Abstract
Improving the quality and developmental competence of in vitro-produced (IVP) embryos is crucial for assisted reproductive technologies. Recently, we demonstrated that TLR2, an innate immune receptor, is expressed in bovine sperm and their activation improves fertilization and subsequent embryo development. However, its role in early embryo development is poorly understood. This study investigated the impact of activation of early embryonic TLR2 on IVP bovine embryo development. The immunofluorescence and western blot analyses confirmed robust TLR2 expression in early embryonic stages. Embryos were treated at 36 h post-insemination (hpi), with 100 ng/ml of TLR2 agonist and cultured in vitro and showed increased blastocyst rates and faster growth speed. Additionally, TLR2 activation slightly increased basal calcium levels and induced autophagy while suppressing cathepsin B activity and DNA damage, leading to reduced apoptosis in embryos. Together, these findings indicate that embryo TLR2 is involved in embryo development competence via a slight increase in the basal cytosolic calcium and subsequent embryo metabolic activities, autophagy induction, and reduction of apoptosis levels. These results provide a promising approach for producing highly competent good-quality embryos for improving the efficiency of IVP bovine embryos.
Similar content being viewed by others
Introduction
Assisted reproductive technology (ART), such as in vitro maturation (IVM) and in vitro fertilization (IVF), offers great potential for improving the reproductive efficiency of domestic animals1. Many studies have been performed to improve the developmental competence of in vitro-produced (IVP) embryos. However, the overall efficiency and viability of IVP embryos remain lower than those of in vivo embryos2,3.
Toll-like receptors (TLRs) family are the main family of pathogen recognition receptors (PRRs), that efficiently recognize virtually all pathogens, and mount an early immune response, resulting in the expression of inflammatory mediators4,5. TLRs are widely expressed throughout the female reproductive tract in mammals6. Among members of the TLRs family, TLR2 and TLR4 are expressed in cumulus cells of cumulus-oocyte complexes (COCs) and play immuno-protective functions critical for cell survival during ovulation and fertilization in mice7,8. Most recently, a study has revealed that human preimplantation embryos express TLRs 9, 5, 2, 6, and 7 during their development from 4-cell embryos to blastocyst9. However, their definite role in embryo development is unidentified.
Calcium (Ca2+) is widely recognized as a versatile signaling messenger that regulates various physiological processes including oocyte activation and early embryonic development in mammals10,11,12. Thus, cytoplasmic Ca2+ is a major signaling nexus during the development of the fertilized ovum and subsequent blastocyst formation13. Induction of a single Ca2+ transient is sufficient to accelerate development at the mouse morula or blastocyst stages14,15. Our most recent investigations in cattle demonstrated that the activation of sperm TLR2 increases Ca2+ influx into sperm, thereby inducing hyperactivation to enhance their penetration to mucus and uterine glands16 as well as improving their fertilization capability and subsequent embryo development17.
In parallel, intracellular Ca2+ plays a crucial role in the induction of autophagy18,19. Autophagy is a fundamental cellular homeostatic process that involves the intracellular degradation of unnecessary cytoplasmic components such as damaged organelles, protein aggregates, and intracellular pathogens via the formation of the autophagosome20,21. Autophagy is essential for preimplantation embryo development in mammals22. It has been shown that autophagy is required for the active elimination of unnecessary proteins and organelles that accumulate within oocytes and early embryos in mice23. The autophagy-deficient oocytes fail to develop beyond the 4 to 8-cell stage after fertilization. Furthermore, the absence of autophagy impairs the embryonal capacity to recycle amino acids for protein neo-synthesis in mice24. Emerging evidence suggests that the ligation of different subsets of TLRs into their ligands activates autophagy25,26,27. In particular, the activation of TLR2 induces autophagy via a MYD88-dependent pathway for capturing cytoplasm-invading microbes28.
On the other hand, strict regulation of the immune response after fertilization is essential for embryo survival and implantation. Thus, early embryos must evade attacks from the maternal immune system to ensure survival within the mother’s body29. However relatively little is known about how early embryos react and respond to different environmental signals. Therefore, we hypothesize that preimplantation embryos express the TLR2 system as a functional element of innate immunity, which accomplishes a physiological role in their developmental process possibly via regulating a basal level of intracellular Ca2+ signaling and the induction of autophagy. To test this hypothesis, initially, we investigated the expression of TLR2 in preimplantation embryos and the impact of their activation on the developmental competence of the IVP embryos. Our initial observations showed that early bovine embryos possess a functional TLR2 system and their activation, via TLR2 agonist, enhanced the developmental competence and blastocyst rate of early embryos. To understand the underlying mechanisms, TLR2-activated embryos were analyzed for cytosolic Ca2+ level, autophagy activity, cathepsin B levels, DNA damage, and apoptosis. To the best of our knowledge, this is the first study to establish the expression of TLR2 in early bovine embryos and their involvement in regulating embryo development and survival involving cytosolic Ca2+ and autophagy.
Results
TLR2 is expressed in early bovine embryos
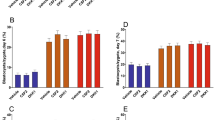
Immunofluorescence (IF) and Western blot (WB) analysis were used to analyze the expression of TLR2 on a developmental series of immature oocytes (referred to hereafter as oocytes), matured oocytes (MII), 4 cells, 8 cells, morula, and Day 7 blastocyst stage embryos. As shown in (Fig. 1a,b), IF and WB analysis revealed that TLR2 protein was abundantly expressed in oocytes, mature oocytes (MII), and different stages of early embryos (4 cells, 8 cells, morula, and blastocyst).
Immunofluorescence (IF) localization and western blot (WB) of TLR2 in bovine in vitro oocytes and early embryos. Bovine embryos were collected on a developmental series of oocytes, matured oocytes (MII), 4-cells, 8-cells, morula, and Day 7 blastocyst (D7 Blast) stage embryos. (a) Representative IF images of the TLR2 expression and localization. The TLR2 protein expression was visualized in red, while DAPI, a nuclear counterstain, was labeled in blue. The overlay depicts the merge of TLR2, DAPI and phase contrast images. The Rabbit IgG isotype was applied as the IgG negative control. MP: metaphase plate, PB: polar body. Scale bar = 100 μm. (b) Western blot analysis was carried out to estimate the expression levels of TLR2 in oocytes and early embryos. The value under representative photographs indicates the total relative fluorescence fold intensity levels of the TLR2 protein level of oocytes and different stages of early embryos relative to that of the oocytes. The relative fold expressions are presented as mean relative fold expression ± SEM of 3 independent experiments.
WB analysis revealed the TLR2 protein band was observed at approximately 95 kDa. This observed molecular weight deviates slightly from the predicted molecular weight of 90.2 kDa for bovine TLR2 based on its amino acid sequence in UniProt. A previous study investigating bovine mammary glands also reported the detection of a TLR2 protein band via WB analysis, although at a slightly lower molecular weight of 86 kDa30. The consistent detection of a band around this size range across different bovine tissues suggests the presence of TLR2 protein, and the observed variations in molecular weight likely reflect post-translational modifications or tissue-specific isoforms.
In MII oocytes, as assessed by IF, TLR2 exhibited a distinct localization pattern. The protein was concentrated in the region surrounding the metaphase plate and the polar body, suggesting a role in meiotic spindle assembly or chromosome segregation. In contrast, during GV oocytes and other early stages of embryo development, TLR2 expression was more diffuse, localizing primarily to the plasma membranes and cytoplasm. No fluorescent signal was observed in the negative control in the IF analysis. The data of WB analyses showed that there were no significant changes in TLR2 levels during oocyte and embryo development (P > 0.05).
