Abstract
The transcription factor Forkhead box protein O1 (FoxO1) is a well-established regulator of glucose and lipid metabolism, yet its role in metabolic dysfunction-associated steatohepatitis (MASH) pathogenesis remains debated. This study investigates hepatocyte-specific FoxO1 mechanisms driving hepatic inflammation in MASH. Using hepatocyte-specific FoxO1-knockout (KO) mice fed a methionine-choline-deficient diet and LPS-treated FoxO1-KO cells, we demonstrated that FoxO1 depletion exacerbates hepatic inflammation, ballooning degeneration, and upregulates TNF-α, CXCL8, and CXCL2 in vivo and in vitro. Transcriptomic analysis revealed that FoxO1 deficiency upregulated pro-inflammatory pathways while suppressing cysteine/methionine metabolism, with a notable reduction in cystathionine γ-lyase (CTH) expression. Luciferase assays confirmed that FoxO1 directly binds the CTH promoter. In MASH mice, reduced CTH levels correlated with elevated TNF-α/CXCL8. Pharmacological CTH inhibition via β-cyano-L-Alanine (BCA) amplified LPS-induced inflammation in THLE-2 cells, while CTH overexpression rescued inflammatory responses in FoxO1-deficient hepatocytes. Our findings unveil FoxO1 as a transcriptional activator of CTH, coupling metabolic adaptation to inflammatory regulation in MASH, and propose the FoxO1-CTH axis as a therapeutic target for inflammatory liver disease.
Similar content being viewed by others
Introduction
Metabolic dysfunction-associated steatohepatitis (MASH) is the advanced stage of MAFLD (metabolic dysfunction-associated fatty liver disease), which progresses from simple steatosis (NAFL) with an inflammatory response. According to epidemiological statistics, the incidence of MAFLD worldwide has reached 30% and is increasing year by year1. Currently, there are more than 300 million MAFLD patients in China, and the growth rate is expected to exceed 30% in the next 10 years2. As a progressive disease, MAFLD-MASH can develop into more severe liver cirrhosis and hepatocellular carcinoma3. Previous publication has reported that the damaged intestinal vascular barrier and increased intestinal permeability lead to gut bacteria and their metabolites migrating into the liver, a prerequisite for inducing the progression of MASH4. Hepatocytes, the primary parenchymal cells in the liver, are responsible for lipid metabolism, protein synthesis, nutrient storage, and liver inflammation5. By sensing microbial products, such as lipopolysaccharide (LPS), hepatocytes can produce and secrete various mediators to regulate liver inflammation through intercellular communication6. Thus, elucidating the regulatory mechanism of inflammatory signals in hepatocytes can offer insights into the treatment of MASH.
The O-class of Forkhead Box Protein 1 (FoxO1) is widely expressed in adipocytes, hepatocytes, intestinal epithelial cells, pancreatic β cells, and immune cells. FoxO1 can regulate the expression of downstream genes as a transcription factor by targeting the promoters of the conserved DNA-binding domain CAAAACAA. In the liver, FoxO1 has emerged as a crucial regulator of many cellular processes, including gluconeogenesis7, glycogenolysis, adipogenesis8, low-density lipoprotein production9, and apoptosis10. FoxO1 depletion decreased the transcription of glucose 6-phosphatase (G6PC) and phosphoenolpyruvate carboxykinase (PEPCK), which consequently inhibited glucose production in the liver11,12. Recent publications have reported on the role of FoxO1 in hepatic inflammation within immune cells. Lymphocyte FoxO1 regulated the Th17/Treg cytokine imbalance in ischemia–reperfusion injury in the liver13. Myeloid FoxO1 depletion attenuated hepatic inflammation and protected mice from developing nonalcoholic steatohepatitis (NASH)14. However, the specific role of hepatocyte FoxO1 in hepatic inflammation remains unclear.
In this study, we established a FoxO1 knockout (FoxO1-KO) HepG2 cell line in vitro and specific hepatocyte FoxO1 knockout mice in vivo to identify the roles of hepatocyte FoxO1 in hepatic inflammatory responses. We found that depletion of FoxO1 enhanced pro-inflammatory responses. Next-generation sequencing (NGS) and KEGG pathway analysis revealed that cysteine and methionine metabolism-associated genes were significantly downregulated in FoxO1-KO cells. Notably, FoxO1 directly targeted the promoter region of cystathionine γ-lyase (CTH), an important enzyme involved in cysteine and methionine metabolism. Moreover, we verified that FoxO1 regulated hepatic inflammatory responses through targeting CTH.
