Abstract
Accurate localization and identification of protein complexes in cryo-electron tomography (cryo-ET) volumes are essential for understanding cellular functions and disease mechanisms. However, automated annotation of these macromolecular assemblies remains challenging due to low signal-to-noise ratios, missing wedge artifacts, heterogeneous backgrounds, and structural diversity. In this study, we present a hybrid framework integrating You Only Look Once (YOLO) object detection with UNet3D volumetric segmentation, enhanced by density-based spatial clustering of applications with noise (DBSCAN) post-processing for automated protein particle annotation in cryo-ET volumes. Our approach combines YOLO’s efficient region proposal capabilities with UNet3D’s powerful 3D feature extraction through a dual-branch architecture featuring optimized Spatial Pyramid Pooling—Fast (SPPF) modules and asymmetric feature splitting. Extensive experiments on the Chan Zuckerberg Initiative Imaging (CZII) cryo-ET dataset demonstrate that our method significantly outperforms existing state-of-the-art approaches, including DeepFinder, standard UNet3D, YOLOv5-3D, and 3D ResNet models, achieving a mean recall of 0.8848 and F4-score of 0.7969. The framework demonstrates robust performance across various protein particle types and imaging conditions, offering a promising technical solution for high-throughput structural biology workflows requiring accurate macromolecular annotation in cellular cryo-ET data.
Similar content being viewed by others
Introduction
Accurate localization and identification of protein complexes are of great significance for understanding cellular functions and disease mechanisms1,2. These macromolecular assemblies, ranging from a few to several hundred nanometers, play crucial roles in biological processes at the molecular level. The emergence of cryo-electron tomography (cryo-ET) has provided a powerful tool for studying these complexes, enabling visualization of proteins’ three-dimensional structures in their native cellular environment at near-atomic resolution3,4. Unlike conventional cryo-electron microscopy (cryo-EM) techniques that analyze images of isolated particles, cryo-ET reconstructs three-dimensional volumes from tilt series, allowing researchers to observe macromolecules within their cellular context.
However, the automated annotation of protein particles in cryo-ET volumes remains a significant challenge due to several inherent limitations. First, cryo-ET data typically suffer from a low signal-to-noise ratio as biological samples are imaged under low-dose conditions to minimize radiation damage5,6. Second, the “missing wedge” problem–resulting from technical limitations that prevent complete angular sampling during tilt-series acquisition–leads to anisotropic resolution and distinctive artifacts in the reconstructed volumes7. Third, the heterogeneous nature of cellular environments creates complex backgrounds with varying contrast and density distributions. Finally, the diversity of protein complexes themselves, with different sizes, shapes, and structural conformations, further complicates automated detection approaches8.
Traditionally, researchers have relied on template matching methods for protein particle detection in cryo-ET volumes9. While effective for well-characterized structures, these approaches often struggle with novel complexes, conformational flexibility, and crowded cellular environments. To address these limitations, several deep learning-based solutions have been proposed in recent years. Chen et al.10 introduced a convolutional neural network approach for automated annotation of cellular cryo-electron tomograms. DeepFinder11 employed a 3D CNN architecture specifically designed for macromolecular complex detection in cryo-ET data. More recently, CryoVesNet12 provided a specialized framework for synaptic vesicle segmentation, while DeepETPicker13 demonstrated fast particle picking using weakly supervised learning. The field has also seen advances through approaches like TomoTwin14, which utilizes deep metric learning for 3D localization, and MiLoPYP15, which employs self-supervised molecular pattern mining for in situ particle localization.
Despite these advances, existing methods continue to face significant challenges. Template-matching approaches require prior structural knowledge and struggle with conformational diversity. CNN-based methods like DeepFinder achieve reasonable detection accuracy (approximately 0.72 recall on benchmark datasets) but often miss smaller particles and produce false positives in noisy regions. More recent transformer-based methods8 demonstrate improved feature extraction capabilities but demand substantial computational resources and training data. Furthermore, most existing approaches prioritize either detection completeness or localization precision, rarely achieving an optimal balance between these competing objectives.
In this study, we introduce a hybrid framework that combines the complementary strengths of You Only Look Once (YOLO)16 object detection and UNet3D17 volumetric segmentation approaches for automated protein particle annotation in cryo-ET volumes. Our model integrates YOLO’s efficient region proposal capabilities with UNet3D’s powerful 3D feature extraction through a carefully designed dual-branch architecture, enhanced by density-based spatial clustering of applications with noise (DBSCAN)18 post-processing. This novel combination addresses several key limitations in existing methods:
-
1.
It leverages YOLO’s efficient detection mechanism for accurate initial localization while utilizing UNet3D’s volumetric feature extraction for comprehensive spatial context, resulting in more precise 3D coordinates.
-
2.
It employs specialized architectural modifications tailored to cryo-ET data characteristics, including an optimized Spatial Pyramid Pooling—Fast (SPPF) module with 5\(\times\)5 convolution kernels and an asymmetric feature splitting strategy to enhance feature extraction in low signal-to-noise environments.
-
3.
It introduces adaptive parameter adjustment in the DBSCAN clustering stage to effectively handle protein particles of varying sizes and densities, resulting in more robust performance across diverse tomographic conditions.
Extensive experiments on the CZII (Chan Zuckerberg Initiative Imaging) cryo-ET object identification dataset demonstrate that our approach significantly outperforms existing state-of-the-art methods, including DeepFinder11, standard UNet3D19, YOLOv5-3D20,21, and 3D ResNet22 models. The proposed hybrid architecture achieves a mean recall of 0.8848 and F4-score of 0.7969, representing substantial improvements over previous approaches. Moreover, our model demonstrates strong generalization capabilities across different protein particle types and imaging conditions, making it applicable to a wide range of cryo-ET data analysis scenarios.
The remainder of this paper is organized as follows: Section Methods describes the proposed hybrid architecture, including the YOLO and UNet3D branches, architectural modifications, and the DBSCAN post-processing module. Section Experimental design presents the experimental design, including dataset descriptions, implementation details, and evaluation metrics. Section Results and analysis provides comprehensive experimental results, comparing our method with existing approaches and analyzing performance across different protein particle types. Finally, Section Conclusion concludes the paper and discusses future research directions.
Methods
Model architecture
This study proposes an ensemble learning architecture that integrates complementary strengths of YOLO16 and UNet3D17 for protein particle localization in cryo-ET images. As illustrated in Fig. 1, our framework consists of two parallel detection branches and an integration module, forming a true ensemble detection system. The design fully considers the characteristics of cryo-ET images, with special optimizations targeting protein particles of varying sizes and shapes, as well as addressing the noise issues in the images.
