Abstract
Multiple severe acute respiratory syndrome (SARS-CoV-2) exposures either due to infection or vaccination result in differential immune responses. Limited data exist on community-level SARS-CoV-2 variant exposures in India. We assessed antibody levels in 211 unvaccinated and 349 vaccinated individuals with and without prior exposure, followed for ~ 2 years (May 2021–October 2023) with baseline blood, weekly saliva for RT-PCR, and blood draws at ~ 6, 12, and 24 months. Humoral and cellular immune responses following primary (N = 8) and secondary (N = 11) Omicron infections in unvaccinated, previously exposed (N = 25), and unexposed vaccinated (N = 14) individuals were evaluated using Meso Scale Discovery and flow cytometry assays at Day 0, 30, and 90. Previously exposed individuals had higher anti-spike IgG levels at baseline (median 57,732 vs. 11,359, p < 0.001 in unvaccinated; 93,827 vs. 26,883, p < 0.001 in vaccinated) and 99% seropositivity at 2 years. Secondary Omicron infection elicited higher anti-spike IgG levels (17,320 vs. 3,930, p < 0.01) and stronger CD8 T cell responses (median CD8 AIM 0.026 vs. 0.001, p = 0.01 at Day90) than primary Omicron infection. Vaccinated individuals showed comparable CD4 and CD8 T cell responses irrespective of prior exposure, but previously exposed individuals had more durable antibodies at Day90 (p < 0.05 in previously exposed). Anti-spike IgG levels and %ACE-2 inhibition to Omicron were lower in both groups compared to other variants. High seropositivity was observed in both vaccinated and unvaccinated individuals after multiple exposures, though antibody levels were higher in individuals with prior exposure. These findings underscore preserved T cell responses to Omicron, although with lesser magnitude among unvaccinated.
Similar content being viewed by others
Introduction
The emergence of the Omicron variant (B.1.1.529) of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in late 2021 marked a significant evolutionary shift in the COVID-19 pandemic. Omicron’s unique constellation of mutations, particularly in the spike protein, conferred increased transmissibility and immune evasion capabilities, enabling fit to rapidly displace previous variants of concern (VOCs) and fuel unprecedented surges in outbreaks worldwide1,2. Although Omicron was associated with reduced disease severity compared with previous VOCs3,4,5,6, its ability to evade the antibody responses elicited by vaccination or prior exposure raised concerns about waning immunity and potential repeat infections7,8,9,10. Several Omicron sub-lineages with further mutations have been reported after the Omicron wave, leading to reinfections11,12.
Tracking population-level antibody kinetics is useful for elucidating the evolution of immune responses based on infection, re-infection, vaccination rollout, and SARS-CoV-2 variant emergence. Longitudinal cohort studies facilitate in-depth characterization of antibody responses, which is especially useful for determining antibody levels in vaccine trials13. Prior studies reported that primary Omicron infection elicits a poor neutralizing antibody response, and the cross-reactivity to pre-Omicron variants depends greatly on previous exposure or vaccination14,15. Although several studies have explored Omicron-specific immune responses and population-level antibody kinetics in other countries16,17,18,19,20, the associated immunological landscape in India has not been extensively characterized. Differences in demographic characteristics, vaccination rollout strategies, and natural immunity backgrounds may lead to differential immune responses compared to those of other countries.
Yadav et al.21 assessed the anti-spike IgG and T-cell responses to wild-type (WT) spike proteins after primary Omicron infection in 19 Indian individuals and observed cross-reactive T-cell responses in only 59% of the participants. Following the Omicron wave, a substantial proportion of the Indian population remained unvaccinated22, and previous waves have conferred variable levels of natural immunity. The Indian population exhibits considerable heterogeneity in prior SARS-CoV-2 exposure, vaccination status (including type of vaccine received), demographic structure, and environmental factors all of which could influence immune responses and reinfection risk23. Considering the heterogeneity of the Indian population and the risks associated with COVID-19 outbreaks, the magnitude and cross-reactivity of antibody and T-cell responses following Omicron infection among unvaccinated and vaccinated individuals with varying levels of SARS-CoV-2 exposure require detailed characterization. In addition, it is crucial to track potential antigen imprinting effects, where the initial exposure influences subsequent immune responses, to optimize vaccine strategies designed specifically for the Indian population.
In this study, we investigated the SARS-CoV-2-specific antibody, humoral, and cellular immune profiles in a south Indian community following the Omicron wave. We used data from a unique longitudinal cohort study in India that systematically tracked community-level antibody levels against SARS-CoV-2, including weekly RT-PCR data for > 2 years for both vaccinated and unvaccinated individuals. Concurrently, we comprehensively profiled the humoral and cellular immune responses following Omicron infection, aiming to identify potential complementary and potentially longer-lasting defense against severe disease. The findings of this study may provide insights into the potential immune landscape associated with the emergence of newer Omicron subvariants.
