Abstract
Several aspects of the relationships between VME indicator taxa and associated fauna have been previously explored mainly on rocky habitat respect the soft bottom. However, the soft bottom hosts many VMEs indicator taxa for which the ecological role remained still unexplored. This habitat hosts many associated species, a lot of them also of commercial value making this habitat more vulnerable to fishing pressure. The present study investigates the variables that shape the distribution of associated fauna and their relationship with VME indicator species in the deep soft bottom of the Mediterranean Sea. Analyzing 74 ROV (Remotely Operated Vehicle) transects, VMEs indicator taxa and fish and crustacean density were extracted and analyzed together with environmental, morphological and anthropogenic variables. Through Redundancy Analysis (RDA), our findings reveal a clear relationship between several fish and crustacean species of commercial value and VME indicator taxa, like Isidella elongata and Pennatuloidea. By applying multivariate statistical approaches to an integrative dataset, the study provides a community-level perspective on the factors shaping fish and crustacean assemblages in potentially vulnerable habitats. The result highlights the necessity to better investigate the relationship among deep sea species in a wide prospectives to give powerful data for their conservation.
Similar content being viewed by others
Introduction
Deep-sea is home to a remarkable diversity of life, including a wide range of habitat-forming species, like sponges and cnidarians, which create complex three-dimensional structures providing essential ecological services for the functioning of benthic ecosystems1,2. These species, in fact, can provide refuge3, nursery areas, and foraging grounds to a wide range of deep-sea animals, playing a crucial role in the life cycle of many species, including those of commercial value4, thus building ecosystems more vulnerable to fishing activities (e.g. bottom trawling)5,6,7,8,9,10. As consequence, these ecosystems are considered a hot-spot of biodiversity9,11 and most of them are classified as Vulnerable Marine Ecosystems (VMEs)4,10,12. According to FAO guidelines (2009), the identification of VMEs is based on key criteria: uniqueness or rarity, functional significance of the habitat, fragility, life-history traits, and structural complexity. The potential presence of VMEs could be identified by the presence of several taxa, defined as VME indicator taxa, that are characterized by common traits like slow growth rate, low fecundity, late age of maturity, habitat-forming and low recovery rates4,13. These traits highlight the vulnerability of these species to anthropogenic impacts like bottom trawling, ocean acidification, and climate change5,14. The removal or degradation of VME indicator taxa may lead to cascading effects on associated communities and the productivity of local fisheries. Many studies have focused on the distribution of VME indicator taxa through habitat suitability modelling15,16,17 however significant knowledge gaps remain regarding their ecological roles in the deep-sea and the associated fauna they host. Particularly, in the Mediterranean Sea the knowledge about the abundance, distribution, and ecological functioning of soft-bottom habitat-formers and the fauna they support is lacking18,19. While hard-bottom VMEs have been extensively studied7,20,21, soft-bottom habitats remain comparatively understudied, despite covering vast areas of the seafloor. Historically perceived as homogeneous and ecologically simpler22 these environments host a variety of tridimensional habitat built by VME indicator species like sea pens and gorgonians10,19. The Mediterranean deep soft-bottom habitats host many VME indicator species (e.g. octocorals) that contribute to enhance the complexity of the substrate modifying its ecology. The sea pen field can influence the current flow at the bottom surface increasing the particle retention and food availability for the other sessile or associated species17. The gorgonian Isidella elongata serves as a key habitat for species of both commercial and conservation importance, such as Aristeus antennatus, Aristeomorpha foliacea, Nephrops norvegicus, and Galeus melastomus14, while the sea pens reducing predation risk for juvenile fish like Sebastes spp18. The increasing of habitat complexity in soft sediments is not solely driven by cnidarians. Other benthic suspension feeders, such as the crinoid Leptometra phalangium also play a role in structuring soft-bottom communities, supporting aggregations of young-of-the-year hake (Merluccius merluccius), greater forkbeard (Phycis blennoides), and deep-water rose shrimp (Parapenaeus longirostris)13,23. Despite their importance, critically knowledge gaps remain regarding the ecological roles of these VME indicator taxa and the associated fauna they can support, especially on soft bottoms19. Therefore, the main aim of this study is to improve our knowledge on the ecological role of deep-sea VME indicator taxa inhabiting soft bottoms in the optic of their conservation and management. The main objectives of this study are: (1) to assess how the distribution of associated fauna (crustaceans and fish) varies in relation to different VMEs indicator taxa, and (2) to determine how the environmental and anthropogenic variables influence that correlation.