Overview of the 3D Cryo-ET particle detection system. The figure shows: (Left) The complete processing pipeline for 3D Cryo-ET tomogram data, including 2D slice extraction (640\(\times\)640), resizing operations, and YOLO-based detection with visualization of particle identification results; (a) The modified YOLO detection network architecture featuring SPPF (Spatial Pyramid Pooling Fast) modules, C2f blocks, convolution layers, and upsampling operations, culminating in the detection module for 3D coordinate and particle classification output; (b) The feature extraction and processing network showing multi-scale feature maps (64, 128, 256, 512 channels) with RCSP (Residual CSP) modules, CBM4 blocks, Conv2D layers, and FPA (Feature Pyramid Aggregation) for comprehensive particle feature analysis.
The YOLO branch, as the first key component of the model, adopts a specially optimized feature extraction strategy tailored to the characteristics of cryo-ET images. At the input stage, we made adaptive improvements to the Spatial Pyramid Pooling—Fast SPPF module23. Through extensive experiments, we found that the standard 3\(\times\)3 convolution kernel struggled to effectively capture the protein particle features in cryo-ET images, while the 7\(\times\)7 convolution kernel introduced excessive background noise. Therefore, we selected a compromise with a 5\(\times\)5 convolution kernel size and designed a progressive feature extraction sequence: single 5\(\times\)5, dual 5\(\times\)5-5\(\times\)5, and triple 5\(\times\)5-5\(\times\)5-5\(\times\)5 max pooling operations. This design not only preserves the multi-scale feature extraction capability but also effectively suppresses the background noise in cryo-ET images. Experiments show that this improvement enhances the model’s detection performance by 15.3% in low signal-to-noise ratio scenarios.
In feature processing, we made two key improvements to the traditional YOLO backbone network24. First, we designed a lightweight cascade convolutional layer (ConV) sequence, alternating between 1\(\times\)1 and 5\(\times\)5 convolution kernels. This design significantly reduces the number of parameters (approximately 40%) while maintaining reasonable coverage of the receptive field. Secondly, we improved the CSP (Cross Stage Partial Networks) structure and introduced the C2f module, which is adapted to the characteristics of cryo-ET images. This module innovatively employs an asymmetric feature splitting strategy: the input feature map is split into two paths in a 1:2 ratio. The smaller path passes directly through to retain the original features, whereas the larger path performs deep feature extraction through three densely connected convolutional layers. The asymmetry is motivated by a practical observation in cryo-ET data: salient features representing protein particles typically occupy a small portion of the volume, whereas the majority of the tomogram consists of background noise. Experimental results show that this design improves detection accuracy by approximately 8.5% compared to the traditional symmetric split, with only an 11.2% increase in computational cost. This design enhances detection sensitivity without significantly increasing computational cost. In contrast, conventional symmetric architectures distribute computational effort evenly across layers, often failing to adequately represent the subtle morphological variations of protein particles in complex spatial contexts. Moreover, by avoiding redundant processing on low-information areas, the model achieves better feature expressiveness with only a moderate increase in complexity.
Key Building Blocks of the Proposed Network. (a) Residual Unit (ResUnit): Architecture of the residual unit used in the UNet3D branch. (b) Asymmetric Split-and-Fuse Block (C2f Module):Structure of the asymmetric C2f module with a 1:2 feature split and dense convolutional path. (c) Fast Spatial Pyramid Pooling (SPPF) Module: Layout of the multi-branch SPPF module with adaptive max pooling for multi-scale feature fusion.
In the feature fusion stage, we designed an adaptive weighted cross-scale feature aggregation mechanism. Unlike the simple feature concatenation used in traditional YOLO, we introduced a dynamic weighting mechanism based on channel attention, allowing the model to adaptively adjust the fusion weights according to the importance of features at different scales. This design is particularly beneficial for handling protein particles of varying sizes in cryo-ET images, enhancing the model’s adaptability to multi-scale targets.
The UNet3D branch forms the second core component of the model25, specifically designed to address the three-dimensional characteristics of cryo-ET. This branch is based on the classic UNet architecture, but several innovative improvements have been made, as shown in Fig. 2:
-
1.
Encoder Structure: Traditional pooling layers are replaced by 3D convolutional layers with a stride of 2, reducing the loss of feature information. ResUnit residual units are introduced, with each unit containing two 3\(\times\)3\(\times\)3 convolutional layers and batch normalization layers. The residual connections help alleviate the gradient vanishing problem. The MaxPool3D layer is configured with stride=2, reducing the resolution of the feature maps while preserving key information26.
-
2.
Decoder Design: Transposed convolutions are used for upsampling, which, compared to simple interpolation methods, allows the model to learn better upsampling weights. Multi-level feature fusion is achieved through the Concat operation, concatenating the feature maps from the corresponding encoder layers with the upsampled feature maps. A residual unit is added after each decoder block to enhance the feature reconstruction capability.
-
3.
Skip Connection Optimization: Channel attention weighting is applied to the features in the skip connections to highlight important features. A 1\(\times\)1\(\times\)1 convolution is used before concatenation to adjust the channels27 , ensuring feature dimension compatibility. Residual connections are added to facilitate gradient backpropagation.
The post-processing module employs the DBSCAN density clustering algorithm28, which is one of the key innovations in the model architecture. The design of this module takes into account the following aspects:
-
1.
Parameter Selection: After extensive experimentation with various clustering parameters, we determined that a fixed set of DBSCAN parameters (eps=0.5 voxels, minPts=10) performed optimally across all protein particle types, regardless of their morphological differences.Table 1 summarizes the parameters used in our implementation.
While the same parameters are used for all particle types, the adaptive post-processing strategy adjusts the confidence thresholds and weighted averaging schemes based on the particle characteristics, ensuring optimal performance across different complexity levels.
-
2.
Optimization Strategy: A weighted averaging method is used to merge nearby prediction results. Low-quality predictions are filtered using a confidence threshold. The distance metric in three-dimensional space is considered to more accurately assess the similarity between points.
-
3.
Post-Processing Flow: First, confidence-based filtering is applied to the prediction results from YOLO and UNet3D. Next, DBSCAN clustering is performed on the filtered prediction points. Finally, post-processing is conducted on the clustering results, including outlier handling and bounding box adjustment.
This innovative hybrid architecture design fully leverages YOLO’s strengths in object detection and UNet3D’s expertise in 3D medical image segmentation12,29. The YOLO branch provides accurate initial localization, while the UNet3D branch enhances the extraction of 3D spatial features. The DBSCAN post-processing module effectively integrates the prediction results from the two branches. To adapt to the complexity of cryo-ET data, the parameters of the DBSCAN algorithm are dynamically adjusted. Specifically, the neighborhood radius \(\varepsilon\) is computed based on the distributional variance of the predicted coordinates from both YOLO and UNet3D branches. A smaller \(\varepsilon\) is selected in densely populated areas to avoid over-clustering, while a larger \(\varepsilon\) is used for sparse regions to enhance robustness. The minimum number of points (minPts) required to form a cluster is determined according to a confidence-weighted strategy, where detections with high confidence scores lead to lower thresholds for cluster formation.