Materials and methods
Study design and participants
The study cohort comprised 1,229 adults aged 18–90 years from the Vellore Health and Demographic Surveillance System (VHDSS). This cohort was established to determine SARS-CoV-2 infections and reinfections at the community level after the peak Delta wave in India in May 2021. The participants were monitored for 2 years (May 2021–October 2023). We followed the protocol outlined for the risk of COVID-19 re-infection and its predictors (CORES) cohort study24. A baseline blood sample was obtained at the time of recruitment, and weekly salivary samples were collected for SARS-CoV-2 detection using RT-PCR. A randomly selected subset of positive RT-PCR samples with cycle threshold (Ct) values of less than 25 underwent whole genome sequencing (WGS) to identify the SARS-CoV-2 lineages causing infection. In addition, three follow-up blood samples were collected—approximately 5–6 months, just prior to the Omicron wave (November 2021–December 2021), 12th month from baseline (May 2022–October 2022), and 24th month from baseline (May 2023–October 2023)—for antibody testing using Meso Scale Discovery panels (MSD; Meso Scale Diagnostics, Rockville, MD, USA). The follow-up period was divided into pre-Omicron (May 19, 2021–December 20, 2021), Omicron-I (December 21, 2021–June 6, 2022), and Omicron-II periods (June 7, 2022–October 31, 2023) based on the 6-monthly blood sampling and the WGS. The 12th month blood samples reflected the level of exposure to the Omicron variant, and the 24th month blood samples reflected the exposure to the Omicron sub-lineages. Of the 1,229 individuals, 1,020 provided samples across all periods. Participants who received one vaccine dose before recruitment or after the Omicron wave were excluded because their exposure status could not be determined. Therefore, data from 560 individuals were used to analyze antibody kinetics over 2 years of follow-up—all were unvaccinated at recruitment, and their demographic characteristics are described in Table S1. The two major vaccines administered in India were adenoviral-vectored vaccine (Covishield) and a whole virion-inactivated vaccine (Covaxin), both of which are based on WT strain. Among the 560 individuals, 349 individuals received two doses of the vaccine after recruitment at a median of 98 days before the Omicron wave (December 2021–March 2022), which was either Covishield or Covaxin, whereas 211 individuals remained unvaccinated during the entire follow-up period. During the Omicron wave, blood samples and peripheral blood mononuclear cells (PBMCs) were obtained from a subset of unvaccinated (n = 19) and vaccinated individuals (n = 39) after RT-PCR positivity on days 0, 30, and 90 to assess the humoral and cellular immune responses among unvaccinated or double-vaccinated seropositive (with prior SARS-CoV-2 exposure, SP) or seronegative (without prior SARS-CoV-2 exposure, SN) individuals (Fig. 1). Antibody responses were assessed among all individuals, whereas cellular immune responses across all three time points following Omicron infection were assessed in 16 unvaccinated and vaccinated individuals (Fig. 1). This study was approved by the Institutional Review Board of Christian Medical College, Vellore (IRB min no: 13585) and was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. Written informed consent was obtained from all participants.
Flowchart depicting the samples collected and analyzed for antibody and cellular immune responses in the COVID-19 re-infection and its predictors (CORES) cohort participants. “+” denotes “positive”, D0- Day 0, D30- Day 30 and D90- Day 90, ACE2- Angiotensin-converting enzyme2, RT-PCR- reverse transcriptase polymerase chain reaction, ELISPOT- Enzyme-Linked ImmunoSpot.
Definitions
Participants were categorized as seropositive or seronegative if their anti-spike IgG concentrations were ≥ 1,960 AU/mL and < 1,960 AU/mL, as measured using the MSD platform, as per the manufacturer’s instructions.
Secondary Omicron infection. Unvaccinated (UNV) participants who were previously exposed, as evidenced by the presence of anti-spike IgG (SP) antibodies at the time of recruitment, and subsequently tested RT-PCR positive during the Omicron wave.
Primary Omicron infection. Unvaccinated (UNV) participants who were not previously exposed, as evidenced by the absence of anti-spike IgG (SN) antibodies at the time of recruitment, who tested RT-PCR positive during the Omicron wave.
Previously exposed and vaccinated. Previously exposed individuals, as evidenced by the presence of anti-spike IgG (SP) antibodies at recruitment, who received two doses of SARS-CoV-2 vaccine and tested RT-PCR positive during the Omicron wave.
Previously unexposed and vaccinated. Previously unexposed individuals, as evidenced by the absence of anti-spike IgG (SN) antibodies at recruitment, who received two doses of SARS-CoV-2 vaccine and subsequently tested RT-PCR positive during the Omicron wave.
Laboratory procedures
Antibody assays
The MSD-based multiplex electrochemiluminescence assay was used to simultaneously detect IgG antibodies against the SARS-CoV-2 spike (S), spike receptor-binding domain (RBD), spike N terminal domain (NTD), and nucleocapsid (NC) antigens using a V-PLEX SARS-CoV-2 Panel 1 kit according to the manufacturer’s protocol at baseline and 6, 12, and 24 months22. This quantitative assay has an early sensitivity (days 0–14 of infection) of 84%, late sensitivity (day 15+) of 98%, and specificity of 99%. All samples were tested at an initial dilution of 1:5,000. Any samples that were above or below the detection range were retested at lower (1:1,000) or higher dilutions (up to 1:50,000), and the results were expressed as arbitrary units/ml (AU/ml). A V-PLEX SARS-CoV-2 Panel 24 IgG kit was used to determine the antibody levels against the WT, Delta, and Omicron variants in post-RT-PCR serum samples at D0, D30, and D90.
Surrogate neutralization assay
An angiotensin-converting enzyme-2 (ACE-2)-binding inhibition assay was performed using the MSD V-PLEX SARS-CoV-2 Panel 13 (ACE-2) kit according to the manufacturer’s protocol (dilution of 1:50) to detect antibodies that block the binding of ACE-2 to the RBD of the WT strain and spike antigens from the Delta (B.1.617.2) and Omicron (B.1.1.529) variants at baseline and at 6 and 12 months. To determine cross-reactivity and antigen imprinting, post-RT-PCR serum samples from D0, D30, and D90 were tested using a similar protocol against the spike proteins of the WT, Delta, Omicron variants using the MSD V-PLEX Panel 24 kit.