Activation of TLR2 during in vitro culture (IVC) increased the blastocyst rate of bovine embryos
Our preliminary dose–response experiments aimed to determine the optimal concentration of TLR2 agonist for modulating early bovine embryo development in vitro. TLR2 agonist treatment commenced at 36 hpi to precede the major embryonic genome activation (EGA) phase. This pre-EGA timing aimed to assess TLR2’s influence on establishing a favorable cellular milieu and to specifically analyze its early effects on embryonic development. The initial findings (Supplementary Tables S1a and S1b) revealed a significant enhancement in blastocyst development rate when embryos were cultured with 100 ng/ml of TLR2 agonist, initiated at 36 h post-insemination (hpi), compared to the untreated control group. Notably, lower concentrations of the agonist tested did not elicit a statistically significant improvement in blastocyst rates. Furthermore, exposure to a higher concentration of 200 ng/ml did not modulate blastocyst development, while a concentration of 1000 ng/ml resulted in a significant reduction in blastocyst rate compared to the control (Supplementary Table S1a). These observations suggest a weak to moderate level of TLR2 stimulation, achieved with 100 ng/ml of the agonist, appears to exert a beneficial effect on early embryonic development. In contrast, excessive TLR2 activation may trigger detrimental downstream signaling pathways, leading to compromised developmental competence. Based on these preliminary data indicating a clear optimum, the concentration of 100 ng/ml TLR2 agonist was selected and consistently applied in all subsequent experiments to investigate the underlying mechanisms of TLR2 signaling in early bovine embryos.
We further investigated whether TLR2 agonist supplementation affected the subsequent post-blastulation period (early blastocyst, expanded blastocyst, and hatching/hatched blastocyst) of IVP embryos. An Early Blastocyst (BL) is defined as a blastocyst with a clearly visible blastocoel cavity and an intact zona pellucida. The inner cell mass (ICM) and trophectoderm (TE) are initiating differentiation. Expanded Blastocyst (ExB) is characterized by a substantially enlarged blastocoel that occupies a significant portion of the embryo, resulting in an increased overall size and a thinning zona pellucida. The ICM is typically more compact, and the TE is a thin layer surrounding the expanded blastocoel. The Hatching/Hatched Blastocyst (HB) category includes blastocysts exhibiting either partial or complete extrusion from the zona pellucida (Fig. 2a,b). The rate of the blastocyst expansion and hatching was tested in control (0.05% ethanol) and 100 ng/ml TLR2 agonist groups (Fig. 2a,c). While the TLR2 agonist treatment did not significantly alter the proportions of early and hatched blastocysts, a notable increase was observed in the percentage of total and, particularly, expanded blastocysts (Fig. 2c), suggesting a positive impact on the progression to more advanced developmental stages.
TLR2 activation increases blastocyst rate in IVP bovine embryos. Equally divided 4-cell embryos were collected 36 h post-insemination(hpi), cultured for 7 days in culture medium supplemented without (0.05% ethanol control) or with 100 ng/ml of TLR2 agonist, and blastocyst rate was analyzed. (a) (Left) Representative photographs of blastocysts that developed after the addition of 0 (Control), or 100 ng/ml TLR2 agonist to the culture medium. Scale bar = 500 μm. (Right) Representative images of blastocysts at different stages: early blastocyst (BL), expanded blastocyst (ExB), and hatched/ hatching blastocyst (HB). (b) Representative time-lapse photographs of the main embryonic development phases developed after the addition of 100 ng/ml TLR2 agonist to the culture medium. The embryonic developmental process from 36 to 162 hpi was captured with time-lapse imaging. Images were captured at 6 h intervals. (c) Day 7 blastocyst rates of different stages of the embryo, between control vs. TLR2 agonist groups. The number of blastocysts in each group is mentioned below the respective bars. Experiments were repeated 5 times. The data are expressed as mean ± S.E.M. and values with asterisks were considered significant variance between the ‘control (n = 297)’ and ‘TLR2 agonist (n = 294)’ groups at *P < 0.05. (d) Evolution of the blastocyst rate of each time point from 120 hpi. Data show mean ± S.E.M of 5 independent experiments. Control(n = 75) vs. TLR2 agonist(n = 75). *P < 0.05; **P < 0.01.
TLR2 activation during IVC enhanced early embryonic developmental rate
The above results demonstrated that the IVP embryo has a higher number of expanded blastocysts after being supplemented with the TLR2 agonist. To determine whether such an effect was attributed to the higher speed of embryo growth induced by TLR2 activation, we performed a time-lapse live imaging microscopy of IVC embryos. The rate of blastocyst formation at different time points was analyzed. The first early blastocyst was formed starting from 132 hpi in both control and TLR2 agonist-treated groups (Fig. 2b). The data showed a significantly higher ratio of TLR2 agonist-treated embryos started to form blastocysts after 138 hpi up to 162 hpi compared to the control group (Fig. 2d).
Activation of early embryonic TLR2 triggered a weak innate immune response in a Day 7 blastocyst
The ligation of TLRs into their specific ligands results in the induction of inflammatory mediators via TLRs signaling pathway4,5. Therefore, to investigate whether the TLR2 system is functional in early bovine embryos, we stimulated the Day 7 blastocyst with TLR2 agonist, and the mRNA expression of inflammatory cytokines was analyzed using RT-PCR. Interestingly, our data showed that 100 ng/ml of TLR2 agonist triggered the mRNA expression of TNFA in the Day 7 blastocysts, but this upregulation was not sustained in expanded blastocysts (Fig. 3a,b). Moreover, both stages of embryos have a higher mRNA expression of type 1 interferons (Interferon-tau (IFNT)) in the TLR2 agonist group than those in the control group. Especially, the exposure to TLR2 agonist decreased the expression of caspase-3 in the expanded blastocyst, but not in the early blastocyst (Fig. 3a,b).
The impact of TLR2 activation on the relative mRNA expression of pro-inflammatory cytokines (TNFA), IFNT, apoptosis-related gene Caspase-3 in an individual Day 7 blastocyst treated by TLR2 agonist. (a) At 36 hpi, embryos were co-cultured with TLR2 agonist (0 or 100 ng/ml) for 7 days, then Day 7 early blastocysts (BL) were collected for further analysis. The mRNA transcription levels of TNFA, IFNT, and Caspase-3 were quantified by real-time PCR assay. Data were presented as mean ± S.E.M of 5 independent experiments. The asterisk denotes a significant variance (*P < 0.05; **P < 0.01) between the ‘control (n = 22)’ and ‘TLR2 agonist (n = 26)’ groups. (b) At 36 hpi, embryos were co-cultured with TLR2 agonist (0, and 100 ng/ml) for 7 days, then Day 7 expanding blastocysts (ExB) were collected for further analysis. The mRNA transcription levels of TNFA, IFNT, and Caspase-3 were quantified by real-time PCR assay. Data are presented as mean ± S.E.M of 5 independent experiments. The asterisk denotes a significant variance (*P < 0.05) between the ‘control (n = 17)’ and ‘TLR2 agonist (n = 17)’ groups.
Activation of early embryonic TLR2 induced a delayed increase in the basal level of cytosolic Ca2+ in day 7 blastocyst
Previous reports showed that an increase in the intracellular Ca2+ level hastens embryo development in preimplantation mouse embryos14,15. In addition, we recently reported that TLR2 regulates Ca2+ influx into sperm17. Based on these, we hypothesized that the activation of embryo TLR2 hastens embryo developmental speed through increasing cytosolic calcium levels. To assess the effect of TLR2 agonist treatment on Ca2+ levels, we employed a Fluo4-AM assay. At 36 hpi, embryos were exposed to the TLR2 agonist, and the cytosolic Ca2+ levels were monitored every 5 min for a total of 1 h (h). Our results showed that TLR2 activation did not induce any acute alterations in Ca2+ levels during the first hour of TLR2 activation (Supplementary Fig. S1a). We then extended our analysis to later time points. Embryos were treated with the TLR2 agonist at 36 hpi and measured the Ca2+ levels at various time points: 1, 2, 24 and 48 h post-treatment. Again, no significant acute alterations in the cytosolic Ca2+ levels were detected (Supplementary Fig. S1b). Finally, we examined Ca2+ dynamics in Day 7 blastocysts that had been continuously exposed to the TLR2 agonist from the 36 hpi till blastocyst formation. These embryos exhibited a slight elevation in cytosolic Ca2+ levels compared to the control embryos, particularly in the ExB and HB stages (Fig. 4a,b).