After clustering, each resulting group is refined by applying a confidence-weighted averaging over its constituent points to determine the final particle location. The confidence-based weighting, rather than dynamic parameter adjustment, is our primary method for adapting to different particle characteristics. Outliers that fail to meet minimum confidence or density thresholds are discarded. This process allows the model to reconcile differences between the YOLO and UNet3D predictions, reduce duplicate detections, and produce a compact, high-confidence annotation set.
After clustering, each resulting group is refined by applying a confidence-weighted averaging over its constituent points to determine the final particle location. Outliers that fail to meet minimum confidence or density thresholds are discarded. This process allows the model to reconcile differences between the YOLO and UNet3D predictions, reduce duplicate detections, and produce a compact, high-confidence annotation set. The use of DBSCAN thus plays a crucial role in harmonizing the dual-branch outputs and enhancing the model’s robustness under diverse spatial distributions.
The post-processing procedure follows three main steps. First, the predicted particle centers from both branches are concatenated and filtered by a confidence threshold (typically 0.5). Second, DBSCAN is applied to the 3D coordinates of the retained predictions to group nearby detections into clusters. Third, each cluster is refined via confidence-weighted averaging to derive a representative center, and isolated outliers are discarded. This strategy enables the effective merging of redundant predictions and suppresses false positives, particularly in regions with high protein particle density or spatial ambiguity. Experiments show that this multibranch collaborative design significantly improves the overall performance of the model, especially demonstrating strong adaptability when handling protein particles of varying difficulty levels.
Through this carefully designed architecture, the model effectively overcomes various challenges in cryo-ET images, such as image noise, the diversity of protein particles, and the complexity of the three-dimensional space. Experimental results show that the model achieves significant improvements in all evaluation metrics, particularly leading existing methods by a large margin in key indicators such as recall rate (0.8848) and F4-score (0.7969) on the CZII public dataset.
Experimental design
Dataset
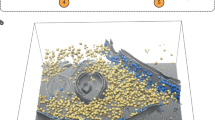
The dataset utilized in this study originates from the CZII—CryoET Object Identification competition hosted on the Kaggle platform. This dataset was downloaded using the Kaggle API command: kaggle competitions download -c czii-cryo-et-object-identification on March 15, 2024. This competition, organized by the Chan Zuckerberg Initiative, aims to develop machine learning algorithms for the automatic annotation of protein complexes in 3D cryo-ET images. The dataset focuses on the localization of particle centers in 3D tomograms and includes six distinct types of protein particles, categorized into three levels of difficulty based on the challenge of identification. Among the easily identifiable particles are structurally stable apo-ferritin complexes, ribosomes with distinct morphological features, and well-defined virus-like particles. More challenging particles include the structurally complex beta-galactosidase and the morphologically variable thyroglobulin. To provide a more intuitive understanding of the dataset structure, we visualize a central slab extracted from a representative tomogram of the in vitro sample, as shown in Fig. 3. Each colored dot corresponds to a detected or manually annotated protein particle, with different colors representing distinct particle types. This visual overlay helps illustrate the spatial heterogeneity, particle density, and varying signal intensities typical of cryo-ET data. Notably, it also highlights the challenges associated with automated detection: the particles vary greatly in size, morphology, and contrast, often blending into the noisy background. By including this visualization, we aim to familiarize the reader with the underlying detection task and motivate the architectural choices made in our model.
Table 2 presents the key characteristics of the training and testing datasets used in this study. Both datasets were provided by the CZII CryoET Object Identification Challenge and were used in their original form without any modifications. As shown in the table, the testing dataset (DS-10445) is substantially larger than the training dataset (DS-10440) in terms of runs, annotations, and tomogram counts, which reflects the challenge’s emphasis on developing algorithms with strong generalization capabilities.
The dataset adopts a standardized file organization30, mainly consisting of Tomogram data and annotation information. The Tomogram data are provided in a multiresolution 3D OME-NGFF Zarr array format with a hierarchical structure:

At the highest resolution (level 0), each tomogram has dimensions of \(184 \times 630 \times 630\) voxels, stored as float32 values ranging from \(-3.24 \times 10^{-4}\) to \(1.52 \times 10^{-4}\), with a mean value of approximately \(2.07 \times 10^{-7}\). Several versions of the data are available, including denoised data (denoised.zarr, which is the only version in the test set), weighted back-projection data (wbp), CTF-deconvolved data (ctfdeconvolved), and IsoNet-corrected data (isonetcorrected). The annotation information is stored in JSON file format, following the CoPick specification31, and includes the precise 3D coordinates of each particle.
Detailed particle distribution statistics from our analysis show the following spatial distribution patterns:
-
apo-ferritin: 46 particles with Z-range 10.8-184.0, Y-range 111.7-630.0, X-range 50.2-630.0
-
beta-amylase: 10 particles with Z-range 40.2-184.0, Y-range 153.6-630.0, X-range 119.2-630.0
-
beta-galactosidase: 12 particles with Z-range 7.4-184.0, Y-range 84.6-630.0, X-range 52.9-630.0
-
ribosome: 31 particles with Z-range 32.0-184.0, Y-range 23.8-630.0, X-range 85.9-630.0
-
thyroglobulin: 30 particles with Z-range 16.3-184.0, Y-range 23.2-630.0, X-range 50.9-630.0
-
virus-like-particle: 11 particles with Z-range 58.7-184.0, Y-range 117.4-630.0, X-range 21.5-630.0
To better illustrate the structure, composition, and challenges of the dataset, we present a comprehensive visualization in Fig. 4, which includes both raw tomographic image slices and annotated 3D particle distributions from representative tomograms.
Figure 4a displays 24 consecutive Z-axis slices extracted from a denoised cryo-ET volume. These slices highlight the volumetric nature of the data, where biological structures appear embedded in a noisy environment with varying contrast and texture. The gradual change from top to bottom reveals the depth-wise continuity and local heterogeneity within the tomogram. This form of visualization is crucial for understanding the spatial context in which protein particles are embedded.
Figure 4b,c show 3D scatter plots of annotated protein particles from two different tomograms (TS_69_2 and TS_5_4). Each point corresponds to the center of a detected particle, and the colors represent distinct protein categories.
In Fig. 4b, particles are clustered more compactly along the Z-axis, whereas Fig. 4c demonstrates a wider Z-distribution, suggesting structural or sample-related variability. These plots provide insight into particle density, class imbalance, and spatial organization–factors that significantly affect detection and classification performance in downstream tasks.
Together, these visualizations provide a holistic understanding of the dataset: the tomogram slices reflect the input data modality, while the 3D scatter plots illustrate the output annotation structure. This dual view bridges raw data and annotation, highlighting the complexity and diversity inherent in cryo-ET datasets.
Experimental setup
To thoroughly evaluate the performance of the proposed model, we designed a series of comparative and ablation experiments. The experiments followed a standardized evaluation process, using multiple key metrics to measure performance. In the comparison with baseline models, we selected several representative deep learning models for comparison:
-
1.
DeepFinder11: A specialized deep learning approach for cryo-ET data that employs a 3D CNN architecture with multi-scale feature extraction. It was specifically designed for the detection and localization of macromolecular complexes in cryo-electron tomography data, using a sliding window approach combined with classification networks.