Materials and methods
Cell culture and reagents
The human hepatocyte cell line HepG2, including wild-type (WT) and FoxO1-KO cells, was bought from Saiye (Suzhou) Biotechnology Co., Ltd15. THLE-2 cells were obtained from Procell Life Science & Technology Co., Ltd. These cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Cat. No: 164210-50, Procell Life Science & Technology, China) and 1% antibiotic (Art. No.: 15070063, Gibco). The cells were collected for performing MTT assays, qRT-polymerase chain reaction (PCR), western blotting, and dual-luciferase reporter gene assays after treatment with 0, 1, or 2 μg/mL LPS (Cat. # L2880-10MG, Sigma-Aldrich), or 1 mM β-cyano-L-Alanine (BCA, No.10010947, Cayman) for 24 h. For recovery assay, an siRNA targeting FoxO1 or an NC siRNA at a final concentration of 10 nM was co-transfected into THLE-2 cells with an empty vector (EV) or CTH expression plasmid for 48 h, followed by treatment with or without LPS for 24 h.
Generation of hepatocyte-specific FoxO1 knockout mice
Conditional FoxO1 knockout C57BL/6N mice were developed at Cyagen Biosciences Inc. (Suzhou, China) using the Cre-loxP recombination system. FoxO1 flox mice were generated by CRISPR-Cas9 targeted insertion of loxP recombination sites into introns flanking exon 2 of FoxO1, excision of exon 2 (FoxO1 Δ2) should cause severe carboxy-terminal deletion of the FoxO1 protein and/or elimination of the mRNA by nonsense-mediated decay. The Cas9 protein, two sgRNAs (sgRNA1: GGGCCCATAGGGTAACATAAAGG; sgRNA2: GCAAACTGATAAACGGAGGGAGG), and the targeting vector (containing exon 2 flanked by two loxP sites and the two homology arms) were co-injected into fertilized eggs. The injected embryos were cultured in KSOM medium overnight, and those that developed to the two-cell stage were transferred into the oviduct of pseudo-pregnant ICR female mice. The F0 founder mice were identified by PCR analysis using a pair of primers (F1: 5′-AATAGATCCTGCTTCATCTCCCTTT-3′, R1: 5′-GAGATCGCAAGTTGATGGAGGAAA-3′) and sequencing, which were bred to wild-type mice to test germ-line transmission and F1 animal generation. The genotype F1 mice were also confirmed by PCR. FoxO1-flox mice were then cross-mated with albumin-Cre mice to generate FoxO1Δhep mice, of which the FoxO1 gene was functionally inactivated due to the deletion of exon 2 through Cre-loxP recombination. The experiments were conducted using homozygous conditional FoxO1 knockout mice.
MASH mouse model
Male C57BL/6 mice (8 weeks old), including WT and hepatocyte-specific FoxO1-KO mice, were obtained from Saiye (Suzhou) Biotechnology Co., Ltd. These mice were housed in a controlled environment (22 °C with a 12/12 h light/dark cycle) and provided water and standard rodent diet. After adaptive feeding, the mice were fed with a methionine choline-deficient diet (MCD, TP36225, Trophic Animal Feed High-Tech Co., Ltd, China) for 6 weeks to induce the MASH model. Methionine/choline supplementation (MCS, TP36225, Trophic Animal Feed High-Tech Co., Ltd, China) diet was used in the control groups. At the end of the experiment, liver and serum samples were collected for pathological and ELISA analysis, respectively. The NAFLD activity scores were used for evaluation as previous publication16. Serum levels of TNFα and CXCL8 were detected by ELISA following the manufacturer’s instructions (Beyotime).
Transcriptome sequencing
Total RNA was extracted using a Trizol reagent kit (Cat. # 15596026CN, Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. RNA quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and checked using RNase-free agarose gel electrophoresis. After the total RNA was extracted, eukaryotic mRNA was enriched using Oligo (dT) beads. Then, the enriched mRNA was broken into short fragments using the fragmentation buffer and reverse transcribed into cDNA using the NEBNext Ultra RNA Library Prep Kit for Illumina (NEB #7530, New England Biolabs, Ipswich, MA, USA). The purified double-stranded cDNA fragments were end-repaired, and a base was added and ligated to Illumina sequencing adapters. The ligation reaction was purified using AMPure XP Beads (1.0X). Amplification was performed via the PCR process. The resulting cDNA library was sequenced using Illumina Novaseq6000 at Gene Denovo Biotechnology Co. (Guangzhou, China). Significantly altered genes (|Fold Change|≥ 2, P< 0.05) underwent KEGG pathway enrichment analysis17,18.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from treated cells using Trizol and reverse transcribed into cDNA (Prime Script RT Reagent Kit (Cat. # RR037A, Takara, Otsu, Shiga, Japan)). Then, qRT-PCR was performed using the SYBR® Premix ExTaqTM (Cat. # RR820A, Takara, Otsu, Shiga, Japan). GAPDH was selected as the endogenous control gene. Subsequently, the relative gene expression levels were calculated using the comparative Ct method. All the primer sequences are shown in Table 1.