Results
The VME indicator taxa that characterized the analyzed ROV transects (Fig. 1) were the bamboo coral I. elongata, sea pens (Pennatula spp., Funiculina quadrangularis, Virgularia mirabilis, Protoptilum carpenterii, and Kophobelemnon stelliferum), and the crinoid L. phalangium (Fig. 2, Table S2).
A total of 39 associated species were included in the analysis, precisely 10 species of crustaceans and 29 species of fish (3 Chondrichthyes and 26 Osteichthyes) (Fig. 3; Table 1). The RDA analysis identified clear patterns in the distribution of fish and crustacean assemblages in relation to environmental (Chl-a, temperature), morphological (rugosity, slope, depth), anthropogenic (BTFE) variables, and density of VME indicator taxa. After VIF, salinity was removed from the final set of explanatory variables.
Underwater video frame of the dominant associated fauna taxa observed in the selected transects. (a) Merluccius merluccius, (b) Helicolenus dactylopterus, (c) Polyprion americanus, (d) Phycis blennoides, (e) Nezumia sp., f) Scyliorhinus canicula, (g) Capros aper, (e) Galeus melastomus, (i) Lepidorhombus boscii, (l) Nephrops norvegicus, (m) Aristeus antennatus, (n) Plesionika sp.
For fish’s model (p-value 0.001) (Fig. 4), the first two RDA axes explained a substantial portion of variation of the total variance (RDA1: 67.7%, RDA2: 13.2%). Several species showed significant associations with the explanatory variables. Helicolenus dactylopterus, Coelorinchus caelorhincus, Lepidorhombus boscii and Chlorophthalmus agassizi were closely associated with the presence of L. phalangium and sea pens species, suggesting a strong association with structured soft-bottom habitats. Species like Nezumia spp., Galeus melastomus, Phycis blennoides were positioned on the right side of the biplot, showing positive associations with I. elongata and higher temperature, Chl-a and depth values. Whereas Hymenocephalus italicus and Nettastoma melanura were positioned along RDA2, showing a positive correlation with the BTFE suggesting possible resilience or opportunistic behavior in disturbed areas. Other species such as Etmopterus spinax, Scyliorhinus canicula, Capros aper, Stomias boa and Polyprion americanus, showed a more central or intermediate distribution along RDA1 and RDA2, potentially reflecting broad ecological tolerances or the use of multiple habitat types. Overall, the results highlight that fish assemblage structure is influenced by both abiotic gradient and the presence of VME indicator taxa.
The RDA on crustaceans’ assemblages (p-value 0.02) (Fig. 5) explained 70.0% of the variation across the first two axes (RDA1: 49.9%, RDA2: 20.8%). Species distributions were primarily driven by morphological variables (depth, slope, rugosity), and the presence of key VME indicator taxa. A distinct cluster of commercially important deep-sea species, including Plesionika spp., Aristeus antennatus, Parapenaeus longirostris and Nephrops norvegicus, were all oriented in the same direction, showing a positive associated with the presence of Pennatuloidea and the bamboo coral I. elongata. These species are associated with the negative side of both RDA1 and RDA2. Additionally, Aristaeomorpha foliacea, another commercially important species, appears to be primarily correlated with I. elongata (on the negative axis of RDA2). In contrast, Geryon longipes appears to be strongly associated with depth, positioned along the positive side of RDA1, and shows no association with VME taxa, suggesting a preference for deep but more homogenous soft sediments. Paromola cuvieri, Munida sp. and Bathynectes maravigna are oriented towards a greater slope and rugosity, indicating a link to a more articulated substrate. These results support that VME indicator taxa such as I. elongata and sea pens significantly contribute to structuring crustacean assemblages on soft bottoms, especially for species of high commercial value. This emphasizes the role of soft-bottom VME taxa in enhancing ecological heterogeneity and supporting biodiversity in deep-sea environments.
Discussions
Vulnerable Marine Ecosystems (VMEs) indicator taxa, whether on rock or soft-bottom, enhance the three-dimensionality of the habitat, influencing the distribution of the associated fauna9. This is particularly important on otherwise monotonous soft-bottom environments, increasing the possibility of finding areas for refuge, spawning and nursery for a wide range of species3,14,23,24,25.
