: A pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database. EClinicalMedicine 59, 101967 (2023).
Rothman, K. J., Lanes, S. & Sacks, S. T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 13, 519–523 (2004).
Evans, S. J., Waller, P. C. & Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 10, 483–486 (2001).
Rivkees, S. A. & Szarfman, A. Dissimilar hepatotoxicity profiles of propylthiouracil and methimazole in children. J. Clin. Endocrinol. Metab. 95, 3260–3267 (2010).
Ang, P. S., Chen, Z., Chan, C. L. & Tai, B. C. Data mining spontaneous adverse drug event reports for safety signals in Singapore - A comparison of three different disproportionality measures. Expert Opin. Drug Saf. 15, 583–590 (2016).
Gatti, M., Antonazzo, I. C., Diemberger, I., De Ponti, F. & Raschi, E. Adverse events with sacubitril/valsartan in the real world: Emerging signals to target preventive strategies from the FDA adverse event reporting system. Eur. J. Prev. Cardiol. 28, 983–989 (2021).
Gastaldon, C. et al. Withdrawal syndrome following discontinuation of 28 antidepressants: Pharmacovigilance analysis of 31,688 reports from the who spontaneous reporting database. Drug Saf. 45, 1539–1549 (2022).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin. Res. ed.) 315, 629–634 (1997).
An, Y. C. et al. Genetic variants associated with response to anti-CGRP monoclonal antibody therapy in a chronic migraine Han Chinese population. J. Headache Pain 25, 149 (2024).
Idzerda, N., Pena, M. J. & Heerspink, H. Personalized medicine in diabetic kidney disease: A novel approach to improve trial design and patient outcomes. Curr. Opin. Nephrol. Hypertens 27, 426–432 (2018).
Schnermann, J. One physiology does not fit all: A path from data variability to “physiogenetics”. Am. J. Physiol. Renal Physiol. 309, F29-32 (2015).
Ilan, Y. Overcoming compensatory mechanisms toward chronic drug administration to ensure long-term, sustainable beneficial effects. Mol. Therapy Methods Clin. Dev. 18, 335–344 (2020).
Lee, K. M. et al. A gender hypothesis of sex disparities in adverse drug events. Soc. Sci. Med. 339, 116385 (2023).
Meslamani, A. Z. A. Adverse drug event reporting among women: Uncovering disparities in underserved communities. Expert Opin. Drug Saf. 23, 543–545 (2024).
Palleria, C. et al. Limitations and obstacles of the spontaneous adverse drugs reactions reporting: Two “challenging” case reports. J. Pharmacol. Pharmacotherapeutics 4, S66–S72 (2013).
Unger, J. M. et al. Sex differences in risk of severe adverse events in patients receiving immunotherapy, targeted therapy, or chemotherapy in cancer clinical trials. J. Clin. Oncol. 40(13), 1474–1486 (2022).
Lipton, R. B. et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 68, 343–349 (2007).
Vetvik, K. G. & MacGregor, E. A. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol. 16, 76–87 (2017).
Zucker, I. & Prendergast, B. J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 11, 32 (2020).
Holm, L., Ekman, E. & JorsäterBlomgren, K. Influence of age, sex and seriousness on reporting of adverse drug reactions in Sweden. Pharmacoepidemiol. Drug Saf. 26, 335–343 (2017).
D’Incau, P. et al. No differences between men and women in adverse drug reactions related to psychotropic drugs: A survey from France, Italy and Spain. Fundam. Clin. Pharmacol. 28, 342–348 (2014).
Strom, B. L. Comment: Comparison of FDA reports of patient deaths associated with sildenafil and injectable alprostadil. Ann. Pharmacother. 35, 1143 (2001).
Mangoni, A. A. & Jackson, S. H. Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br. J. Clin. Pharmacol. 57, 6–14 (2004).
Sera, L. C. & McPherson, M. L. Pharmacokinetics and pharmacodynamic changes associated with aging and implications for drug therapy. Clin. Geriatr. Med. 28, 273–286 (2012).
Masoud, A. T. et al. Efficacy of calcitonin gene-related peptide (CGRP) receptor blockers in reducing the number of monthly migraine headache days (MHDs): A network meta-analysis of randomized controlled trials. J. Neurol. Sci. 427, 117505 (2021).
Wang, X., Wen, D., He, Q., You, C. & Ma, L. Efficacy and safety of monoclonal antibody against calcitonin gene-related peptide or its receptor for migraine patients with prior preventive treatment failure: a network meta-analysis. J. Headache Pain 23, 105 (2022).
Edvinsson, L. The CGRP pathway in migraine as a viable target for therapies. Headache 58(Suppl 1), 33–47 (2018).
Sorby-Adams, A. J., Marcoionni, A. M., Dempsey, E. R., Woenig, J. A. & Turner, R. J. The role of neurogenic inflammation in blood-brain barrier disruption and development of cerebral oedema following acute central nervous system (CNS) injury. Int. J. Mol. Sci. 18, 1788 (2017).
Durham, P. L. Diverse physiological roles of calcitonin gene-related peptide in migraine pathology: Modulation of neuronal-glial-immune cells to promote peripheral and central sensitization. Curr. Pain Headache Rep. 20, 48 (2016).
Thomas, J. L., Schindler, E. A. & Gottschalk, C. Meningeal lymphatic vessel dysfunction driven by CGRP signaling causes migraine-like pain in mice. J. Clin. Invest. 134, e182556 (2024).
Propson, N. E., Roy, E. R., Litvinchuk, A., Köhl, J. & Zheng, H. Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging. J. Clin. Invest. 131, e140966 (2021).
Fox, F. E. et al. Calcitonin gene-related peptide inhibits proliferation and antigen presentation by human peripheral blood mononuclear cells: Effects on B7, interleukin 10, and interleukin 12. J. Invest. Dermatol. 108, 43–48 (1997).
Umeda, Y. Inhibition of immune responses by calcitonin gene-related peptide. Ann. N. Y. Acad. Sci. 657, 552–554 (1992).
Barbosa Bomfim, C. C. et al. CGRP inhibits SARS-CoV-2 infection of bronchial epithelial cells, and its pulmonary levels correlate with viral clearance in critical COVID-19 patients. J. Virol. 98, e0012824 (2024).
Li, Y. et al. Neuropeptide calcitonin gene-related peptide promotes immune homeostasis of bacterial meningitis by inducing major histocompatibility complex class II ubiquitination. J. Infect. Dis. 229, 855–865 (2024).
Hazell, L. & Shakir, S. A. Under-reporting of adverse drug reactions: A systematic review. Drug Saf. 29, 385–396 (2006).
Schneeweiss, S. & Avorn, J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J. Clin. Epidemiol. 58, 323–337 (2005).
Author information
Authors and Affiliations
Contributions
Junchen Chen was considered as first author; Yong Li was considered as corresponding author; Junchen Chen: Writing – original draft, Investigation.Shunqiu Huang and Yashi Chen: Formal analysis, Methodology. Cheng Luo: Methodology. Yong Li: Writing – review & editing, Conceptualization.All authors provided input and suggestions and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, J., Huang, S., Chen, Y. et al. Comprehensive safety analysis of adverse events associated with eptinezumab in migraine treatment. Sci Rep 15, 24491 (2025). https://doi.org/10.1038/s41598-025-09490-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09490-1