Western blotting
Proteins were extracted from treated HepG2 WT and FoxO1-KO cells using RIPA Lysis and Extraction Buffer (Cat. # 89900, Thermo Scientific) supplemented with protease inhibitors (Cat. # KGB5101-100, KeyGEN BioTECH). The same amount of protein in each sample was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a PVDF membrane. Protein blotting was performed at 4 ℃ using 5% skimmed milk powder for blocking, after which proteins were incubated with the primary antibody (diluted 1:1000). The proteins were incubated with a secondary antibody (diluted 1:5000) 24 h later at room temperature for one hour. The primary antibodies used are as follows: inducible nitric oxide synthase (iNOS) (Cat. # 20609, CST), TNFα (Cat. # 8184S, CST), CTH (# ab151769, Abcam). The secondary antibodies are goat anti-rabbit IgG and human ads HRP (Cat. # 7074, CST). Finally, the enhanced chemiluminescence detection reagent (Santa Cruz) was used to visualize the bands.
Dual-luciferase reporter gene assay
A human CTH-luciferase reporter plasmid was constructed by synthesizing the CTH promoter region (− 1000 ~ + 27) and inserting it into the pGL6-Basic vector (Beyotime Biotechnology). HepG2 WT and FoxO1 KO cells were cultivated separately to achieve a density of 70%. Currently, pGL6-CTH Luc and pRL-TK (as a control for transfection efficiency) were co-transfected into cells using Lipofectamine 3000 (Cat. # L3000075, Thermo Fisher Scientific). After incubating the cells for 6 h, the liquid was replaced with a fresh culture medium, and the cells were cultured for 48 h. Luciferase assays were performed with cell lysates prepared using the Dual-Luciferase reporter assay kit (Cat. # E1910 and E1960, Promega) per the manufacturer’s protocols.
Statistical analysis
At least three independent experiments were performed in this study. Results were analyzed using GraphPad Prism (La Jolla, CA, USA) software, and all data were compared between the two groups using Student’s t-test. Differences were considered statistically significant if the P value was less than 0.05. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Results
Depletion of hepatocyte FoxO1 aggravated MASH progression in vivo
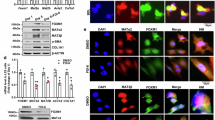
To investigate the effects of hepatocyte FoxO1 on MASH, we constructed hepatocyte-specific FoxO1 knockout mice and detected knockout efficiency by qPCR and Western Blot (Fig. 1A, B). The animal model was induced by feeding with the MCD diet, and the MCS diet was used as a control. Pathological results showed that hepatocyte FoxO1 knockout significantly aggravated lobular inflammation, ballooning change, and collagen fiber deposition in both the control diet group (MCS) and MASH modeling group (MCD) (Fig. 1C, D). Furthermore, serum levels of ALT and AST were significantly higher in FoxO1-KO mice than in the WT group (Fig. 1E, F). These data suggested that Depletion of hepatocyte FoxO1 aggravated liver injury and inflammation in the MASH mouse model.
Depletion of hepatocyte FoxO1 aggravated MCD diet-induced MASH in vivo. (A and B) The knockout efficiency of FoxO1 in liver tissue was detected by qPCR and western blotting. (C) Representative images of H&E staining, Oil red staining, and Masson staining in WT and hepatocyte-specific FoxO1-KO mice. (D) Non-alcoholic fatty liver disease activity scores NAFLD activity scores were calculated. (E and F) Serum levels of alanine transaminase (ALT) and aspartate aminotransferase (AST). Statistical analysis was performed using the Student’s t-test. *, P < 0.05; ***, P < 0.001.(n = 5).
FoxO1 depletion enhanced inflammatory cytokines and chemokines in vitro
To dissect the molecular pathways underlying the observed phenotype, we established the FoxO1 knockout HepG2 cell line (referred to as FoxO1-KO) and detected knockout efficiency by Western Blot (Fig. 2A). Considering that bacterial compontent from gut is a critical trigger for MASH progression4, the cell model was established in a LPS-induced manner. To compare the whole cellular responses between WT and FoxO1-KO HepG2 cells, we treated HepG2 cells in duplicate with 2 µg/mL LPS for 24 h. Subsequently, we isolated total cellular RNA for transcriptome sequencing. Principal component analysis showed that the cellular transcriptome profiles of three duplicates were highly similar but had diverged between WT and FoxO1-KO cells (Fig. 2B). A volcano plot showed that a total of 559 genes were differentially regulated (DEGs), meeting the criteria of false discovery rate below 0.05 and absolute fold change ≥ 2. Among these genes, 228 were up-regulated and 331 were down-regulated in FoxO1-KO cells, as compared to WT cells (Fig. 2C). In addition, we found that the expression of pro-inflammatory molecules, including the interleukins and their receptors (IL32, IL1R2, IL21R), chemokine (C-X-C motif) ligand (CXCL) family (CXCL2 and CXCL8), and tumor necrosis factor (TNF) ligand and receptor superfamily (TNFRSF19 and TNFRSF21) (Fig. 2D), was significantly increased in FoxO1-KO cells. To confirm the NGS data, RT-PCR and Western Blot analysis were used to detect the mRNA and protein levels of pro-inflammatory cytokines. Compared to WT HepG2, TNFa, CXCL8, and CXCL2 mRNA levels were substantially increased in both control and LPS-treated FoxO1-KO cells (Fig. 3A–C). Furthermore, FoxO1 depletion enhanced the protein levels of TNFα and inducible nitric oxide synthase (iNOS), which are directly attributable to the inflammatory process (Fig. 3D). Taken together, these results suggest that FoxO1 depletion enhanced pro-inflammatory responses in hepatocytes in vitro.