Our study highlights the little-explored relationship between soft-bottom VME indicator taxa and their associated fauna in the deep Mediterranean Sea. By applying a constrained ordination method (RDA), we were able to detect significant associations between environmental, anthropogenic and biological gradients and species composition. In particular, we found that several species of high commercial and conservation interest exhibit a dependence on the presence of VME indicator taxa, reinforcing what has already been observed in other studies7,8,14,26,27,28,29. The main commercial fish observed in the study area (i.e. the Shortnose green eye C. agassizii, the Blackbelly rosefish H. dactylopterus, and the Four-spot megrim L. boscii), were associated with the crinoid L. phalangium and the sea pens (Fig. 4). The blackmouth shark G. melastomus, as well as P. blennoides and Nezumia spp., are preferentially associated with the bamboo coral I. elongata and high value of temperature and Chl-a (Fig. 4). These two environmental variables are among the main drivers which influence the Mediterranean deep-sea fish assemblages, especially in areas where upwellings take place30as recently described for the Strait of Sicily31.
The same was observed for crustacean species of commercial interest. The red shrimp A. antennatus, the deep-water rose shrimp P. longirostris, Plesionika spp., the Norway lobster N. norvegicus and A. rissoana are associated with Pennatuloidea and I. elongata, as also observed in other Mediterranean studies14,24,25,26confirming the positive relationships between soft-bottom VME indicator taxa and associated fauna observed in the present study.
For example, Carbonara et al.25 observed a strong relationship between the presence of I. elongata and various species of fish and crustaceans, specifically, a close connection between I. elongata and nursery/spawning areas of A. foliacea and A. antennatus. Indeed, the branches of I. elongata are also used as spawning grounds by small oviparous sharks (i.e. G. melastomus and Scyliorhinus spp.)8,14,25.
The association between VME indicator taxa and related fauna can be attributed to several ecological factors. Passive suspension feeders, such as those of the superfamily Pennatuloidea and other octocorals, often inhabit areas with high zooplankton abundance, providing a rich trophic resource. Indeed, habitats of I. elongata have been associated with elevated zooplankton densities26. This abundance supports planktonic fish and fosters the growth of benthic and nekto-benthic populations, which could serve as prey for benthic feeders and scavengers29. Additionally, the structural complexity provided by VME indicator taxa, offers shelter and nursery habitats for various fish species, enhancing local biodiversity. Such complexity creates favorable conditions for a diverse community of predators, including crustaceans, bony fish, elasmobranchs, and cephalopods14,25,27,32. Recent studies have emphasized the importance of these habitats in supporting fish populations. Fishing effort revealed distinct patterns between fish and crustacean assemblages. In the fish RDA model, BTFE emerged as a significant predictor, with species such as N. melanura and H. italicus showing positive associations. Conversely, several species of commercial and ecological value, including C. agassizii, H. dactylopterus, L. boscii, P. blennoides, G. melastomus and C. caelorhincus, were positioned on the opposite side of the BTFE vector in the ordination space. This pattern suggests that prolonged trawling activity may have already filtered out more sensitive fish species, leading to communities dominated by taxa that tolerate or even benefit from altered conditions. Moreover, the RDA biplot for fish clearly shows that a greater number of fish species are associated with higher densities of VME indicator taxa, and tend to occur in areas with lower fishing effort. This suggests that changes in assemblage structure are not driven solely by direct reductions in fishing intensity, but also by the increased presence of VME taxa, which are themselves vulnerable to trawling. In contrast, the crustacean assemblages showed no significant response to BTFE. As demonstrated in the study by Milisenda et al.33in regions with deep rose shrimp populations, extensive trawling can drastically alter the demersal ecology, leading to a decrease in other demersal species (such as fish) and an increase in scavenging taxa such as crustaceans, which also benefit from bioturbated habitats34.
These findings highlight the urgent need for conservation efforts in these ecosystems. Since these VME indicator taxa are in areas suitable for trawling and support high levels of biodiversity, fishing activities affect them. Bottom trawling represents one of the most anthropogenic impacts on VMEs35,36. This activity can cause damage to VME indicator taxa living on both soft and hard substrates. Hard substrates are mainly impacted by sediment resuspension phenomena37,38. In soft-bottom, the main impact is the removal of the benthic species13,39especially when it comes to delicate creatures (such sea pens, the crinoid L. phalangium, and the bamboo coral I. elongata), where even minimal fishing effort can result in large mortality rates by uprooting and crushing colonies that are anchored in the sediment13,40,41.
