Abstract
Sleep disturbances impair neurocognitive function, but their impact on early psychomotor recovery after day surgery remains unclear. This study aimed to investigate the impact of preoperative sleep disturbance on postoperative psychomotor recovery in patients undergoing hysteroscopic day surgery under general anesthesia, with a focus on psychomotor function and emergence from anesthesia. A prospective cohort study was conducted, including 110 patients scheduled for elective hysteroscopic day surgery. Participants were divided into two groups based on their Pittsburgh Sleep Quality Index (PSQI) scores: the Non-Sleep Disturbance (NSD) group (PSQI ≤ 5) and the Sleep Disturbance (SD) group (PSQI > 5). Psychomotor recovery was assessed using the Digit Symbol Substitution Test (DSST) and Trail Making Tests (TMT-A and TMT-B) at 30, 60, and 90 min post-extubation. Secondary outcomes included anesthesia-related time parameters, postoperative adverse events, and sleep quality on postoperative days 1 and 3, evaluated using the Athens Insomnia Scale (AIS). At 30 min post-extubation, the NSD group demonstrated significantly better psychomotor performance than the SD group (DSST: P = 0.0312; TMT-A: P = 0.0008; TMT-B: P = 0.0146). However, percentage change analysis for TMT-A revealed no significant differences between groups at any time point. No significant differences were observed between groups at 60–90 min for DSST or TMT-B. The SD group had a shorter anesthesia induction time (P < 0.0001), but no differences were found in eye-opening or extubation times. Both groups achieved similar Aldrete scores in the PACU (P = 0.7699). Postoperative sleep quality (AIS scores) and adverse events, including pain, nausea, and dizziness, were comparable between the groups. Patients with preoperative sleep disturbances undergoing general anesthesia exhibit delayed psychomotor recovery in the early postoperative phase, particularly within 30 min post-extubation. Continuous monitoring during this critical period is essential to ensure patient safety and optimize recovery outcomes in day surgery settings.
Similar content being viewed by others
Introduction
The widespread adoption of day surgeries in China has led to an increase in procedures that allow for rapid recovery and prompt discharge. This trend significantly improves the efficiency of healthcare resource utilization and addresses the growing demand for faster patient recovery1,2,3,4. Traditionally, recovery from day surgery is assessed using tools such as the Aldrete score and the Post Anesthetic Discharge Scoring System (PADSS)5,6,7. Furthermore, evaluating emergence from anesthesia involves assessing both the speed of awakening and the restoration of psychomotor functions. While tools like the Aldrete score and PADSS are routinely used clinically to determine discharge readiness5,6,7neuropsychological tests such as the Trail Making Test (TMT) and Digit Symbol Substitution Test (DSST) are established research tools for objectively quantifying psychomotor recovery8,9. Understanding the timeline for complete psychomotor recovery is essential for advising patients on their post-procedure activities and ensuring their overall safety.
Meanwhile, sleep disturbances are becoming increasingly prevalent, with over 300 million people in China reportedly suffering from long-term sleep issues, according to the “China Sleep Research Report” by the Chinese Sleep Research Society. Those afflicted by sleep disturbances often experience sluggish responsiveness, memory impairments, difficulty concentrating, fatigue, daytime sleepiness, and heightened anxiety. These symptoms adversely impact brain function, leading to longer reaction times, emotional instability, and reduced reasoning and decision-making capabilities10,11,12,13,14,15increasing the risk of accidents in daily life. Research has identified sleep disturbances as an independent risk factor for postoperative delirium (POD)16,17with perioperative sleep disturbances further elevating this risk18. Additionally, sleep deprivation is recognized as a risk factor for postoperative cognitive decline in human studies, with animal models suggesting it increases susceptibility to postoperative neurocognitive disorder (PND)19. Sleep deprivation is known to heighten sensitivity to anesthetic agents, shorten induction times, and prolong emergence from anesthesia, while contributing to the release of accumulated sleep debt during recovery20,21,22. We hypothesized that preoperative sleep disturbances would delay postoperative psychomotor recovery in patients undergoing hysteroscopic day surgery under general anesthesia.