The effects of FoxO1 on hepatic inflammation in vitro were detected by transcriptome sequencing. (A) FoxO1 gene knock-out efficiency was detected by Western blot. (B) Principal component analysis (PCA) revealed the relationships between samples in the LPS-induced WT and FoxO1-KO groups. (C) A volcano plot showed differentially expressed genes (DEGs), along with their statistical significance values and fold changes. Up-regulated or down-regulated DEGs are shown in red or yellow dots, respectively. (D) A heat map-based representation of inflammation-related DEGs in LPS-treated WT and FoxO1-KO cells.
Effects of FoxO1 on hepatic inflammation were confirmed in WT and FoxO1-KO cells. WT and FoxO1-KO cells were treated with 0, 1, or 2 µg/mL LPS for 24 h. (A–C) The relative mRNA expression levels of TNFa, CXCL8, and CXCL2 were detected via qRT-PCR. GAPDH was used as a reference gene. (D) The protein expression levels of iNOS and TNFα were detected by western blotting. β-tubulin was used as the control for loading. The results are presented in triplicate as means ± SD values (n = 3). Statistical analysis was performed using the Student’s t-test. *, P < 0.05; **, P < 0.01.
FoxO1 depletion decreased the expression of cysteine and methionine metabolism-associated genes
To explore the mechanisms by which FoxO1 regulates hepatic inflammation, we analyzed enriched pathways in up-regulated and down-regulated gene sets against the KEGG pathway database. The up-regulated genes are involved in the production of TNF, NF-kappa B, NOD-like receptor, and the MAPK signaling pathway, which confirms the critical role of FoxO1 in mediating host inflammatory responses (Fig. 4A). Notably, the topmost significantly enriched KEGG pathway among the downregulated DEGs was that associated with cysteine and methionine metabolism (Fig. 4B). The P value of cysteine and methionine metabolism enrichment was 0.00005. For this pathway analysis, FoxO1 depletion significantly inhibited the expression of seven genes (Fig. 4C). A previous study showed that cysteine exhibited anti-inflammatory effects in human coronary arterial endothelial cells19. Thus, we hypothesized that FoxO1 might regulate hepatic inflammation through the cysteine and methionine metabolism-associated pathway.
FoxO1 could target the promoter region of cystathionine γ-lyase
We then performed qRT-PCR and western blotting to verify the role of FoxO1 in CTH expression. Consistent with the transcriptome sequencing results, the mRNA and protein levels of CTH were decreased in FoxO1-KO cells (Fig. 5A, B). To identify the gene targeted directly by FoxO1, the promoter sequences of the above seven cysteine and methionine metabolism-associated genes were downloaded from NCBI, and transcription factor binding sites were analyzed by JASPAR (https://jaspar.genereg.net/). The predicted data showed that the potential binding sites of FoxO1 were in regions with positions ranging from − 388 to − 379, − 31 to − 18, and − 2 to + 10 of the CTH promoter (Fig. 5C). To validate whether FoxO1 could directly bind to the CTH promoter, a fragment of the CTH promoter region (− 1000 ~ + 27) was cloned into the pGL6 basic luciferase vector (pGL6-luc). Empty pGL6-CTH-luc and pGL6-luc vectors were transfected into WT or FoxO1-KO cells. We found that the promoter activity of CTH was significantly lower in FoxO1-KO cells than in WT cells. However, the transiently co-transfected FoxO1 expression plasmid could facilitate the recovery of luciferase activity in FoxO1-KO cells (Fig. 5D).
FoxO1 targets the promoter region of CTH. (A) The relative CTH mRNA expression levels in HepG2 cells were detected via qRT-PCR. GAPDH was used as an internal control. (B) The protein levels of CTH were detected via Western blot. β-actin was used as a loading control. (C) Predicted binding sites of FoxO1 in the promoter region of CTH are highlighted in yellow. (D) WT and FoxO1-KO cells were transfected with the control vector or pGL6-CTH-Luc, using pRL-TK as a control to assess transfection efficiency. FoxO1-KO cells were also transfected with or without FoxO1 expression plasmids (overexpression, OE). Cells were collected after 48 h and analyzed for dual-luciferase activities. Mean ± standard error of the mean (SEM) values were derived from three independent experiments. Statistical analysis was performed using the Student’s t-test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To further verify the correlation between FoxO1 and CTH from the cell experiments to the in vivo model, the expression of CTH and cytokines was detected in the MASH mouse model. Consistent with the in vitro data, the expression of CTH was significantly reduced in the liver tissue of FoxO1-KO mice (Fig. 6A, B). Additionally, serum levels of proinflammatory cytokines, TNFα and CXCL8, were increased dramatically in FoxO1-KO mice (Fig. 6C, D), which suggested that hepatocyte FoxO1 contributed to liver inflammation in vivo. Taken together, these results suggest that FoxO1 regulates CTH expression by directly targeting its promoter region.
