Abstract
TOPLESS/TOPLESS-RELATED (TPR) proteins are conserved transcriptional co-repressors vital for plant growth and development. However, the functions of soybean TPR (GmTPR) gene family members and their roles in photoperiod responses remain largely unexplored. In this study, we identified 12 TPR genes in the soybean genome, distributed across 11 chromosomes. Phylogenetic analysis classified GmTPRs into three subfamilies (Class I–III) by comparing them with Arabidopsis TPR proteins. Collinearity analysis revealed that 6 GmTPR genes are collinear with 3 AtTPR genes, resulting in 10 pairs of collinear genes, with no tandem duplications found. Analysis of physicochemical properties, motif composition, and gene structure indicated significant differences among GmTPR members. Subcellular localization confirmed that GmTPRs predominantly reside in the nucleus, suggesting regulatory functions. Additionally, analysis of cis-regulatory elements revealed significant enrichment of light-responsive elements in GmTPR promoters, indicating possible regulation by light. Tissue-specific expression analysis showed that GmTPR genes are mainly expressed in flowers and seeds. RNA-seq and RT-qPCR analyses revealed distinct expression patterns of GmTPRs between long and short photoperiods, highlighting their responsiveness to photoperiod changes. This study provides a comprehensive analysis of the GmTPR family, emphasizing their critical role in photoperiod responses.
Similar content being viewed by others
Introduction
The growth and development of plants depend on the precise regulation of gene expression1. Co-regulatory transcription factors play a significant role in controlling gene expression in plants2,3. Co-repressors, as a type of transcriptional regulatory factor, are unable to bind to DNA independently; instead, they function by forming multiprotein complexes with other regulatory factors to induce a repressive chromatin state, thereby epigenetically silencing target genes4,5.
TPR proteins are evolutionarily highly conserved transcriptional co-repressors, which include TPL, TPR1, TPR2, TPR3, and TPR4 in Arabidopsis6,7. They represent a subfamily of the Groucho/Thymidine uptake 1 (Gro/Tup1) family8,9,10. Members of the Gro/Tup1 family possess an N-terminal glutamine-rich LisH domain (PF08513) and a C-terminal WD40 domain (PF00400). Notably, the TPR subfamily is uniquely distinguished by the presence of a CTLH domain (PF10607)4,11. In plants, TPR proteins constitute a well-conserved family of transcriptional corepressors. The first TPR mutant, topless-1 (tpl-1), was identified in Arabidopsis and is associated with a semi-dominant embryonic developmental mutation. At restrictive temperatures (29 °C), the most pronounced polarity changes occur, characterized by the conversion of the embryonic shoot pole into a second root pole. This apical embryonic patterning defect requires the redundant function of 5 members of the TPR family12,13. Beyond their role in apical embryo development, research has shown that TPR co-repressor factors are also involved in numerous other developmental processes. TPR proteins interact with the conserved Ethylene-Responsive Factor Associated Amphiphilic Repression (EAR) motif in transcription factors through their LisH and CTLH domains, playing a critical role in regulating of development, stress responses, and hormone signaling1.
To date, the TPR family has been reported in several species, including Arabidopsis thaliana9 allotetraploid rapeseed (Brassica napus)14 and tomato (Solanum lycopersicum)15. Research has gradually revealed the composition and functions of TPR gene families, indicating that TPR proteins play critical roles in a wide array of plant processes, particularly in plant development. For example, in maize (Zea mays), the zinc-finger transcription factor RAMOSA1 interacts with the TPR factor REL2 to suppress indeterminate meristem fate, underscoring the importance of TPR co-repressors in meristem maintenance16. Additionally, a direct interaction has been reported between the CTLH domain of TPR and the EAR domain of IAA12, which mediates the repression of ARF transcription by AUX/IAA, a mechanism that regulates a crucial developmental cell fate switch during early embryogenesis1,2. Furthermore, the TPR co-repressor family is known to be a component of the central circadian oscillator mechanism. TPR proteins interact with PSEUDO RESPONSE REGULATORS (PRRs) at the promoters of CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) to inhibit transcription and modulate circadian rhythms17. Notably, the absence of TPR disrupts the function of several pathways that repress Flowering Locus T (FT), including TEM1, AP2, and AGL15. The FT repressor TOE1 recruits TPR to inhibit FT expression in the leaves of wild-type plants, playing a crucial role in the transition to flowering6. Co-repressors typically bind to histone deacetylases (HDACs), forming larger heterocomplexes. In plants, the TPR proteins co-localizes with HDACs, suggesting that these may work together to regulate gene expression18. In Arabidopsis, HDACs play a crucial role in the regulation of flowering, primarily by influencing the interactions among photoperiod, plant hormones, and transcription factors19. Therefore, the regulatory role of TPR in the photoperiod signaling pathway deserves further investigation to explore its potential mechanisms in flowering and plant development.