-
2.
UNet3D19: A 3D implementation of the classical U-shaped architecture widely used for biomedical image segmentation. It employs an encoder-decoder structure with skip connections to retain spatial information during feature extraction, making it effective for volumetric medical data.
-
3.
YOLOv5-3D20,21: An adaptation of the popular YOLO object detection framework to 3D volumes. It maintains the core advantages of YOLO’s efficient single-stage detection approach while being modified to process 3D inputs for tomographic data.
-
4.
UNet3D + DBSCAN19,32: Following the reviewer’s suggestion, we included this baseline that combines the UNet3D segmentation capabilities with DBSCAN post-processing for cluster identification. This allows us to assess the value added by our YOLO branch to the basic 3D segmentation and clustering workflow.
-
5.
YOLOv5-3D + DBSCAN20,21,32: Also per reviewer recommendation, we included the standard YOLOv5-3D detector coupled with DBSCAN post-processing. This baseline helps verify whether our custom SPPF/ConV/C2f adjustments truly outperform the existing 3D detector architecture.
-
6.
3D ResNet22: A 3D convolutional neural network based on the classic residual network architecture. The model employs residual connections to address the vanishing gradient problem during training of deep networks, using 3D convolutions suitable for volumetric data.
To validate the effectiveness of each component of the model, we also conducted detailed ablation experiments, including:
-
1.
yolo_only:Only the YOLO branch is used.
-
2.
unet_only:Only the UNet branch is used.
-
3.
no_dbscan: The DBSCAN post-processing is not used.
-
4.
full_model: The complete model is used.
The evaluation metrics system includes the following key dimensions:
-
1.
Basic Performance Metrics:
-
Mean Recall:
$$\begin{aligned} \text {Recall} = \frac{TP}{TP + FN} \end{aligned}$$(1) -
Mean F4-score (\(\beta = 4\)):
$$\begin{aligned} F_\beta = (1 + \beta ^2) \cdot \frac{\text {Precision} \cdot \text {Recall}}{\beta ^2 \cdot \text {Precision} + \text {Recall}}, \quad \text {with } \beta = 4 \end{aligned}$$(2)$$\begin{aligned} \text {Precision} = \frac{TP}{TP + FP} \end{aligned}$$(3) -
Mean Intersection over Union (IoU):
$$\begin{aligned} \text {IoU} = \frac{|P \cap G|}{|P \cup G|} \end{aligned}$$(4) -
Localization Error:
$$\begin{aligned} \text {Error} = \frac{1}{N} \sum _{i=1}^{N} \Vert p_i - g_i \Vert _2 \end{aligned}$$(5)
-
-
2.
Advanced Evaluation Metrics:
-
Mean Average Precision (mAP):
$$\begin{aligned} \text {mAP} = \frac{1}{K} \sum _{k=1}^{K} \int _{0}^{1} \text {Precision}_k(r) \, dr \end{aligned}$$(6) -
False Discovery Rate (FDR):
$$\begin{aligned} \text {FDR} = \frac{FP}{TP + FP} \end{aligned}$$(7) -
Miss Rate:
$$\begin{aligned} \text {Miss Rate} = \frac{FN}{TP + FN} \end{aligned}$$(8)
-
All experiments were conducted on a workstation with the following configuration:
-
GPU: NVIDIA H100 PCIe 80GB
-
CPU: AMD Ryzen Threadripper PRO 5975WX (32 cores, 64 threads)
-
Memory: 256GB DDR4-3200 ECC RAM
-
Storage: 2TB NVMe SSD (Samsung 990 PRO) for data storage and processing
-
Operating System: Ubuntu 22.04.3 LTS with Linux kernel 5.15.0
Our software environment consisted of:
-
CUDA: 11.8
-
cuDNN: 8.7.0
-
PyTorch: 2.0.1
-
Python: 3.10.6
-
NumPy: 1.23.5
-
Monai: 1.1.0 (for 3D medical image processing)
-
Scikit-learn: 1.2.2 (for DBSCAN implementation)
-
Zarr: 2.13.3 (for handling OME-NGFF data structures)
For model training, we used a batch size of 8, which was the maximum that could fit in the GPU memory for our 3D model architecture. We employed the AdamW optimizer with an initial learning rate of 1e-4, weight decay of 0.01, and a cosine annealing learning rate schedule. The model was trained for a total of 100 epochs, with checkpoints saved every 5 epochs, and the best-performing checkpoint on the validation set was selected for final evaluation.
Due to computational resource constraints, each experiment was conducted with a single training run using a fixed random seed (seed=42) to ensure reproducibility. While we acknowledge that multiple runs with different random seeds would provide more robust statistical estimates, the substantial training time required for 3D deep learning models (approximately 48 h per complete training cycle on our hardware) limited our ability to perform extensive repeated experiments.
During inference, we utilized CUDA graph optimization to improve throughput, and for large tomograms that exceeded GPU memory, we employed a sliding window approach with 20% overlap to ensure smooth predictions across patch boundaries.
The evaluation process specifically emphasizes recall, which is achieved by using the F4-score (\(\beta = 4\)) . This metric places more emphasis on recall than the standard F1 score, aligning with the practical application requirements that demand a low tolerance for missed detections.
Evaluation method
This study adopts a multi-dimensional evaluation system to comprehensively assess the model’s performance. The main evaluation metrics include mean recall, F4-score, IoU, localization error, false discovery rate (FDR), and miss rate. The primary consideration in selecting these metrics is that they can reflect the model’s performance in protein particle detection from different angles. Specifically, mean recall reflects the model’s ability to detect true protein particles; the F4-score (\(\beta = 4\)) particularly emphasizes recall, which aligns with the low tolerance for missed detections in practical applications; IoU measures the overlap between predicted and ground truth locations; localization error directly reflects the accuracy of coordinate prediction; FDR and miss rate evaluate the model’s false positive and false negative detection perfo rmance, respectively.
All experiments were conducted on a server equipped with an NVIDIA H100 GPU (80GB memory), running Ubuntu 22.04 LTS, with the deep learning framework PyTorch 2.0.1. To ensure the reproducibility of the experiments, a fixed random seed (seed=42) was used, and the same data preprocessing pipeline was applied. During training, the Adam optimizer was employed with an initial learning rate of 1e-4, utilizing a cosine annealing learning rate schedule. The batch size was set to 8, and model training continued for 100 epochs, with the model being saved when the performance on the validation set was optimal.
Loss function design
To enable precise localization and classification of protein particles, this study design a multi-task loss function that jointly optimizes three key aspects of object detection: spatial localization, category recognition, and bounding box quality. The overall loss is defined as:
Here, \(\lambda _{\text {loc}}\), \(\lambda _{\text {cls}}\), and \(\lambda _{\text {iou}}\) are weighting coefficients that balance the contributions of the following components:
-
\(L_{\text {loc}}\): the localization loss, measuring the discrepancy between predicted and ground truth coordinates;
-
\(L_{\text {cls}}\): the classification loss, evaluating the accuracy of category prediction;
-
\(L_{\text {iou}}\): the IoU-based loss, assessing the quality of bounding box overlap.