In particular, the bamboo coral I. elongata has a rigid carbonaceous skeleton that breaks when touched by bottom trawls; the colonies of sea pen F. quadrangularis are characterized by a delicate axial rod and is not able to withdraw sink into the sediment, unlike other sea pens species (e.g. V. mirabilis, P. carpenterii). The co-existence with commercial species, the presence of specific morphological structures, as well as their particular life history traits, make these two species extremely vulnerable and, for that, they have been listed by the IUCN Red List of Threatened species as “Critically Endangered”42.
Conclusion
The use of an extensive and high-resolution dataset opens the possibility to study how several VME indicator taxa, and environmental variables potentially influence the distribution of the deep fish and crustaceans across extensive soft bottom areas. Moreover, the few studies that have investigated fish-VME relationships on soft bottoms usually relied on bottom trawl data. Our use of georeferenced ROV data allows for a direct spatial link between fish and crustacean distributions and VME indicator taxa, offering insights into actual co-occurrence and habitat use patterns in situ. Several species of commercial value are found to be associated with VME taxa confirming the potential use of this habitat during their life cycle. The result of the study highlights the necessity to better investigate the relationship among deep sea species in a wide prospectives in order to give powerful data for their conservation. By applying multivariate statistical approaches to an integrative dataset, including VMEs, environmental variables, edaphic factors, and fishing effort, we provide a community-level perspective on the factors shaping fish and crustacean assemblages in potentially vulnerable habitats. The study therefore goes beyond simple species associations and offers an ecologically coherent framework for understanding habitat-fauna-environment interactions in a poorly studied ecosystem type. Moving forward, the integration of multidisciplinary approaches and the collaboration between scientific research and policy-making will be essential to achieve effective management and long-term sustainability of marine resources.
Materials and methods
Study area and data collection
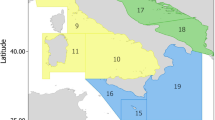
The research was conducted in the northern sector of the Strait of Sicily, with the centroid of the study area located at 38°0.587’N, 11°19.329’E (Fig. 1). The area, part of the MedWind project framework, spans a total surface of approximately 1651 km². Acoustic mapping was performed between August 12th and September 6th, 2021, using two multibeam echosounders: a Kongsberg EM2040 for depths ranging from 150 to 300 m, and a Kongsberg EM712-MK2 for deeper zones reaching 1000 m. The resulting data were processed to generate a Digital Terrain Model (DTM) with a spatial resolution of 5 m.
ROV-based visual surveys were subsequently carried out between September 11th and November 17th 2021, over a 67-day period, from the MainportGeo research vessel. The remotely operated vehicle used was a Tomahawk Light Work Class ROV equipped with dual manipulators, four types of cameras (full HD, standard color and black-and-white, and a 6 K high-definition camera), laser scaling devices, a sampling box, beacon, DVL system, and a Seabird Microcat SBE 37. A total of 140 transects were completed across a depth range of 135 to 885 m, covering a total distance of 129.5 km (mean transect length: 929 m ± 257 SD).
During the video surveys, all observations were georeferenced and recorded using the Ocean Floor Observation Protocol (OFOP) software which logged time, date, ROV and vessel positions, depth, substrate characteristics, and encountered species. In parallel with the ROV deployments, oceanographic data (temperature and salinity) were collected at 97 randomly selected stations using a Rosette sampler and a Sea-Bird Scientific SBE 911 Plus V2 CTD probe. Of the 140 transects collected during the ROV surveys, a subset of 74 transects were selected for analysis based on their dominant soft-bottom substrate, which averaged approximately 90% coverage (Fig. 1 and Table S1).
Explanatory variables
The explanatory variables included in the models to assess the influence on the distribution patterns of associated fauna were classified into different groups: VME indicator taxa, morphological (slope, depth, rugosity, aspect), environmental (temperature, salinity and Chl-a), and anthropogenic (Bottom Trawling Fishing Effort – BTFE) (Figure S1).
