Abstract
Coronary heart disease (CHD) and type 2 diabetes mellitus (T2DM) frequently coexist, significantly elevating the risk of adverse cardiovascular outcomes and mortality. The C-reactive protein-triglyceride-glucose index (CTI), a novel composite marker reflecting both inflammation and insulin resistance, has been associated with poor prognosis in various populations. However, its relationship with all-cause and cardiovascular mortality in patients with coexisting CHD and T2DM remains unclear. Data were obtained from 759 adults with both CHD and T2DM enrolled in the National Health and Nutrition Examination Survey (NHANES) 1999–2010 cycles, with mortality follow-up through December 31, 2019. CTI was calculated and categorized into quartiles. Kaplan-Meier survival analysis, multivariable Cox proportional hazards models, and restricted cubic spline (RCS) analyses were performed to assess the association between CTI levels and risks of all-cause and cardiovascular mortality. Pearson correlation analyses were conducted to evaluate the associations between CTI and key metabolic and inflammatory markers. Subgroup analyses were performed to assess the robustness and consistency of the associations across different clinical strata. During a median follow-up of 109 months, 520 all-cause deaths and 213 cardiovascular deaths were recorded. Higher CTI levels were independently associated with increased risks of both all-cause (HR: 1.68; 95%CI: 1.47–1.92) and cardiovascular mortality (HR: 1.70; 95%CI: 1.38–2.10) after full adjustment (P for trend < 0.001). RCS analysis confirmed a linear dose-response relationship. The association was consistent across subgroups, with stronger effects observed in patients with HbA1c < 7%. CTI also showed significant correlations with multiple metabolic and inflammatory markers.nteraction analyses further revealed that the association between CTI and all-cause mortality was more pronounced in younger individuals (P for interaction = 0.012), while no significant effect modification was observed across cardiovascular-kidney-metabolic stages or frailty status. Findings remained robust in survey-weighted analyses, and CTI also showed strong correlations with a range of metabolic and inflammatory biomarkers, supporting its integrative prognostic utility. Elevated CTI is independently associated with higher all-cause and cardiovascular mortality risks in patients with CHD and T2DM. CTI may serve as a valuable biomarker for risk stratification in this high-risk population. Further studies are warranted to validate its clinical utility.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality worldwide, with coronary heart disease (CHD) and type 2 diabetes mellitus (T2DM) being two of the most prevalent and interrelated contributors to this global burden1. Epidemiological studies have shown that approximately 30–40% of patients with CHD also have T2DM, and individuals with T2DM face a two- to fourfold increased risk of cardiovascular events compared to those without diabetes2,3. The coexistence of CHD and T2DM not only amplifies the risk of adverse cardiovascular outcomes but also complicates clinical management, leading to significantly worse long-term prognosis4,5.
Chronic systemic inflammation and insulin resistance (IR) are well-established pathophysiological mechanisms driving the progression of atherosclerosis and subsequent cardiovascular events, particularly in patients with both CHD and T2DM6,7. C-reactive protein (CRP), a sensitive marker of systemic inflammation, and the triglyceride-glucose (TyG) index, a surrogate marker of insulin resistance, have each been independently associated with increased cardiovascular risk8,9,10. However, relying on a single biomarker may not fully capture the complex interplay between metabolic dysfunction and inflammatory processes in high-risk cardiometabolic populations.
The C-reactive protein-triglyceride-glucose index (CTI) has recently emerged as a novel composite marker that integrates both inflammatory and metabolic components, providing a more comprehensive assessment of cardiovascular risk11. While previous studies have demonstrated the prognostic value of CTI in populations with hypertension, cerebrovascular disease, and cancer12,13,14, evidence regarding its association with mortality outcomes in patients with coexisting CHD and T2DM remains limited.