The basal level of cytosolic calcium activity and the fluorescence analysis of autophagy activity in TLR2 agonist treated bovine Day 7 blastocysts. (a) Representative images of Fluo-4 AM fluorescence (green) in Day 7 embryos treated with control (0.05% ethanol) or 100 ng/ml TLR2 agonist. Scale bar = 500 μm. (b) (Left) Quantification of the relative levels of basal cytosolic Ca2+ of embryos in control and TLR2 agonist embryos. (Right) Quantification of the relative levels of basal cytosolic Ca2+ in control and TLR2 agonist at different stages (BL, ExB, and HB) of embryonic development. The asterisk denotes a significant variance between the ‘control (n = 93)’ and ‘TLR2 agonist (n = 110)’ groups. (c) Representative images of autophagy dye (green) in Day 7 embryos treated with control (0.05% ethanol) or 100 ng/ml TLR2 agonist. Scale bar = 500 μm. (d) (Left) Quantification of the relative levels of autophagy levels in control and TLR2 agonist. (Right) Quantification of the relative levels of autophagy activity in control and TLR2 agonist at different stages (BL, ExB, and HB) of embryonic development. Examples of a quantitative assessment of autophagy (green) using ImageJ. Experiments were repeated 5 times. The data are expressed as mean ± S.E.M. and the student’s t-test was utilized for statistical analyses. The asterisk denotes a significant variance between the ‘control (n = 96)’ and ‘TLR2 agonist (n = 111)’ groups. *P < 0.05; **P < 0.01; ****P < 0.0001 vs. control group.
Activation of early embryonic TLR2 increased autophagy levels in Day 7 blastocyst
The fact that either TLR2 and/or Ca2+ signaling pathways regulate autophagy together with the ability of autophagy to improve the quality and development of embryos prompted us to investigate the impact of TLR2 activation on the autophagy levels of the bovine blastocyst. The TLR2 agonist group showed higher autophagic activities in early, expanded and hatching/hatched blastocysts compared to the control group (Fig. 4c,d). These results suggest that TLR2 activation improves the quality and development of early embryos via induction of autophagy.
Activation of early embryonic TLR2 suppressed intracellular cathepsin B activity in Day 7 blastocyst
Cathepsin B activity is inversely correlated with the quality and developmental competence of bovine embryos31,32. Therefore, we investigated cathepsin B levels in TLR2 agonist-treated embryos as a biomarker for embryo quality. Our results showed a lower fluorescence intensity of cathepsin B activity detected in the TLR2 agonist-treated embryos compared to those in the control group (Fig. 5a,b), especially in the early and expanded blastocysts. These results suggest that TLR2 activation improves the quality of IVP blastocyst via suppression of cathepsin B expression.
Intracellular cathepsin B activity in TLR2 agonist treated bovine Day 7 blastocysts. (a) Representative images of intracellular cathepsin B activity in control (0.05% ethanol, n = 49) and 100 ng/ml TLR2 agonist (n = 56). Scale bar = 50 μm. (b) (Left) Relative fluorescence intensity of cathepsin B activity. (Right) Relative fluorescence intensity of cathepsin B activity at different stages: BL, ExB, and HB. Experiments were repeated 3 times. The data are expressed as mean ± S.E.M. and the student’s t-test was utilized for statistical analyses. *P < 0.05; **P < 0.01; ***P < 0.001 vs. control group.
Activation of TLR2 decreased the levels of DNA damage in early bovine embryos
Gamma-H2A.X immunofluorescence staining was used to detect the effect of TLR2 agonist on DNA damage during embryo development. The levels of positive gamma-H2A.X cells in the TLR2 agonist group were lower than those in the control group (Fig. 6a,b). These results suggest that TLR2 activation suppresses the levels of DNA damage in early embryos thereby improving the quality and development of IVP embryos.
Immunofluorescence analysis of gamma-H2A.X in TLR2 agonist treated bovine Day 7 blastocysts. (a) Representative images of DNA damage in embryos. Staining for gamma-H2A.X (green) and DNA (blue). Scale bar = 50 μm. (b) (Left) Relative fluorescence intensity of gamma-H2A.X positive signals after TLR2 agonist treatment. (Right) Relative fluorescence intensity of gamma-H2A.X activity at different stages: BL, ExB, and HB. The asterisk denotes a significant variance between the ‘control (n = 86)’ and ‘TLR2 agonist (n = 108)’ groups. Data show mean ± S.E.M (n = 5), *P < 0.05.
Activation of TLR2 reduced apoptosis in early bovine embryos
To further explore the effects of TLR2 activation on early embryos, apoptosis-related indexes were analyzed. Initially, both differential staining and TUNEL assay as effective methods were performed for evaluating in vitro culture conditions of bovine embryos33. Results showed a decrease in the average number of apoptotic cells and dead cell index (DCI) in the TLR2 agonist-treated group compared with the control, especially in expanded blastocysts (Fig. 7a–c). Moreover, Although the cell number increased gradually with embryonic development, there was no significant difference between ICM and TE (Fig. 7b,c).
Differential staining of the inner cell mass (ICM) and trophectoderm (TE), and TUNEL assay of TLR2 agonist-treated bovine Day 7 blastocysts. (a) Representative images of Hoechst 33,342 (Total DNA, blue), PI (TE cell, red), and transferase-mediated dUTP nick end labeling (TUNEL, apoptotic nuclei, green) stained Day 7 in vitro–produced bovine embryos. The merged image was TUNEL, PI and Total DNA. Scale bar = 50 μm. (b) The effects of TLR2 activation on the number of ICM, TE, and apoptosis-related indexes. (c) The effect of TLR2 activation on the number of ICM, TE, and apoptosis-related indexes at different stages: BL, ExB, and HB. The asterisk denotes a significant variance between the ‘control (n = 66)’ and ‘TLR2 agonist (n = 86)’ groups. Data show mean ± S.E.M (n = 5), *P < 0.05; **P < 0.01; ***P < 0.001 vs. control group.
Discussion
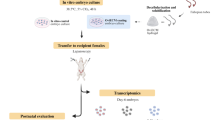
The current study sheds new light on the functional role of the TLR2 system in regulating early embryo development possibly via induction of calcium levels, autophagy and reduction of apoptosis during the preimplantation period in cattle. Here, we adapted an in vitro culture model to investigate the impact of TLR2 on the developmental competence of early bovine embryos. The obtained results illustrated that bovine embryos possess a well-developed TLR2 system. Further, the activation of TLR2 with its specific agonist before EGA increased the developmental competence of IVP bovine embryos. In particular, TLR2 activation increased cytosolic Ca2+ levels, induced autophagy, and reduced DNA damage and apoptosis, thereby supporting embryo development and survival under in vitro culture conditions. The working hypothesis for the impact of TLR2 activation on the developmental competence of early bovine embryos is illustrated in Fig. 8.
Schematic illustration for the working hypothesis of the functional role of the TLR2 system in regulating early embryo development. Toll-like receptor 2 (TLR2) is expressed in bovine early embryos. Activation of TLR2 with its specific agonist (Pam3Cys) increases the developmental competence of IVP bovine embryos. (1) Activation of bovine TLR2 accelerates embryo development, possibly via inducing weak innate immune responses (TNFA, IFNT), and lowering apoptosis-related genes to improve the quality of the embryo. (2) Increased cytosolic Ca2+ which subsequently accelerates the developmental process and blastocyst formation in IVP bovine embryos. (3) Meanwhile, TLR2 activation induced the autophagic activity of embryos. (4) Also, TLR2 activation suppressed apoptosis-related genes, cathepsin B activity and DNA damage thereby decreasing the apoptosis levels in IVP embryos. Together, these findings indicate that activation of early embryonic TLR2 enhances cytosolic calcium, and autophagy and reduces apoptosis levels thereby supporting embryo development and survival under in vitro culture conditions.