SARS-CoV-2-specific T cell responses
PBMCs were isolated on a Ficoll–Paque density gradient medium (Histopaque-1077, Sigma), washed with 1X phosphate-buffered saline (PBS), and resuspended in 90% fetal bovine serum (FBS)/10% DMSO (D2650, Sigma–Aldrich USA) and stored in liquid nitrogen till the time of testing. Cryopreserved PBMCs were thawed, washed, counted, and rested for 4 h in glutamine-containing RPMI-1640 medium supplemented with 10% FBS and 1% penicillin–streptomycin (complete medium, 72400047, Gibco–Thermo Fisher) at 2 × 106 cells/mL in six-well plates. We harvested and replated at 1.5 × 106 cells/mL and pre-incubated with 1 µg/mL anti-CD40 (130-094-133, Miltenyi Biotec, Germany) for 15 min at 25 °C before adding 1 µg/mL of peptide pools for the WT, Delta, and Omicron variant spikes (15-mer peptides spanning the entire spike region, Table S2) and 5 µg/mL of protein transport inhibitor cocktail (00-4980-93, eBiosciences). Peptide fragments were synthesized as crude material by TC Peptide Lab (San Diego, California, USA), dissolved in DMSO (10–20 mg/mL), and assembled into spike megapools for WT, Delta, and Omicron variants by combining and lyophilizing overlapping 15-mer peptides, then re-dissolving at 1 mg/mL. The negative control wells were exposed solely to dimethyl sulfoxide, without the inclusion of peptides. For the positive control, 1 × 106 cells were stimulated with 5 µg/mL phytohemagglutinin (PHA, L9379, Sigma–Aldrich USA) without anti-CD40 pre-incubation; 1.25 µL anti-CXCR5 (741316, BD Biosciences) was added at the time of stimulation and incubated for 16 h at 37 °C in an atmosphere containing 5% CO2. All cultures were performed in U-bottom plates at a final volume of 200 µL.
Enumeration of SARS-CoV-2-specific T cells
To quantify SARS-CoV-2-specific T cells, cultured PBMCs were washed with 1× phosphate-buffered saline, stained with Live/Dead fixable viability stain 780 (FVS 780) at 4 °C for 30 min, and incubated with a cocktail containing anti-CD3, CD4, CD8, CD19, CD14, CD56, CD45RA, and CCR7 in a staining buffer containing 2% FBS for 30 min at 4 °C. The cells were then fixed and permeabilized using a Cytofix/Cytoperm Fixation/Permeabilization Kit (BD Biosciences, Franklin Lakes, NJ, USA) for 20 min at 4 °C and treated with a cocktail containing anti-IL-2, IFN-γ, TNF-α, CD69, CD154, CD137, and CD287 in 1× Perm/Wash and Brilliant Stain Buffer Plus. Cells were analyzed on a FACSymphony (A3, BD Biosciences, San Jose, California, United States) system, and average of 0.5–0.7 × 106 cells) (Table S3). The results were expressed as frequencies of activation-induced marker-positive (AIM+) and cytokine positive (IL-2+/IFN-γ+/TNF-α+) cells on gated CD4 (CD19–CD14–CD56–CD4+) and CD8 cells (CD19–CD14–CD56–CD8+) after subtracting the frequencies of AIM+/cytokine + cells in matched unstimulated controls. For each stimulation, 1.5 million PBMCs were seeded and an average of 200,000 CD4 and 70,000 CD8 positive live T cells were acquired by flow cytometry, ensuring sufficient events for reliable detection of low-frequency responses. AIM + cells were identified as CD69 + CD154 + for CD4, circulating T follicular helper (cTfh) AIM + cells were identified as CD69 + CD154 + on (CD4 + CXCR5+) cells and CD69 + CD137 + for CD8. The memory gates were applied to CD4+/CD8 + T cells on CCR7 and CD45RA markers, which were classified as central memory (CCR7 + CD45RA–), effector memory (CCR7–CD45–), and T cell effector memory on CD45RA+ (Temra) on (CD8 + CD45RA + CCR7–) cells. SARS-CoV-2-specific IFN-γ + CD69 + T cells were further gated into the memory markers to assess the SARS-CoV-2-specific memory T cells. The multifunctional profile of CD4 + T cells was determined using the Boolean gate function on SARS-CoV-2-specific IL-2+/IFN-γ+/TNF-α + cells. All flow cytometry data were analyzed using FlowJo, LLC (version 10.8.1). The negative values obtained after subtracting the background were normalized to zero. The gating strategy is shown in Fig. S1.
Enumeration of RBD-specific antibody-secreting cells from polyclonally stimulated memory B cells
PBMCs were thawed and 5 × 106 cells were stimulated with 1 µg/mL R848 and 10 ng/mL IL-2 (3850-4APW-R1-1, Mabtech, Sweden) and incubated for 5 days. On the 6th day, cells were seeded after being pre-blocked with cRPMI for 30 min to assess total and RBD-specific IgG levels. The assay protocol was adapted from that of Rouers et al.25, and spot counts were enumerated using an automated Enzyme-Linked ImmunoSpot (ELISPOT) reader (AID iSpot, Advanced imaging Devices, Gmbh, Strassberg) (Fig. S2). The RBD-specific spots were expressed as a percentage of all IgG spots.