Therefore, this study aimed to investigate the association between CTI levels and both all-cause and cardiovascular mortality in patients with CHD and T2DM using data from the National Health and Nutrition Examination Survey (NHANES). NHANES is a nationally representative, population-based cohort with standardized protocols and long-term follow-up, enabling robust estimation of risk in diverse U.S. populations. By leveraging this rich dataset and rigorous analytic strategies—including survey-weighted methods—our objective was to evaluate the prognostic value (rather than causal inference) of CTI in predicting mortality outcomes. This study seeks to provide novel insights into the potential utility of CTI as a clinically applicable biomarker for risk stratification in this high-risk population.
Methods
Study design and population
This study utilized data from the NHANES 1999–2010 cycles, a nationally representative, cross-sectional survey conducted by the Centers for Disease Control and Prevention (CDC) in the United States. NHANES is designed to assess the health and nutritional status of the general U.S. population, with an emphasis on various chronic diseases, demographic data, and lifestyle factors. The study was conducted in accordance with the ethical guidelines of the NHANES Research Ethics Review Board, and all participants provided written informed consent. The study design and reporting were strictly aligned with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
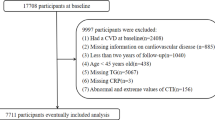
Participants aged ≥ 20 years with diagnosed CHD and T2DM at baseline were included. CHD was defined by self-reported physician diagnosis of angina, myocardial infarction, or coronary heart disease. T2DM was defined as fasting plasma glucose (FPG) ≥ 7.0 mmol/L, glycated hemoglobin (HbA1c) ≥ 6.5%, or self-reported diabetes diagnosis with antidiabetic treatment. Individuals were excluded if they had missing data for C-reactive protein, Triglycerides (TG), fasting plasma glucose, or mortality follow-up information.
A total of 759 participants who met both the CHD and T2DM diagnostic criteria were included in the final analysis. During the follow-up period, 520 participants died from all causes, and 213 died from cardiovascular causes. The study design is illustrated in (Fig. 1).
Assessment of the CTI
The CTI was calculated using the following formula, integrating markers of systemic inflammation and insulin resistance:
CTI = 0.412×Ln(CRP) + Ln(TG [mg/dL]×FPG [mg/dL]/2)15.
The CTI value for each participant was calculated, and participants were categorized into quartiles (Q1-Q4) based on CTI distribution, with Q1 serving as the reference group.
Follow-up and outcomes
Death data were obtained through linkage to the National Death Index (NDI), which provides cause-of-death information for individuals included in the NHANES dataset. The follow-up period for each participant began at the baseline survey and continued until either the date of death or the study cutoff date, December 31, 2019, whichever occurred first. This study assessed both all-cause mortality and cardiovascular mortality. All-cause mortality was defined as death from any cause (010), while cardiovascular mortality specifically referred to death due to heart disease (054–064) or cerebrovascular disease (070).
Covariates
Baseline covariates were selected based on clinical relevance and prior literature regarding cardiovascular risk factors: Demographic factors: Age, Gender, Race, Education level, Marital status, and Poverty-income ratio (PIR). Clinical comorbidities: Hypertension, Heart failure, Stroke, Cancer, Body mass index (BMI), Waist circumference (WC), Systolic and Diastolic blood pressure (SBP, DBP), Drink, Cardiovascular-Kidney-Metabolic syndrome (CKM stage 0–4) and Frailty status (defined using the frailty index [FI] as: FI ≤ 0.10 as non-frail; 0.11–0.21 as pre-frail; >0.21 as frail). Laboratory measurements: HbA1c, Total cholesterol (TC), High-density lipoprotein cholesterol (HDL-C), Serum creatinine (Cr), Blood urea nitrogen (BUN), Uric acid (UA), Albumin (ALB), White blood cell count (WBC), Hemoglobin (HGB), Platelet count (PLT), Mean corpuscular volume (MCV), Lymphocyte count (LYMPH), estimated Glomerular filtration rate (eGFR, calculated using the CKD-EPI equation), triglycerides, fasting plasma glucose, C-reactive protein.