The OFOP analysis shows that the selected transects were dominated by three VME indicator taxa (I. elongata, Pennatuloidea and L. phalangium). These taxa were counted (N), and their abundance standardized to one linear kilometer, calculating their density (N * Km−1).
Morphological variables were derived from a high-resolution Multi-beam depth data (5 m) obtained through multibeam echosounder surveys. Slope, rugosity, and aspect were computed using the “terrain” function from the R package raster43. Slope reflects the steepness of the seabed, ranging from 0° (flat) to 90° (vertical), and is known to influence benthic habitat distribution by enhancing local current flows and affecting fishing gear accessibility44,45. Rugosity was calculated as the elevation difference between neighboring cells and provides a measure of seafloor complexity, with higher values indicating more rugged or rocky terrains. Aspect describes the orientation of the slope and is relevant for assessing exposure to prevailing currents46.
Environmental variables were modeled using spatial interpolation techniques. Bottom temperature and salinity data were collected from 97 CTD stations during the survey period and interpolated via co-kriging, with bathymetry included as a co-variable to improve prediction accuracy16. Chlorophyll-a concentration data were retrieved from the Copernicus Marine Service and represent the annual mean for 2021, spatially matched to the study area.
BTFE was estimated from Automatic Identification System (AIS) data for 2021, processed following the methodology described by Russo et al. (2016)47. The spatial distribution of bottom trawling activity was reconstructed by identifying fishing events through vessel speed and bathymetric filters, and cumulative Bottom Trawling Fishing Effort (BTFE) was calculated as the total number of fishing hours per 1 km² grid cell.
Response variables
The associated fauna observed during the video surveys, fish and crustacean species, was treated as response variables in the statistical models. For both fish and crustaceans, the sighting density distribution of each species was calculated (N * Km−1). Next, the quartiles of these two distributions were calculated, and species falling below the first quartile (both fish and crustaceans) were removed from the analysis to minimize noise and improve the robustness of the results. However, for crustaceans, the limited number of species observed posed challenges in terms of data interpretation. To address this issue and reduce the differences in density between species (e.g., high abundance versus rare observed species), the final dataset for crustaceans was converted into a presence/absence format. This approach allowed equal weighting of all species in the analysis, ensuring a more balanced representation of the crustacean taxa.
Data analysis
To assess the relationship between associated fauna (fish and crustaceans), and explanatory variables, a Redundancy Analysis (RDA) was performed. This multivariate ordination method assumes a linear relationship between species responses and the ordination axes48. In this study, each of the 74 transects was considered as a single analytical unit. For each transect, the mean values of environmental (temperature, salinity, chlorophyll-a), morphological (depth, slope, rugosity, aspect), and anthropogenic (BTFE) variables were calculated. Before proceeding with RDA, to reduce multicollinearity among explanatory variables and improve model performance, a Variance Inflation Factor (VIF) analysis was conducted. Variables exhibiting VIF values greater than 3 were considered highly correlated and were excluded from further analysis49.
All analysis were conducted using the “vegan” package in R50. To evaluate the contribution of each explanatory variable in shaping the associated faunal assemblage, we applied the envfit() function50 to all predictor variables (VME, morphological, environmental, and anthropogenic). This approach projects each variable onto the RDA ordination and tests its statistical significance via permutation. Only variables with p-values < 0.05 were considered significantly associated with community structure and were retained for interpretation and graphical representation. The percentage of variance explained by each RDA axis was calculated using constrained eigen values50. This method ensures that only the model-constrained portion of variance is reported, excluding unconstrained residual variation.
To determine which species of associated fauna were significantly associated with the RDA axes, we calculated Pearson correlations between each species and the RDA axes (RDA1 and RDA2), and then we used two-sided tests to check if the correlation between species and RDA axes were significantly different from zero, either positive or negative.
RDA biplots were generated using ggplot251 and ggrepel52 R packages. Species scores, site scores, and centroids of explanatory variables were extracted with the scores() function. The RDA axes were annotated with the percentage of variance they explained to facilitate interpretation of the ordination.
Data availability
The datasets presented in this article are not readily available because they are part of an ongoing project. The datasets will be accessible at project closure and requests for access should be sent to TR at teresa.romeo@szn.it.
References
Buhl-Mortensen, L. et al. Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins: biological structures and biodiversity. Mar. Ecol. 31, 21–50 (2010).