This study aims to investigate the relationship between preoperative sleep disturbances and postoperative psychomotor recovery in patients undergoing day surgery. By understanding these dynamics, we hope to provide better guidance for post-procedure care and improve patient outcomes.
Methods
Design and study subjects
This study was a prospective cohort clinical trial approved by Ningbo No.2 Hospital (Approval Code: SL-NBEY-KY-2024-039-01) and registered with the Chinese Clinical Trial Registry (ChiCTR 2400082693, date of registration: 03/04/2024). The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines and followed the principles of the 2013 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all participants prior to enrollment.
Inclusion and exclusion criteria
Participants were recruited between April and September 2024 from Ningbo No.2 Hospital. All were scheduled for elective hysteroscopic day surgery. The inclusion criteria were: (1) general anesthesia via laryngeal mask airway; (2) age between 18 and 65 years; (3) The American Society of Anesthesiologists (ASA) status of I or II; (4) voluntary participation with signed informed consent. Exclusion criteria included: (1) neurological or psychiatric disorders; (2) language barriers; (3) illiteracy; (4) pregnancy; (5) allergy to anesthetics, dependence on addictive substances, or alcoholism; (6) use of drugs affecting the central nervous system; (7) Body mass index (BMI) > 30 kg/m².
Exposure factor: preoperative sleep quality
Preoperative sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI).
Preoperative sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI), a validated 19-item questionnaire evaluating seven domains of sleep quality (e.g., latency, duration, efficiency) over the past month. Each domain is scored 0–3, with a global score > 5 indicating clinically significant sleep disturbance23.Based on PSQI scores, participants were categorized into two groups: Non-Sleep Disturbance (NSD) group (PSQI ≤ 5) and Sleep Disturbance (SD) group (PSQI > 5). Participants were enrolled in a 1:1 ratio between the two groups. Participants were recruited consecutively between April and September 2024. To ensure comparable enrollment timelines between groups: All eligible patients in SD group were enrolled immediately upon identification. For each SD patient enrolled, the next chronologically eligible NSD patient was selected from the surgical schedule.
Study protocol
Upon entering the operating room, patients were monitored with electrocardiogram, blood pressure, pulse oxygen saturation, and bispectral index (BIS). Anesthesia induction began with 3 min of pure oxygen inhalation, followed by an intravenous injection of sufentanil (0.3 µg/kg). Five minutes later, propofol (2 mg/kg) was administered intravenously. Loss of consciousness was confirmed with BIS < 60, followed by intravenous rocuronium (0.4 mg/kg) for laryngeal mask insertion. Once the laryngeal mask was properly positioned, mechanical ventilation was initiated with a total gas flow of 2 L/min, oxygen concentration at 40%, tidal volume of 8 mL/kg, and ventilation rate of 12 breaths/min, maintaining Pressure of End-Tidal Carbon Dioxide(PetCO₂)between 35 and 45 mmHg. Anesthesia was maintained with sevoflurane concentration at a concentration of 1.5% and remifentanil infusion rate of 0.1–0.2 µg/kg/min, adjusted based on surgical needs and patient physiological response. BIS was maintained between 40 and 60, and vasoactive drugs were used to ensure hemodynamic stability. In cases of hypotension, defined as a systolic blood pressure below 80 mmHg, norepinephrine (4 µg) was administered immediately. For instances of bradycardia, characterized by a heart rate below 50 bpm for over 1 min, atropine (0.5 mg) was used. To prevent postoperative nausea and vomiting, ondansetron (4 mg) was intravenously administered prior to the completion of surgery. Upon surgical completion, anesthetic agents sevoflurane and remifentanil were discontinued. Muscle relaxation reversal was achieved through the administration of neostigmine (1 mg) combined with atropine (0.5 mg). The laryngeal mask was removed once the patient regained consciousness, BIS ≥ 80, spontaneously opened eyes, protective reflexes returned, and was responsive to verbal commands. Patients were then transferred to the post-anesthesia care unit (PACU). PACU nurses, blinded to the patient group allocations, assessed patients every 5 min using the Aldrete score. Patients were permitted to return to the ward once they reached an Aldrete score of ≥ 9.