Preimplantation embryos are greatly affected by surrounding environmental conditions while undergoing critical early developmental events, including EGA, the transition when the embryo’s own genome begins transcription after relying on maternal factors, epigenome remodeling, and blastocyst formation.34. Therefore, early embryos should be equipped with a functional system for recognizing and signaling various entities to which they might be exposed. It has been shown that TLRs genes are widely expressed in human preimplantation embryos with an active pathway in response to TLRs ligands9. Our IF and WB results revealed that TLR2 proteins are highly expressed in oocytes, matured oocytes (MII), and early bovine embryos (4 cells, 8 cells, morula, and blastocysts). Notably, the activation of TLR2 before EGA in bovine embryos enhanced developmental competence and blastocyst formation during in vitro culture.
The time-lapse imaging system results demonstrate that TLR2-activated embryos exhibit accelerated development, reaching the blastocyst stage approximately 6–12 h faster than control embryos. This is evidenced by a significantly higher blastocyst rate observed from 138 to 162 hpi. Furthermore, at the Day 7 endpoint, the TLR2 agonist treatment significantly increased the total blastocyst rate and, importantly, the proportion of expanded blastocysts. While the percentage of early blastocysts remained unchanged, the trend towards a higher proportion of hatching/hatched blastocysts further suggests a positive effect on later developmental progression. Collectively, these results indicate that TLR2 activation specifically promotes the timely progression to more advanced and developmentally competent stages where these later blastocysts are widely recognized as having higher developmental potential and a stronger correlation with successful implantation35.
In order to understand the underlying mechanisms, we initially investigated whether the TLR2 system is functional in early bovine embryos. Our results showed that the ligation of TLR2 with their specific agonist induced a slight upregulation of the mRNA transcription of the inflammatory cytokine, TNFA, in the individual Day 7 blastocyst suggesting that embryos possess a functional TLR2 signaling pathway. Interestingly it has been shown that minute levels of TNFA play an important physiological role in cell proliferation and embryo differentiation in mice36. However high concentrations of TNFA, observed during uterine infection, might be detrimental to embryos through inhibiting cell proliferation and induction of apoptosis in mice37. In our model, the addition of precise amounts of TLR2 agonist during IVC suppressed the mRNA expression of apoptosis-related genes (Caspase-3) in blastocyst suggesting that such activation of embryo TLR2 improved the viability of early embryo. Furthermore, our observations showed that the TLR2 activation induced mRNA expression of IFNT, the pregnancy recognition signal in ruminants, in the blastocyst. The release of IFNT is highly correlated with the quality and developmental competence of in vitro-derived bovine embryos38,39. Previous studies have shown that bovine blastocysts of higher quality exhibit increased IFNT levels compared to those of lower quality40. At day 7, the observed decrease in TNFA mRNA levels in the expanded blastocyst stage strongly suggests a genuine down-regulation of TNFA during later development. We have no clear explanation for this phenomenon, but this dynamic shift in TNFA gene expression during the expansion process appears to be specifically regulated because IFNT is maintained at higher levels in TLR2-activated embryos. Collectively, the findings highlight a precise, stage-specific regulatory mechanism for TNFA in the developing embryo.
The ability of early bovine embryos to express an active TLR2 system and their tendency to improve embryo developmental competence upon activation prompted us to hypothesize that the early embryonic TLR2 system is involved in some metabolic processes regulating embryo development and survival. One of those processes which is directly associated with embryo development is Ca2+ influx14,15. Most recently we reported that the activation of sperm TLR2 increases Ca2+ influx into sperm which enhances their penetration into uterine glands16 and their fertilizing capability and subsequent embryo development in cattle17.
The results presented in this study demonstrate that TLR2 agonist treatment does not induce any acute response in cytosolic Ca2+ levels in early-stage embryos, while it induces a chronic and delayed increase in Ca2+ dynamics at later developmental stages. The lack of an acute Ca2+ response to TLR2 agonist treatment in early embryos could be attributed to several factors. One possibility is that the machinery required for Ca2+ mobilization in response to TLR2 signaling is not yet fully developed at these early stages41. Alternatively, the expression levels of key signaling molecules involved in TLR2-mediated Ca2+ signaling might be insufficient to induce an acute significant Ca2+ response12. Rather, the slight and chronic increase in calcium levels might be attributed to the slight increase in basal metabolic activities rather than the direct effect of TLR2 activation on calcium uptake12,42. However, the underlying molecular mechanisms by which TLR2 regulates Ca2+ stores and delayed, stage-specific effects of TLR2 agonist on Ca2+ dynamics and metabolism in preimplantation embryos remain to be investigated.
Another physiological process that controls embryo quality and development is autophagy. It has been shown that TLRs, especially the TLR2 system, regulate autophagy in mammals25,26,27,28. In addition, the increase in intracellular Ca2+ triggers autophagy18,19. The induction of autophagy improves embryo viability in cloned mouse embryos43 likely through degrading unrequired maternal RNAs and proteins after genomic activation as well as providing nutrients and energy20,21,23. In bovine embryos, good-quality embryos displayed higher autophagy activity31,32. These findings are consistent with our observations that TLR2 activation induced autophagy in blastocysts. Therefore, we suggest that the activation of TLR2 signaling pathway in bovine embryos induces autophagy to maintain cellular homeostasis thereby improving embryo quality, development competence, and blastocyst formation. However, the downstream protein components of the TLR2 signaling pathway that regulates the autophagy process in early bovine embryos require further investigation.
Autophagosome is formed by the fusion of autophagic vacuoles and lysosomes, allowing the degradation of the cargo by lysosomal enzymes, especially cathepsin B44,45. Cathepsin B expression is inversely correlated with the quality and developmental competence of bovine embryos and its over-expression induces apoptosis in embryos31,32. Therefore, we investigated Cathepsin B expression after TLR2 activation in embryo. As expected, our finding showed lower cathepsin B activity in TLR2 agonist-treated embryos. Additionally, TLR2 stimulation decreased the number of apoptotic cells and increased the total cell number which further verified our hypothesis. Besides, low levels of DNA damage were observed in TLR2 agonist-treated IVP embryos. Together, these results suggest that the early embryonic-TLR2 activation induces autophagy and reduces cathepsin B activity which inhibits early apoptosis thereby generating a good-quality and developmentally competent embryo.
The present study revealed a novel role for TLR2 in promoting bovine embryonic development and growth, extending its known functions beyond pathogen recognition. While prior research has predominantly investigated TLR2’s involvement in host defense7,8, our findings demonstrate that its optimal and weak activation can exert beneficial effects on early embryonic competence. The observed enhancements in blastocyst rates and developmental speed, coupled with the modulation of basal calcium levels, autophagy induction, and apoptosis reduction, highlight the potential importance of the TLR2 system and innate immune signaling in establishing a favorable cellular environment for successful preimplantation development. These results suggest that embryonic TLR2 may participate in sensing unidentified maternal-endogenous molecules, allowing for its finely tuned activation at an optimal level to influence critical developmental processes. Further investigation into the downstream signaling pathways and the in vivo relevance of these findings is crucial for understanding the physiological role of TLR2 in embryonic development and the establishment of early pregnancy in cattle.
The findings presented in this study have significant translational potential for improving ART in cattle. The understanding of the role of TLR2 in embryonic development can inform the development of novel strategies to enhance embryo quality and survival rates. For instance, the TLR2 agonist could be used to manipulate embryonic development in a controlled manner, potentially leading to improvements in embryo production and transfer efficiency. Further, insights into the interaction between TLR2 and the innate immune system provide valuable information for optimizing culture conditions and reducing the risk of infections during in vitro embryo production.
While our findings demonstrate a promising approach to enhance the developmental competence of IVP bovine embryos through early TLR2 activation, it is crucial to acknowledge that this study primarily focused on the preimplantation stage. Therefore, the direct implications of these in vitro improvements for subsequent pregnancy establishment rates, calf viability, and the long-term health of offspring remain to be fully elucidated. Future research is warranted to investigate the in vivo effects of this early embryonic intervention. Embryo transfer experiments with detailed monitoring of pregnancy outcomes, placental development, fetal growth, and the health status of resulting calves are essential to fully assess the translational potential of our findings.