Soybean, as a short-day plant, is highly sensitive to photoperiods. This sensitivity significantly impacts soybean’s adaptability to cultivation and is one of the key factors in maximizing soybean yield20,21. The genetic variation of major gene loci and quantitative trait loci that control flowering and maturity enables soybeans to exhibit a broad range of ecological adaptability. To maximize soybean yield across different regions, multiple genetic loci or genes play crucial roles in regulating soybean maturity and adaptability, some of which have been extensively identified and molecularly characterized22,23,24,25,26. However, the members and functions of the TPR gene family in soybean have yet to be systematically analyzed. Investigating the role of TPR genes in the regulation of photoperiod in soybean could provide valuable insights to enhance the adaptability of this important crop.
In this study, we conducted a comprehensive identification of 12 TPRs in the soybean genome. Subsequently, we analyzed their evolutionary relationships, gene structures, phylogenetic relationships, and protein compositions, as well as the expression responses of each GmTPR member under different photoperiods and the subcellular localization of some members.
Materials and methods
Identification and general characterization of TPR proteins in Glycine max
The soybean protein sequence (Wm82.a4.v1) was obtained from Phytozome (https://phytozome-next.jgi.doe.gov/). A BLAST analysis was conducted using the amino acid sequences of five known Arabidopsis TPR proteins, and a Hidden Markov Model (HMM) was constructed to predict the members of the soybean TPR gene family (E-value < 10−5). All predicted members were further screened using the SMART online platform (http://smart.embl-heidelberg.de/) to identify complete TPR domains. Genes encoding proteins containing one LisH domain, one CTLH domain, and two WD40-related domains were classified as members of the TPR family. Additionally, TBtools-II was employed to calculate the properties of TPR family members, including the number of amino acids and the isoelectric point (pI)27,28. AlphaFold3 (http://alphafoldserver.com) was used to predict the tertiary structures of GmTPR proteins.
Phylogenetic and synteny analysis of the GmTPR family
ClustalW was used for the multiple sequence alignment of all TPR protein sequences28. A phylogenetic tree of GmTPRs was constructed using MEGA1129,30, and a comprehensive phylogenetic tree that includes Arabidopsis, soybean, rice, and Medicago truncatula. Both phylogenetic trees were constructed using the Maximum Likelihood algorithm with 1000 bootstrap repeats31. Gene duplication events and synteny analysis (Glycine max vs. Arabidopsis; Glycine max vs. Glycine max) were performed using the default parameters of TBtools-II software28.
Gene structure, conserved motif, and cis-acting elements
Based on the GFF files and chromosome length information, TBtools was utilized to analyze the chromosome distribution of all GmTPR gene family members, as well as the exon/intron structures of each GmTPR27. The conserved motifs of all GmTPR proteins were analyzed by the MEME tool (http://meme-suite.org/tools/meme). Conserved domains within all GmTPR proteins were identified using the CD-Search tool (https://www.ncbi.nlm.nih.gov/cdd/) from the NCBI database. Cis-acting elements in the promoter sequences (upstream of 2000 bp) of the GmTPR gene family were predicted using the PlantCARE website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). TBtools-II was used to visualize results28.
Subcellular localization of GmTPRs
Plant-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/) was used to predict the subcellular localization of all GmTPR proteins. Subsequently, four GmTPRs were cloned and transiently overexpressed in tobacco leaves for subcellular localization experiments to validate the predictions. The GmTPRs were amplified and subsequently ligated into the fusion expression vector pSuper1300-MAS-EGFP following digestion, leading to the successful construction of the expression vector. Recombinant plasmids were introduced into Agrobacterium GV3101. Agrobacterium containing the pSuper1300-MAS-GmTPR1/5/9/11-EGFP expression vectors, as well as Agrobacterium carrying the empty control vector pSuper1300-MAS-EGFP, were cultured. The bacterial cells were collected by centrifugation at 4000 rpm for 15 min, and the supernatant was discarded. Subsequently, the bacterial cells were resuspended in 1 mL of tobacco transformation solution (OD600 = 0.7–1.0). After resuspension, the mixture was allowed to stand at room temperature or 28 °C for 2 h before injecting into tobacco leaves. Approximately 2–3 days post-injection, the lower epidermis of the tobacco leaves was peeled off to prepare temporary sections. The subcellular localization of the fusion proteins was then examined using a confocal microscope (Nikon Combined A1 R HD25 and N-SIM Systems), with images captured simultaneously.
RNA-seq data analysis of gene expression
Based on RNA-seq data (TPM) of GmTPRs extracted from SoyOmics (https://ngdc.cncb.ac.cn/soyomics/transcriptome/), we investigated the expression patterns of GmTPR genes in different soybean tissues. The transcriptomic data for soybeans were obtained from the publicly accessible database of the National Center for Biotechnology Information (NCBI) (Accession number: GSE94228). After removing adapter sequences and low-quality reads from the RNA-seq data using fastp (v.0.23.0)32 we aligned the cleaned RNA-seq data to the Wm82.a4.v1 genome using HISAT2 (v.2.1.2)33 with default parameters. We then quantified and normalized using StringTie (v.1.3.5)34 with default settings. The heatmap in TBtools-II was used for further expression analysis28.