Outcome measurement
The primary outcome was psychomotor function, assessed using the Digit Symbol Substitution Test (DSST) score 30 min after laryngeal mask removal. Secondary outcomes included DSST assessments at 60 and 90 min, along with Trail Making Test A (TMT-A) and Trail Making Test B (TMT-B) at 30, 60, and 90 min after laryngeal mask removal.
Standard instructions were used to guide the patients through a practice session before conducting the actual test. The DSST involved assigning digits to symbols over 90 s, with the number of correct completions recorded24,25. TMT-A and TMT-B, based on versions developed at Huashan Hospital Affiliated to Fudan University, participants to connect numbers 1 to 25 in sequence, and the completion time was recorded. In TMT-A, shapes alternated between squares and circles, while in TMT-B, each number corresponded to two shapes (a square and a circle) and the test required alternating connections, ensuring that each number is connected to the correct shape26,27,28.
Additional secondary outcomes included LOC time ((loss of consciousness, from propofol administration to BIS reaching 60), anesthesia time (from anesthesia induction to anesthesia withdrawal), surgery time (from invasive endoscopic operation start to endoscope removal/final skin suture), eye opening time(from anesthesia withdrawal to first eye opening), and extubation time (from anesthesia withdrawal to laryngeal mask removal). Intraoperative drug use included remifentanil dosage (µg) and end-tidal concentration of sevoflurane (%). Postoperative pain was assessed using the Visual Analog Scale (VAS) upon PACU arrival. Analgesia was administered reactively: intravenous parecoxib sodium (40 mg) was given if VAS ≥ 4. If pain persisted (VAS ≥ 4 after 15 min), intravenous tramadol (50 mg) was added. Total consumption of parecoxib sodium and tramadol was recorded. Postoperative sleep disturbances was evaluated on postoperative days 1 and 3 using the Athens Insomnia Scale (AIS). Adverse events included hypoxemia, dizziness, and nausea/vomiting.
Blinding
This study employed assessor, intraoperative management, and data analysis blinding. Postoperative evaluations were conducted by an independent researcher (KJ T) blinded to patients’ preoperative sleep status. The anesthesiologists managing intraoperative care were also blinded to group assignments. Data analysis was performed using coded data by independent analysts (CW and XY L) unaware of group assignments until analysis was completed.
Statistical analysis
The primary endpoint of this study was psychomotor recovery, measured by DSST scores at 30 min post-anesthesia, which was used to calculate the sample size. Sample size was determined with PASS 15.0 software, setting power (1-β) at 0.8 and significance level (α) at 0.05 (two-sided). A sample size of 42 per group was sufficient to detect a 5-point DSST difference (standard deviation of 8) between groups29with an anticipated dropout rate of 20%, requiring at least 53 patients per group. The final sample size was set at 110.
All statistical analyses were performed using Prism 9.5 software (GraphPad Software, San Diego, CA, USA). Categorical data were expressed as counts and percentages, analyzed using Chi-square or Fisher’s exact test. Continuous data were presented as mean ± standard deviation or median (interquartile range), analyzed using Mann-Whitney U or t-tests. Mean group differences and relative risk (RR) were reported with 95% confidence interval (95% CI). Psychomotor recovery was evaluated using repeated measures ANOVA to compare DSST and TMT results at 30, 60, and 90 min post-anesthesia. Mauchly’s test was used to check sphericity, and the Greenhouse-Geisser correction was applied if violated. Post-hoc tests employed Bonferroni correction. Statistical significance was set at a two-sided P < 0.05.