We consider embryonic quality to be a complex concept about the embryo’s ability to develop to advanced stages with appropriate timing, exhibit cellular health (low apoptosis, minimal DNA damage), and possess the necessary cellular mechanisms, such as modulated calcium signaling and autophagy, and physiological characteristics for successful implantation and subsequent development. Our data collectively indicate an improvement in these aspects following TLR2 agonist treatment.
In conclusion, our study demonstrates that TLR2 is expressed and functional in early bovine embryos. Stimulation of TLR2 with a specific agonist at early embryonic stage enhances the quality and developmental competence of IVP embryos. We propose that these improvements are mediated, in part, by an increase in the basal levels of cytosolic calcium, autophagy induction, reduced DNA damage, and enhanced cellular viability. The observed faster developmental rate, alongside enhanced embryo quality, suggests that TLR2 activation may promote a more efficient developmental trajectory, though further research is needed to establish a causal relationship between the activation of the TLR2 signaling pathway and the observed beneficial effects on embryo development.
Methods
Ethics
All experiments were conducted following the Guiding Principles for the Care and Use of Research Animals Promulgated by Obihiro University of Agriculture and Veterinary Medicine, Japan. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Obihiro University of Agriculture and Veterinary Medicine (Permit number 19–111).
Experimental design
The design and framework of multiple investigations performed in the present study were illustrated in Supplementary Fig. S2. Initially, the expression of TLR2 in oocytes, matured oocytes (MII), and different developmental stages of the early embryo (4-cells, 8-cells, morula and blastocyst) were analyzed using immunofluorescence assay. Further, western blot analysis was conducted to confirm the TLR2 protein expression in the above stages. Next, we adapted an IVC model to examine the potential impact of TLR2 activation on the development competence of IVP bovine embryos. Initially, a dose-dependent experiment was conducted. At 36 hpi, embryos were exposed to different concentrations of Pam3Cys, a specific TLR2 agonist, for the activation of embryo TLR2. Pam3CSK4 is the TLR1/2 synthetic lipopeptide representing the acylated amino terminus of bacterial lipopeptides, which activates Toll-like receptors 2 and 1 (TLR2/1), and recognizes mainly molecules from gram-positive pathogens. Our initial observations showed that only 100 ng/ml of TLR2 agonist improved blastocyst rates of IVP embryos. Therefore, this concentration of TLR2 agonist,100 ng/ml, was used for the subsequent experiments. A time-lapse imaging system was conducted to investigate the impact of TLR2 activation on the speed of embryo development. To understand the underlying mechanisms, we first investigated whether the TLR2 system is functional in early embryos by analyzing the gene expression of proinflammatory cytokines in TLR2 agonist-treated blastocysts using RT-PCR assay. Subsequently, we analyzed the impact of TLR2 activation on the proposed mechanisms involved in the development of preimplantation embryos including cytosolic Ca2+ levels, autophagy activity, cathepsin B levels, DNA damage and apoptosis in IVP day 7 blastocysts.
Oocyte collection and in vitro maturation (IVM)
Bovine ovaries were collected from a local slaughterhouse (Obihiro, Hokkaido, Japan) in 0.9% saline with 1% penicillin–streptomycin (Gibco, Grand Island, USA) at approximately 35 °C and immediately transported to the laboratory within 2 h. The IVM was performed according to the previously mentioned protocol46. Briefly, COCs were aspirated from individual visible antral follicles of 2–6 mm in diameter using a 10 ml syringe attached to an 18 G needle, then washed three times in oocyte collection medium (OCM; Research Institute for the Functional Peptides Co., Ltd.) supplemented with 10% fetal bovine serum (FBS, Biowest). Approximately 40–50 oocytes with a homogenous cytoplasm and surrounded by at least three layers of compact cumulus cells were used for in vitro maturation in 500 μl high-performance-modified 199 media (HP-M199; Research Institute for the Functional Peptides Co., Ltd.) supplemented with 10 ng/ml epidermal growth factor (Sigma) and 10% FBS for 22 h at 38.5 °C in 5% CO2 with humidified air. The collection and in vitro maturation media were supplemented with 10% FBS to provide essential nutrients, growth factors, and protective elements known to support oocyte health and maturation, consistent with established protocols.
Sperm preparation and in vitro fertilization
Three semen straws from 3 different Holstein bulls (to mitigate the inherent biological variability associated with individual sires) were thawed at 37 °C for 30 s, pooled, and washed twice using SP-TALP47 by centrifugation at 1500 rpm for 5 min at room temperature. Then, sperm concentration was adjusted to 2 × 106 sperm/ml in fertilization medium IVF100 (Research Institute for the Functional Peptides, Yamagata, Japan). Finally, 15 COCs and sperm were co-incubated for 6 h at 38.5 °C in 5% CO2 in humidified air.
In vitro culture (IVC)
Six hours post-insemination (hpi), cumulus cells were removed by repeated pipetting. Presumptive zygotes (n = 50–55) were transferred to 400 µl BO-IVC medium (IVF-bioscience, Poland) under mineral oil in 4-well dishes at 38.5 °C in a humidified atmosphere of 5% O2, 5% CO2, and 90% N2 and kept for 7 days in IVC medium till blastocyst formation.
Immunofluorescence analysis of TLR2 expression
The expression of TLR2 protein in oocytes, matured oocytes (MII), and different stages of early embryos (4-cells, 8-cells, morula and blastocyst) were assessed by the immunofluorescence assay as previously described48. Briefly, embryos were washed three times in PBS containing 0.1% polyvinyl alcohol (PBS-PVA) and then fixed in 4% paraformaldehyde (PFA) at room temperature (RT) for 30 min. Embryos were transferred into the drop of 0.1% Tween-20 (Sigma, P1379) in PBS-PVA for 30 min for permeabilization. Next, embryos were incubated in 5% BSA/PBS at RT for 2 h and incubated with primary antibody (1:100, 10 µg/ml, rabbit polyclonal anti-TLR2 antibody, orb11487, Biorbyt, Cambridge, UK, homology is 93% compared to bovine) at 4 °C overnight. Then, the embryos were incubated with secondary antibody (1:200, 10 µg/ml, goat anti-rabbit IgG labeled with Alexa Flour 546, Invitrogen, Thermo Fisher Scientific, USA) at RT for 2 h. The Rabbit IgG isotype (1:500, 10 µg/ml, Invitrogen, Thermo Fisher Scientific) was applied as the IgG negative control. Finally, the embryos were mounted with DAPI-containing VECTASHIELD antifade mounting medium (H-1200; Vector Laboratories, USA) on glass slides. Images were captured using a fluorescence microscope (Keyence, BZ-X800, Osaka, Japan). This experiment was repeated 3 times using nine oocytes and embryos from each stage.
Western blot analysis
The TLR2 protein in oocytes, mature oocytes, and different stages of early embryos (4-cells, 8-cells, morula and blastocyst) was confirmed by the western blotting as previously described with modifications49,50. When collecting oocytes and mature (MII) oocytes, COCs were denuded of cumulus cells by pipetting. Oocytes, matured oocytes (MII), and different stages of early embryos were washed three times in 100 µl drops of PBS containing 1 mg/ml polyvinylpyrrolidone (PBS-PVP) by transferring from drop to drop. Groups of 50 oocytes, matured oocytes or embryos for each repeat were placed into 200 µl tubes and stored at − 80 °C. This experiment was repeated 3 times.
Cell lysates were prepared from frozen oocytes, matured oocytes (MII), and embryos by pipetting in radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with protease inhibitors. TLR2 protein expression in the lysates was analyzed by SDS–PAGE. Separated protein transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, USA) were blocked with Bullet Blocking One (Nacalai Tesque, Kyoto, Japan), and the membranes were incubated with the rabbit polyclonal anti-TLR2 antibody (1:2000, orb11487, Biorbyt, Cambridge, UK). The membranes were then incubated with secondary antibody, horseradish peroxidase labeled goat anti-rabbit IgG (1:5000, Vector Laboratories, Burlingame, CA, USA), and immunoreactive bands were detected using enhanced chemiluminescence (EMD Millipore, Burlingame, MA, USA). Signals were detected using C-DiGit Blot Scanner (LI-COR, Lincolin, NE, USA) and then band density was assessed with Image studio DiGit software (version 5.2) and intensity was converted to relative fluorescence fold intensity.