Real-time quantitative polymerase chain reaction (RT-qPCR) analysis of the expression levels of GmTPR genes
The soybean variety William 82 (Wm82) was used in the experiment, plants were grown on long days (LD, 16 h light/8 h dark) or short days (SD, 8 h light/16 h dark) under cool white light and at about 25 °C. Fully expanded trifoliolate leaves were harvested from seedlings 14 days after emergence (DAE) at 0, 4, 8, 12, 16, and 24 h post-treatment. RNA was extracted using the Eastep™ Super Total RNA Extraction Kit from Promega. One microgram of total RNA was reverse transcribed into first-strand cDNA using the SPARKscript II RT Plus Kit (with gDNA Eraser, SparkJade). The expression levels of soybean TPRs were analyzed by quantitative real-time PCR (RT-qPCR) using an ABI QuantStudio real-time PCR system with a SYBR Green master mix (CWBIO). The constitutively expressed GmACT11 (Glyma.11G100100) was used as a quantitative internal control, and three biological replicates were applied for quantitative real-time PCR (qRT-PCR) analysis. Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆Ct methods35,36. Primers are listed in Supplementary Table 1.
Results
Identification and characterization of TPR genes in soybean
A total of 12 members of the TPR gene family were identified in soybean using BLAST and Hidden Markov Model (HMM) searches with 5 AtTPR proteins from Arabidopsis thaliana. These members were subsequently designated as GmTPR1-12, based on their homologous relationships with the corresponding AtTPR family members. Additionally, we analyzed the 12 GmTPR proteins for molecular weight (MW), theoretical isoelectric point (pI), subcellular localization, and other physicochemical properties (Table 1).
Regarding the physicochemical parameters, GmTPR9 has the longest coding sequence (CDS) at 3,408 bp and a protein length of 1,135 amino acids. In contrast, GmTPR12 possesses the shortest CDS length at 3,165 bp, with a protein length of 1054 amino acids. The molecular weights of the GmTPR proteins range from 117.79 to 126.45 kDa, with an average molecular weight of 123.71 kDa. The isoelectric points (pI) of the GmTPR proteins vary from 5.63 to 6.97, yielding an average value of 6.55 (Table 1). Additionally, GmTPR1, GmTPR2, GmTPR7, and GmTPR8 were classified as unstable proteins, exhibiting instability indices greater than 40. Furthermore, all members of the TPR family are classified as hydrophilic proteins, displaying average hydrophilicity values of approximately − 0.27 and an aliphatic index of less than 100 (Table 1). Using the AlphaFold3 tool for predictions, our 3D homology modeling results indicated that GmTPR possesses significant structural similarity. The protein is primarily composed of α-helices (12.81–20.67%), extended chains (12.65–22.11%), and random coils (65.09–68.17%) (Fig. S1).
Evolutionary expansion analysis of the GmTPR family
To investigate the evolutionary relationships among TPR homologs in different plants, a phylogenetic tree (Fig. 1) was constructed using the amino acid sequences of the TPR from soybean (12), Arabidopsis (5), rice (3), maize (5), and Medicago truncatula (8). The evolutionary analysis revealed three distinct groups, designated as Group I, II, and III. Of these, Group I contains the highest number of members, including 6 from soybean, 3 from Arabidopsis, 3 from maize, 2 from rice, and 6 from Medicago truncatula. Group II is characterized by unique branches that separate legume plants from grasses and crucifers, reflecting a correlation between the evolutionary trajectories of the TPR family and the species themselves. Notably, Group III comprises only 2 GmTPR members (GmTPR11 and GmTPR12), suggesting that this subgroup may be unique to soybeans and fulfill specific functional roles. Each GmTPR member (except for GmTPR11 and GmTPR12) from soybean is closely related to at least one MedtrTPR member, indicating relative conservation of evolutionary relationships.
Phylogenetic analysis of GmTPR proteins from Glycine Max, Zea mays, Arabidopsis, rice and Medicago truncatula. Phylogenetic tree was constructed with MUSCLE using the maximum likelihood (ML) method. The bootstrap values from 1,000 replications are indicated on the branches. The different colored labels the different class of GmTPRs.
Chromosomal distribution and synteny analysis of the GmTPR family
A chromosome distribution map of GmTPRs based on annotation information from the soybean genome. The distribution of the 12 GmTPRs is uneven across 11 of the 20 soybean chromosomes, with 2 genes located on chr13. Conversely, Chr03, 04, 06, 07, 08, 10, 15, 17, 19, and 20 each contain only one gene (Fig. S2).
