Abstract
Neuroblastoma (NB) is a common pediatric solid tumor, particularly in children under 2 years. While survival rates for low- and intermediate-risk NB have improved, the prognosis for high-risk patients remains poor. Inflammation plays a crucial role in tumor progression, and systemic inflammation markers have prognostic value in various cancers. This study investigates the prognostic significance of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), and albumin-to-fibrinogen ratio (AFR) in NB patients. A retrospective analysis was conducted on 166 NB patients diagnosed between January 2013 and November 2024 at Hebei Provincial Children’s Hospital. Preoperative blood parameters, including neutrophil, lymphocyte, platelet counts, albumin, and fibrinogen levels, were used to calculate NLR, SII, and AFR. ROC curves assessed the relationship between these markers and patient outcomes. Kaplan-Meier survival analysis and Cox regression were performed to evaluate their prognostic value. NLR and SII had high predictive value for mortality, with ROC AUCs of 0.90 and 0.89, respectively. AFR had lower predictive value (AUCs of 0.73 and 0.74). Kaplan-Meier analysis showed that high NLR, low AFR, and high SII correlated with poorer overall survival (OS) and event-free survival (EFS). Both univariate and multivariate Cox regression identified NLR and SII as independent risk factors for OS and EFS. This study demonstrates the prognostic value of NLR, SII, and AFR in the evaluation of NB prognosis, particularly highlighting the efficacy of NLR and SII in predicting mortality and recurrence risks.
Similar content being viewed by others
Introduction
Neuroblastoma (NB) is a tumor originating from the neural crest, accounting for 8–10% of all childhood cancers1. It is the most common extracranial solid tumor in children and the third most common pediatric cancer overall, following hematologic malignancies and brain tumors2. Due to its highly aggressive nature, nearly half of neuroblastoma patients present with metastatic disease at diagnosis3contributing to NB being responsible for approximately 15% of all childhood cancer-related deaths4. One of the hallmark features of neuroblastoma is its clinical heterogeneity, with significant variability in disease progression and prognosis among patients5. Prognosis largely depends on disease stage, which is classified according to the International Neuroblastoma Risk Group (INRG) classification system. This system stratifies neuroblastoma into very low, low, intermediate, or high-risk categories6. While low- and intermediate-risk patients generally have a favorable prognosis, those with high-risk neuroblastoma still face a poor outcome, with long-term survival rates below 50%7.
Advancements in research have led to multimodal treatment strategies that significantly improve the prognosis of high-risk neuroblastoma patients8 but these treatments can also result in severe complications, such as growth and developmental disorders, secondary malignancies, neutropenia, and infections9,10. To minimize the risk of late-stage complications, it is crucial to accurately assess the risk stratification of neuroblastoma patients and adjust treatment intensity accordingly11. Prognostic risk stratification for neuroblastoma is vital for personalized therapy, with risk factors such as age, tumor histological type, tumor differentiation, and MYCN amplification playing essential roles in determining prognosis11. However, genetic testing remains time-consuming and expensive, and histological classification and disease staging are only available after preoperative biopsy or surgical resection. Therefore, there is a need for more rapid and cost-effective clinical markers to assess prognosis and guide subsequent treatment decisions12.
Inflammation plays a pivotal role in the initiation and progression of tumors13. Full blood cell count and protein measurement are convenient and low-cost methods that indirectly reflect the level of inflammation. Currently, systemic inflammatory markers such as the Neutrophil-to-Lymphocyte Ratio (NLR), Systemic Immune-Inflammation Index (SII), and Albumin-to-Fibrinogen Ratio (AFR) have proven to be effective prognostic tools in various adult cancers, including gastric cancer, colorectal cancer, lung cancer, prostate cancer, and breast cancer14,15,16,17,18. Additionally, the prognostic value of inflammatory immune indices has been validated in some pediatric solid tumors12,19. However, to date, no study has analyzed the prognostic value of the three systemic inflammatory markers—NLR, SII, and AFR—in neuroblastoma (NB) patients. This study aims to evaluate and compare the prognostic significance of NLR, SII, and AFR in NB.