Activation of TLR2 in embryos
Pam3Cys, a synthetic TLR2 ligand (ab142085, Abcam) was used to specifically and selectively activate the embryo TLR251. A stock solution of 100 μg/ml of TLR2 in 50% ethanol was prepared. For experiments involving the application of the TLR2 agonist, a corresponding control group was included. Control embryos were cultured under identical in vitro conditions but were treated with the vehicle (50% ethanol) used to dissolve the agonist at the same final concentration as the agonist used in that specific experiment. This vehicle control was implemented to ensure that any observed effects were specifically due to TLR2 activation and not a consequence of the vehicle itself. Initially, to identify the effects of TLR2 activation on embryo developmental competence, dose-dependent experiments were performed. After 36 hpi, only normally divided embryos were used. Different concentrations (0, 1, 10, 100, and 1000 ng/ml) of Pam3Cys, prepared from a 100 μg/ml stock solution in 50% ethanol, were supplemented from 36 hpi till Day 7 post-insemination and the blastocyst formation was analyzed. In addition, a narrow range of concentrations (0, 50, 100, and 200 ng/ml) of Pam3Cys was also performed. Our initial observations showed that 100 ng/ml of Pam3Cys greatly improved blastocyst rates of IVP embryos. Therefore, this concentration of TLR2 agonist,100 ng/ml, was used for subsequent experiments.
Time-lapse imaging
A time-lapse imaging system was used to track morphologic changes and the speed of development of embryos and their associations with TLR2 activation. Embryos were treated with 0.05% ethanol (as a control group) or 100 ng/ml of TLR2 agonist at 36 hpi and incubated at 38.5 °C in a humidified atmosphere of 5% O2, 5% CO2, and 90% N2 incubator (Astec, VCI/VMI series) with the time-lapse observation system. Meanwhile, the system was set at the plane with the best focus of the image. Embryo development was tracked by capturing images every 6 h automatically till 162 h post-insemination. Obtained embryo images were exported and manually reviewed. The number and time at which embryos reached the blastocyst stage was recorded.
RNA extraction, cDNA synthesis, and quantitative real-time PCR
Briefly, individual Day 7 blastocyst was stored in 10 μl extraction buffer and incubated at 42 °C for 30 min. RNA was extracted using an Arcturus PicoPure RNA Isolation Kit (Cat. no. 12204–1; Arcturus, Foster, CA, USA) following the manufacturer’s protocol. 10 μl purified total RNA was reverse-transcribed using the QuantiTect® Reverse Transcription kit in a final volume of 20 μl. Quantitative real-time PCR was carried out by an iCycler iQ (Bio-Rad Laboratories, Japan) using QuantiTect SYBR Green PCR Master Mix (QIAGEN GmbH, Germany) as previously described29. The amplification program was run with an initial activation step (15 min at 95 °C), followed by 40 cycles of PCR (15 s at 95 °C, 15 s at 51.3 ~ 58.9 °C, and 30 s extension at 72 °C). The sequences of used primers were described in Supplementary Table S2. The efficiencies of the q-PCR amplifications for investigated genes were verified. The calculated cycle threshold (Ct) values were normalized using Histone H2A as the internal standard. Fold changes in relative gene expression were determined using the Delta-Delta comparative threshold method.
Measuring cytosolic Ca2+
Cytosolic Ca2+ levels were assessed using Fluo-4 AM (F311, Dojindo, Japan) as previously described52 with minor alterations. For this experiment, the control group was treated with 0.05% ethanol, and the treatment group was exposed to 100 ng/ml Pam3Cys (dissolved in 0.05% ethanol). First, embryos at 36 hpi and Day 7 blastocyst were washed three times in PBS-PVA and then loaded with 5 μM Fluo-4 AM for 40 min at 38.5 °C in the dark. Next, embryos were washed three times with D-PBS to remove free Fluo-4 AM. Subsequently, the stained embryos were transferred to a drop of D-PBS on a glass-bottom imaging dish (Iwaki, Japan), Images were captured by the fluorescence microscope (Keyence, BZ-X800, Osaka, Japan), and the BZ-X FITC (OP-87763) filters were set for green wavelengths. The images of cytosolic Ca2+ levels were captured under the same exposure time and fluorescence intensity and processed using image J for intensity measurements. In brief, the defined cells were selected and the background was excluded by setting the threshold above the background level. This threshold was maintained at the same level for all analyzed images and the relative fluorescence intensity was calculated.
Detection of autophagy activity
The autophagic activity was detected using a Cyto-ID Autophagy Detection kit (Enzo Life Sciences, USA) as previous reported53. In the autophagy assays, control embryos were treated with 0.05% ethanol, while the treatment group received 100 ng/ml Pam3Cys (in 0.05% ethanol) from 36 hpi until the time of analysis. Briefly, Day 7 blastocyst were washed in PVA-PBS and incubated with the reaction mix (1 μl CYTO-ID Green Detection Reagent in 500 μl 1 × Assay Buffer) at 38.5 °C for 30 min. After incubation, the blastocysts were transferred to 5 μl drops of PVA-PBS on a glass-bottom imaging dish and covered with mineral oil. Then images were captured using a fluorescence microscope (Keyence, BZ-X800, Osaka, Japan), and the BZ-X FITC (OP-87763) filter was set for green wavelengths. All images of autophagy activity were obtained using the same exposure time and the autophagy intensity was quantified with ImageJ software. In brief, the defined cells were selected and the background was excluded by setting the threshold above the background level. This threshold was maintained at the same level for all analyzed images and the relative fluorescence intensity was calculated.
Detection of intracellular activity of Cathepsin B in blastocysts
In this assay, control embryos were treated with 0.05% ethanol, while the treatment group received 100 ng/ml Pam3Cys (in 0.05% ethanol) from 36 hpi until the time of analysis. Magic Red cathepsin B detection kit (Immunochemistry Technologies LLC, Bloomington, MN, USA) was used for the detection of Cathepsin B activity of Day 7 blastocyst according as previously described32. In brief, Day 7 blastocysts from each group were washed in PVA-PBS 3 times and then incubated in 500 μl of PBS with 2 μl of reaction mix at 38.5 °C, and 5% CO2 for 30 min. After washing, the freshly stained blastocysts were mounted onto the slide and immediately captured by a fluorescence microscope (Keyence, BZ-X800, Osaka, Japan), and the BZ-X RED (OP-87765) filters were set for detecting intracellular cathepsin B activity. All images were captured using the same exposure time. The average total fluorescence intensity was calculated by Image J software. In brief, the defined cells were selected and the red cytoplasmic background was excluded by altering the threshold. This threshold was maintained at the same level for all analyzed images. The number of red dots was calculated. The total fluorescence intensity = number of the red dots x mean fluorescence intensity.
Immunofluorescence staining and quantitative analysis of gamma-H2AX
In this assay, control embryos were treated with 0.05% ethanol, while the treatment group received 100 ng/ml Pam3Cys (in 0.05% ethanol) from 36 hpi until the time of analysis. DNA damage was evaluated by gamma-H2A.X immunofluorescence staining as previously described54 with minor alterations. Briefly, Day 7 blastocysts were washed 3 times and fixed in 2% PFA for 20 min before being kept in a blocking solution (0.3% BSA, 0.01% Tween-20, and 0.02% NaN3 in PBS) at 4 °C for 15 min. Embryos were permeabilized with a permeabilization solution (0.3% BSA, 0.1% Triton X-100, and 0.02% NaN3 in PBS) for 15 min, then washed 3 times with blocking solution and kept for another 15 min before incubating with anti-gamma H2A.X (phospho S139) antibody (1:100) at 4 °C overnight. Thereafter, embryos were incubated with a secondary antibody (goat anti-rabbit fluorescein isothiocyanate (FITC)-conjugated, 1:200) for 1 h at 4 °C. Finally, Day 7 blastocysts were mounted onto a glass slide using a Vectashield mounting medium (Vector Laboratories Inc., CA, USA) containing DAPI for DNA labeling. The embryo fluorescence was measured by the fluorescence microscope (Keyence, BZ-X800, Osaka, Japan). BZ-X FITC (OP-87763) and BZ-X DAPI (OP-87762) filters were set to observe autophagy and DNA activity, respectively. The fluorescence intensity was measured using Image J software. In brief, the defined cells were selected and the background was excluded by setting the threshold above the background level. This threshold was maintained at the same level for all analyzed images and the relative fluorescence intensity was calculated.