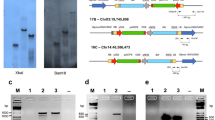
Tandem and segmental duplications play a significant role in the formation of gene families during evolution, influencing the functional divergence of genes37,38. To explore this further, we analyzed the collinearity relationships of the GmTPR gene family both within and across species (Fig. 2). The collinearity analysis between the soybean and Arabidopsis genomes revealed that 6 GmTPR genes are collinearly related to 3 AtTPR genes, resulting in a total of 10 pairs of collinear genes (Fig. 2a). Specifically, AtTPL and AtTPR1 exhibit collinearity with GmTPR1, GmTPR2 and GmTPR4, GmTPR10, while AtTPR3 displays collinearity with GmTPR6 and GmTPR8 (Fig. 2a). This indicates functional conservation among the TPR genes across different species.
Collinear analysis of GmTPR proteins and TPR proteins from different plants. a Chromosomal distribution and syntenic relationship prediction between Glycine max and Arabidopsis thaliana TPR genes. b Distribution and synteny analysis of GmTPR genes. The soybean chromosomes are presented in different colors. Colors indicate different classes of GmTPRs.
The analysis of collinearity within the GmTPR gene family revealed that no tandem duplication events have occurred. However, there are 22 pairs of collinear genes identified within GmTPRs (Fig. 2b). Given the size of the GmTPR family, segmental duplication appears to play a crucial role in its expansion. Previous studies on genomic duplication events between soybean and its ancestral species, Glycine soja39 suggest that all GmTPRs originated from whole genome duplication (WGD) in wild soybean. These findings collectively indicate that the GmTPR gene family in soybean has undergone significant gene duplication throughout evolution, contributing to the expansion of the GmTPR family.
Gene structure, domains, conserved motifs of GmTPR family
To systematically analyze the relationship between the function and evolution of the GmTPR genes, we investigated the gene structures, domains, and conserved motifs of all GmTPRs (Fig. 3)30. The GmTPR gene family consists of either 24 or 25 exons; specifically, GmTPR1 and GmTPR10 contain 24 exons, while the other members possess 25. The presence of repeated domains is a common characteristic of evolutionary processes; all 12 members of the GmTPR family contain 1 LisH domain, 1 CTLH domain, and 2 WD40-related domains. Notably, GmTPR5-8 each possess 1 additional CTLH-related domain. Furthermore, GmTPR3 and GmTPR4 not only exhibit the typical domains characteristic of the GmTPR family but also contain a unique PHA03247 domain. The motif structure of the GmTPR family is largely consistent, encompassing all ten identified motifs (Fig. S3); however, GmTPR11 and GmTPR12 each feature an additional motif 6 compared to the other family members. The conservation and variation observed in these structures and motifs highlight both the functional conservation of the soybean TPR family and the functional diversification among its members.
Analysis of cis-regulatory elements in GmTPR promoters
To explore the regulatory patterns of GmTPR gene expression, the PlantCARE server was utilized to analyze the cis-elements in the promoter regions of 12 GmTPR genes in soybean. This analysis identified a total of 38 cis-acting elements, including light-responsive elements, hormone-responsive elements, environmental stress response elements, and those associated with developmental and metabolic processes (Fig. 4). Among the 12 GmTPR genes, at least 3 types of light-responsive elements were found in their promoter regions, with the Box4 light-responsive element present in all GmTPR gene promoters. This observation indicates a prominent light response characteristic within the GmTPR family. Additionally, MeJA-responsive elements, specifically the CGTCA-motif and TGACG-motif, along with ABA-responsive elements, were more frequently detected in some GmTPR gene promoters than other hormone-related elements. These findings suggest that GmTPR genes may play a role in MeJA and ABA signaling pathways and potentially respond to environmental stress. The analysis also revealed five types of stress-responsive elements within the promoters of GmTPR genes. Most members contained long terminal repeat (LTR) elements associated with cold stress and antioxidant response elements (ARE). Other identified stress-related elements included defense and stress response elements enriched with TC repeat sequences, transcription factor binding sites (GC-motif), and MYB binding sites related to drought induction (MBS). In contrast, very few elements related to growth and development were detected in the promoter regions of GmTPR genes.
Subcellular localization of GmTPR proteins
Predicting the subcellular localization of all members using Plant-mPLoc indicates that they are located in the nucleus (Table 1). The subcellular localization of GmTPR proteins representing distinct phylogenetic branches (GmTPR1, GmTPR5, GmTPR9, GmTPR11) was assessed through translational fusion with green fluorescent protein (GFP) and subsequent transient expression experiments in tobacco protoplasts (Fig. 5). Microscopic analysis demonstrated that the GmTPRs-GFP fusion proteins were exclusively localized in the nucleus. This nuclear localization is indicative of the proteins’ potential roles in the precise regulation of gene expression and the integration of various biological signals. Collectively, these findings support the hypothesis that GmTPR proteins are involved in transcriptional regulatory activities.