Statistical analysis
Antibody levels across the blood samples over 2 years were compared using nonparametric Friedman tests with Dunn’s correction. We compared the secondary and primary Omicron infection groups and the previously exposed and unexposed-vaccinated groups in terms of the magnitude of antibody, T cell, and B cell levels following Omicron infection using Mann–Whitney U tests. All paired samples across the three time points following infection were statistically compared using nonparametric analysis of variance, and the Freidman test with Dunn’s correction for multiple comparisons was applied for adjusted p-values. The correlation matrix between antibody and cellular immune profiles was constructed using nonparametric Spearman rank correlation coefficients. All analyses were performed using GraphPad Prism (version 10.0.0, GraphPad Software, San Diego, CA, USA), and all statistical tests were two-sided with statistical significance set at p < 0.05. Principal component analysis (PCA) was performed using R software (version 4.2.2, R Foundation for Statistical Computing, Vienna, Austria).
Results
Seropositive unvaccinated individuals exhibited higher antibody levels and a higher ACE2 Inhibition percentage
Among the 211 unvaccinated individuals during the 2-year follow-up, 170 were previously exposed (SP) and 41 were unexposed (SN) at recruitment. Among the 170 SP individuals, 113 (66%) tested positive based on RT-PCR, among whom 81 tested positive once and 32 tested positive twice during the 2-year follow-up. Among the 41 SN individuals, 27 (65%) tested positive, among whom 14 and 13 tested positive once and twice, respectively. By the end of the 24-month follow-up, seropositivity to the spike antigen was 99% in the SP group (169/170) and 95% (39/41) in the SN group (Fig. S3). The SP group exhibited a higher magnitude of anti-spike and anti-RBD IgG levels than the SN group (median (interquartile range (IQR)) anti-spike IgG levels of 57,732 (30,304 − 102,233) vs. 11359 (6,381 − 22,528) AU/ml in SP and SN individuals at 24 months, respectively, p < 0.05, Fig. 2a–c). A similar pattern was observed with percentage ACE-2 inhibition levels against WT, Delta, and Omicron at 1 year of follow-up (p < 0.05, Fig. 2d-f). No significant difference was observed in antibody levels for the 6th month pre-Omicron blood samples among individuals who tested positive and negative during the Omicron wave (December 2021–March 2022, Fig. S4a). A small number of individuals classified as unexposed based on anti-spike IgG had detectable anti- nucleocapsid IgG antibodies at baseline, possibly reflecting prior asymptomatic infection with waning anti-spike antibodies or very recent infections with early anti-N seroconversion (Fig. 2c).
Antibody levels to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) among the unvaccinated study participants over 24 months who were seropositive and seronegative at baseline. (a) Anti-spike IgG levels. (b) Anti-spike receptor-binding domain (RBD) IgG levels. (c) Anti-nucleocapsid IgG levels. Percentage angiotensin-converting enzyme-2 (ACE-2) inhibition levels to SARS-CoV-2 among the unvaccinated study participants over 24 months who were seropositive and seronegative at baseline. (d) Percentage ACE-2 inhibition levels to wild type (WT). (e) Percentage ACE-2 inhibition levels to Delta. (f) Percentage ACE-2 inhibition levels to the Omicron variant. The data are represented as individual values with median and interquartile range. Data were analyzed for statistical significance using with Mann–Whitney U test. P-values are indicated by asterisks as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 and ns, no significant difference. AU/ml- arbitrary units/ml. N = 170 for Seropositive at baseline; N = 41 for Seronegative at baseline.
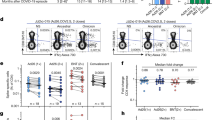
We analyzed antibody kinetics and cellular immune responses in a subset of unvaccinated individuals who tested positive during the Omicron wave. Following Omicron infection, antibody levels against Omicron were lower than those against other variants, irrespective of prior exposure (p < 0.05, Fig. 3a). Primary Omicron infected individuals showed lower antibody levels against all variants than secondary Omicron infected individuals [median (interquartile range [IQR]), 17,320 (14,901–39,239) vs. 3,930 (184–8,593) on D0 (p < 0.01), 10,960 (5,454–13,050) vs. 1,457 (260–4,258) on D30 (p = 0.0057), and 3,575 (2,926–5,386) vs. 646 (212–3,183) on D90 (p = 0.02) in secondary vs. primary Omicron-infected individuals]. Similar to the binding antibody levels, primary Omicron-infected individuals showed lower neutralizing antibody levels against Omicron and weaker responses to pre-Omicron variants than secondary Omicron-infected individuals (p < 0.05). In contrast, the secondary Omicron-infected individuals showed a higher percentage of ACE-2 inhibition for all variants, including Omicron (p < 0.05) at D0 and D30; however, by D90, the levels for Omicron in the SP group were comparable to those in the primary Omicron-infected group [median [IQR], 18.45 (0.00–24.56) vs. 0.00 (0.00–2.79) at D90, p = 0.05; Fig. 3a, b, Table S4].