Materials and methods
Patients
This retrospective study analyzed the clinical data of consecutive neuroblastoma patients who were diagnosed and underwent surgical treatment at Hebei Provincial Children’s Hospital between January 2013 and November 2024. Inclusion criteria were: pathological diagnosis of neuroblastoma, undergoing tumor resection with curative intent, and availability of complete preoperative blood test results within one week prior to surgery, as well as follow-up data. Exclusion criteria included severe infections or immunodeficiencies, presence of other malignancies, incomplete serological data, and incomplete follow-up records. A total of 166 patients were included. Demographic information, risk classification, and laboratory markers were retrospectively collected. Considering the potential impact of neoadjuvant chemotherapy, blood samples were drawn from all patients in a fasting state within one week prior to surgery, with all patients being afebrile (axillary temperature < 37.28 °C) and without signs of active infection or chronic inflammation at the time of blood sampling. The measured parameters included neutrophil, lymphocyte, and platelet counts, as well as levels of albumin and fibrinogen. The following indices were calculated: Neutrophil-to-Lymphocyte Ratio (NLR) = neutrophil count/lymphocyte count; Albumin-to-Fibrinogen Ratio (AFR) = albumin level/fibrinogen level; Systemic Immune-Inflammation Index (SII) = platelet count × neutrophil count/lymphocyte count. Informed consent was obtained from all participants, and the study protocol was approved by the Medical Research Ethics Committee of Hebei Provincial Children’s Hospital (Ethics number: 202136).
Follow-up of patients
All patients were followed up every three months for the first three years after surgery, followed by semi-annual follow-up thereafter. During the follow-up period, clinical history, physical examination, peripheral tumor biomarker levels, and abdominal and pelvic imaging (CT or ultrasound) were assessed according to the NCCN Clinical Practice Guidelines in Oncology. Overall survival (OS) was defined as the time from initial treatment to death, while event-free survival (EFS) was defined as the time from initial treatment to the first occurrence of disease progression or relapse.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism 9.5.1.733 for Windows. Continuous variables were expressed as mean ± standard deviation or median (interquartile range [IQR]), while categorical variables were expressed as frequencies and percentages, with comparisons made using the chi-square test. Receiver operating characteristic (ROC) curves were generated for NLR, AFR, and SII to evaluate their association with recurrence and mortality. The optimal cutoff values for these markers were determined using the Youden index, which is calculated as sensitivity + specificity − 1. Kaplan-Meier survival curves were used to estimate OS and EFS, and log-rank tests were applied to compare differences between groups. Univariate and multivariate Cox regression analyses were performed to assess the impact of predictive factors on OS and EFS. Variables with a P value < 0.05 in the univariate analysis were included in the multivariate analysis. NLR, AFR, and SII were incorporated as continuous variables in the multivariate Cox regression model for evaluation. To improve the readability of hazard ratios (HRs), SII values were divided by 100, so the HR for SII indicates the effect of an increase of 100 units of SII on OS and EFS. A P value of < 0.05 was considered statistically significant.
Results
Patient characteristics
This study included 166 patients diagnosed with neuroblastoma (NB), as summarized in Table 1. The median age of the patients was 27 months (range: 12–60 months). Of these, 69 patients (41.57%) were younger than 18 months, while 97 patients (58.43%) were aged 18 months or older. There were 87 male patients (52.41%) and 79 female patients (47.59%). According to the International Neuroblastoma Risk Group (INRG) risk classification, 109 patients (65.66%) were categorized as non-high-risk, while 57 patients (34.34%) were classified as high-risk. The majority of patients (118, 71.08%) received chemotherapy, while 48 patients (28.92%) did not. Recurrence occurred in 27 patients (16.27%), while 139 patients (83.73%) did not experience recurrence. The overall survival rate was 86.75% (144 patients), with a mortality rate of 13.25% (22 patients).