Pham, C. K. et al. The importance of deep-sea vulnerable marine ecosystems for demersal fish in the Azores. Deep Sea Res. Part. Oceanogr. Res. Pap. 96, 80–88 (2015).
Carluccio, A., Capezzuto, F., Maiorano, P., Sion, L. & D’Onghia, G. Cold-water coral ecosystems along the Apulian coasts: biodiversity and urgency of conservation measures in the anthropocene. J. Nat. Conserv. 84, 126785 (2025).
FAO. International Guidelines for the Management of Deep-Sea Fisheries in the High Seas (2009).
Ramirez-Llodra, E. et al. Deep, diverse and definitely different: unique attributes of the world’s largest ecosystem. Biogeosciences 7, 2851–2899 (2010).
FAO. The State of Mediterranean and Black Sea Fisheries (2018).
D’Onghia, G., Sion, L. & Capezzuto, F. Cold-water coral habitats benefit adjacent fisheries along the Apulian margin (central Mediterranean). Fish. Res. 213, 172–179 (2019).
30 Cold-Water Corals as Shelter, Feeding and Life-History Critical Habitats for Fish Species: Ecological Interactions and Fishing Impact. in Coral Reefs of the World 335–356. Springer International Publishing, Cham, (2019). https://doi.org/10.1007/978-3-319-91608-8_30
Ortega, M., Castro-Cadenas, M. D., Steenbeek, J. & Coll, M. Identifying and prioritizing demersal fisheries restricted areas based on combined ecological and fisheries criteria: the Western mediterranean. Mar. Policy. 157, 105850 (2023).
Lozano, P. et al. Environmental variables and bottom trawling effort affect the complexity and distribution of soft-bottom octocorals and burrowing megafauna communities in the Gulf of Cádiz. Deep Sea Res. Part. Oceanogr. Res. Pap. 206, 104269 (2024).
Danovaro, R. et al. Deep-Sea biodiversity in the mediterranean sea: the known, the unknown, and the unknowable. PLoS ONE. 5, e11832 (2010).
Report of the Workshop on. Deep-sea Species Identification, Rome, 2–4 December (2009).
Incidental Catch of Vulnerable Species in Mediterranean and Black Sea Fisheries - A Review. FAO, (2021). https://doi.org/10.4060/cb5405en
Mastrototaro, F. et al. Isidella elongata (Cnidaria: Alcyonacea) facies in the Western mediterranean sea: visual surveys and descriptions of its ecological role. Eur. Zool. J. 84, 209–225 (2017).
Lauria, V. et al. Species distribution models of two critically endangered deep-sea octocorals reveal fishing impacts on vulnerable marine ecosystems in central mediterranean sea. Sci. Rep. 7, 8049 (2017).
Palummo, V. et al. Effect of environmental and anthropogenic factors on the distribution and co-occurrence of cold-water corals. Front. Mar. Sci. 10, 1272066 (2023).
Bastari, A., Pica, D., Ferretti, F., Micheli, F. & Cerrano, C. Sea pens in the mediterranean sea: habitat suitability and opportunities for ecosystem recovery. ICES J. Mar. Sci. 75, 1722–1732 (2018).
Baillon, S., Hamel, J. F., Wareham, V. E. & Mercier, A. Deep cold-water corals as nurseries for fish larvae. Front. Ecol. Environ. 10, 351–356 (2012).
Miatta, M. & Snelgrove, P. V. R. Sea pens as indicators of macrofaunal communities in deep-sea sediments: evidence from the Laurentian channel marine protected area. Deep Sea Res. Part. Oceanogr. Res. Pap. 182, 103702 (2022).
Ross, S. W., Rhode, M. & Quattrini, A. M. Demersal fish distribution and habitat use within and near Baltimore and Norfolk canyons, U.S. Middle Atlantic slope. Deep Sea Res. Part. Oceanogr. Res. Pap. 103, 137–154 (2015).
Arnaud-Haond, S. et al. Two pillars of cold-water coral reefs along Atlantic European margins: prevalent association of Madrepora oculata with Lophelia pertusa, from reef to colony scale. Deep Sea Res. Part. II Top. Stud. Oceanogr. 145, 110–119 (2017).
Danovaro, R., Snelgrove, P. V. R. & Tyler, P. Challenging the paradigms of deep-sea ecology. Trends Ecol. Evol. 29, 465–475 (2014).