Tissue-specific expression analysis of GmTPRs during different developmental stages of soybeans
To elucidate the potential functions of GmTPR family members, we analyzed an expression dataset derived from various tissues at different developmental stages and organ types. Our results revealed significant tissue-specific expression patterns among the GmTPR genes (Fig. 6). Notably, most GmTPR members exhibited elevated expression levels in floral tissues, with GmTPR11 and GmTPR12 as exceptions. Specifically, GmTPR3, GmTPR5, GmTPR8, GmTPR9, and GmTPR10 exhibited the highest expression levels during the V3 (The soybean plant has typically developed three fully expanded true leaves) stage of flowering, while GmTPR1 and GmTPR2 peaked on the day of flowering (Day 0). In contrast, GmTPR4 and GmTPR7 reached their maximum expression levels during the flowering withering stage. In contrast, GmTPR11 and GmTPR12 demonstrated the highest expression during seed development, significantly surpassing the expression levels of other GmTPR genes. Interestingly, the GmTPR genes generally exhibited lower expression levels in roots and stems, particularly in leaves; however, besides its robust expression in floral tissues during the V3 stage, GmTPR6 also exhibited a striking, primary root-specific elevation at the VE5 stage (at 5 days after emergence), underscoring its functional specificity. However, the overall expression of GmTPR members progressively declined during the seed-filling process, with expression levels diminishing as seeds approach maturity. This highlights the possibility that GmTPR genes play a crucial role not only during flowering but also in the early stages of seed development.
Gene expression pattern of the GmTPRs in soybean tissues. The color corresponding to each tissue above the heatmap represents the average expression level of all GmTPR members in the respective soybean tissue across different developmental stages. The color gradient is consistent with the heatmap scale.
Photoperiod-dependent expression of GmTPR genes
To investigate the expression differences of GmTPR genes under varying photoperiods, we analyzed diurnal transcriptome data from soybean unifoliate leaves40. Under LD conditions with 16 h of light, the diurnal expression patterns of GmTPR genes were diverse (Fig. 7a). Notably, the expression levels of GmTPR1, GmTPR2, GmTPR3, GmTPR5, GmTPR9 and GmTPR10 exhibited a downward trend at the onset of light, reaching a minimum after eight hours of light before subsequently increasing. Strikingly, while GmTPR5 and GmTPR9 displayed an expression inflection point at 12 h of light exposure followed by sustained decline, GmTPR1-4 showed delayed inflection points at 16 h. Upon darkness initiation, all these genes demonstrated progressive downregulation. Notably, GmTPR12 did not exhibit an inflection point during the light period; instead, it peaked four hours after the onset of darkness, marking a unique observation. In contrast, the expression levels of GmTPR6, GmTPR7, and GmTPR8 showed a continuous increase during the 12 to 16 h of light, followed by a decline, with expression levels still decreasing after eight hours of darkness.
Expression analysis of GmTPR genes in the indicated seedlings over a 24 h long-day cycle. a The expression levels of GmTPR genes under LD conditions as shown by RNA-seq. b Real-time quantitative polymerase chain reaction (RT-qPCR) analysis of the expression levels of GmTPR genes under LD conditions. ZT for zeitgeber time (hours after light on). The levels of GmTPR genes were normalized directly to the constitutively expressed GmACT11. Error bars indicate SD of three biological repeats; white and dark bars below the x-axis to mark light and dark periods, respectively.
To validate the expression patterns identified through RNA-seq analysis, we conducted quantitative PCR (RT-qPCR) to assess the expression changes of twelve GmTPR genes in the leaves of two-week-old soybean (Wm82) under both long and short photoperiods at various time points (Fig. 7b). Under LD conditions, while some minor variations in gene expression were observed; Specifically, GmTPR3 and GmTPR4 show a continuous increase in expression throughout the light period. Notably, GmTPR3 displayed upregulation after four hours of darkness, followed by a subsequent decrease. In contrast, GmTPR1 and GmTPR2 were downregulated during the light phase but exhibited upregulation when exposed to darkness. This upregulation reached an inflection point after four hours of darkness before declining. Overall, the expression patterns of the other GmTPR members (GmTPR5, GmTPR10, GmTPR11, GmTPR12) were generally consistent with those observed in the RNA-seq analysis, although the timing of the minimum expression point varied, and the subsequent increases were more gradual. Under SD conditions with 8 h of light (Fig. S4), the expression trends of soybean GmTPRs differed slightly. GmTPR6, GmTPR7, GmTPR8, GmTPR9, and GmTPR10 exhibited an overall declining trend in expression during the light period. Notably, after transitioning into the night, their expression patterns contrasted with those observed under LD conditions, where GmTPR expression levels increased. Most of these genes showed no further increase in expression after 12 h of darkness, followed by a subsequent downregulation. GmTPR1 and GmTPR2 exhibited minimal changes in expression during the light period and the first eight hours of darkness, but also displayed an inflection point in expression after twelve hours of darkness. Collectively, soybean GmTPRs exhibited divergent expression patterns under photoperiod conditions, implicating their involvement in diurnal regulation.