Among the 166 patients, 144 (86.7%) were in the survival group, and 22 (13.3%) were in the mortality group. Compared with the survival group, the mortality group had a shorter median overall survival (20.00 [IQR 16.00–35.25] vs. 54.00 [35.00–73.25]; p < 0.001) and event-free survival (EFS) (12.50 [9.25–19.75] vs. 54.00 [34.50–73.25]; p < 0.001). Additionally, the mortality group exhibited larger tumor diameter (8.35 [7.03–9.78] vs. 5.50 [3.95–8.60]; p = 0.004), higher neutrophil-to-lymphocyte ratio (NLR) (4.26 [2.56–10.26] vs. 0.91 [0.53–1.42]; p < 0.001), lower average platelet count (AFR) (10.36 [7.34–18.81] vs. 19.28 [15.35–25.44]; p < 0.001), higher systemic immune-inflammation index (SII) (1342.08 [618.65–2004.99] vs. 249.44 [140.49–394.67]; p < 0.001), and higher lactate dehydrogenase (LDH) levels (816.50 [370.50–2108.25] vs. 301.50 [236.75–487.25]; p < 0.001).
For categorical variables, the mortality group had higher proportions of high INRG Scale (95.45% vs. 25.00%; p < 0.001), high NLR (77.27% vs. 10.42%; p < 0.001), low AFR (59.09% vs. 9.72%; p < 0.001), high SII (90.91% vs. 22.22%; p < 0.001), and metastatic status (95.45% vs. 50.00%; p < 0.001). The mortality group also had a higher rate of chemotherapy utilization (95.45% vs. 67.36%; p = 0.007). No statistically significant differences were found in LDH stratification (p = 0.060) or sex (p = 0.500).
Regarding therapeutic approaches, in this study, 57 high-risk patients underwent induction chemotherapy, autologous stem cell transplantation, surgical resection, and postoperative chemotherapy; 46 intermediate-risk patients received induction chemotherapy and surgical resection; and 35 low-risk patients as well as 28 very low-risk patients underwent surgical resection alone. Unfortunately, our study is not yet equipped to perform GD2 immunotherapy.
ROC curve analysis
Receiver Operating Characteristic (ROC) curves were constructed to assess the predictive capabilities of NLR, AFR, and SII for mortality and recurrence, as shown in Fig. 1. The Area Under the Curve (AUC) values are provided in Table 2. For predicting mortality, the AUC values for NLR, AFR, and SII were 0.90, 0.73, and 0.89, respectively. For predicting recurrence, the AUC values were 0.88, 0.74, and 0.82, respectively. Both NLR and SII demonstrated high AUC values for predicting both mortality and recurrence, whereas AFR showed lower AUC values. Based on the cutoff values determined by the Youden index, the inflammatory markers were categorized into high and low groups: high NLR, high AFR, high SII, and low NLR, low AFR, low SII.
Association between NLR, AFR, SII, and survival rates
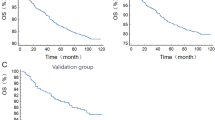
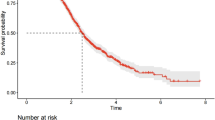
Kaplan-Meier survival curves were generated using the cutoff values for the three inflammatory markers (Figs. 2 and 3). The analysis revealed that patients with low NLR, high AFR, and low SII had significantly lower overall survival (OS) and event-free survival (EFS) compared to those with high NLR, low AFR, and high SII.
Univariate and multivariate Cox regression analysis
The three inflammatory markers were included in both univariate and multivariate Cox regression analyses as continuous and categorical variables. Table 3 and Supplemental Table S1 summarize the results of univariate and multivariate Cox regression analyses for NLR, AFR, and SII (per 100 units) as continuous variables. Multivariate analysis revealed that NLR (HR = 1.12, 95% CI: 1.06–1.19) and SII (per 100 units, HR = 1.04, 95% CI: 1.02–1.07) as continuous variables were independent predictors of OS. For EFS, NLR (HR = 1.13, 95% CI: 1.07–1.20), AFR (HR = 0.94, 95% CI: 0.89–0.99), and SII (per 100 units, HR = 1.05, 95% CI: 1.02–1.07) were independent prognostic factors.