Humoral and cellular immune responses following Omicron infection among unvaccinated individuals with and without pre-Omicron exposure. Antibody levels (a) and percentage ACE-2 inhibition levels (b) to SARS-CoV-2 variants among secondary (SP + UNV) and primary Omicron-infected (SN + UNV) unvaccinated individuals at D0, 30, and 90 (N = 11 for Secondary Omicron infected; N = 8 for Primary Omicron infected). (c-j) Variant spike-specific B cell and T cell responses elicited by Omicron infection among previously exposed and unexposed unvaccinated individuals. (c) RBD-specific spot-forming antibody-secreting cells (ASCs) expressed as a percentage of total IgG-secreting cells; (d) RBD-specific spot-forming ASCs (e-g) CD4 T cell responses (h, i) CD8 T cell responses following Omicron infection at Day 0, 30, 90 in Secondary Omicron infected (black) and Primary Omicron infected (pink). Spike-specific (e) activation-induced marker-positive (AIM+) CD4 T cells (f). Any one of the three cytokines interferon-γ (IFN-γ)/tumor necrosis factor-α (TNF-α)/interleukin-2 (IL2) + CD4 T cells (g) AIM + CD4 circulating T follicular helper (cTfh) cells (h) AIM + CD8 T cells (i). Any one of the three cytokines IFN-γ/TNF-α/IL2 + CD8 T cells (j) Proportion of polyfunctional CD4 T cells in secondary and primary Omicron-infected, unvaccinated individuals assessed on D0, 30, 90 following Omicron infection. The data are represented as median with interquartile range. Data were analyzed for statistical significance using Mann–Whitney U test. Background subtracted data were analyzed in all cases. P-values are indicated by asterisks as *p < 0.05, **p < 0.01. N = 8 for Secondary Omicron infected (SP + UNV); N = 8 for Primary Omicron infected (SN + UNV) for T and B cell analysis. WT- wild type.
In contrast to the humoral immune responses, both groups showed comparable RBD-specific antibody-secreting cell (ASC) levels within total IgG [median [IQR], 0.03 (0.01–0.03) vs. 0.01 (0.00–0.02) in secondary vs. primary Omicron infected individuals, p = 0.1; Fig. 3c] and RBD-specific ASC [median [IQR], 631 (268–893) vs. 194 (22–303) in secondary vs. primary Omicron infected individuals, p = 0.06; Fig. 3d]. Consistent with the B cells, the CD4 T cell responses (AIM+, cytokine+, peripheral T follicular helper (pTfh) AIM) were comparable at all time points following Omicron infection [median [IQR] for Omicron-specific CD4 cytokine + responses, 0.01 (0.00–0.06) vs. 0.00 (0.00–0.07) on D0 (p = 0.6), 0.08 (0.01–0.13) vs. 0.05 (0.005–0.07) on D30 (p = 0.3), and 0.01 (0.00–0.09) vs. 0.02 (0.00–0.13) on D90 (p = 0.6) in secondary vs. primary Omicron infected individuals; Fig. 3e–g]. Unlike CD4 T cell responses, Omicron-specific AIM + CD8 T cell levels were higher among secondary Omicron infected individuals on D30 and D90 [median [IQR], 0.01 (0.00–0.05) vs. 0.001 (0.00–0.002) on D30 (p = 0.02) and 0.026 (0.005–0.3) vs. 0.001 (0.00–0.007) on D90 (p = 0.01) in SP vs. SN individuals; Fig. 3h, i]. Notably, the IFN-γ-specific CD8 T cell responses were higher among secondary Omicron-infected individuals than among primary Omicron-infected individuals at D90 [median [IQR], 0.01 (0.007–0.02) vs. 0.00 (0.00–0.001), p = 0.005; Fig. S5, Table S5]. These findings suggested that prior exposure to the pre-Omicron variant boosted the CD8 T cell responses to Omicron, particularly IFN-γ + responses at D90 post-infection.
We found that the magnitude of Omicron-specific AIM + or cytokine + CD4 T cells was lower than that for the WT strain in both secondary and primary Omicron infected individuals [median [IQR] for AIM + CD4 T cells, 0.07 (0.01–0.2) vs. 0.001 (0.00–0.02)] on D0 (p = 0.01) and 0.08 (0.04–0.14) vs. 0.03 (0.00–0.06) on D30 (p = 0.03); for cytokine + CD4 T cells, 0.1 (0.04–0.22) vs. 0.01 (0.00–0.009) on D90 (p = 0.03) for WT vs. Omicron in secondary Omicron infected individuals; Fig. S6a-c). This confirms the cross-recognition ability of CD4 + T cells against pre-Omicron variants. There was also no difference in the magnitude of CD8 T-cell responses across the variants in either group (Fig. S6d, e).
We assessed the percentage of variant-specific polyfunctional CD4 T cells using the Boolean gate function of FlowJo software. Irrespective of prior exposure, the proportions of three-cytokine + and two-cytokine + samples increased at D30 and declined by D90 (Fig. 3j). We determined antigen-specific memory T-cell levels across variants in secondary Omicron-infected individuals and found that Tem levels were higher than Tcm levels (p < 0.05) up to D90. In contrast, the primary Omicron-infected individuals showed higher Tcm than Tem levels (Fig. S7 a, b). Antigen-specific CD8 memory phenotypes were predominantly associated with Tem and Temra in both groups up to D90 following Omicron infection (Fig. S7 c, d).
Overall, these findings suggested that, similar to the humoral immune response, prior exposure to the pre-Omicron variants markedly enhanced the magnitude of the CD8 T cell response in secondary Omicron-infected individuals, whereas CD4 T cell and memory B cell levels were comparable with polyfunctional CD4 T cell levels against variants in both groups up to D30.