The effects of hepatocyte FoxO1 on CTH and inflammatory cytokines in vivo. (A) The relative CTH mRNA expression levels in mouse liver tissues were detected via qRT-PCR. GAPDH was used as an internal control. (B) The protein levels of CTH in vivo were detected via Western blot. β-actin was used as the control for loading. (C and D) Serum levels of TNFα and CXCL8 were detected by ELISA. Statistical analysis was performed using the Student’s t-test. *, P < 0.05.
Hepatocyte FoxO1 depletion exacerbates inflammatory responses through CTH
Previous studies reported that CTH regulated inflammatory responses in human keratinocytes and rat kidney tissues20,21. To investigate the role of CTH in hepatic inflammation, β-cyano-L-Alanine (BCA), a CTH-specific reversible inhibitor, was used to identify its functions. As shown in Fig. 7A, LPS stimulation reduced the mRNA level of CTH, while co-treatment with 1 mM BCA further downregulated its expression relative to both control and LPS-only groups. Furthermore, BCA treatment promoted the expression of pro-inflammatory cytokines, TNFα, CXCL8, CXCL2, and IL-32 at the mRNA level (Fig. 7B–E). To verify whether FoxO1 regulates the expression of inflammatory cytokines through CTH, we transiently transfected CTH overexpression plasmids into FoxO1-KO cells and detected CTH mRNA level by qRT-PCR (Fig. 7F). As expected, CTH overexpression significantly inhibited the expression of TNFα, CXCL8, CXCL2, and IL-32 in both control and LPS-treated FoxO1-KO cells (Fig. 7G–J).
FoxO1 regulates inflammation responses through CTH. (A–E) WT HepG2 cells were treated with 2 µg/mL LPS alone or a combination of LPS and 1 mM BCA for 24 h. Relative expression levels of CTH, TNFα, CXCL8, CXCL2, and IL-32 were evaluated by qRT-PCR. (F–J) WT and FoxO1-KO cells were transfected with an empty vector or CTH expression plasmid. Cells were treated with LPS after 24 h, and relative mRNA levels of CTH, TNFα, CXCL8, CXCL2, and IL-32 were detected via qRT-PCR. GAPDH was used as a reference gene. Data represent the mean ± SEM values of three independent experiments. ns, no significance; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; as determined by the Student’s t-test.
To validate the effect of FoxO1-CTH on hepatic inflammation, another liver cell line, THLE-2 cells, was used to further confirm the effects of FoxO1 and CTH on hepatic inflammation. An siRNA targeting FOXO1 was co-transfected with or without CTH expression plasmid into THLE-2 cells, followed by treatment with or without LPS for 24 h. Consistent with the above results, knockdown of FoxO1 enhanced LPS-induced TNFα, CXCL8, and IL-32 expression. Over-expression of CTH restores the mRNA level of pro-inflammatory cytokines (Fig. 8A–C). Taken together, these data suggest that FoxO1 regulates the expression of hepatic inflammatory cytokines through CTH.
FoxO1 regulates hepatic inflammation through CTH in THLE-2 cells. (A–C) THLE-2 cells were co-transfected with si FOXO1 and/or CTH expression plasmid. Forty-eight hours later, cells were treated with 2 µg/mL LPS after 24 h, and relative mRNA levels of TNFα, CXCL8, and IL-32 were detected via qRT-PCR. GAPDH was used as a reference gene. Data represent the mean ± SEM values of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Discussion
In the last three decades, MAFLD has emerged as the most widespread chronic liver disease. The high global incidence and prevalence rates of MAFLD-MASH pose an increasingly heavy burden on global public health3. Clinical evidence has shown that the intrahepatic expression of FoxO1 is correlated with advanced liver disease progression 22. Nonetheless, the mechanism underlying the role of FoxO1 in hepatic inflammation remains unclear. Herein, we demonstrated that hepatocyte FoxO1 depletion aggravated the progression of MASH in vivo. Furthermore, FoxO1-KO in the cell line disrupted the expression of cysteine and methionine metabolism-associated genes, and particularly inhibited CTH expression levels. This effect contributed to increased levels of hepatic inflammation. In summary, we unveiled CTH as a new target of FoxO1 in the regulation of hepatic inflammation.