Variables with > 20% missing data were excluded. For variables with less than 20% missing data, we applied random forest imputation (missForest algorithm) to ensure data completeness. This method outperforms traditional approaches such as Multiple Imputation by Chained Equations (MICE) in handling nonlinear relationships, complex interactions, and mixed-type variables16. All variables were obtained through standardized NHANES protocols; definitions and documentation are available at https://www.cdc.gov/nchs/nhanes/.
Statistical analysis
Baseline characteristics were described according to mortality status. Continuous variables were presented as medians with interquartile ranges (IQR) and compared using the Wilcoxon rank-sum test. Categorical variables were expressed as counts and percentages, with comparisons made using the Chi-square test. Kaplan-Meier survival curves and log-rank tests were used to evaluate differences in survival across CTI quartiles. Cox proportional hazards regression models were constructed to assess the association between CTI and mortality outcomes. Three models were developed: Model 1: Unadjusted. Model 2: Adjusted for demographic factors (age, gender, race, education, poverty-income ratio, drink, cardiovascular-kidney-metabolic syndrome, body mass index, waist circumference). Model 3: Further adjusted for clinical comorbidities and laboratory variables (heart failure, frailty status, systolic blood pressure, triglycerides, HbA1c, estimated Glomerular filtration rate, HDL-C). The proportional hazards assumption was verified using Schoenfeld residuals. Restricted cubic spline (RCS) analyses with four knots were conducted to explore potential non-linear relationships between CTI and mortality risk, adjusting for covariates in Model 3. Subgroup analyses were performed stratified by age, gender, body mass index, HbA1c, estimated glomerular filtration rate, and comorbidities. To evaluate potential effect modification, two approaches were used. First, multiplicative interaction terms (e.g., CTI × categorical subgroup variable) were included in the Cox models to test for statistical interaction using Wald statistics, with P values reported. Second, for continuous effect modifiers (age, body mass index, HbA1c, and estimated glomerular filtration rate), additional effect-based interaction analyses were conducted by incorporating CTI × continuous variable terms into multivariable Cox regression models. Marginal effects and hazard ratios of CTI across the continuous variable spectrum were estimated and visualized using model-based marginal plots, enabling a more granular assessment of effect heterogeneity. Given the complex, multistage probability sampling design of NHANES, we incorporated weighted analyses in this study to enhance the national representativeness of our estimates. All model-based analyses accounted for the NHANES survey design, including sample weights, strata, and primary sampling units. Weighted Kaplan–Meier survival curves, Cox proportional hazards models, and subgroup analyses were performed using the survey (version 4.4-2) package in R. All statistical analyses were conducted using R software (version 4.2.2). A two-sided P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics of the participants
A total of 759 participants with coexisting CHD and T2DM were included in the final analysis. During a median follow-up of 109 months, 520 (68.5%) participants died from all causes, and 213 (28.1%) deaths were attributed to cardiovascular causes. As shown in Table 1, compared to survivors, non-survivors of all-cause mortality were older, more likely to be non-Hispanic White, and had a higher prevalence of heart failure and stroke (all P < 0.001). Additionally, they exhibited lower body mass index, diastolic blood pressure, hemoglobin, platelet count, lymphocyte count, and estimated glomerular filtration rate levels, but higher concentrations of blood urea nitrogen, serum creatinine, and C-reactive protein (all P < 0.05). Similar trends were observed among cardiovascular non-survivors. Notably, CTI levels were significantly elevated in both all-cause and cardiovascular death groups (P < 0.001), whereas TyG index levels did not differ significantly between survivors and non-survivors.
Importantly, the distribution of frailty status also varied significantly according to mortality status (P < 0.001 for all-cause and P = 0.049 for cardiovascular mortality), with a higher proportion of frail individuals among non-survivors. Additionally, a trend toward more advanced CKM stages was observed among non-survivors, although the difference was not statistically significant. These findings suggest that both frailty and cardiovascular-kidney-metabolic syndrome progression may contribute to adverse outcomes and warrant further stratified analyses.