Seropositive vaccinated individuals exhibited higher antibody levels and a higher rate of ACE2 Inhibition
Among the 349 individuals who were vaccinated with either two doses of viral vector or whole virion-inactivated vaccine after recruitment (median, 45 d after recruitment) and before the Omicron wave (median, 98 d), 309 were previously exposed (SP) and 40 were unexposed (SN, Fig. 4). Among the exposed individuals, 139 tested positive once and 69 tested positive twice. In the unexposed group, 29 and 11 tested positive once and twice, respectively. Previously exposed-vaccinated individuals had higher antibody levels against the spike and RBD of SARS-CoV-2 than previously unexposed individuals at 24 months (p < 0.05, Fig. 4a, b). In the previously exposed group, the antibody levels for spike and RBD at 24 months were comparable to those at baseline, whereas the levels for nucleocapsid were significantly higher, reflecting the differences in exposure during and after the Omicron wave (July 2022–October 2023, Fig. S8). Previously exposed individuals had broader neutralizing antibody against SARS-CoV-2 variants than unexposed individuals (p < 0.05, Fig. 4d, e), but they became comparable for Omicron at 12 months (Fig. 4f). There was a significant difference in antibody levels for the 6th month pre-Omicron blood samples between individuals that tested positively and negatively during the Omicron wave (December 2021–March 2022, Fig. S4b). Among participants with prior seropositivity, 149 RT-PCR-confirmed infections occurred in the Covishield group and 59 in the Covaxin group. Among seronegative individuals, 17 infections were reported in the Covishield group and 15 in the Covaxin group during follow-up.
Antibody levels to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) among the vaccinated study participants over 24 months who were seropositive and seronegative at baseline. (a) Anti-spike IgG levels. (b) Anti-spike receptor-binding domain (RBD) IgG levels. (c) Anti-nucleocapsid IgG levels. Percentage angiotensin-converting enzyme-2 (ACE-2) inhibition levels to SARS-CoV-2 among the vaccinated study participants over 24 months who were seropositive and seronegative at baseline. (d) Percentage ACE-2 inhibition levels to wild-type (WT). (e) Percentage ACE-2 inhibition levels to Delta variant. (f) Percentage ACE-2 inhibition levels to the Omicron variant. The data are represented as individual values with median and interquartile range. Data were analyzed for statistical significance using with Mann–Whitney U test. P-values are indicated by asterisks as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 and ns, no significant difference. AU/ml- arbitrary units/ml. N = 309 for Seropositive at baseline; N = 40 for Seronegative at baseline.
Vaccinated individuals showed comparable antibody levels and cellular immune responses following Omicron infection, irrespective of previous exposure
Following Omicron infection, the antibody levels against the variants were comparable on D0 and D30 among vaccinated individuals irrespective of prior exposure, except for the Delta variant [median [IQR] antibody levels against Delta variant, 186886 (65515–260821) vs. 59901 (23939–103166) on D0 (p = 0.008) and 137010 (81920–224183) vs. 58849 (37667–111662) on D30 (p = 0.009) in exposed vs. unexposed individuals]. By D90, the previously exposed vaccinated group showed higher binding and percentage ACE-2 inhibition levels than unexposed individuals for all variants, except Omicron (p < 0.05). Compared with the other variants, the antibody and percentage ACE-2 inhibition levels against Omicron were lower and comparable at all time points, irrespective of prior exposure at D0, D30, and D90 [median [IQR] for antibody levels against Omicron, 14614 (3521–21184) vs. 7841 (2567–11168) on D0 (p = 0.5), 12310 (6375–17583) vs. 9445 (4059–14375) on D30 (p = 0.2), and 7253 (3135–10019) vs. 4818 (1403–8716) on D90 (p = 0.2) in prior exposed vs. unexposed-vaccinated individuals] (Fig. 5a, b, Table S6).
Humoral and cellular immune responses following Omicron infection among vaccinated individuals with and without pre-Omicron exposure. Antibody levels (a) and percentage ACE-2 inhibition levels (b) to SARS-CoV-2 variants among previously exposed (SP + V) and unexposed (SN + V) vaccinated individuals at D0, 30, and 90 (N = 25 Previously exposed-vaccinated; N = 14 for previously unexposed-vaccinated). (c-j) Variant spike-specific B cell and T cell responses elicited by Omicron infection among previously exposed and unexposed vaccinated individuals. (c) RBD-specific spot-forming ASCs expressed as a percentage of total IgG-secreting cells, (d) RBD-specific spot-forming ASCs, (e-g) CD4 T cell responses, (h, i) CD8 T cell responses following Omicron infection at D0, 30, 90 in previously exposed (blue) and previously unexposed vaccinated (red). Spike-specific (e) AIM + CD4 T cells. (f) Any one of the three cytokines IFN-γ/TNF-α/IL2 + CD4 T cells, (g) AIM + cTfh CD4 T cells, (h) AIM + CD8 T cells. (i) Any one of the three cytokines IFN-γ/TNF-α/IL2 + CD8 T cells. (j) Proportion of polyfunctional CD4 T cells in previously exposed and unexposed vaccinated individuals assessed on D0, 30, 90 following Omicron infection. The data are represented as median with interquartile range. Data were analyzed for statistical significance using Mann–Whitney U test. Background subtracted data were analyzed in all cases. P-values are indicated by asterisks as *p < 0.05, **p < 0.01. N = 8 for Previously exposed-vaccinated (SP + V); N = 8 for Previously unexposed-vaccinated (SN + V) for both T and B cell analysis.