Hepatocytes act as central drivers of inflammation in MAFLD, rather than merely serving as passive victims of inflammatory processes. During MAFLD progression to MASH, these cells produce diverse cytokines and immunomodulators that critically regulate both hepatic inflammation and repair mechanisms. The expression of IL-32 was markedly upregulated in NAFLD patients, and its expression correlated with liver damage and steatosis23. This process is crucial for the inflammatory response as it facilitates the production of IL-1β and tumor necrosis factor-alpha (TNF-α). Thus, IL-32 was considered to be a novel candidate biomarker of MAFLD. Furthermore, hepatocytes can also produce various chemokines that recruit immune cells in response to stress to the liver. CXCL2 is an important gene associated with MASH progression24. In a concanavalin A (ConA)-induced hepatitis model, hepatocyte CXCL2 regulated liver inflammatory signals by recruiting neutrophils25. The intrahepatic expression of CXCL8 is elevated in NAFLD patients, which enhances neutrophil recruitment by triggering the AKT/mTOR/STAT signaling pathway26. Our study observed that pro-inflammatory cytokines and chemokines, including TNF-α, IL-32, CXCL2, and CXCL8, were up-regulated in FoxO1-KO cells.
FoxO1 has garnered widespread attention recently due to its crucial role in immune and metabolic disorders. Several studies have reported the diverse effects of FoxO1 on the inflammatory responses of different cell types. FoxO1 skewed macrophage polarization towards the pro-inflammatory M1 phenotype, exacerbating NASH progression14. However, lymphocyte FoxO1 reduced the polarization of Th17 cells and protected the liver from injury in liver ischemia and reperfusion injury (IRI)13,27. In a CCL4-induced liver fibrosis model, Pan et al. revealed that a hepatocyte FoxO1 deficiency reduced inflammation and protected against liver fibrosis28. Nevertheless, in a diet-induced NAFLD mouse model, hepatic FoxO1/3/4 deletion accelerated the pathogenesis of liver fibrosis and enhanced liver inflammation29. These controversial observations indicate that FoxO1 has diverse effects on liver inflammatory responses in different models. In this study, we presented clear evidence showing that hepatocyte FoxO1 mediates LPS-induced inflammation in FoxO1-KO cells.
CTH is a crucial enzyme in the methionine transsulfuration pathway, operating in conjunction with other enzymes as a unified system to facilitate cysteine synthesis and simultaneous hydrogen sulfide (H2S) production in mammals30. The anti-inflammatory effects of CTH have been reported in kidney and intestinal ischemia–reperfusion injury, rheumatoid arthritis (RA), and sub-cytotoxic formaldehyde-induced inflammation20,21,31. In intestinal ischemia–reperfusion injury (I/R), inhibition of CTH results in upregulation of proinflammatory cytokines such as IL-6 and TNF-α32. In RA, endogenous CTH exhibits powerful anti-inflammatory effects by modulating the transcription factor Sp-1 to suppress the expression of Jumonji domain-containing protein 3 (JMJD3), thereby affecting histone methylation modifications associated with transcriptional repression31. In osteoarthritis (OA) models, CTH expression is negatively correlated with levels of IL-1β and other inflammatory markers; CTH overexpression suppresses inflammation and promotes cartilage repair33. In bovine mammary epithelial cells (MAC-T), LPS treatment suppresses CTH expression and H₂S production, leading to enhanced inflammation, whereas overexpression of CTH or administration of NaHS (an H₂S donor) attenuates inflammation by inhibiting IL-8 expression34. With regard to chronic liver disease, Xu et al. found that the CTH protein level was significantly down-regulated in the liver of patients with NAFLD, especially in the fibrotic areas35. Furthermore, CTH was shown to promote a post-translational modification (sulfhydration) of the farnesoid X receptor (FXR), a well-known metabolic nuclear receptor that regulates lipid and glucose metabolism, cholesterol secretion, and bile acid biosynthesis36. Our finding provides more evidence regarding the anti-inflammatory role of CTH in hepatocytes. Importantly, CTH was identified to be a direct target of FoxO1.
In conclusion, the presented data verified that FoxO1 is a key regulator of hepatic inflammatory responses in MASH progression. The anti-inflammatory role of FoxO1 mainly stems from its modulation of cysteine and methionine metabolism. Moreover, we demonstrated for the first time that FoxO1 is directly bound to the promoter region of CTH, thereby inhibiting inflammatory cytokine and chemokine expression. Our study has provided novel insights into the molecular mechanism by which hepatic FoxO1 regulates inflammatory responses, which provides a potential therapeutic approach for controlling inflammation in the progression of MASH.
Data availability
The datasets generated and analyzed during the current study are available in the GEO database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE293003), with the accession number GSE293003. All data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Vaz, K. et al. Current understanding and future perspectives on the impact of changing NAFLD to MAFLD on global epidemiology and clinical outcomes. Hepatol. Int. 17, 1082–1097. https://doi.org/10.1007/s12072-023-10568-z (2023).