Kaplan-Meier survival curves across CTI quartiles
Kaplan-Meier survival curves (Fig. 2) revealed significant differences in both all-cause (Fig. 2A) and cardiovascular mortality (Fig. 2B) across quartiles of the CTI. Participants in the highest CTI quartile exhibited the lowest cumulative survival probabilities. Log-rank tests confirmed significant trends, with progressively higher mortality rates observed from Q1 to Q4 for both outcomes (P for trend < 0.001). These findings suggest a dose-response relationship between elevated CTI and increased mortality risk in patients with CHD and T2DM.
To enhance national representativeness, weighted Kaplan-Meier analyses accounting for the NHANES complex survey design were additionally conducted. The weighted results demonstrated consistent survival trends with the unweighted analysis, further supporting the robustness of our findings (Supplementary Fig. S1).
Cox regression analysis of CTI and mortality outcomes
Multivariate Cox proportional hazards regression models were used to evaluate the association between CTI and mortality (Table 2). After adjusting for potential confounders, CTI was independently associated with increased risk of both all-cause and cardiovascular mortality. For each unit increase in CTI, the hazard ratios (HRs) were 1.68 (95% CI: 1.47–1.92, P < 0.001) for all-cause mortality and 1.70 (95% CI: 1.38–2.10, P < 0.001) for cardiovascular mortality in the fully adjusted model. When CTI was analyzed as a categorical variable, participants in the highest quartile (Q4, ≥ 9.24) had a 3.07-fold increased risk of all-cause death (HR: 3.07, 95% CI: 2.17–4.34, P < 0.001) and a 2.75-fold increased risk of cardiovascular death (HR: 2.75, 95% CI: 1.60–4.72, P < 0.001) compared to those in the lowest quartile (Q1, < 8.18). A significant dose-response relationship was observed across CTI quartiles (P for trend < 0.001).
To ensure the national representativeness of our findings, we additionally performed weighted Cox regression analyses that accounted for the complex sampling design of NHANES. The results remained robust and consistent, with similar effect estimates observed for both continuous and categorical CTI measures (Supplementary Table S1).
Restricted cubic spline analysis of CTI and mortality.
Restricted cubic spline (RCS) analyses were conducted to explore the potential nonlinear relationship between CTI and mortality risk (Fig. 3). In fully adjusted models, a linear and dose-dependent association was observed between CTI levels and both all-cause (Fig. 3A) and cardiovascular mortality (Fig. 3B). The risk of death increased steadily as CTI rose, with no significant evidence of nonlinearity (P for nonlinearity > 0.05 for both outcomes). These findings suggest a proportional relationship between elevated CTI and increased mortality risk in patients with CHD and T2DM.
Subgroup analyses of the CTI-mortality relationship
Subgroup analyses were conducted to examine the robustness of the association between CTI and mortality across clinically relevant strata (Fig. 4). For all-cause mortality (Fig. 4A), the positive association between higher CTI and increased mortality risk remained significant in nearly all subgroups, including those stratified by age, gender, heart failure, stroke, drinking status, body mass index, HbA1c, and estimated glomerular filtration rate, with no significant effect modifications observed (all P for interaction > 0.05). Notably, the association remained significant across all stages of cardiorenal-metabolic syndrome (CKM stage), as well as across frailty categories (frail, pre-frail, and non-frail), although the interaction terms for CKM stage and frailty status were not statistically significant (P for interaction = 0.357 and 0.505, respectively). These findings suggest that the prognostic value of CTI is broadly consistent regardless of comorbidity burden or frailty level.
For cardiovascular mortality (Fig. 4B), similar trends were observed. The association between CTI and cardiovascular death remained robust across most subgroups. While slightly higher effect sizes were observed in participants with better renal function (eGFR ≥ 60 mL/min/1.73 m²) and lower HbA1c levels, none of the subgroup interaction tests reached statistical significance (all P for interaction > 0.05). The association was also maintained across different CKM stages and frailty status levels, further highlighting the generalizability of CTI as a risk indicator across diverse clinical phenotypes.