Results
Patient characteristics, surgery, and anesthesia
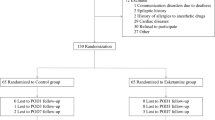
A total of 447 patient records were screened, and 349 met the inclusion criteria. Based on PSQI scores, patients were stratified into two groups: NSD (PSQI ≤ 5, n = 292) and SD (PSQI > 5, n = 57). In the NSD group, 237 patients were not approached for the study due to the large sample size and to maintain a balanced 1:1 group ratio, and 55 were enrolled. In the SD group, 2 patients declined participation, leaving 55 enrolled. During follow-up, 1 patient in the NSD group was lost to follow-up, resulting in 54 completing the study. In the SD group, 3 patients were lost to follow-up, and 2 were excluded due to changes in the anesthesia plan, leaving 50 completing the study. In total, 104 patients completed the study and were included in the final analysis (Figure. 1).
The NSD and SD groups showed no significant differences in demographics such as age, height, weight, and BMI, as well as ASA classification and education level (P > 0.05 for all). Both groups had similar rates of hypertension and diabetes, and there were no significant differences in surgery or anesthesia duration (P > 0.5 for all). No significant differences were observed in remifentanil usage or sevoflurane concentration (P > 0.5 for both) (Table 1). A significant difference was found in the PSQI scores, with the SD group scoring higher, confirming their classification based on sleep quality (P < 0.0001).
Psychomotor recovery
In both groups, there was a notable decline in DSST, TMT-A, and TMT-B scores 30 min after extubation compared to baseline levels. However, at 60 and 90 min post-extubation, these scores significantly improved, exceeding the baseline levels (Fig. 2).
Comparison of Postoperative Psychomotor Recovery Between NSD and SD Groups. Abbreviations: A: DSST (Digit Symbol Substitution Test); B: TMT-B (Trail Making Test B); C: TMT-A (Trail Making Test A); D: Percentage Change in TMT-A from Baseline. Within-group comparisons to baseline: aP < 0.05, bP < 0.01, cP < 0.001, dP < 0.0001. Between-group comparisons: *P < 0.05, **P < 0.01, ***P < 0.001.
At baseline, there was no significant difference in DSST scores between the NSD and SD groups (P = 0.9376). However, at 30 min postoperatively, the NSD group scored significantly higher than the SD group (P = 0.0312). No significant differences were observed at 60 and 90 min (P = 0.7520 and P = 0.6841, respectively) (Fig. 2 A).
For TMT-A completion times, the NSD group consistently demonstrated significantly shorter times than the SD group across all postoperative intervals: baseline (P = 0.0206), 30 min (P = 0.0008), 60 min (P = 0.0022), and 90 min (P = 0.0363) (Fig. 2 C). To address the baseline differences, we assessed the impact of surgery and anesthesia on TMT-A using percentage change, calculated as (postoperative time - baseline time)/baseline time. The analysis revealed no significant differences in the percentage change from baseline in TMT-A between the groups at any time point (Fig. 2D).
Regarding TMT-B completion times, there was no significant difference at baseline (P = 0.1021). At 30 min, the NSD group had significantly shorter times (P = 0.0146), but no significant differences were found at 60–90 min (P = 0.1277 and P = 0.1053, respectively) (Fig. 2B).
The aldrete scores and anesthesia-related time parameters
Upon arrival at the PACU, the number of patients with an Aldrete score ≥ 9 was similar between the NSD (88.89%) and SD (86%) groups (Table 2) (P = 0.7699). At 15 and 30 min in the PACU, all patients in both groups achieved an Aldrete score ≥ 9.
Regarding anesthesia-related time parameters, the SD group had a significantly shorter induction time compared to the NSD group (P < 0.0001). However, there were no significant differences in eye opening time or extubation time between the groups.