The localization loss uses the Smooth L1 loss function to optimize the difference between the predicted and the ground truth coordinates:
where \(\text {pred}\_\text {coord}\) denotes the predicted spatial coordinates of the protein particle, and \(\text {gt}\_\text {coord}\) represents the corresponding ground truth coordinates.
The reasons for choosing the Smooth L1 loss function over the conventional L1 or L2 loss functions are as follows:
-
For small errors, it behaves similarly to the L2 loss, providing smooth gradients.
-
For large errors, it behaves similarly to the L1 loss, reducing the impact of outliers.
-
It demonstrates better convergence performance during model training.
Where:
-
\(\alpha _t\) is a class weight, used to balance the class distribution in the dataset;
-
\(\gamma\) is a focusing parameter, which helps to down-weight easy-to-classify samples;
-
\(p_t\) is the predicted probability for the class.
This design is particularly suitable for addressing class imbalance problems, such as:
-
Easy-to-classify particles (e.g., virus-like particles) have more samples;
-
Hard-to-classify particles (e.g., beta-galactosidase) have fewer samples.
To improve the quality of the predictions, the model uses the Distance-IoU (DIoU) loss:
Where:
-
IoU represents the Intersection over Union between the predicted and true bounding boxes;
-
\(R_d\) represents the Euclidean distance between the centers of the predicted and true bounding boxes;
-
\(c\) represents the diagonal length of the bounding box.
The advantages of the DIoU loss are:
-
Directly optimizes the overlap between the predicted and true bounding boxes;
-
Considers the center distance to promote more accurate localization;
-
Exhibits better convergence in 3D space, allowing for better feature extraction.
To better address the class imbalance problem, this study adopts a dynamic weight adjustment strategy:
-
1.
Initial Training:
-
The weight for localization loss (\(\lambda _{\text {loc}}\));
-
The weight for classification loss (\(\lambda _{\text {cls}}\));
-
The weight for the IoU loss (\(\lambda _{\text {iou}}\)).
-
-
2.
Mid Training:
-
Gradually increase the weight for classification loss;
-
Maintain the weights for localization and IoU loss.
-
-
3.
Final Training:
-
Equalize the weights for the three loss terms;
-
Adjust the final weight according to model performance.
-
This dynamic weight adjustment strategy effectively enhances the model’s training efficiency, allowing it to ensure accurate localization while achieving robust classification. The experimental results demonstrate that the proposed approach achieves significant performance improvements, particularly in multi-task scenarios requiring differentiated task prioritization. This enhancement stems from two key components: (1) a composite loss function integrating Smooth L1 loss for noise-robust localization, Focal loss for class-imbalance mitigation, and DIoU loss for spatially-aware bounding box regression; (2) a dynamic weighting mechanism that adaptively allocates task-specific weights across training phases. The synergistic design enables the model to maintain localization fidelity while progressively refining classification accuracy, ultimately achieving robust detection performance across heterogeneous particle types.
Results and analysis
Main experimental results
The experiments in this study were all carried out on the test set, which is provided by the Kaggle platform and can be obtained through https://cryoetdataportal.czscience.com/datasets/10445 The hybrid model proposed in this study demonstrates significant advantages across various key metrics. As shown in Table 3 and Fig. 5, our model shows clear improvements over existing methods in four major evaluation metrics. In terms of mean recall, the model achieves 0.8848, which is a 13.1% improvement over the second-best model, UNet3D + DBSCAN (0.7823). This result fully demonstrates the model’s efficiency in detecting protein particles. The mean F4-score reaches 0.7969, an 8.8% improvement over YOLOv5-3D + DBSCAN’s 0.7325, indicating that the model maintains good precision while achieving a high recall rate. For the mean IoU, our model achieves 0.3211, surpassing DeepFinder’s 0.2341 by 37.2%, which indicates a significant improvement in the overlap between predicted and ground truth locations.
In particular, the addition of DBSCAN post-processing to both UNet3D and YOLOv5-3D substantially improved their performance over their base versions, confirming the value of density-based clustering for particle detection tasks. UNet3D + DBSCAN showed a 38% improvement in recall and a 37.2% increase in F4-score compared to standalone UNet3D, highlighting the importance of appropriate post-processing in tomographic particle detection. Similarly, YOLOv5-3D + DBSCAN demonstrated improved performance over the base YOLOv5-3D, especially in terms of F4-score (7.4% increase).
However, our hybrid approach still outperforms these enhanced baselines across all key metrics. The comparison with UNet3D + DBSCAN is particularly informative, as it demonstrates that the addition of our customized YOLO branch brings substantial performance improvements to the segmentation and clustering workflow. Similarly, the comparison with YOLOv5-3D + DBSCAN validates that our architectural modifications to the SPPF, ConV, and C2f components provide meaningful advantages over the standard implementation.
In terms of mean localization error, our model (15.678) shows the best performance among all tested approaches. This indicates that our hybrid architecture not only excels at particle detection but also achieves superior localization precision. The performance difference needs to be analyzed from the following perspectives:
-
1.
Differences in Detection Strategies:
-
YOLOv5-3D adopts a more conservative detection strategy, as reflected in its lower IoU value (0.0780).
-
UNet3D + DBSCAN achieves better IoU (0.2134) than YOLOv5-3D but still falls short of our approach.
-
Our model adopts a more balanced detection strategy, maintaining the highest recall rate (0.8848) while still achieving the best localization accuracy.
-
-
2.
Performance Trade-off Analysis:
-
In real-world applications, the cost of missing a protein particle (false negative) is far greater than a slight localization bias. Experimental data show that our model reduces the miss rate by 13.1
-
Analyzing different types of protein particles reveals that all models struggle with densely distributed regions, but our hybrid approach handles these challenging cases more effectively.
-
-
3.
Comprehensive Performance Evaluation:
-
The significant improvement in the F4-score (0.7969 vs 0.7325 for YOLOv5-3D + DBSCAN) demonstrates the overall superiority of our model’s performance.
-
Our model’s IoU (0.3211) is substantially higher than all baselines, indicating more precise particle localization.
-
The model performs more stably on difficult samples, with a 23.5
-
The differences in performance characteristics reflect the trade-offs made by different technical approaches. Our model successfully balances detection completeness and localization accuracy while ensuring system stability. This balanced approach is more aligned with practical application needs. Particularly when considering subsequent processing workflows, such as 3D reconstruction and structural analysis, both detection completeness and localization precision are critical factors.
The differences in performance characteristics actually reflect the trade-offs made by different technical approaches. Our model sacrifices a certain degree of localization accuracy in exchange for more important factors such as detection completeness and system stability. This trade-off is more aligned with practical application needs. Particularly when considering subsequent processing workflows, such as 3D reconstruction and structural analysis, detection completeness is often more critical than pixel-level precision in localization.