To explore potential interactions with continuous clinical variables, multiplicative interaction models were constructed for age, body mass index, HbA1c, and estimated glomerular filtration rate (Fig. 5A–H). Age demonstrated a statistically significant interaction with CTI on all-cause mortality (P for interaction = 0.012), with a stronger association in younger individuals. No significant interactions were found for body mass index, HbA1c, or estimated glomerular filtration rate in either outcome.
Additionally, complex survey-weighted subgroup analyses based on NHANES design (Supplementary Fig. S2–S3) yielded results consistent with the unweighted findings, further confirming the stability and robustness of CTI in predicting mortality risk across key subpopulations.
Subgroup analysis of the association between CTI and all-cause and cardiovascularmortality in different populations. (A) All-cause mortality; (B) Cardiovascular mortality; CTI,C-reactive protein-triglyceride-glucose index; BMI, Body mass index; HbA1c, Glycatedhemoglobin; eGFR, estimated glomerular filtration rate.
Interaction effects between CTI and continuous clinical variables on mortality risk. (A-D) Association of CTI with all-cause mortality across levels of age (A), BMI (B), HbA1c (C), and eGFR (D). (E-H) Association of CTI with cardiovascular mortality across levels of age (E), BMI (F), HbA1c (G), and eGFR (H). BMI, Body mass index; HbA1c, Glycated hemoglobin; eGFR, estimated glomerular filtration rate; HR: Hazard Ratio.
Correlation analysis of CTI and clinical indicators
A correlation heatmap was constructed to evaluate the associations between CTI and various demographic, clinical, and biochemical parameters (Fig. 6). As expected, CTI showed significant positive correlations with its core components—C-reactive protein, triglycerides, and fasting plasma glucose. In addition, moderate positive correlations were observed between CTI and body mass index, systolic blood pressure, uric acid, and blood urea nitrogen, suggesting potential links with metabolic dysregulation, renal impairment, and systemic inflammation.
Conversely, CTI was negatively correlated with estimated glomerular filtration rate, HDL-C, and albumin, indicating that elevated CTI levels may reflect deteriorated renal function, poor nutritional status, and adverse lipid profiles. These findings suggest that CTI exhibits concordant variation with multiple conventional mortality-related factors, reinforcing its biological plausibility as a clinically meaningful prognostic biomarker.
Pearson correlation matrix among baseline variables. PIR, Poverty-income ratio; BMI, Body mass index; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; TG, Triglycerides; FPG, Fasting plasma glucose; Cr, Serum creatinine; TC, Total cholesterol; HDL-C, High-density lipoprotein cholesterol; ALB, Albumin; BUN, Blood urea nitrogen; HbA1c, Glycated hemoglobin; UA, Uric acid; eGFR, estimated glomerular filtration rate; WBC, White blood cell count; HGB, Hemoglobin; PLT, Platelet count; MCV, Mean corpuscular volume; LYMPH, Lymphocyte count; CRP, C-reactive protein; TyG, Triglyceride-glucose; CTI, C-reactive protein-triglyceride-glucose index.
Discussion
In this nationally representative cohort study, we identified a significant and independent association between elevated CTI levels and increased risks of both all-cause and cardiovascular mortality in patients with CHD and T2DM. These associations persisted after comprehensive adjustment for demographic, clinical, and biochemical confounders, and exhibited a clear linear dose-response relationship. Subgroup analyses further confirmed the robustness of these findings across various clinical strata, particularly among individuals with better glycemic control (HbA1c < 7%). Furthermore, the predictive utility of CTI remained consistent across stages of cardiorenal-metabolic syndrome and frailty status, with no significant effect modification observed. Despite the absence of statistical interaction, the consistent direction and magnitude of associations suggest that CTI may serve as a stable and generalizable prognostic marker, even among individuals with advanced cardiometabolic or functional impairment. This highlights CTI’s potential to capture the cumulative burden of systemic inflammation and metabolic dysregulation, particularly in high-risk populations where conventional markers may be insufficient.