Postoperative sleep quality and other secondary measures
Comparative analysis of the AIS scores between the two groups on postoperative days 1 and 3 revealed no statistically significant differences. Only one patient in the NSD group had an AIS score greater than 6 on the first postoperative day (Table 3), indicating insomnia, while all other patients scored 6 or below. There were no significant differences between the groups in terms of insomnia incidence, VAS scores for pain, nausea, and dizziness.
Discussion
The primary objective of this study was to evaluate the impact of preoperative sleep disturbance on recovery profiles during emergence from general anesthesia. Our findings demonstrated that while sleep disturbance did not prolong awakening time, it significantly delayed psychomotor recovery in the early emergence phase. Patients with sleep disturbance exhibited impaired cognitive and motor function at 30 min post-extubation, highlighting the need for enhanced monitoring and tailored recovery strategies in this population.
Clinically, many hospitals and healthcare institutions use an Aldrete score of 9 or above as a threshold for safely transferring patients from the recovery room to a ward or discharge. During the early postoperative recovery phase, though both groups achieved Aldrete scores of 9 or more at 15 min post-surgery, differences in psychomotor function were observed. The study revealed that patients in the SD group gradually approached the NSD group’s performance on the DSST and TMT-B at 60 and 90 min postoperatively. However, at the critical 30-minute mark post-surgery, the psychomotor performance of the SD group was still significantly lower compared to the NSD group. Research indicates that the prefrontal cortex plays a crucial role in arousal control during emergence from general anesthesia. While healthy brains may experience a transient decrease in cognitive function immediately following anesthesia, cognitive abilities generally recover over time. Previous studies have shown that cognitive task performance of healthy volunteers returned to baseline levels within three hours after deep anesthesia emergence, although recovery rates may vary for different cognitive functions30. In our study, both the SD groups and NSD groups exhibited a decline in all three assessment scales at 30 min post-recovery compared to preoperative baselines, indicating ongoing recovery of psychomotor function. This finding underscores the importance of continuous monitoring during the early postoperative phase, particularly for patients with sleep disturbance.
Patients in the SD group consistently failed to reach the performance levels of the NSD group in the TMT-A postoperatively. Considering baseline differences, we employed the percentage change to assess psychomotor recovery after anesthesia. The percentage change post-general anesthesia showed no significant differences between the two groups. However, two-way ANOVA revealed a significant interaction effect, indicating that the impact of group and time point on TMT-A scores was not independent, with distinct patterns of change observed between groups at various time points. Notably, a highly significant difference was seen between groups at 30 min postoperatively, which diminished over time at 60 and 90 min. This suggests that for tasks like the TMT-A, which heavily rely on alertness and attention, patients with sleep disturbance similarly experience a greater negative impact on recovery in the early stages of awakening from general anesthesia.
Preoperatively, patients with sleep disturbance required more time to complete TMT-A, indicating pre-existing impairments in spatial memory, attention, processing speed, and executive function. TMT-A primarily evaluates basic attention and visual scanning abilities. Sleep disturbance may initially impact these fundamental cognitive functions, resulting in poorer performance on straightforward tasks. In contrast, the DSST and TMT-B require greater cognitive resources, such as working memory, executive function, and task-switching ability. Patients with sleep disturbance might mobilize more cognitive resources in these complex tasks to compensate for sleep deficits, achieving performance levels similar to the NSD group. Studies indicate that individuals with sleep disturbance show significant declines on simple attention tests, such as response time and error rate, followed by moderate effects on complex attention and working memory. Higher-order reasoning and crystallized intelligence accuracy are less affected31. We cautiously hypothesize that such neurobiological mechanisms could partially explain the observed patterns, though direct neural evidence is lacking in our study. Further neuroimaging research should explicitly test whether this compensatory model modulates anesthetic effects in sleep-deprived populations.