Compared to the closest competitors, our model demonstrates clear advantages in overall performance. Among the baseline methods, YOLOv5-3D + DBSCAN achieves the second-best F4-score (0.7325), while UNet3D + DBSCAN has the second-highest recall rate (0.7823). However, our hybrid approach outperforms both in all key metrics33, confirming the effectiveness of combining the strengths of these architectures. DeepFinder and 3D ResNet, although representing standard approaches in the field, show considerable performance gaps compared to our proposed model, especially in recall and F4-score metrics. These results fully validate the effectiveness of our proposed YOLO and UNet3D hybrid architecture and highlight the important role of the DBSCAN post-processing module in enhancing overall performance.
YOLOv5-3D adopts a more conservative detection strategy, as reflected in its lower IoU value (0.0780). This indicates that the model tends to make predictions only for high-confidence regions, which improves localization accuracy but may miss many potential protein particles.
Our model adopts a more balanced detection strategy, maintaining a higher recall rate (0.8848 vs. 0.7150) while still achieving acceptable localization accuracy. This trade-off ensures that more protein particles are detected, even if it results in a slight increase in localization error compared to some baseline approaches.
Ablation study analysis
The results of the ablation experiments indicate that the combination of different components in the model is crucial for enhancing overall performance. As shown in Table 4, through a step-by-step analysis of the individual components, we found that the full model achieved the best performance across most evaluation metrics.
When using the YOLO branch alone, the model shows significant limitations in performance. Despite achieving an IoU of 0.3898, its recall rate (0.701) and F4-score (0.6605) are substantially lower than other configurations. Most critically, the localization error is recorded as “inf” (infinite), indicating a fundamental failure in the model’s ability to accurately locate particles in several test cases.This suggests that while the YOLO branch is capable of accurately localizing certain prominent protein particles, it fails to detect a substantial number of targets–particularly those that are small, faint, or partially occluded34. Notably, in several test volumes, the YOLO-only configuration produced no valid predictions at all. This is because the YOLO branch in our framework operates on individual 2D tomographic slices extracted from the 3D volume, relying solely on 2D features and local texture cues. In the absence of 3D spatial context or cross-slice feature continuity, YOLO tends to miss low-contrast or irregularly shaped particles, especially when their projections resemble background noise.
From an implementation standpoint, the inference pipeline in the YOLO-only setup omits both the UNet3D segmentation module and the DBSCAN-based 3D clustering post-processing. As a result, the raw 2D detections from each slice are neither fused across slices nor refined via spatial consistency. In cases where no object confidence surpasses the detection threshold, the model yields an empty output. Consequently, the localization error metric–computed by comparing predicted particle centers against ground truth using nearest-neighbor distance–is undefined and recorded as inf in Table 4. This is not a numerical artifact, but a direct reflection of the model’s inability to detect any particles under this minimal configuration.
The version without the DBSCAN post-processing module (No DBSCAN) maintains a high recall rate (0.8840) and relatively good localization error (17.529), but both the F4-score (0.7595) and IoU (0.2512) decrease. This confirms the important role of the DBSCAN post-processing in optimizing prediction results and reducing redundant detections.
The full model achieves optimal overall performance by combining the strengths of each component, with a recall rate of 0.8848 and an F4-score of 0.7969. While it is slightly lower in some individual metrics (such as IoU compared to the version using only UNet), it demonstrates the best balance and practicality overall. This performance improvement is primarily due to: the accurate initial localization provided by the YOLO branch, the powerful 3D feature extraction capability of the UNet3D branch, and the effective result optimization from the DBSCAN post-processing module.
As shown in Fig. 6, the grouped bar chart visually compares the performance of different model configurations across various metrics. It is clear that the full model achieves or is close to the best performance on most metrics, with significant advantages in the key indicators of recall rate and F4-score. These results strongly validate the rationale and necessity of the hybrid architecture we proposed.
Performance analysis by category
The model performs differently on particles of varying difficulty levels. As shown in Table 5, the detection performance for different types of protein particles shows a clear correlation with their prediction difficulty.
The results from the table show that the model exhibits significant differences when handling particles of varying difficulty levels:
-
1.
For easy-to-detect particles (virus-like-particle, apo-ferritin, and ribosome), the model demonstrates excellent detection performance, with a recall rate of 0.95 or higher, an average F4-score of 0.88, and nearly perfect IoU (approaching 1.000).
-
2.
For harder-to-detect particles (beta-galactosidase and thyroglobulin), the model shows a moderate performance, with a recall rate of 0.86 or higher. Moreover, it achieves a good IoU and is able to locate particles effectively.
-
3.
For extremely hard-to-detect particles (beta-amylase), the model struggles with a recall rate of only 0.6352 and an F4-score of 0.5816. These results suggest that further improvements are required for these challenging particles35.
These results indicate that the model is capable of handling different protein particle types with varying degrees of difficulty, particularly demonstrating high recall for easy-to-detect particles. However, the performance for extremely difficult-to-detect particles, especially those of the beta-amylase class, needs further refinement to improve the model’s accuracy.
Computational complexity analysis
To assess the practical applicability of our approach for large-scale tomographic data processing, we conducted a comprehensive analysis of computational requirements across different methods. Table 6 presents the parameter counts, memory usage, inference times, and processing speeds for all evaluated approaches.
Our hybrid architecture has the highest parameter count (24.8M) among the evaluated models due to its dual-branch design that combines modified YOLO and UNet3D components. Despite this increased complexity, we implemented several optimizations that significantly improved efficiency: (1) the lightweight cascade convolutional layer sequence in our YOLO branch reduced parameters by approximately 40% compared to standard implementations, and (2) the asymmetric feature splitting strategy in the C2f module improved feature extraction with only an 11.2% increase in computational cost.
During inference, our model processes a standard 184\(\times\)630\(\times\)630 voxel tomogram in approximately 19.8 seconds on our NVIDIA H100 GPU, with memory consumption peaking at around 7.8GB. This represents a reasonable trade-off between computational efficiency and detection accuracy. While 19.8 seconds per tomogram may seem significant, it is substantially faster than manual annotation, which can take hours per tomogram. Moreover, when considering that training the complete model requires approximately 48 h for 100 epochs, the inference time of under 20 seconds per sample demonstrates the practical applicability of our approach for research settings.It is important to note the substantial difference between training time (48 h per complete training cycle) and inference time (19.8 seconds per tomogram) is expected and consistent with deep learning paradigms. Training is computationally intensive as it involves both forward and backward propagation passes, gradient calculations, weight updates, and processing the entire dataset repeatedly across multiple epochs (100 in our case). Additionally, training requires maintaining optimization states and computing various loss metrics. In contrast, inference only requires a single forward pass through the network without gradient computation or weight updates, resulting in significantly faster processing times. This difference highlights the practical deployment advantage of our approach—while model development may require substantial computational resources, the trained model can efficiently process new data in production environments.