Lu, R., Liu, Y. & Hong, T. Epidemiological characteristics and management of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis in China: A narrative review. Diabetes Obes. Metab. 25(Suppl 1), 13–26. https://doi.org/10.1111/dom.15014 (2023).
Thomas, J. A., Kendall, B. J., El-Serag, H. B., Thrift, A. P. & Macdonald, G. A. Hepatocellular and extrahepatic cancer risk in people with non-alcoholic fatty liver disease. Lancet Gastroenterol. Hepatol. 9, 159–169. https://doi.org/10.1016/S2468-1253(23)00275-3 (2024).
Mouries, J. et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 71, 1216–1228. https://doi.org/10.1016/j.jhep.2019.08.005 (2019).
Seki, E. & Schwabe, R. F. Hepatic inflammation and fibrosis: Functional links and key pathways. Hepatology 61, 1066–1079. https://doi.org/10.1002/hep.27332 (2015).
Wree, A., Holtmann, T. M., Inzaugarat, M. E. & Feldstein, A. E. Novel drivers of the inflammatory response in liver injury and fibrosis. Semin. Liver Dis. 39, 275–282. https://doi.org/10.1055/s-0039-1685515 (2019).
Schmoll, D. et al. Regulation of glucose-6-phosphatase gene expression by protein kinase Balpha and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and independent effects of insulin on promoter activity. J. Biol. Chem. 275, 36324–36333. https://doi.org/10.1074/jbc.M003616200 (2000).
Fernandes, G. W. et al. The Foxo1-inducible transcriptional repressor Zfp125 causes hepatic steatosis and hypercholesterolemia. Cell Rep. 22, 523–534. https://doi.org/10.1016/j.celrep.2017.12.053 (2018).
Kamagate, A. et al. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J. Clin. Invest. 118, 2347–2364. https://doi.org/10.1172/JCI32914 (2008).
Park, S. J., Sohn, H. Y., Yoon, J. & Park, S. I. Down-regulation of FoxO-dependent c-FLIP expression mediates TRAIL-induced apoptosis in activated hepatic stellate cells. Cell. Signal. 21, 1495–1503. https://doi.org/10.1016/j.cellsig.2009.05.008 (2009).
Matsumoto, M., Pocai, A., Rossetti, L., Depinho, R. A. & Accili, D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell. Metab. 6, 208–216. https://doi.org/10.1016/j.cmet.2007.08.006 (2007).
Wu, Y. et al. Novel mechanism of Foxo1 phosphorylation in glucagon signaling in control of glucose homeostasis. Diabetes 67, 2167–2182. https://doi.org/10.2337/db18-0674 (2018).
Ren, H. Z., Xia, S. Z., Qin, X. Q., Hu, A. Y. & Wang, J. L. FOXO1 alleviates liver ischemia-reperfusion injury by regulating the Th17/Treg ratio through the AKT/Stat3/FOXO1 pathway. J. Clin. Transl. Hepatol. 10, 1138–1147. https://doi.org/10.14218/JCTH.2021.00551 (2022).
Lee, S. et al. Myeloid FoxO1 depletion attenuates hepatic inflammation and prevents nonalcoholic steatohepatitis. J. Clin. Invest. https://doi.org/10.1172/JCI154333 (2022).
Cheng, J. et al. FOXO1 induced fatty acid oxidation in hepatic cells by targeting ALDH1L2. J. Gastroenterol. Hepatol. https://doi.org/10.1111/jgh.16662 (2024).
Brunt, E. M. et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 53, 810–820. https://doi.org/10.1002/hep.24127 (2011).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592. https://doi.org/10.1093/nar/gkac963 (2023).
Hasegawa, S. et al. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin. Exp. Immunol. 167, 269–274. https://doi.org/10.1111/j.1365-2249.2011.04519.x (2012).
Lee, E. et al. Cystathionine metabolic enzymes play a role in the inflammation resolution of human keratinocytes in response to sub-cytotoxic formaldehyde exposure. Toxicol. Appl. Pharmacol. 310, 185–194. https://doi.org/10.1016/j.taap.2016.09.017 (2016).
Wang, P., Isaak, C. K., Siow, Y. L. & K, O. Downregulation of cystathionine beta-synthase and cystathionine gamma-lyase expression stimulates inflammation in kidney ischemia-reperfusion injury. Physiol. Rep. https://doi.org/10.14814/phy2.12251 (2014).
Fernandez-Galan, E. et al. Liver FoxO1 overexpression is positively associated with the degree of liver injury in cirrhotic patients. Adv. Lab. Med. 4, 218–226. https://doi.org/10.1515/almed-2023-0014 (2023).
Baselli, G. A. et al. Liver transcriptomics highlights interleukin-32 as novel NAFLD-related cytokine and candidate biomarker. Gut 69, 1855–1866. https://doi.org/10.1136/gutjnl-2019-319226 (2020).