When we determined the RBD-specific IgG-secreting cell levels (percentage of total IgG) following stimulation of memory B cells on D90 and found no difference between the exposed and unexposed-vaccinated groups [median [IQR], 0.05 (0.03–0.05) vs. 0.04 (0.02–0.06), p = 0.5; Fig. 5c, d]. Regarding the variant-specific CD4 + and CD8 + AIM and cytokine + T cell responses, no differences were observed between groups on D0, D30, and D90 [median [IQR] for Omicron-specific AIM + CD4 T cells, 0.05 (0.00–0.25) vs. 0.09 (0.007–0.2) on D0 (p = 0.7), 0.05 (0.00–0.14) vs. 0.07 (0.005–0.13) on D30 (p = 0.8), and 0.01 (0.00–0.12) vs. 0.02 (0.00–0.03) on D90 (p = 0.8); Fig. 5e–i]. These results indicated that T cell responses to Omicron were comparable in vaccinated individuals, regardless of prior exposure (Table S7).
Regarding the levels of individual cytokines (Fig. S10), only WT-specific responses, particularly IFN-γ levels in CD4 cells, were higher among individuals with prior exposure before vaccination than among individuals unexposed prior to vaccination at D90 (Fig. S10a). This suggests that subsequent exposure after vaccination boosts CD4 + T-cell responses to the WT strain, a critical factor for cross-reactivity. Surprisingly, we also found higher levels of WT-specific IL-2 + CD8 T cells on D0 [median [IQR], 0.005 (0.00–0.02) vs. 0.04 (0.01–0.06), p = 0.03] and D30 [median [IQR], 0.006 (0.002–0.02) vs. D90 0.03 (0.03–0.05), p = 0.006] in unexposed than exposed-vaccinated individuals (Fig. S10d).
In contrast to unvaccinated individuals who showed declining polyfunctional CD4 T cell levels by D90, the proportion of three-cytokine + CD4 T cells showed an increasing trend at D90 in both groups (Fig. 5j). The previously exposed group showed higher IFN-γ + IL-2 + levels against Delta and Omicron at D90 [median [IQR], 0.001 (0.0006–0.006) vs. 0.00 (0.00–0.0003) for Delta (p = 0.001) and 0.001 (0.0004–0.003) vs. 0.00 (0.00–0.0009) for Omicron (p = 0.01) than the unexposed-vaccinated individuals.
Previously exposed-vaccinated individuals showed higher Tcm than Tem levels at all time points (Fig. S11a, b). In contrast, the unexposed-vaccinated individuals showed higher Tem than Tcm levels. CD8 memory responses were predominantly associated with Tem and Temra in both groups up to D90 following Omicron infection (Fig. S11c, d).
Vaccinated individuals showed higher CD4 T cell responses to the WT strain following Omicron infection, irrespective of prior exposure (Fig. S6a-c), whereas the magnitude of CD8 T cell responses was comparable between variants in both groups (Fig. S6d, e).
Multiparametric analysis
A multiparametric analysis was performed using both a correlation matrix and PCA to assess immunogenicity following exposure. The correlation matrix, assessing cellular and humoral immune responses to Omicron on D0, D30, and D90 (Figs. 6a–n and 7a–m), showed a strong correlation between the anti-spike IgG (Omicron), anti-NC IgG, and neutralization antibody antigen levels (Omicron) in all groups, except the vaccinated group without prior exposure (r = 0.4, p = 0.05, Fig. 7m).
Correlation analysis of Omicron-specific cellular and humoral immune parameters in secondary and primary Omicron-infected groups. (a), (f), (k) Correlation matrix of D0, D30, and D90 samples of secondary Omicron-infected individuals. (b), (g), (l) Correlation matrix of D0, D30, and D90 samples of primary Omicron-infected individuals. The black square in each matrix indicates a significant association between immune parameters. Spearman rank-order correlation values (r) are shown from white (–1.0) to black/pink for secondary Omicron-infected and primary Omicron-infected, respectively (1.0); r values are indicated by color and square size. p-values are indicated by asterisks as *p < 0.05, **p < 0.01, ***p < 0.001. nAb is a neutralizing antibody. (c–e), (h–j), (m–n) The association of indicated parameters is represented by a scatterplot. Black indicates secondary Omicron infection; pink indicates primary Omicron infection. The Spearman rank-order correlation values (r) and p-values are shown. N = 8 for Secondary Omicron infected (SP + UNV); N = 8 for Primary Omicron infected (SN + UNV) for correlation analysis.
Correlation analysis of Omicron-specific cellular and humoral immune parameters in previously exposed and unexposed vaccinated groups following Omicron infection. (a), (e), (i) Correlation matrix of D0, D30, and D90 samples of previously exposed-vaccinated individuals. (b), (f), (j) Correlation matrix of D0, D30, and D90 samples of unexposed-vaccinated individuals. The black square in each matrix indicates significant association between the immune parameters. Spearman rank-order correlation values (r) are shown from white (−1.0) to blue/red for previously exposed/unexposed vaccinated individuals, respectively (1.0); r values are indicated by color and square size. p-values are indicated by asterisks as *p < 0.05, **p < 0.01, ***p < 0.001. (c-d), (g-h), (k-m) The association of indicated parameters is represented by scatterplot. Blue indicates previously exposed-vaccinated; red indicates previously unexposed-vaccinated. Spearman rank-order correlation values (r) and p-values are shown. N = 8 for Previously exposed-vaccinated (SP + V); N = 8 for Previously unexposed-vaccinated (SN + V) for correlation analysis.