Differential staining and detection of apoptotic nuclei by TUNEL assay
In this assay, control embryos were treated with 0.05% ethanol, while the treatment group received 100 ng/ml Pam3Cys (in 0.05% ethanol) from 36 hpi until the time of analysis. Day 7 blastocysts were differentially stained for evaluation of inner cell mass (ICM) cells, trophectoderm (TE), and apoptosis cells as described previously33. Briefly, Day 7 blastocysts were washed 3 times and permeabilized with 30 μg/ml PI (Propidium iodide) and 0.2% Triton X-100 for 20 s. Following permeabilization, embryos were transferred to 4% PFA containing 30 μg/ml bisbenzimide (Hoechst 33,342) and kept for 20 min at RT. Next, embryos were transferred to a drop (50 μl) of in situ cell death detection kit solution (Roche, Germany) for 45 min at 38.5 °C, 5% CO2 in the dark according to the manufacturer’s instructions. Finally, embryos were washed and mounted on glass slides in mounting medium (H-1000.Vectashield) and examined under the fluorescent microscope (Keyence, BZ-X800, Osaka, Japan). The BZ-X FITC (OP-87763), BZ-X RED (OP-87765), and BZ-X DAPI (OP-87762) filters were set for green, red, and blue wavelengths, respectively. The number of ICM, TE cells, and dead cells (green) were counted. The dead cell index (DCI, %), and TE/ICM were calculated. DCI = the number of dead cells/ the total cell number.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA). One-way analysis of variance (ANOVA) followed by Bonferroni’s post-comparison test (> two groups) or two-sample t-test (two groups) was used to compare the mean differences among the groups. Each experiment was repeated three to five times. Data are expressed as mean ± S.E.M. Data were considered to be statistically significant at * P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Brackett, B. G. et al. Normal development following in vitro fertilization in the cow. Biol. Reprod. 27(1), 147–158. https://doi.org/10.1095/biolreprod27.1.147 (1982).
Han, H. I., Lee, S. H. & Park, C. K. Development of In vitro embryo production system using collagen matrix gel attached with vascular endothelial growth factor derived from interleukin-1 beta-treated porcine endometrial tissue. Tissue Eng. Part C Methods 23(7), 396–403. https://doi.org/10.1089/ten.TEC.2017.0071 (2017).
Déniz, F. P., Encinas, C. & Fuente, J. Morphological embryo selection: An elective single embryo transfer proposal. JBRA Assist Reprod. 22(1), 20–25. https://doi.org/10.5935/1518-0557.20180015 (2018).
Medzhitov, R. & Janeway, C. A. Jr. Innate immunity: The virtues of a nonclonal system of recognition. Cell 91(3), 295–298. https://doi.org/10.1016/s0092-8674(00)80412-2 (1997).
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388(6640), 394–397. https://doi.org/10.1038/41131 (1997).
Aflatoonian, R. & Fazeli, A. Toll-like receptors in female reproductive tract and their menstrual cycle dependent expression. J. Reprod. Immunol. 77(1), 7–13. https://doi.org/10.1016/j.jri.2007.03.014 (2008).
Shimada, M., Hernandez-Gonzalez, I., Gonzalez-Robanya, I. & Richards, J. S. Induced expression of pattern recognition receptors in cumulus oocyte complexes: Novel evidence for innate immune-like functions during ovulation. Mol. Endocrinol. 20(12), 3228–3239. https://doi.org/10.1210/me.2006-0194 (2006).
Liu, Z., Shimada, M. & Richards, J. S. The involvement of the Toll-like receptor family in ovulation. J. Assist Reprod. Genet. 25(6), 223–228. https://doi.org/10.1007/s10815-008-9219-0 (2008).
Aboussahoud, W. S. et al. The expression and activity of Toll-like receptors in the preimplantation human embryo suggest a new role for innate immunity. Hum. Reprod. 36(10), 2661–2675. https://doi.org/10.1093/humrep/deab188 (2021).
Berridge, M. J., Lipp, P. & Bootman, M. D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1(1), 11–21. https://doi.org/10.1038/35036035 (2000).
Wang, C. & Machaty, Z. Calcium influx in mammalian eggs. Reproduction 145(4), R97–R105. https://doi.org/10.1530/REP-12-0496 (2013).
Miao, Y. L. & Williams, C. J. Calcium signaling in mammalian egg activation and embryo development: The influence of subcellular localization. Mol. Reprod. Dev. 79(11), 742–756. https://doi.org/10.1002/mrd.22078 (2012).
Armant, D. R. Intracellular Ca2+ signaling and preimplantation development. Adv. Exp. Med. Biol. 843, 151–171. https://doi.org/10.1007/978-1-4939-2480-6_6 (2015).
Stachecki, J. J., Yelian, F. D., Leach, R. E. & Armant, D. R. Mouse blastocyst outgrowth and implantation rates following exposure to ethanol or A23187 during culture in vitro. J. Reprod. Fertil. 101(3), 611–617. https://doi.org/10.1530/jrf.0.1010611 (1994).
Stachecki, J. J., Yelian, F. D., Schultz, J. F., Leach, R. E. & Armant, D. R. Blastocyst cavitation is accelerated by ethanol- or ionophore-induced elevation of intracellular calcium. Biol. Reprod. 50(1), 1–9. https://doi.org/10.1095/biolreprod50.1.1 (1994).
Akthar, I. et al. Activation of sperm Toll-like receptor 2 induces hyperactivation to enhance the penetration to mucus and uterine glands: A trigger for the uterine inflammatory cascade in cattle. Front. Immunol. 14, 1319572. https://doi.org/10.3389/fimmu.2023.1319572 (2023).
Ma, D., Marey, M. A., Shimada, M. & Miyamoto, A. Toll-like receptor 2 is involved in calcium influx and acrosome reaction to facilitate sperm penetration to oocytes during in vitro fertilization in cattle. Front. Cell Dev. Biol. 10, 810961. https://doi.org/10.3389/fcell.2022.810961 (2022).
Cárdenas, C. et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142(2), 270–283. https://doi.org/10.1016/j.cell.2010.06.007 (2010).
Høyer-Hansen, M. et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol. Cell 25(2), 193–205. https://doi.org/10.1016/j.molcel.2006.12.009 (2007).
Mizushima, N., Yamamoto, A., Matsui, M., Yoshimori, T. & Ohsumi, Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell 15(3), 1101–1111. https://doi.org/10.1091/mbc.e03-09-0704 (2004).
Mizushima, N. & Levine, B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 12(9), 823–830. https://doi.org/10.1038/ncb0910-823 (2010).
Song, B. S. et al. Induction of autophagy promotes preattachment development of bovine embryos by reducing endoplasmic reticulum stress. Biol. Reprod. 87(1), 8–11. https://doi.org/10.1095/biolreprod.111.097949 (2012).
Tsukamoto, S. et al. Autophagy is essential for preimplantation development of mouse embryos. Science 321(5885), 117–120. https://doi.org/10.1126/science.1154822 (2008).
Tsukamoto, S., Kuma, A. & Mizushima, N. The role of autophagy during the oocyte-to-embryo transition. Autophagy 4(8), 1076–1078. https://doi.org/10.4161/auto.7065 (2008).
Xu, Y. et al. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity 27(1), 135–144. https://doi.org/10.1016/j.immuni.2007.05.022 (2007).
Delgado, M. A., Elmaoued, R. A., Davis, A. S., Kyei, G. & Deretic, V. Toll-like receptors control autophagy. EMBO J. 27(7), 1110–1121. https://doi.org/10.1038/emboj.2008.31 (2008).