Discussion
A total of 12 TPR genes were identified in soybean, compared to only 5 in Arabidopsis. This discrepancy may be attributed to gene duplication events that facilitated the expansion of the soybean TPR gene family7. Previous studies indicate that the number of genes in the TPR family generally remains stable, typically comprising 4 to 6 homologous members; for instance, there are 6 members in tomato, 5 in Arabidopsis, and 3 in rice15,41,42. The identification of 12 members within the soybean TPR gene family—with no evidence of tandem gene duplication—suggests that segmental duplication may be a significant mechanism driving this expansion. Further research on genomic duplication events in soybean and its ancestral species, Glycine soja, supports the notion that all GmTPR genes originated from WGD in wild soybean39. This evidence bolsters the hypothesis that the GmTPR family in soybean has undergone substantial gene duplication throughout evolution, leading to the family expansion observed today, which is retained through multiple WGD events.
Phylogenetic analysis revealed that GmTPR11 and GmTPR12 belong to a lineage that has experienced gene loss in Arabidopsis. Notably, members from Class III, specifically GmTPR11 and GmTPR12, possess one fewer exon compared to other subfamilies of soybean TPR genes; additionally, GmTPR12 features an extra motif (motif 6). These structural differences may have implications for their functional specificity. Further investigation shows that, unlike other soybean TPR members, GmTPR11 and GmTPR12 exhibit lower expression levels in floral tissues but the highest expression levels in seeds. This differential tissue-specific expression highlights the functional variation of GmTPR genes within the soybean TPR family. Moreover, GmTPR6 displays a significant increase in expression during the VE5 stage of root development, suggesting a potential role in promoting root growth and enhancing the plant’s capacity to absorb water and nutrients, thereby facilitating overall growth. The observed patterns of conservation and variation in the structures and motifs of these genes underscore both the fundamental conservation of functions within the soybean TPR family and the functional diversification among its members.
The significant enrichment of photore-responsive elements in the promoter regions of soybean TPR genes, along with their tissue-specific expression patterns, suggests the conserved functions of this gene family in soybean. Previous research has highlighted the significant roles of TPRs in various plant hormone signaling pathways. For instance, Szemenyei et al. reported the critical function of TPRs in repressing auxin-responsive genes13. Moreover, Causier and Kuhn identified the responsive roles of TPRs in other non-classical auxin signaling mechanisms6,43. Interestingly, the overall expression of GmTPR genes gradually decreases during the seed-filling phase, with expression levels declining as seeds approach maturity. This trend suggests that these genes may be associated with the enhancement of seed maturation processes and storage functions.
Notably, GmTPR genes exhibit distinct expression profiles, with the majority demonstrating higher expression levels in floral tissues. Additionally, RT-qPCR analysis reveals that under varying photoperiod conditions, plants can adjust their flowering time and growth strategies by regulating the expression of the GmTPR genes in response to external light environments. This adaptability allows plants to thrive and reproduce more successfully in dynamic environments. The GmTPR genes may interact with other genes in the flowering regulation network (such as those involved in combinatorial regulation and light perception), thus affecting the physiological rhythms of plants by modulating photoperiod responses. The expression pattern of GmTPRs offers crucial insights into how plants respond to varying photoperiods, which is vital for researching plant adaptability in natural ecosystems. This hypothesis is further supported by previous studies showing that TOPLESS-related complexes inhibit flowering in Arabidopsis. TPR proteins were initially defined as key components of auxin signal transduction and response pathways, and their functions have been explored at various stages of plant growth and development. TPRs can influence different biological processes in plants by recruiting specific target genes or interacting with transcription factors containing short repressive domains (RD). Previous studies have identified hundreds of TPR interacting factors within transcription-related protein families6 suggesting that TPRs are broadly involved in major signaling pathways and developmental processes throughout the plant life cycle. The formation of an inhibitory complex through interactions among PRRs, histone deacetylase 6, and TPR represents a critical component of the biological clock17. As corepressors, TPL proteins and CDF1 bind to the CO and FT promoters in the morning to assemble a repressive transcriptional complex, thereby inhibiting CO and FT expression and regulating seasonal flowering44. TPR2, together with AFP2, forms the CO-AFP2-TPR2 complex by bridging CO, and, through its interaction with AFP2, recruits HDACs to the FT promoter, thereby reducing histone acetylation and repressing nighttime FT transcription; this complex regulates photoperiodic flowering by modulating CO protein stability and histone deacetylation at the FT chromatin45. Our results demonstrate that when soybean is planted under different photoperiods, the expression levels and trends of TPR vary at the same time point. These data support the hypothesis of functional diversification of TPR proteins in photoperiod response pathways, suggesting that GmTPRs may recruit other factors in light response pathways to influence gene expression. However, the mechanisms and impacts of their roles in soybean photoperiod response require further in-depth investigation.