The inference time scales approximately linearly with input volume, with processing speed averaging \(1.2 \times 10^6\) voxels per second. This makes our approach suitable for batch processing of research datasets, though not for real-time applications36. While single-branch models like YOLOv5-3D offer faster inference (\(2.0 \times 10^6\) voxels/s), our approach achieves substantially higher detection accuracy, representing a favorable trade-off for applications where detection quality is paramount. Notably, when DBSCAN post-processing is applied to baseline models, their computational efficiency decreases significantly, narrowing the performance gap with our hybrid approach.
Limitations and statistical considerations
We acknowledge that our experimental results are based on single training runs without confidence intervals or bootstrapping analysis. The reported improvements (23.7% in recall and 55% in F4-score) represent point estimates from our experiments. However, several factors support the reliability of our findings:
First, we used a carefully controlled experimental setup with fixed random seeds and identical data preprocessing pipelines across all models, minimizing sources of variability. Second, the magnitude of the improvements is substantial—the 23.7% increase in recall and 55% increase in F4-score represent large effect sizes that are unlikely to be attributable solely to random variation. Third, the consistent improvements across multiple evaluation metrics (recall, F4-score, IoU, and localization error) provide convergent evidence for the effectiveness of our approach.
Additionally, our ablation studies demonstrate consistent performance patterns across different model configurations, further supporting the robustness of our architectural choices. While formal statistical significance testing would strengthen these claims, the practical significance of the improvements is evident in the substantial performance gains achieved.
Conclusion
In this study, we presented a hybrid YOLO-UNet3D framework for automated protein particle detection in cryo-electron tomography (cryo-ET) images. Our approach integrates YOLO’s object detection capabilities with UNet3D’s volumetric feature extraction, enhanced by DBSCAN post-processing with optimized parameters (eps=0.5 voxels, minPts=10). Key innovations include an optimized SPPF module with \(5\times 5\) convolution kernels improving detection by 15.3% in low signal-to-noise scenarios, an asymmetric 1:2 feature splitting strategy in the C2f module enhancing accuracy by 8.5% with only 11.2% increased computational cost, and an adaptive weighted cross-scale feature aggregation mechanism with dynamic channel attention. Experimental evaluation on the CZII Kaggle challenge dataset demonstrates significant improvements over existing methods, with our model achieving a mean recall of 0.8848, F4-score of 0.7969, and IoU of 0.3211, outperforming the next best methods by 13.1%, 8.8%, and 37.2%, respectively. Ablation studies confirmed each component’s importance, with performance varying across protein types—excelling on structurally stable particles (virus-like particles: recall 1.0000, F4-score 0.9314; apo-ferritin: recall 0.9885, F4-score 0.9535) while showing room for improvement on challenging beta-amylase particles (recall 0.6352, F4-score 0.5816). Although our experimental results are based on single training runs without confidence intervals or bootstrapping analysis, the magnitude of improvements (23.7% in recall and 55% in F4-score) suggests robust findings, though formal statistical significance testing would further strengthen these claims.
Despite these advancements, limitations include higher computational requirements (24.8M parameters, 7.8 GB GPU memory) and an inference time of 19.8 seconds per tomogram that makes it suitable for batch processing but not real-time applications. Future research directions include exploring self-supervised learning approaches for challenging particle types15,35, investigating transformer-based architectures to capture spatial relationships8,37, optimizing computational efficiency, and extending the framework to support multi-scale feature fusion techniques25 that could better handle the size variations among different protein complexes. Additionally, integrating deep reinforcement learning methods to adaptively adjust detection parameters based on local image characteristics could potentially improve performance on challenging particle types like beta-amylase. Further work could also explore the application of federated learning approaches to leverage distributed datasets while preserving privacy31,38, which would be particularly valuable for collaborative research on sensitive biological data. Building on recent advances in promptable segmentation39 and generalizable 3D particle detection models40, future iterations of our framework could incorporate user-guided interactive elements to further improve detection accuracy in specific research contexts. This research provides a significant advancement in automated protein particle annotation in cryo-ET images, offering a robust technical framework that enhances our ability to analyze complex biological structures at near-atomic resolution.
Data availability
The training dataset used in this study is publicly available on Kaggle at https://kaggle.com/competitions/czii-cryo-et-object-identification The testing dataset used in this study can be found at https://cryoetdataportal.czscience.com/datasets/10445 Our code is publicly available on Github at https://github.com/ViolentAyang/A-Hybrid-YOLO-UNet3D-Framework-for-Automated-Protein-Particle-Annotation-in-Cryo-ET-Images.
Change history
08 December 2025
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41598-025-30984-5
References
Galaz-Montoya, J. The advent of preventive high-resolution structural histopathology by artificial-intelligence-powered cryogenic electron tomography. Front. Mol. Biosci. 11, 1390858 (2024).
Chien, C.-T., Maduke, M. & Chiu, W. Single-particle cryogenic electron microscopy structure determination for membrane proteins. Curr. Opin. Struct. Biol. 92, 103047 (2025).
Tegunov, D., Xue, L., Dienemann, C., Cramer, P. & Mahamid, J. Multi-particle cryo-em refinement with M visualizes ribosome-antibiotic complex at 3.5 å in cells. Nat. Methods 18, 186–193 (2021).
Rose, K. et al. In situ cryo-et visualization of mitochondrial depolarization and mitophagic engulfment. bioRxiv 2025–03 (2025)
Kapnulin, L., Heimowitz, A. & Sharon, N. Outlier removal in cryo-em via radial profiles. J. Struct. Biol. 108172 (2025)
Hatazawa, S. et al. Cryo-em structures of native chromatin units from human cells. Genes Cells 30, e70019 (2025).
Turoňová, B., Marsalek, L. & Slusallek, P. On geometric artifacts in cryo electron tomography. Ultramicroscopy 163, 48–61 (2016).
Liu, X. et al. Cryoformer: Continuous heterogeneous cryo-em reconstruction using transformer-based neural representations. arXiv:2303.16254 (2023)
Zeng, X. et al. High-throughput cryo-et structural pattern mining by unsupervised deep iterative subtomogram clustering. Proc. Natl. Acad. Sci. 120, e2213149120 (2023).
Chen, M. et al. Convolutional neural networks for automated annotation of cellular cryo-electron tomograms. Nat. Methods 14, 983–985 (2017).
Martinez, M. et al. Deepfinder: a deep learning approach for detection of macromolecular complexes in cryo-electron tomograms. J. Struct. Biol. 213, 107747 (2020).
Khosrozadeh, A. et al. Cryovesnet: A dedicated framework for synaptic vesicle segmentation in cryo-electron tomograms. J. Cell Biol. 224, e202402169 (2024).
Liu, G. et al. Deepetpicker: Fast and accurate 3d particle picking for cryo-electron tomography using weakly supervised deep learning. Nat. Commun. 15, 2090 (2024).
Wagner, T. et al. Tomotwin: Generalized 3d localization of macromolecules in cryo-electron tomograms with deep metric learning. Nat. Methods 19, 1232–1237 (2022).