Colloca, F., Carpentieri, P., Balestri, E. & Ardizzone, G. D. A critical habitat for mediterranean fish resources: shelf-break areas with Leptometra phalangium (Echinodermata: Crinoidea). Mar. Biol. 145, 1129–1142 (2004).
Pierdomenico, M. et al. Effects of trawling activity on the bamboo-coral Isidella elongata and the sea pen Funiculina quadrangularis along the Gioia Canyon (Western mediterranean, Southern tyrrhenian Sea). Prog Oceanogr. 169, 214–226 (2018).
Carbonara, P. et al. Exploring a deep-sea vulnerable marine ecosystem: Isidella elongata (Esper, 1788) species assemblages in the Western and central mediterranean. Deep Sea Res. Part. Oceanogr. Res. Pap. 166, 103406 (2020).
Cartes, J. E., LoIacono, C., Mamouridis, V., López-Pérez, C. & Rodríguez, P. Geomorphological, trophic and human influences on the bamboo coral Isidella elongata assemblages in the deep mediterranean: to what extent does Isidella form habitat for fish and invertebrates? Deep Sea Res. Part. Oceanogr. Res. Pap. 76, 52–65 (2013).
Mytilineou, C. et al. New cold-water coral occurrences in the Eastern ionian sea: results from experimental long line fishing. Deep Sea Res. Part. II Top. Stud. Oceanogr. 99, 146–157 (2014).
Palummo, V. et al. Improving the knowledge base of vulnerable marine ecosystems’ distribution in the amendolara bank (Ionian Sea). Mediterr. Mar. Sci. 25, 220–230 (2024).
Carluccio, A., Capezzuto, F., Maiorano, P., Sion, L. & D’Onghia, G. Fishes associated with a vulnerable marine ecosystem network in the central mediterranean sea. Fishes 9, 433 (2024).
Mérigot, B. et al. Stability of the relationships among demersal fish assemblages and environmental-trawling drivers at large spatio-temporal scales in the Northern mediterranean sea. Sci. Mar. 83, 153 (2020).
Di Lorenzo, M., Sinerchia, M. & Colloca, F. The North sector of the Strait of sicily: a priority area for conservation in the mediterranean sea. Hydrobiologia 821, 235–253 (2018).
Carbonara, P. et al. Spatio-temporal distribution of Isidella elongata, a vulnerable marine ecosystem indicator species, in the Southern Adriatic sea. Hydrobiologia https://doi.org/10.1007/s10750-022-05022-4 (2022).
Milisenda, G. et al. Discard composition associated with the deep water Rose shrimp fisheries (Parapenaeus longirostris, Lucas 1846) in the south-central mediterranean sea. Mediterr. Mar. Sci. 18, 53 (2017).
Kumar, A. B. & Deepthi, G. R. Trawling and by-catch: implications on marine ecosystem. Curr Sci 90(8), 922–931 (2006).
Consoli, P. et al. The impact of fisheries on vulnerable habitats: the case of trawling on circa-littoral grounds in the Strait of Sicily (central mediterranean Sea). Mar. Biol. Res. 13, 1084–1094 (2017).
IUCN. Thematic Report - Conservation Overview of Mediterranean Deep-Sea Biodiversity: A Strategic Assessment. (2019).
Ragnarsson, S. Á. et al. The impact of anthropogenic activity on Cold-Water corals. In Marine Animal Forests (eds Rossi, S., Bramanti, L., Gori, A., Orejas, C. et al.) 1–35 (Springer International Publishing, 2016). https://doi.org/10.1007/978-3-319-17001-5_27-1.
Bilan, M. et al. Vulnerability of six cold-water corals to sediment resuspension from bottom trawling fishing. Mar. Pollut Bull. 196, 115423 (2023).
Hinz, H. Impact of Bottom Fishing on Animal Forests: Science, Conservation, and Fisheries Management. in Marine Animal Forests (eds. Rossi, S., Bramanti, L., Gori, A. & Orejas Saco Del Valle, C.) 1–19Springer International Publishing, Cham, (2016). https://doi.org/10.1007/978-3-319-17001-5_37-1
Murillo, F. J. New VME indicator Species (excluding Corals and sponges) and some Potential VME elements of the NAFO Regulatory Area. Centro Oceanográfico de Vigo, 1–20 (2011).