In our study, both groups’ DSST scores at the 60-minute and 90-minute intervals were notably improved compared to the preoperative baseline. The short-term repeated use of the DSST for psychomotor recovery assessment inevitably introduces a learning effect. This effect involves learning the symbol positions on the test, which enhances visual scanning speed. Memory of the symbol-digit pairings reduces the frequency of relevant searches, thereby automatically decreasing matching time. Furthermore, most participants adhere strictly to a sequential process of matching followed by writing, whereas some participants match and write simultaneously. In these cases, the search for the next digit occurs during the current digit’s writing process32. Overall, these various learning processes can lead to a reduction in matching time.
In this study, patients in the sleep disturbance group exhibited a significantly shortened time to loss of consciousness, while their eye-opening and extubation times were not prolonged compared to the control group. Research has shown that during anesthesia induction and the period of loss of consciousness, the overall neural temporal autocorrelation gradually increases, whereas functional connectivity progressively decreases. Conversely, upon the recovery of consciousness, cortical temporal autocorrelation abruptly returns to baseline, and both cortical and subcortical neural connectivity rapidly strengthen. These differences cannot be explained by pharmacokinetic effects alone, indicating that the induction and emergence phases of anesthesia follow asymmetric neurodynamics33. This asymmetry may explain why the speed of loss of consciousness and the speed of awakening are not consistent.
Studies examining anesthetic sensitivity in sleep-disturbed individuals reveal contradictory findings: while some animal models show enhanced GABAergic anesthetic effects (e.g., faster loss of righting reflex with propofol/isoflurane)20,22 clinical studies report increased anesthetic requirements (e.g., higher propofol doses)34,35 This discrepancy may stem from methodological differences (e.g., acute vs. chronic sleep disturbance models) or interspecies variations in GABA/glutamate homeostasis. Specifically, chronic sleep disturbances may downregulate GABA receptors, necessitating higher anesthetic doses to achieve inhibition, whereas acute deprivation could transiently potentiate GABAergic effects. The exact mechanisms remain unresolved and warrant further investigation into neuroreceptor adaptations across sleep disturbance subtypes.
This study has several limitations that should be acknowledged. First, it was conducted at a single center with a relatively small sample size, and the study population comprised only women of reproductive age. Additionally, the exclusion of high-risk patients (ASA III-IV), obese individuals, and the elderly may introduce selection bias, limiting the generalizability of the findings across different genders, age groups, and patient populations. Second, preoperative sleep disturbances were assessed using subjective measures, which may be prone to recall bias or underreporting. Incorporating objective sleep monitoring methods, such as polysomnography or actigraphy, in future studies could provide more robust and accurate assessments of sleep quality. Third, our observation period was limited to 90 min post-anesthesia, which restricts our ability to draw conclusions about longer-term recovery dynamics and potential delayed effects. Fourth, while we excluded patients with diagnosed neuropsychiatric disorders, preoperative cognitive screening (e.g., MMSE) was not performed. Undetected mild cognitive impairment could confound psychomotor test results, though baseline group comparability in TMT-B/DSST suggests minimal impact. Finally, this study focused exclusively on patients undergoing hysteroscopic day surgery, which has unique recovery characteristics. The findings may not be generalizable to other surgical procedures or inpatient settings, where recovery dynamics differ.
Conclusion
Patients with preoperative sleep disturbances exhibit delayed psychomotor recovery in the early postoperative phase, particularly within 30 min post-extubation. Future research should address this finding through multi-center studies with objective sleep assessments and longer observation periods to inform evidence-based recovery protocols.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AIS:
-
Athens Insomnia Scale
- ASA:
-
American Society of Anesthesiologists
- BIS:
-
Bispectral Index
- BMI:
-
Body Mass Index
- DSST:
-
Digit Symbol Substitution Test
- LOC:
-
Loss of Consciousness
- NSD:
-
Non-Sleep Disturbance
- PACU:
-
Post-Anesthesia Care Unit
- PADSS:
-
Post Anesthetic Discharge Scoring System
- PETCO₂:
-
End-Tidal Carbon Dioxide
- PND:
-
Postoperative Neurocognitive Disorder
- POD:
-
Postoperative Delirium
- PSQI:
-
Pittsburgh Sleep Quality Index
- SD:
-
Sleep Disturbance
- STT:
-
Shape Trails Test
- TMT:
-
Trail Making Test
- TMT-A:
-
Trail Making Test A
- TMT-B:
-
Trail Making Test B
- VAS:
-
Visual Analog Scale
References
Li, X. et al. Retroperitoneal laparoscopic partial adrenalectomy (RLPA) for 20–40 mm nonfunctional adrenal tumors in the day surgery mode. Front. Endocrinol. 13, 1099818 (2022).