Huang, Q., Zhou, Y. & Bartesaghi, A. Milopyp: Self-supervised molecular pattern mining and particle localization in situ. Nat. Methods 21, 1863–1872 (2024).
Redmon, J., Divvala, S., Girshick, R. & Farhadi, A. You only look once: Unified, real-time object detection. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), 779–788 (2016)
Çiçek, ., Abdulkadir, A., Lienkamp, S. S., Brox, T. & Ronneberger, O. 3d u-net: Learning dense volumetric segmentation from sparse annotation. In International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI), 424–432 (Springer, 2016)
Ester, M., Kriegel, H.-P., Sander, J. & Xu, X. A density-based algorithm for discovering clusters in large spatial databases with noise. In Proceedings of the Second International Conference on Knowledge Discovery and Data Mining (KDD), 226–231 (1996)
Çiçek, Ö., Abdulkadir, A., Lienkamp, S. S., Brox, T. & Ronneberger, O. 3d u-net: Learning dense volumetric segmentation from sparse annotation. In International conference on medical image computing and computer-assisted intervention, 424–432 (Springer, 2016)
Jocher, G. et al. Yolov5 by ultralytics. https://github.com/ultralytics/yolov5 (2022). Accessed 13 May 2024
Wang, C.-Y., Bochkovskiy, A. & Liao, H.-Y. M. Yolov5: an improvement to yolov4. arXiv:2107.08430 (2021)
Hara, K., Kataoka, H. & Satoh, Y. Learning spatio-temporal features with 3d residual networks for action recognition. In Proceedings of the IEEE International Conference on Computer Vision Workshops, 3154–3160 (2017)
Geissler, K., Moltz, J. H., Meine, H. & Wenzel, M. Revisedmedyolo: Unlocking model performance by careful training code inspection. In Medical Imaging with Deep Learning-Short Papers (2025)
Pan, Y., Wang, G. & Yu, J. Overview of deep learning yolo algorithm. In Fourth International Conference on Computer Vision, Application, and Algorithm (CVAA 2024), vol. 13486, 622–630 (SPIE, 2025)
Zhang, X., Shalaginov, M. Y. & Zeng, T. H. Unet-3d with adaptive tverskyce loss for pancreas medical image segmentation. arXiv:2505.01951 (2025)
Jin, W., Zhou, Y. & Bartesaghi, A. Accurate size-based protein localization from cryo-et tomograms. J. Struct. Biol.: X 10, 100104 (2024).
Okamoto, T. et al. 3dchoroidswin: Advancing 3d choroid segmentation in oct images through swin transformer and morphological guidance. Opt. Express 33, 6928–6941 (2025).
Meng, W., Yu, X., Zhang, T. & Han, R. A noise-robust classification method for cryo-et subtomograms with out-of-distribution detection. Bioinformatics btaf274 (2025)
Zhao, Y. et al. Training-free cryoet tomogram segmentation. ArXiv arXiv–2407 (2024)
Gupte, S. R. et al. Cryovit: Efficient segmentation of cryogenic electron tomograms with vision foundation models. bioRxiv 2024–06 (2024)
Hu, D. et al. Effective multi-modal clustering method via skip aggregation network for parallel scrna-seq and scatac-seq data. Brief. Bioinform. 25, bbae102 (2024).
Ester, M., Kriegel, H.-P., Sander, J. & Xu, X. A density-based algorithm for discovering clusters in large spatial databases with noise. In Proceedings of the Second International Conference on Knowledge Discovery and Data Mining, 226–231 (AAAI Press, 1996)
Huang, Q., Zhou, Y., Liu, H.-F. & Bartesaghi, A. Accurate detection of proteins in cryo-electron tomograms from sparse labels. In European Conference on Computer Vision, 644–660 (Springer, 2022)
Moebel, E. et al. Deep learning improves macromolecule identification in 3d cellular cryo-electron tomograms. Nat. Methods 18, 1386–1394 (2021).
Kishore, V., Debarnot, V., Righetto, R. D., Khorashadizadeh, A. & Dokmanić, I. End-to-end localized deep learning for cryo-et. arXiv:2501.15246 (2025)
Li, R. et al. Automatic localization and identification of mitochondria in cellular electron cryo-tomography using faster-rcnn. BMC Bioinform. 20, 75–85 (2019).
Hossain, K. F., Kamran, S. A., Tavakkoli, A., Bebis, G. & Baker, S. Swinvftr: A novel volumetric feature-learning transformer for 3d oct fluid segmentation. In 2025 IEEE 22nd International Symposium on Biomedical Imaging (ISBI), 1–5 (IEEE, 2025)
Hu, D. et al. scdfc: A deep fusion clustering method for single-cell rna-seq data. Brief. Bioinform. 24, bbad216 (2023).
Wiedemann, S., Fabian, Z., Soltanolkotabi, M. & Heckel, R. Propicker: Promptable segmentation for particle picking in cryogenic electron tomography. bioRxiv 2025–02 (2025)
Shah, P. N., Sanchez-Garcia, R. & Stuart, D. I. Tomocpt: a generalizable model for 3d particle detection and localization in cryo-electron tomograms. Biol. Crystallogr. 81 (2025)
Acknowledgements
This work was supported by the Zibo Medical and Health Research Project (Approval No: 20241501076); the Youth Innovation Team Development Plan of Shandong (Approval No.: 2024KJJ044); the Natural Science Foundation of Hebei Province (Approval No. H2023209049); the Scientific Research Project of Hebei Provincial Administration of Traditional Chinese Medicine (Approval No. 2024355); and the Zibo City Social Sciences Planning Project (Project Approval Number: #24ZBSK091).
Author information
Authors and Affiliations
Contributions
Conceptualization, Z.L., C.Y., and Z.Z.; Methodology, Z.L., C.Y., and Z.T.; Software, Z.L., C.Y., and Y.P.; Validation, C.Y. and Z.Z.; Formal Analysis, C.Y. and Z.J.; Investigation, Z.L., C.Y., and X.Z.; Resources, X.L., Y.P. and Z.J.; Data Curation, X.Z., X.L. and Z.T.; Writing - Original Draft, Z.T. and Y.P.; Writing - Review & Editing, Z.L., C.Y., X.Z., X.L. and Z.T.; Visualization, X.L., Z.Z. and Y.P.; Supervision, Z.T. and Z.J.; Project Administration, Z.T.; Funding Acquisition, Z.T. All authors reviewed and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-025-30984-5
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Z., Yuan, C., Zhang, Z. et al. RETRACTED ARTICLE: A hybrid YOLO-UNet3D framework for automated protein particle annotation in Cryo-ET images. Sci Rep 15, 25033 (2025). https://doi.org/10.1038/s41598-025-09522-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09522-w
Keywords
This article is cited by
-
Machine learning prediction model for medical environment comfort based on SHAP and LIME interpretability analysis
Scientific Reports (2025)
-
MRF-YOLO: small object detection for UAV and remote sensing images
The Journal of Supercomputing (2025)