The underlying mechanisms linking CTI to adverse outcomes likely stem from its ability to simultaneously capture two pivotal pathophysiological processes: systemic inflammation and insulin resistance17. Chronic low-grade inflammation, as reflected by elevated C-reactive protein, plays a central role in endothelial dysfunction, plaque instability, and thrombogenesis, thereby accelerating atherosclerotic progression18. Concurrently, insulin resistance contributes to metabolic dysregulation, promoting hyperglycemia, dyslipidemia, and hypertension, all of which synergistically exacerbate cardiovascular risk19,20. While C-reactive protein and the TyG index have individually been associated with cardiovascular events21,22,23, our findings support that CTI, as a composite marker, offers superior risk stratification by integrating these interrelated pathways. This is consistent with previous studies demonstrating CTI’s predictive value in hypertensive populations and stroke risk24,25, underscoring its broader applicability beyond single-pathway biomarkers.
Compared with prior research focusing on TyG-related indices and cardiovascular outcomes26,27,28, our study uniquely highlights the prognostic relevance of CTI in a high-risk cardiometabolic population characterized by the coexistence of CHD and T2DM. Notably, in contrast to the non-significant association observed between the TyG index and mortality risk in our cohort, CTI consistently demonstrated a strong relationship with both all-cause and cardiovascular mortality. These findings suggest that incorporating inflammatory status into metabolic risk assessment enhances the identification of vulnerable individuals who may not be flagged by traditional markers alone.
From a clinical perspective, CTI represents a simple, cost-effective, and easily accessible biomarker that could be integrated into routine evaluations to improve risk stratification and guide personalized management strategies in patients with CHD and T2DM. Given its derivation from commonly available laboratory parameters, CTI holds promise for widespread application, particularly in resource-limited settings29,30. Regular monitoring of CTI may facilitate early identification of patients at elevated risk, enabling timely implementation of targeted interventions aimed at mitigating inflammation and metabolic dysfunction.
Despite these strengths, several limitations warrant consideration. First, the observational nature of this study precludes causal inference, and residual confounding cannot be entirely excluded. Second, reliance on self-reported diagnoses for CHD and comorbidities may introduce misclassification bias. Third, CTI was assessed only at baseline, preventing evaluation of longitudinal changes and their potential prognostic implications. Finally, the NHANES cohort primarily represents the U.S. population, which may limit generalizability to other ethnicities or regions.
Conclusions
In summary, elevated CTI is independently associated with higher risks of all-cause and cardiovascular mortality in patients with CHD and T2DM. CTI may serve as a valuable biomarker for improving risk stratification and guiding clinical management in this high-risk group. Future prospective studies are warranted to confirm these findings and explore whether CTI-guided interventions can improve patient outcomes.
Data availability
The NHANES data were collected by the NCHS, a division of the CDC of the United States. The data are released for research purposes and can be accessed with permission from the NCHS at https://www.cdc.gov/nchs/nhanes/.
References
Wong, N. D. & Sattar, N. Cardiovascular risk in diabetes mellitus: Epidemiology, assessment and prevention. Nat. Rev. Cardiol. 20 (10), 685–695 (2023).
Sattar, N. et al. Twenty years of cardiovascular complications and risk factors in patients with type 2 diabetes: A nationwide Swedish cohort study. Circulation 147 (25), 1872–1886 (2023).
Kong, D. et al. Bayesian network analysis of factors influencing type 2 diabetes, coronary heart disease, and their comorbidities. BMC Public. Health. 24 (1), 1267 (2024).
Rivas-Garcia, L. et al. Association between diet quality and risk of type 2 diabetes mellitus in patients with coronary heart disease: Findings from the CORDIOPREV study. Nutrients 16 (8), 1249 (2024).