Conclusions
In this study, we employed bioinformatics approaches to systematically analyze the soybean TPR gene family, exploring its structural characteristics and comparing the features of this gene family across various plants from an evolutionary perspective. Analysis of the promoter regions of the soybean GmTPR genes revealed a significant enrichment of light-responsive elements. Coupled with transcriptional expression analysis, the study confirmed that different photoperiods indeed regulate GmTPR genes. In summary, our findings provide critical insights for the functional identification of the soybean TPR genes, which play a vital role in regulating plant growth and development as well as photoperiod response pathways, thereby offering new avenues for their functional characterization.
Data availability
The transcriptomic data for soybeans were obtained from the publicly accessible database of the National Center for Biotechnology Information (NCBI) (Accession number: GSE94228). All data generated or analyzed during this study are included in this published article and its supplementary file.
References
Ke, J. et al. Structural basis for recognition of diverse transcriptional repressors by the TOPLESS family of corepressors. Sci. Adv. 1, e1500107 (2015).
Garner, C. M. et al. Opposing functions of the plant TOPLESS gene family during SNC1-mediated autoimmunity. PLoS Genet. 17, e1009026 (2021).
Xu, F. et al. Exportin-4 coordinates nuclear shuttling of TOPLESS family transcription corepressors to regulate plant immunity. Plant Cell 33, 697–713 (2021).
Plant, A. R., Larrieu, A. & Causier, B. Repressor for hire! The vital roles of TOPLESS-mediated transcriptional repression in plants. New Phytol. 231, 963–973 (2021).
Mu, Y. P. et al. Mirids secrete a TOPLESS targeting protein to enhance JA-mediated defense and gossypol accumulation for antagonizing cotton bollworms on cotton plants. Mol. Plant. 17, 1687–1701 (2024).
Causier, B., Ashworth, M., Guo, W. & Davies, B. The TOPLESS interactome: a framework for gene repression in Arabidopsis. Plant Physiol. 158, 423–438 (2012).
Liu, X., Galli, M., Camehl, I. & Gallavotti, A. RAMOSA1 ENHANCER LOCUS2-Mediated transcriptional repression regulates vegetative and reproductive architecture. Plant Physiol. 179, 348–363 (2019).
Huang, L. J. et al. The role of corepressor HOS15-mediated epigenetic regulation of flowering. Front. Plant Sci. 13, 1101912 (2022).
Liu, Z. & Karmarkar, V. Groucho/Tup1 family co-repressors in plant development. Trends Plant Sci. 13, 137–144 (2008).
Lee, J. E. & Golz, J. F. Diverse roles of Groucho/Tup1 co-repressors in plant growth and development. Plant Signal. Behav. 7, 86–92 (2012).
Khan, M. & Djamei, A. TOPLESS corepressors as an emerging hub of plant pathogen effectors. Mol. Plant Microbe Interact. 37, 190–195 (2024).
Szemenyei, H., Hannon, M. & Long, J. A. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319, 1384–1386 (2008).
Long, J. A., Ohno, C., Smith, Z. R. & Meyerowitz, E. M. TOPLESS regulates apical embryonic fate in Arabidopsis. Science 312, 1520–1523 (2006).
Zhang, X. et al. Genome-wide analysis of TOPLESS/TOPLESS-RELATED co-repressors and functional characterization of BnaA9.TPL regulating the embryogenesis and leaf morphology in rapeseed. Plant Sci. 346, 112149 (2024).
Hao, Y. et al. Genome-wide identification, phylogenetic analysis, expression profiling, and protein-protein interaction properties of TOPLESS gene family members in tomato. J. Exp. Bot. 65, 1013–1023 (2014).
Gallavotti, A. et al. The control of axillary meristem fate in the maize ramosa pathway. Development 137, 2849–2856 (2010).
Wang, L., Kim, J. & Somers, D. E. Transcriptional corepressor TOPLESS complexes with pseudoresponse regulator proteins and histone deacetylases to regulate circadian transcription. Proc. Natl. Acad. Sci. U.S.A. 110, 761–766 (2013).
Krogan, N. T., Hogan, K. & Long, J. A. APETALA2 negatively regulates multiple floral organ identity genes in Arabidopsis by recruiting the co-repressor TOPLESS and the histone deacetylase HDA19. Development 139, 4180–4190 (2012).
Chen, X., Ding, A. B. & Zhong, X. Functions and mechanisms of plant histone deacetylases. Sci. China Life Sci. 63, 206–216 (2020).