Murillo, F. J. et al. Morphometry and growth of sea pen species from dense habitats in the Gulf of st. Lawrence, Eastern Canada. Mar. Biol. Res. 14, 366–382 (2018).
Salvati, E. & Bo, M. Carlo Rondinini,Alessia Battistoni,CorradoTeofili. Lista Rossa IUCN dei coralli Italiani. Comitato Italiano IUCN e Ministero dell’Ambiente e dellaTutela del Territorio e del Mare, Roma. (2014).
Hijmans, R. J. raster: Geographic Data Analysis and Modeling. The R Foundation (2010). https://doi.org/10.32614/cran.package.raster
Mohn, C. & Beckmann, A. Numerical studies on flow amplification at an isolated shelfbreak bank, with application to Porcupine bank. Cont. Shelf Res. 22, 1325–1338 (2002).
Wilson, M. F. J., O’Connell, B., Brown, C., Guinan, J. C. & Grehan, A. J. Multiscale terrain analysis of multibeam bathymetry data for habitat mapping on the continental slope. Mar. Geod. 30, 3–35 (2007).
Qin, C. et al. An adaptive approach to selecting a flow-partition exponent for a multiple‐flow‐direction algorithm. Int. J. Geogr. Inf. Sci. 21, 443–458 (2007).
Russo, T. et al. Assessing the fishing footprint using data integrated from different tracking devices: issues and opportunities. Ecol. Indic. 69, 818–827 (2016).
Legendre, P. & Legendre, L. Numerical Ecology, Developments in Environmental Modelling. (2012).
Zuur, A. F., Ieno, E. N., Walker, N., Saveliev, A. A. & Smith, G. M. Mixed Effects Models and Extensions in Ecology with R (Springer New York, 2009). https://doi.org/10.1007/978-0-387-87458-6
Jari Oksanen, F. et al. Eduard Szoecs, & Helene Wagner. Community Ecology Package. (2020).
Hadley Wickham. Ggplot2 Elegant Graphics for Data Analysis. (2016).
Slowikowski, K. Ggrepel: automatically position Non-Overlapping text labels with ‘ggplot2’. R Foundation. https://doi.org/10.32614/cran.package.ggrepel (2016).
Acknowledgements
The author(s) declare financial support was received for this article’s research, authorship, and/or publication. This research was funded by Centro Ricerche ed Infrastrutture Marine Avanzate in Calabria (CRIMAC) - Fondo FSC 2014 -2020 - Piano Stralcio «Ricerca e Innovazione 2015 -2017» – Programma Nazionale Infrastrutture di Ricerca (PNIR), CUP C64I20000320001. This work was also in part funded by the project “National Biodiversity Future Center - NBFC” (CUP D33C22000960007; Project Code: CN_00000033; Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research), funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4 - Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU.The data were collected during an oceanographic survey funded by Renexia company, as part of an environmental impact assessment for developing an offshore wind farm. The Renexia company had no role in designing the study, collecting, analyzing, interpreting the data, drafting the article, or submitting it for publication. Furthermore, the authors are grateful to the anonymous Referees for the valuable feedback, which improved the paper. We would like to thank everyone involved in the oceanographic survey, our colleagues Frine Cardone, Valentina Costa, Bruna Giordano, Antonio Giova, Augusto Passarelli, Francesco Stenico, and Margherita Toma and all the crew members, the survey technicians, and the ROV pilots.
Author information
Authors and Affiliations
Contributions
V.P.: Data curation, Investigation, Analysis, Validation, Writing – original draft. G.M.: Data curation, Analysis, Writing – review & editing. D.P.: Investigation, Writing – review & editing. S.C.: Investigation, Project administration, Writing – review & editing. E.S.: Investigation, Writing – review & editing. L.D.V.: Data curation, Writing – review & editing. P.B.: Data curation, Writing – review & editing. N.S.: Project administration, Writing – review & editing. T.R.: Funding acquisition, Project administration, Writing – review & editing. S.G.: Funding acquisition, Project administration, Writing – review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Palummo, V., Milisenda, G., Pica, D. et al. Ecological role of benthic Vulnerable Marine Ecosystems (VMEs) indicator taxa on soft bottoms. Sci Rep 15, 26654 (2025). https://doi.org/10.1038/s41598-025-10243-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-10243-3