Khawaja, T. et al. Coronary artery calcium, hepatic steatosis, and atherosclerotic cardiovascular disease risk in patients with type 2 diabetes mellitus: Results from the Dallas heart study. Prog Cardiovasc. Dis. 78, 67–73 (2023).
Shaya, G. E., Leucker, T. M., Jones, S. R., Martin, S. S. & Toth, P. P. Coronary heart disease risk: Low-density lipoprotein and beyond. Trends Cardiovasc. Med. 32 (4), 181–194 (2022).
Yang, T. et al. Insulin resistance and coronary inflammation in patients with coronary artery disease: A cross-sectional study. Cardiovasc. Diabetol. 23 (1), 79 (2024).
Arnold, N. et al. C-reactive protein modifies lipoprotein(a)-related risk for coronary heart disease: The biomarcare project. Eur. Heart J. 45 (12), 1043–1054 (2024).
Cui, C. et al. Joint association of TyG index and high sensitivity C-reactive protein with cardiovascular disease: A National cohort study. Cardiovasc. Diabetol. 23 (1), 156 (2024).
Kuppa, A., Tripathi, H., Al-Darraji, A., Tarhuni, W. M. & Abdel-Latif, A. C-Reactive protein levels and risk of cardiovascular diseases: A Two-Sample bidirectional Mendelian randomization study. Int. J. Mol. Sci. 24 (11), 9129 (2023).
Zhou, Y., Lin, H., Weng, X., Dai, H. & Xu, J. Correlation between hs-CRP-triglyceride glucose index and NAFLD and liver fibrosis. BMC Gastroenterol. 25 (1), 252 (2025).
Huo, G. et al. Association between C-reactive protein-triglyceride glucose index and stroke risk in different glycemic status: Insights from the China health and retirement longitudinal study (CHARLS). Cardiovasc. Diabetol. 24 (1), 142 (2025).
Ruan, G. T. et al. Prognostic importance of an indicator related to systemic inflammation and insulin resistance in patients with Gastrointestinal cancer: A prospective study. Front. Oncol. 14, 1394892 (2024).
Zhao, D. F. Value of C-Reactive Protein-Triglyceride glucose index in predicting Cancer mortality in the general population: Results from National health and nutrition examination survey. Nutr. Cancer. 75 (10), 1934–1944 (2023).
Ruan, G. T. et al. A novel inflammation and insulin resistance related Indicator to predict the survival of patients with Cancer. Front. Endocrinol. (Lausanne). 13, 905266 (2022).
Hu, J. & Szymczak, S. A review on longitudinal data analysis with random forest. Brief. Bioinform. 24 (2), bbad002 (2023).
Li, W. et al. Systemic immune inflammation index with all-cause and cause-specific mortality: A meta-analysis. Inflamm. Res. 73 (12), 2199–2216 (2024).
Melnikov, I. et al. Monomeric C-Reactive protein in atherosclerotic cardiovascular disease: Advances and perspectives. Int. J. Mol. Sci. 24 (3), 2079 (2023).
Di Pino, A. & DeFronzo, R. A. Insulin resistance and atherosclerosis: Implications for Insulin-Sensitizing agents. Endocr. Rev. 40 (6), 1447–1467 (2019).
Zhang, B. et al. Association between C-reactive protein-triglyceride glucose index and testosterone levels among adult men: Analyses of NHANES 2015–2016 data. Sex. Med. 13 (1), qfaf012 (2025).
Huang, C. et al. Association between C-reactive protein-triglyceride glucose index and depressive symptoms in American adults: Results from the NHANES 2005 to 2010. BMC Psychiatry. 24 (1), 890 (2024).
Tang, X., Zhang, K. & He, R. The association of triglyceride-glucose and triglyceride-glucose related indices with the risk of heart disease in a National. Cardiovasc. Diabetol. 24 (1), 54. https://doi.org/10.1186/s12933-025-02621-y (2025). Erratum in: Cardiovasc Diabetol. 2025;24(1):174.