To further validate the application of NLR, AFR, and SII in prognosis, we categorized these markers and incorporated them into a regression analysis alongside age, sex, tumor size, risk stratification, metastasis, chemotherapy status, and LDH levels. In univariate analysis, the following factors were significantly associated with poorer overall survival (OS) and event-free survival (EFS): tumor size > 5.75 cm, high-risk classification, distant metastasis, and no chemotherapy. Elevated LDH levels were associated with worse OS but not with EFS (Supplemental Table S2). In multivariate Cox regression analysis, factors with a P value < 0.05 in the univariate analysis were included in the model, and the effects of NLR, AFR, and SII on prognosis were assessed. The results showed that in the OS analysis, NLR, AFR, and SII were all independent prognostic factors (P < 0.001) (Supplemental Table S3). In the EFS analysis, NLR and AFR had P values < 0.001, while SII had a P value of 0.004, indicating that NLR and AFR had a more significant impact on EFS (Supplemental Table S4).
Discussion
Neuroblastoma is one of the most common solid tumors in children, with a higher incidence in children under the age of 2 (approximately 8–10% of all pediatric cancers)1,2. Although modern treatment strategies have significantly improved the survival rates of patients with low- and intermediate-risk neuroblastoma, the prognosis for patients with high-risk neuroblastoma, particularly those at advanced stages, remains poor20. In this study, among the 166 patients, 34.34% were classified as high-risk, with approximately 16% experiencing recurrence and 13.25% succumbing to the disease, suggesting that the prognosis for high-risk neuroblastoma remains unfavorable. The accuracy of prognostic assessment is crucial for developing personalized treatment plans11.
Inflammation is closely associated with tumor initiation and progression, and recent studies have highlighted the prognostic significance of inflammatory markers in various adult and pediatric cancers12,13. In our study, we systematically evaluated the impact of three common inflammatory markers—the neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), and albumin-to-fibrinogen ratio (AFR)—on the prognosis of neuroblastoma patients. Our data demonstrated that both NLR and SII showed high areas under the receiver operating characteristic (ROC) curves for predicting mortality and recurrence, with areas of 0.90 and 0.89, respectively, suggesting that they are highly effective in prognosticating neuroblastoma outcomes. In contrast, AFR demonstrated weaker predictive performance for mortality and recurrence, with ROC areas of 0.73 and 0.74. Although high AFR was associated with better overall survival (OS) and event-free survival (EFS), its predictive efficacy was significantly lower than that of NLR and SII. This result may be attributed to the nature of AFR as a composite indicator of nutritional status and inflammation, which may not fully reflect the tumor’s invasiveness and metastatic potential during severe inflammation.
According to Kaplan-Meier survival curve analysis, patients with high NLR, low AFR, and high SII had significantly lower five-year overall survival (OS) and event-free survival (EFS) compared to those with low NLR, high AFR, and low SII. This finding is consistent with previous studies, where high levels of NLR and SII were closely associated with poor prognosis in tumors, as demonstrated in research on various cancers14,18,21,22. In univariate and multivariate Cox regression analyses, NLR and SII, as continuous variables, were independent risk factors for OS and EFS. Specifically, for each 1-unit increase in NLR, the risk of OS increased by 12% (HR = 1.12), and for each 100-unit increase in SII, the risk of OS increased by 4% (HR = 1.04). In multivariate analysis, NLR, AFR, and SII were all confirmed as independent prognostic factors for OS (P < 0.001), suggesting that inflammatory responses may play an important role in the progression of neuroblastoma. Elevated inflammation markers typically reflect the body’s immune response to the tumor and the degree of inflammatory cell infiltration in the tumor microenvironment. NLR and AFR demonstrated stronger prognostic value in EFS analysis (P < 0.001), while the P value for SII was 0.004, suggesting that NLR and AFR may more sensitively reflect the association with treatment-related events, such as relapse and progression. This difference may be related to the distinct biological characteristics of these inflammatory markers and their mechanisms in the tumor microenvironment. This finding is consistent with previous studies on adult cancers, where high NLR and SII have been identified as independent adverse prognostic factors for several cancer types14,15,16.