Jiang, L. et al. Day surgery program at West China hospital: exploring the initial experience. Cureus 12, e8961 (2020).
Haihan, D. et al. The development of day surgery in China and the effectiveness and reflection of day surgery in ophthalmology-specialized hospitals. Cost Eff. Resour. Alloc. 22, 47 (2024).
Jiang, H., Han, J., Lu, A. & Liu, X. Day surgery management model in china: practical experience and initial evaluation. Int. J. Clin. Exp. Med. 7, 4471–4474 (2014).
Chung, F. Discharge criteria–a new trend. Can. J. Anaesth. = J. Canadien D’anesthesie. 42, 1056–1058 (1995).
Chung, F., Chan, V. W. & Ong, D. A post-anesthetic discharge scoring system for home readiness after ambulatory surgery. J. Clin. Anesth. 7, 500–506 (1995).
Ead, H. From aldrete to PADSS: reviewing discharge criteria after ambulatory surgery. J. Perianesthesia Nursing: Official J. Am. Soc. PeriAnesthesia Nurses. 21, 259–267 (2006).
Rörtgen, D. et al. Comparison of early cognitive function and recovery after desflurane or Sevoflurane anaesthesia in the elderly: a double-blinded randomized controlled trial. Br. J. Anaesth. 104, 167–174 (2010).
Mincer, J. S. et al. Delineating the trajectory of cognitive recovery from general anesthesia in older adults: design and rationale of the TORIE (Trajectory of recovery in the Elderly) project. Anesth. Analg. 126, 1675–1683 (2018).
Yaffe, K. et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. Jama 306, 613–619 (2011).
Yaffe, K., Falvey, C. M. & Hoang, T. Connections between sleep and cognition in older adults. Lancet Neurol. 13, 1017–1028 (2014).
Nilsson, J. P. et al. Less effective executive functioning after one night’s sleep deprivation. J. Sleep. Res. 14, 1–6 (2005).
Alhola, P. & Polo-Kantola, P. Sleep deprivation: impact on cognitive performance. Neuropsych Dis. Treat. 3, 553–567 (2007).
Couyoumdjian, A. et al. The effects of sleep and sleep deprivation on task-switching performance. J. Sleep. Res. 19, 64–70 (2010).
Killgore, W. D. S., Balkin, T. J. & Wesensten, N. J. Impaired decision making following 49 h of sleep deprivation. J. Sleep. Res. 15, 7–13 (2006).
Todd, O. M. et al. Sleep disruption at home as an independent risk factor for postoperative delirium. J. Am. Geriatr. Soc. 65, 949–957 (2017).
Guo, H. et al. Association between preoperative sleep disturbance and postoperative delirium in elderly: A retrospective cohort study. Nat. Sci. Sleep. 16, 389–400 (2024).
Leung, J. M. et al. Preoperative sleep disruption and postoperative delirium. J. Clin. Sleep. Medicine: JCSM: Official Publication Am. Acad. Sleep. Med. 11, 907–913 (2015).
Glumac, S., Kardum, G. & Karanovic, N. Postoperative cognitive decline after cardiac surgery: A narrative review of current knowledge in 2019. Med. Sci. Monit. 25, 3262–3270 (2019).
Mashour, G. A. et al. Isoflurane anesthesia does not satisfy the homeostatic need for rapid eye movement sleep. Anesth. Analg. 110, 1283–1289 (2010).