Shi, C. S. & Kehrl, J. H. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J. Biol. Chem. 283(48), 33175–33182. https://doi.org/10.1074/jbc.M804478200 (2008).
Wang, M. et al. Domain fusion TLR2–4 enhances the autophagy-dependent clearance of Staphylococcus aureus in the genetic engineering goat. Life 11, 78044. https://doi.org/10.7554/eLife.78044 (2022).
Zinnah, M. A. et al. Peptidoglycan disrupts early embryo-maternal crosstalk via suppression of ISGs expression induced by interferon-tau in the bovine endometrium. Biochem. Biophys. Res. Commun. 532(1), 101–107. https://doi.org/10.1016/j.bbrc.2020.08.006 (2020).
Bai, X. et al. Toll-like receptor 2 Is associated with the immune response, apoptosis, and angiogenesis in the mammary glands of dairy cows with clinical mastitis. Int. J. Mol. Sci. 23(18), 10717. https://doi.org/10.3390/ijms231810717 (2022).
Bettegowda, A. et al. Identification of novel bovine cumulus cell molecular markers predictive of oocyte competence: Functional and diagnostic implications. Biol. Reprod. 79(2), 301–309. https://doi.org/10.1095/biolreprod.107.067223 (2008).
Balboula, A. Z. et al. Intracellular cathepsin B activity is inversely correlated with the quality and developmental competence of bovine preimplantation embryos. Mol. Reprod. Dev. 77(12), 1031–1039. https://doi.org/10.1002/mrd.21250 (2010).
Fouladi-Nashta, A. A. et al. Differential staining combined with TUNEL labelling to detect apoptosis in preimplantation bovine embryos. Reprod. Biomed. Online 10(4), 497–502. https://doi.org/10.1016/s1472-6483(10)60827-9 (2005).
Monk, D., Mackay, D. J. G., Eggermann, T., Maher, E. R. & Riccio, A. Genomic imprinting disorders: Lessons on how genome, epigenome and environment interact. Nat. Rev Genet. 20(4), 235–248. https://doi.org/10.1038/s41576-018-0092-0 (2019).
Nishigai, M., Kamomae, H., Tanaka, T. & Kaneda, Y. The Influence of developmental stage and morphological quality of frozen-thawed bovine embryos on pregnancy rate in bovine embryo transfer. J. Reprod. Dev. 45(4), 301–306. https://doi.org/10.1262/jrd.49.23 (1999).
You, K., Gu, H., Yuan, Z. & Xu, X. Tumor necrosis factor alpha signaling and organogenesis. Front. Cell Dev. Biol. 9, 727075. https://doi.org/10.3389/fcell.2021.727075 (2021).
Xu, D. X. et al. Tumor necrosis factor alpha partially contributes to lipopolysaccharide-induced intra-uterine fetal growth restriction and skeletal development retardation in mice. Toxicol. Lett. 163(1), 20–29. https://doi.org/10.1016/j.toxlet.2005.09.009 (2006).
Kubisch, H. M., Larson, M. A. & Roberts, R. M. Relationship between age of blastocyst formation and interferon-tau secretion by in vitro-derived bovine embryos. Mol. Reprod. Dev. 49(3), 254–260. https://doi.org/10.1002/(SICI)1098-2795(199803)49:3%3c254::AID-MRD5%3e3.0.CO;2-N (1998).
Bao, Z. J., Zhao, S., Haq, I. U. & Zeng, S. M. Recombinant bovine interferon-τ enhances in vitro development of bovine embryos by upregulating expression of connexin 43 and E-cadherin. J. Dairy Sci. 97(11), 6917–6925. https://doi.org/10.3168/jds.2014-8106 (2014).
Hernandez-Ledezma, J. J., Mathialagan, N., Villanueva, C., Sikes, J. D. & Roberts, R. M. Expression of bovine trophoblast interferons by in vitro-derived blastocysts is correlated with their morphological quality and stage of development. Mol. Reprod. Dev. 36(1), 1–6. https://doi.org/10.1002/mrd.1080360102 (1993).
Whitaker, M. Calcium at fertilization and in early development. Physiol. Rev. 86(1), 25–88. https://doi.org/10.1152/physrev.00023.2005 (2006).
Campbell, K. & Swann, K. Ca2+ oscillations stimulate an ATP increase during fertilization of mouse eggs. Dev. Biol. 298(1), 225–233. https://doi.org/10.1016/j.ydbio.2006.06.032 (2006).
Shen, X. et al. Induction of autophagy improves embryo viability in cloned mouse embryos. Sci. Rep. 5, 17829. https://doi.org/10.1038/srep17829 (2015).
Galluzzi, L., Pietrocola, F., Levine, B. & Kroemer, G. Metabolic control of autophagy. Cell 159(6), 1263–1276. https://doi.org/10.1016/j.cell.2014.11.006 (2014).
Liu, L. et al. Lysosomal dysfunction and autophagy blockade contribute to IMB-6G-induced apoptosis in pancreatic cancer cells. Sci. Rep. 7, 41862. https://doi.org/10.1038/srep41862 (2017).
Ideta, A. et al. A simple medium enables bovine embryos to be held for seven days at 4 °C. Sci. Rep. 3, 1173. https://doi.org/10.1038/srep01173 (2013).
Parrish, J. J., Susko-Parrish, J., Winer, M. A. & First, N. L. Capacitation of bovine sperm by heparin. Biol. Reprod. 38(5), 1171–1180. https://doi.org/10.1095/biolreprod38.5.1171 (1988).
Talukder, A. K. et al. Oviduct epithelium induces interferon-tau in bovine Day-4 embryos, which generates an anti-inflammatory response in immune cells. Sci. Rep. 8(1), 7850. https://doi.org/10.1038/s41598-018-26224-8 (2018).
Nakamura, K. et al. Effects of miR-98 in intrauterine extracellular vesicles on maternal immune regulation during the peri-implantation period in cattle. Sci. Rep. 9(1), 20330. https://doi.org/10.1038/s41598-019-56879-w (2019).
Fear, J. M. & Hansen, P. J. Developmental changes in expression of genes involved in regulation of apoptosis in the bovine preimplantation embryo. Biol. Reprod. 84(1), 43–51. https://doi.org/10.1095/biolreprod.110.086249 (2011).
Reitermann, A., Metzger, J., Wiesmüller, K. H., Jung, G. & Bessler, W. G. Lipopeptide derivatives of bacterial lipoprotein constitute potent immune adjuvants combined with or covalently coupled to antigen or hapten. Biol. Chem. Hoppe Seyler 370(4), 343–352. https://doi.org/10.1515/bchm3.1989.370.1.343 (1989).
Zhang, L. et al. Mitochondrial Ca2+ overload leads to mitochondrial oxidative stress and delayed meiotic resumption in mouse oocytes. Front. Cell Dev. Biol. 8, 580876. https://doi.org/10.3389/fcell.2020.580876 (2020).
Balboula, A. Z. et al. Inverse relationship between autophagy and CTSK is related to bovine embryo quality. Reproduction 159(6), 757–766. https://doi.org/10.1530/REP-20-0036 (2020).
Dicks, N. et al. Relief of endoplasmic reticulum stress enhances DNA damage repair and improves development of pre-implantation embryos. PLoS ONE 12(11), e0187717. https://doi.org/10.1371/journal.pone.0187717 (2017).
Acknowledgements
This work was funded by Livestock Promotional Funds of the Japan Racing Association (JRA). The authors would like to thank Genetics Hokkaido for their generous supply of the semen straws used in all the experiments. This work was supported by the Station for Management of Common Equipment, Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Japan.
Author information
Authors and Affiliations
Contributions
D.M., M.M., and A.M. conceived the study and initiated the study design. D.M., I.A., T.H., and K.K. performed the experiment. A.M. provided reagents,materials and analysis tools. D.M., I.A., and M.M. described the manuscript. A.M., K.K., K.I., and M.S. revised the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, D., Akthar, I., Hashimoto, T. et al. Toll-like receptor 2 activation of early divided bovine embryo promotes its viability and development competence in vitro. Sci Rep 15, 24678 (2025). https://doi.org/10.1038/s41598-025-09570-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09570-2