A key limitation of our study is the lack of systematic genetic profiling data (MYCN amplification, 1p/11q deletions, 17q gain) in our cohort, primarily due to clinical constraints in resource-limited settings. These factors are established cornerstones of the INRG stratification system11. While we acknowledge this gap, our findings suggest that NLR and SII provide complementary prognostic information accessible without advanced genetic testing. Notably, emerging evidence links MYCN amplification with systemic inflammation23,24suggesting potential biological synergies between our markers and genetic drivers. Future multi-center studies integrating genetic and inflammatory biomarkers are warranted to refine risk stratification. Moreover, the intensity of treatment varied among patients in our study cohort and may have impacted the outcomes. Due to the limitations of this study, we are currently unable to conduct GD2 immunotherapy. The primary treatment modalities for patients were surgical resection and chemotherapy. However, although the multivariate model has adjusted for chemotherapy exposure, future analyses with detailed treatment stratification may help elucidate the interplay between inflammatory markers and specific therapies.
Inflammatory responses play a crucial role in tumor initiation, progression, and metastasis. Numerous studies have shown that inflammation can promote tumor cell proliferation, survival, angiogenesis, and immune evasion, thus accelerating tumor progression25. In the tumor microenvironment (TME), sustained inflammation not only alters immune cell functions but also promotes the secretion of tumor-associated cytokines and chemokines, which support tumor cell growth while suppressing the host’s immune surveillance26. In neuroblastoma, tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) are abundant within the tumor27. The presence of high levels of TAMs not only promotes tumor growth and angiogenesis but also exacerbates local inflammation through the secretion of pro-inflammatory cytokines such as TNF-α and IL-1β, further enhancing the tumor’s invasiveness and metastatic potential by suppressing anti-tumor immune responses25,28.
Tumor immune evasion is one of the key roles of inflammation in tumor progression. The inflammatory microenvironment not only provides opportunities for immune evasion but also weakens the host’s anti-tumor immune responses by altering immune cell functions. Tumor-associated immunosuppressive cells, such as MDSCs, inhibit CD8 + T-cell anti-tumor activity through the secretion of suppressive factors such as IL-10 and TGF-β, thereby enhancing the tumor’s ability to escape immune surveillance29. The integration of NLR, SII, and AFR with INRG risk classification offers a better prediction of patient prognosis. In this study, combining high NLR, low AFR, and high SII in high-risk patients was associated with significantly poorer OS and EFS. This finding suggests that inflammatory markers can complement the INRG classification to refine the risk stratification of neuroblastoma patients and support personalized treatment decisions.
Systemic immune-inflammation markers, as blood-based indicators, are non-invasive and easily accessible, providing a more convenient method for clinical prognosis assessment. In primary healthcare settings, the low cost and easy accessibility of systemic immune-inflammation markers make them an ideal tool for prognostic evaluation. Primary hospitals often lack advanced diagnostic equipment and specialized personnel, whereas testing for systemic immune-inflammation markers can be performed with routine blood tests, significantly reducing the demand for medical resources. Furthermore, the non-invasive nature of these markers eliminates additional harm to patients, further enhancing their applicability in clinical practice. Future research could explore the performance of NLR, AFR, and SII in different clinical subgroups and verify their combined use with other known prognostic factors, such as MYCN amplification and tumor staging. Additionally, with ongoing advancements in technology, new inflammation-related biomarkers and scoring systems may further improve the precision and sensitivity of prognostic evaluations.
There are some limitations in this study. First, it was a single-center retrospective study with a relatively small sample size, and thus the results should be interpreted with caution. Second, the cutoff values for the three systemic immune-inflammation indices in this study were determined using the Youden index within our cohort. Further analysis is needed to determine whether these cutoff values are applicable to other cohorts.Third, nutritional factors also play a significant role in cancer prognosis, and AFR, as an indicator of both nutritional status and inflammation, showed limited prognostic value in this study. Future research will continue to analyze the role of nutritional factors in the prognosis of neuroblastoma patients. Critical genetic prognosticators (MYCN status, chromosome aberrations) were unavailable for the patients, precluding their inclusion in multivariate models. This reflects real-world challenges in universal genetic testing. Therefore, necessitates caution in interpreting the independent prognostic value of inflammatory markers relative to established biomarkers. Large-scale prospective studies could further validate the generalizability of these findings and investigate the role of inflammatory markers across different treatment regimens.