Dai, W. et al. Key genes associated with non-alcoholic fatty liver disease and acute myocardial infarction. Med. Sci. Monit. 26, e922492. https://doi.org/10.12659/MSM.922492 (2020).
Noh, J. R. et al. Small heterodimer partner negatively regulates C-X-C motif chemokine ligand 2 in hepatocytes during liver inflammation. Sci.Rep. 8, 15222. https://doi.org/10.1038/s41598-018-33660-z (2018).
Pan, X. et al. Chemokines in non-alcoholic fatty liver disease: A systematic review and network meta-analysis. Front. Immunol. 11, 1802. https://doi.org/10.3389/fimmu.2020.01802 (2020).
Ren, H. et al. FOXO1 regulates Th17 cell-mediated hepatocellular carcinoma recurrence after hepatic ischemia-reperfusion injury. Cell Death Dis. 14, 367. https://doi.org/10.1038/s41419-023-05879-w (2023).
Pan, Q. et al. Hepatocyte FoxO1 deficiency protects from liver fibrosis via reducing inflammation and TGF-beta1-mediated HSC activation. Cell. Mol. Gastroenterol. Hepatol. 17, 41–58. https://doi.org/10.1016/j.jcmgh.2023.08.013 (2024).
Pan, X., Zhang, Y., Kim, H. G., Liangpunsakul, S. & Dong, X. C. FOXO transcription factors protect against the diet-induced fatty liver disease. Sci. Rep. 7, 44597. https://doi.org/10.1038/srep44597 (2017).
Li, Z. et al. Methionine metabolism in chronic liver diseases: An update on molecular mechanism and therapeutic implication. Signal Transduct. Target Ther. 5, 280. https://doi.org/10.1038/s41392-020-00349-7 (2020).
Wu, W. et al. Cystathionine-gamma-lyase ameliorates the histone demethylase JMJD3-mediated autoimmune response in rheumatoid arthritis. Cell. Mol. Immunol. 16, 694–705. https://doi.org/10.1038/s41423-018-0037-8 (2019).
Zhao, X. H. et al. Cystathionine gamma-lyase (Cth) induces efferocytosis in macrophages via ERK1/2 to modulate intestinal barrier repair. Cell Commun. Signal 21, 17. https://doi.org/10.1186/s12964-022-01030-y (2023).
Wei, K. et al. Cystathionine-gamma-lyase attenuates inflammatory response and pain of osteoarthritis. Int. Immunopharmacol. 120, 110289. https://doi.org/10.1016/j.intimp.2023.110289 (2023).
Lin, T. et al. CTH/H(2)S Regulates LPS-Induced Inflammation through IL-8 signaling in MAC-T cells. Int. J. Mol. Sci. https://doi.org/10.3390/ijms231911822 (2022).
Xu, W. et al. Hepatocellular cystathionine gamma lyase/hydrogen sulfide attenuates nonalcoholic fatty liver disease by activating farnesoid X receptor. Hepatology 76, 1794–1810. https://doi.org/10.1002/hep.32577 (2022).
Sun, L., Cai, J. & Gonzalez, F. J. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 18, 335–347. https://doi.org/10.1038/s41575-020-00404-2 (2021).
Disclosure
The abstract of this paper was presented at the International Digestive Disease Forum (IDDF) as a poster presentation with interim findings (IDDF2024-ABS-0295). The poster’s abstract was archived in ‘Poster Abstracts’ in the journal Gut: Hyperlink with https://doi.org/10.1136/gutjnl-2024-IDDF.35
Funding
The work was supported by the National Natural Science Foundation of China (82200574, 82170585), Funding by Science and Technology Projects in Guangzhou (2023A03J0955), the Science and Technology Planning Project of Guangdong Province (2023A0505010007), Guangdong Weiji Medical Development Foundation Specialized Research Fund for Gastroenterology (K-202401210), The Project of Key Medical Discipline in Guangzhou (2025–2027), Foundation of Guangzhou Key Clinical Specialties (Institute of Clinical Medicine).
Author information
Authors and Affiliations
Contributions
HTC, CH, HQC, and YJZ participated in the formulation of the study design. JMC and SQY performed in vivo and in vitro experiments. CH and YQL conducted RNA-Seq data analysis. HTC, JWC and CH were responsible for conceptualization and data collection, analysis, and interpretation. HTC and CH supervised the study and wrote the manuscript. YJZ and HQC reviewed and modified the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All methods were carried out in accordance with relevant guidelines and regulations. All animal experiment protocols were approved by the Animal Ethics Committee of South China University of Technology, and all experiments were performed in compliance with the ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, HT., Huang, C., Chen, JW. et al. Hepatocyte FoxO1 depletion exacerbates hepatic inflammation in MASH by targeting cystathionine γ-lyase. Sci Rep 15, 26631 (2025). https://doi.org/10.1038/s41598-025-10192-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-10192-x