Lin, X., Liu, B., Weller, J. L., Abe, J. & Kong, F. Molecular mechanisms for the photoperiodic regulation of flowering in soybean. J. Integr. Plant Biol. 63, 981–994 (2021).
Corbellini, M. et al. Geographical adaptability for optimizing the recommendation of soybean cultivars in the Brazilian Cerrado. Sci. Rep. 14, 13076 (2024).
Dissanayaka, A. et al. Quantitative trait locus mapping of soybean maturity gene E5. Breed. Sci. 66, 407–415 (2016).
Fang, C. et al. A recent retrotransposon insertion of J caused E6 locus facilitating soybean adaptation into low latitude. J. Integr. Plant Biol. 63, 995–1003 (2021).
Dong, L. et al. Genetic basis and adaptation trajectory of soybean from its temperate origin to tropics. Nat. Commun. 12, 5445 (2021).
Dong, L. et al. Parallel selection of distinct Tof5 alleles drove the adaptation of cultivated and wild soybean to high latitudes. Mol. Plant. 15, 308–321 (2022).
Lu, S. et al. Natural variation at the soybean J locus improves adaptation to the tropics and enhances yield. Nat. Genet. 49, 773–779 (2017).
Wang, Y. et al. Genome-wide analysis of VQ motif-containing proteins in Moso bamboo (Phyllostachys edulis). Planta 246 (1), 165–181 (2017).
Chen, C. et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 13 (8), 1194–1202 (2020).
Thompson, J. D., Gibson Tj Fau - Higgins, D. G. & Higgins, D. G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinformatics. Chapter 2, Unit 2.3 (2003). https://doi.org/10.1002/0471250953.bi0203s00.
Hall, B. G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 30, 1229–1235 (2013).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33 (7), 1870–1874 (2016).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Kim, D., Landmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods. 12, 357–360 (2015).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295 (2015).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 (2001).
Kanai, M., Yamada, T., Hayashi, M., Mano, S. & Nishimura, M. Soybean (Glycine max L.) triacylglycerol lipase GmSDP1 regulates the quality and quantity of seed oil. Sci. Rep. 9, 8924 (2019).
Zhao, W. et al. Genome-wide identification and characterization of circular RNAs by high throughput sequencing in soybean. Sci. Rep. 7, 5636 (2017).
Zhao, Z. et al. A comprehensive analysis of the WRKY family in soybean and functional analysis of GmWRKY164-GmGSL7c in resistance to soybean mosaic virus. BMC Genom. 25, 620 (2024).
Du, J. et al. Pericentromeric effects shape the patterns of divergence, retention, and expression of duplicated genes in the paleopolyploid soybean. Plant. Cell. 24, 21–32 (2012).
Li, M. et al. Comprehensive mapping of abiotic stress inputs into the soybean circadian clock. Proc. Natl. Acad. Sci. U.S.A. 116, 23840–23849 (2019).
Wang, W. et al. OsbHLH061 links TOPLESS/TOPLESS-RELATED repressor proteins with POSITIVE REGULATOR OF IRON HOMEOSTASIS 1 to maintain iron homeostasis in rice. New Phytol. 234, 1753–1769 (2022).
Sun, B. et al. A novel transcriptional repressor complex MYB22-TOPLESS-HDAC1 promotes rice resistance to brown planthopper by repressing F3’H expression. New Phytol. 239, 720–738 (2023).
Kuhn, A. et al. Direct ETTIN-auxin interaction controls chromatin States in gynoecium development. eLife 9, e51787 (2020).
Goralogia, G. S. et al. CYCLING DOF FACTOR 1 represses transcription through the TOPLESS co-repressor to control photoperiodic flowering in Arabidopsis. Plant J. 92, 244–262 (2017).
Chang, G. et al. ABI5-BINDING PROTEIN2 coordinates CONSTANS to delay flowering by recruiting the transcriptional corepressor TPR2. Plant Physiol. 179, 477–490 (2019).
Funding
The work was supported in part by the Taishan Scholars Program (tsqn202211301), Natural Science Foundation of Shandong Province (ZR2021YQ16, ZR2022QC262, ZR2023QC085), Yuandou Scholars Program, Weifang Science and Technology Plan (2023ZJ1063), and project SYS202206 supported by the Shandong Provincial Natural Science Foundation.
Author information
Authors and Affiliations
Contributions
Xiao Luo, C.S., and S.Y. conceived the study. W.R., C.S., and S.Y. performed most of the experiments and bioinformatic analysis. Y.F., C.H., X.L., and Y.Z. performed part of the experiments. W.R., C.S., and Xiao Luo drafted and improved the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The plant material and methods involved in this study were carried out in compliance with relevant institutional, national, and international guidelines and legislation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, S., Ren, W., Song, C. et al. Global identification and characterization of soybean TPR genes with expression analysis under photoperiod variations. Sci Rep 15, 24644 (2025). https://doi.org/10.1038/s41598-025-10368-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10368-5