Conclusion
In summary, this study has validated the potential value of NLR, SII, and AFR in the prognostic assessment of patients with neuroblastoma. Particularly, NLR and SII, as systemic inflammatory markers, can effectively predict mortality and recurrence risk in neuroblastoma. The assessment based on inflammatory response is characterized by its speed, simplicity, and cost-effectiveness, thus providing strong support for the clinical management of neuroblastoma.
Data availability
The data underlying this study are not publicly available because they contain information that could compromise the privacy and confidentiality of the research participants. The dataset includes sensitive information that could potentially identify individuals. Therefore, sharing the data publicly would violate ethical guidelines and participant consent agreements. However, the authors are willing to provide access to the data upon reasonable request and subject to appropriate ethical and legal considerations. Requests for data access should be directed to Chenglong Zhang at 2199889466@qq.com. Any data sharing will be contingent upon approval from the relevant institutional review board or ethics committee and the fulfillment of any necessary data sharing agreements.
Abbreviations
- NLR:
-
Neutrophil-to-Lymphocyte Ratio
- AFR:
-
Albumin to fibrinogen ratio
- SII:
-
Systemic Immune-Inflammation Index
- NB:
-
Neuroblastoma
- INRG:
-
International Neuroblastoma Risk Group
- OS:
-
Overall survival
- EFS:
-
Event-free survival
References
Castleberry, R. P. & Neuroblastoma Eur. J. Cancer 33, 1430–1437 ; (1997). discussion 1437–1438.
Swift, C. C., Eklund, M. J., Kraveka, J. M. & Alazraki, A. L. Updates in diagnosis, management, and treatment of neuroblastoma. Radiographics 38, 566–580 (2018).
Maris, J. M., Hogarty, M. D., Bagatell, R., Cohn, S. L. & Neuroblastoma Lancet 369, 2106–2120 (2007).
Nguyen, R. & Thiele, C. J. Immunotherapy approaches targeting neuroblastoma. Curr. Opin. Pediatr. 33, 19–25 (2021).
Rivera, Z., Escutia, C., Madonna, M. B. & Gupta, K. H. Biological insight and recent advancement in the treatment of neuroblastoma. IJMS 24, 8470 (2023).
Cohn, S. L. et al. The international neuroblastoma risk group (INRG) classification system: an INRG task force report. J. Clin. Oncol. 27, 289–297 (2009).
Maris, J. M. Recent advances in neuroblastoma. New. Engl. J. Med. 362, 2202–2211 (2010).
Wienke, J. et al. The immune landscape of neuroblastoma: challenges and opportunities for novel therapeutic strategies in pediatric oncology. Eur. J. Cancer. 144, 123–150 (2021).
Mody, R. et al. Irinotecan, temozolomide, and Dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: A report from the children’s oncology group. J. Clin. Oncol. 38, 2160–2169 (2020).
Zhao, Q. et al. Role and toxicity of radiation therapy in neuroblastoma patients: A literature review. Crit. Rev. Oncol. Hematol. 149, 102924 (2020).
Tolbert, V. P. & Matthay, K. K. Neuroblastoma: clinical and biological approach to risk stratification and treatment. Cell. Tissue Res. 372, 195–209 (2018).
Nayak, A., McDowell, D. T., Kellie, S. J. & Karpelowsky, J. Elevated preoperative Neutrophil-Lymphocyte ratio is predictive of a poorer prognosis for pediatric patients with solid tumors. Ann. Surg. Oncol. 24, 3456–3462 (2017).
Diakos, C. I., Charles, K. A., McMillan, D. C. & Clarke, S. J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 15, e493–503 (2014).
Jomrich, G. et al. High systemic Immune-Inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann. Surg. 273, 532–541 (2021).
Zhang, J., Zhang, H. Y., Li, J., Shao, X. Y. & Zhang, C. X. The elevated NLR, PLR and PLT May predict the prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Oncotarget 8, 68837–68846 (2017).