Li, C. et al. The triglyceride-glucose index and its obesity-related derivatives as predictors of all-cause and cardiovascular mortality in hypertensive patients: Insights from NHANES data with machine learning analysis. Cardiovasc. Diabetol. 24 (1), 47 (2025).
Tang, S. et al. C-reactive protein-triglyceride glucose index predicts stroke incidence in a hypertensive population: A National cohort study. Diabetol. Metab. Syndr. 16 (1), 277 (2024).
Liu, J. et al. Deep learning-based segmentation of acute ischemic stroke MRI lesions and recurrence prediction within 1 year after discharge: A multicenter study. Neuroscience 565, 222–231 (2025).
Dang, K. et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018. Cardiovasc. Diabetol. 23 (1), 8 (2024).
Tao, L. C., Xu, J. N., Wang, T. T., Hua, F. & Li, J. J. Triglyceride-glucose index as a marker in cardiovascular diseases: Landscape and limitations. Cardiovasc. Diabetol. 21 (1), 68 (2022).
Che, B. et al. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: An analysis of UK biobank data. Cardiovasc. Diabetol. 22 (1), 34 (2023).
Ren, Y. et al. Association between the C-reactive protein-triglyceride-glucose index and endometriosis: A cross-sectional study using data from the National health and nutrition examination survey, 1996–2006. BMC Womens Health. 25 (1), 13 (2025).
Xu, M., Zhang, L., Xu, D., Shi, W. & Zhang, W. Usefulness of C-reactive protein-triglyceride glucose index in detecting prevalent coronary heart disease: Findings from the National health and nutrition examination survey 1999–2018. Front. Cardiovasc. Med. 11, 1485538 (2024).
Acknowledgements
The authors gratefully acknowledge the National Health and Nutrition Examination Survey (NHANES) for providing open-access data used in this study. We also extend our sincere thanks to all participants involved in the survey for their valuable contributions.
Funding
None.
Author information
Authors and Affiliations
Contributions
Ya’nan Hu, Qiang Zhou and Xuejin Chen collected the relevant information; Haoran Li and Lele Wang performed the statistical analysis; Shizhong Cheng performed the data visualization; Nan Tang wrote the manuscript, which was subsequently revised by Qingdui Zhang, Ji Hao, and Chunmei Qi. All the authors contributed to the discussion and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
NHANES was conducted with approval by the National Center for Health Statistics Ethics Review Board, and obtained informed written consent from all the individuals involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Author details.
1Department of Cardiology, The Second Affiliated Hospital of Xuzhou Medical University, Xuzhou, 221000, China.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, N., Chen, X., Li, H. et al. Association of C reactive protein triglyceride glucose index with mortality in coronary heart disease and type 2 diabetes from NHANES data. Sci Rep 15, 24687 (2025). https://doi.org/10.1038/s41598-025-10184-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10184-x
Keywords
This article is cited by
-
Associations of metabolic indicators and inflammation-related indices with adverse cardiovascular events in US adults: NHANES 1999–2018
Lipids in Health and Disease (2026)
-
Prognostic value of the CRP–TyG index for in-hospital and 1-year mortality in patients with decompensated heart failure
BMC Cardiovascular Disorders (2026)
-
Study of the prognostic value of the systemic immune-inflammation index combined with the triglyceride-glucose index in coronary heart disease: a retrospective study based on the MIMIC database
BMC Cardiovascular Disorders (2026)
-
Association between C-reactive protein-triglyceride glucose index (CTI) and cardiovascular and all-cause mortality risk among the elderly population: insights from three datasets
Clinical Research in Cardiology (2026)
-
C-reactive protein–triglyceride glucose index predicts mortality in cardiovascular-kidney-metabolic syndrome stage 0–3: a prospective cohort study
Diabetology & Metabolic Syndrome (2025)