Qian, H., Zhou, Q., Cui, N. & Zhang, S. Sleep deprivation increases the anesthetic potency of Sevoflurane regardless of duration. J. Integr. Neurosci. 21, 135 (2022).
Tung, A., Szafran, M. J., Bluhm, B. & Mendelson, W. B. Sleep deprivation potentiates the onset and duration of loss of righting reflex induced by Propofol and isoflurane. Anesthesiology 97, 906–911 (2002).
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 (1989).
Jaeger, J. Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J. Clin. Psychopharmacol. 38, 513 (2018).
Daderwal, M. C., Sreeraj, V. S., Suhas, S., Rao, N. P. & Venkatasubramanian, G. Montreal cognitive assessment (MoCA) and digit symbol substitution test (DSST) as a screening tool for evaluation of cognitive deficits in schizophrenia. Psychiatry Res. 316, 114731 (2022).
Zhao, Q. et al. The shape trail test: application of a new variant of the trail making test. PLoS One. 8, e57333 (2013).
Wei, M. et al. Diagnostic accuracy of the Chinese version of the Trail-Making test for screening cognitive impairment. J. Am. Geriatr. Soc. 66, 92–99 (2018).
Llinàs-Reglà, J. et al. Trail Mak. Test. Assessment 24, 183–196 (2017).
Jehu, D. A., Davis, J. C., Madden, K., Parmar, N. & Liu-Ambrose, T. Minimal clinically important difference of executive function performance in older adults who fall: A secondary analysis of a randomized controlled trial. Gerontology 68, 771–779 (2021).
Mashour, G. A. et al. Recovery of consciousness and cognition after general anesthesia in humans. eLife 10, e59525 (2021).
Lim, J. & Dinges, D. F. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol. Bull. 136, 375–389 (2010).
Cornelis, C. et al. Preserved learning during the Symbol-Digit substitution test in patients with schizophrenia, Age-Matched controls, and elderly. Front. Psychiatry. 5, 189 (2014).
Huang, Z. et al. Asymmetric neural dynamics characterize loss and recovery of consciousness. NeuroImage 236, 118042 (2021).
Erden, V. et al. Insomnia May increase anesthetic requirement. J. Clin. Anesth. 34, 367–372 (2016).
Qiu, Y. et al. The effect of preoperative sleep quality on the target plasma concentration of Propofol and postoperative sleep in patients undergoing painless gastroscopy. BMC Anesthesiol. 23, 9 (2023).
Funding
This study was funded by the Medical Scientific Research Foundation of Zhejiang Province (2023KY272, 2025KY263, 2025KY1453), Ningbo Public Service Techology Foundation (2024S153), Ningbo Medical and Health Brand Discipline (PPXK2024-05), Ningbo Leading Medical & Health Discipline (2022-B10).
Author information
Authors and Affiliations
Contributions
Cha Wang: Conceptualization, Data curation, Formal analysis, Investigation, Writing-original draft; Keji Tao: Conceptualization, Methodology, Investigation, Formal analysis; Xiujun An: Investigation, Visualization; Jianliang Sun: Supervision, Resources; Xiaoyu Li: Writing-review & editing, Visualization; Jinye Gu: Writing-review & editing; Junping Chen: Writing-review & editing; Bo Lu: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing-review & editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This clinical study was approved by the Ethics Committee of Ningbo No. 2 Hospital (Approval No. SL-NBEY-KY-2023-039-01) and has been registered in the Clinical Trial Registration Center of China (Registration No. ChiCTR2400082693). All patients received comprehensive information about the study and provided written informed consent prior to participation.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, C., Tao, K., An, X. et al. Association between preoperative sleep disturbance and psychomotor recovery profiles after general anesthesia in day surgery: a prospective cohort study. Sci Rep 15, 25207 (2025). https://doi.org/10.1038/s41598-025-10198-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10198-5