Xie, H. et al. The inflammatory burden index is a superior systemic inflammation biomarker for the prognosis of non-small cell lung cancer. J. Cachexia Sarcopeni. 14, 869–878 (2023).
Meng, L., Yang, Y., Hu, X., Zhang, R. & Li, X. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: a systematic review and meta-analysis. J. Transl Med. 21, 79 (2023).
W, G. et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: an updated meta-analysis of 17079 individuals. Cancer Medicine 8,4135–4148 (2019).
Kunc, M. et al. Systemic inflammatory markers and serum lactate dehydrogenase predict survival in patients with Wilms tumour. Arch. Med. Sci. 18, 1253–1261 (2022).
Jm, M., Md, H., Sl, C. & Neuroblastoma R, B. Lancet (London England) 369,2106–20 (2007).
Nie, D., Gong, H., Mao, X. & Li, Z. Systemic immune-inflammation index predicts prognosis in patients with epithelial ovarian cancer: A retrospective study. Gynecol. Oncol. 152, 259–264 (2019).
Chovanec, M. et al. Systemic immune-inflammation index in germ-cell tumours. Brit J. Cancer. 118, 831–838 (2018).
L, B., Rm, Y. & Ya, D. The Tumor Microenvironment in Neuroblastoma: New Players, New Mechanisms of Interaction and New Perspectives. Cancers 12, (2020).
R, H. et al. Targeting EP2 receptor with multifaceted mechanisms for high-risk neuroblastoma. Cell Reports 39, 111000 (2022).
Greten, F. R. & Grivennikov, S. I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51, 27–41 (2019).
Zhao, H. et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Sig Transduct. Target. Ther. 6, 263 (2021).
Yang, L. & Lin, P. C. Mechanisms that drive inflammatory tumor microenvironment, tumor heterogeneity, and metastatic progression. Semin Cancer Biol. 47, 185–195 (2017).
Wang, M., Chen, S., He, X., Yuan, Y. & Wei, X. Targeting inflammation as cancer therapy. J. Hematol. Oncol. 17, 13 (2024).
Decock, J., Comito, G., Zaravinos, A. & Editorial Tumor microenvironment, inflammation, and resistance to immunotherapies. Front. Oncol. 13, 1215332 (2023).
Acknowledgements
We would like to express our sincere gratitude to all the people and organisations who supported and helped us in this study. Firstly, we would like to thank the Hebei Provincial Health Committee for funding this project, without which this study would not have been possible. We would also like to thank the researchers who participated in the data analysis, your hard work made this study a satisfactory one. Finally, we would like to thank all the anonymous reviewers and editors for their valuable comments and suggestions on this paper.
Funding
This study was finded by grants from Medical Science Research Project Plan of Hebei Provincial Health Commission
20220039.
Author information
Authors and Affiliations
Contributions
Chenglong Zhang:Writing – original draft, Investigation, Conceptualization, Formal analysisJianlei Geng :Writing – review & editing, ResourcesXiaoyu Wang: Writing – review & editing, ConceptualizationYu Tian: Writing – review & editing, Formal analysisHuizhong Niu:Writing – review & editing, SupervisionPengju Zhang:Writing – review & editing, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval and consent to participate and publish statement
The study was approved by the Medical Research Ethics Committee of Hebei Children’s Hospital (approval number: 202136). All participants were provided with a detailed description of the study, including its objectives, methods, and potential risks. Written informed consent was obtained from all participants prior to their inclusion in the study. Participants were informed that their participation was voluntary and that they had the right to withdraw from the study at any time without any penalty. Consent for publication was obtained from all participants for the use of their data and images in this study. All personal identifying information has been anonymized to protect the privacy of the participants. For participants under the age of 18, written informed consent was obtained from their parents or legal guardians.All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Medical Research Ethics Committee of Hebei Children’s Hospital.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, C., Wang, X., Tian, Y. et al. Inflammatory markers including NLR, AFR and SII as prognostic factors in neuroblastoma. Sci Rep 15, 24101 (2025). https://doi.org/10.1038/s41598-025-10209-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-10209-5