Antibody and CD4 + T-cell levels were strongly correlated in secondary Omicron infected and previously exposed vaccinated individuals (Fig. 6a, f, k, and 7i). This reflects the concomitant induction of this two-prong immune response following Omicron exposure. Omicron spike-specific CD4 AIM + T and CD8 AIM + T cell levels were strongly correlated only in secondary Omicron-infected individuals (r = 0.8, p = 0.03 on D0; r = 0.9, p = 0.003 on D30; and r = 0.8, p = 0.01 on D90). No correlation was observed between antibody, T, or B cell levels in previously unexposed-vaccinated individuals (Fig. 7b, f, j, m). PCA mapping was performed on D0 and D30 following Omicron infection to assess the clustering of immunological profiles following different exposures (Fig. 8).
Principal Component Analysis (PCA) following Omicron infection in all four groups based on all immune parameters obtained at Day 0 (a) and Day 30 (b). Arrows indicate the prominent immunological parameters. Ellipses represent the clustering of each group. Blue indicates previously exposed-vaccinated (SP + V), red indicates unexposed-vaccinated (SN + V), black indicates Secondary Omicron infection (SP + UNV), and pink indicates Primary Omicron infection (SN + UNV). W- WT, D- Delta, Om- Omicron.
The D0 (Fig. 8a) and D30 (Fig. 8b) PCA biplot showed that cellular immune parameters were closely associated with humoral immune parameters clustered together. At D30, clustering in the primary Omicron-infected group (SN + UNV) showed a distinct immunological profile compared to the other groups. The other three groups had similar immunological profiles with greater heterogeneity. Overall, strong relationships were observed between various key immune response components following exposure.
Discussion
We tracked SARS-CoV-2-specific antibody levels in a south Indian community over 2 years and characterized the humoral and cellular immune responses following Omicron infection in vaccinated and unvaccinated individuals, with or without pre-Omicron variant exposure. Both unvaccinated and vaccinated previously exposed individuals showed higher antibody levels at the end of follow-up than individuals without prior exposure. Following the Omicron wave, the percentage ACE-2 inhibition for Omicron at the 12th month of follow-up was lower than that for Delta and WT in previously exposed-vaccinated and unvaccinated individuals. This implied exposure to pre-Omicron variants greatly boosted the immune response; thus, immune imprinting may constrain the adaptive immune response to newer SARS-CoV-2 variants. At the end of the 2-year follow-up, almost 99% of the study participants had antibodies against the spike protein of SARS-CoV-2. These findings are consistent with those of a seroprevalence study conducted in other countries26. Among participants with prior seropositivity, 149 RT-PCR-confirmed infections occurred in the Covishield group and 59 in the Covaxin group. Among seronegative individuals, 17 infections were reported in the Covishield group and 15 in the Covaxin group during follow-up. While we performed exploratory comparison based on vaccine type, the small sample sizes in the covaxin subgroups, along with a high rate of intercurrent infections during follow-up, limited our ability to draw definitive conclusions about immunogenicity differences between vaccine types.
Unvaccinated individuals with primary Omicron infections exhibited lower binding and negligible neutralizing antibody responses compared with those with secondary infections, suggesting that prior exposure enhances cross-reactive Omicron-specific immunity. By 90 days post-infection, the neutralizing antibodies in secondary Omicron infections declined to levels comparable to those in primary infections, likely due to transient Omicron-induced responses or the faster decay of cross-reactive antibodies. Similarly, Medic et al. observed higher BA.2 neutralizing antibody titers in previously exposed individuals27,28,29. CD4 T cell responses (AIM+, cytokine+, cTfh+) were similar between primary and secondary Omicron infections; however, the magnitude of CD4 T cell responses was lower in unvaccinated individuals than in vaccinated individuals, irrespective of prior exposure, indicating that infection elicits a weaker T cell response than vaccination. This could be explained by the use of spike-specific peptides, and the inclusion of other epitopes may have resulted in greater magnitude of CD4 + T-cell responses. This has been demonstrated in other studies, where non-spike-specific peptides, in addition to spike-specific peptides, elicited a robust T cell response20,30. In addition, the magnitude of CD4 T cell responses for Omicron spike-specific responses include CD4 + AIM responses ranges from 0.04 to 0.1% in infected individuals and 0.06–0.3% in vaccinated individuals31. CD4 + cytokine-producing cells are typically reported between 0.03 and 0.3% in infected individuals and 0.005–0.05% in recipients of inactivated or Ad26.CoV2.S vaccines32.
In contrast, the CD8 T cell responses, including AIM + and IFN-γ + cell levels, were higher in secondary infections at both D30 and D90, suggesting that prior exposure primes the immune system for stronger responses to re-infection, potentially leading to asymptomatic or milder outcomes. Similarly, previous studies have reported strong CD4 T-cell responses despite poor humoral immunity in primary infections and enhanced CD8 T-cell responses following secondary infections30,33,34. Both primary and secondary Omicron infections elicited cross-reactive T-cell responses against WT and Delta variant, highlighting the role of T-cell immunity in mitigating severe infection. Indeed, T-cell responses are largely conserved against evolving SARS-CoV-2 variants, even when neutralizing antibody responses wane31,32. Though the levels of CD8 T cell responses are higher in secondary than in primary Omicron infection, the magnitude of CD8 T cell responses were lower than CD4, as reported in other studies32,35.
Memory T cell phenotype analysis revealed that secondary Omicron infections promoted Tem cell dominance, suggesting stronger circulating antiviral effector responses that could prevent future infections. In contrast, primary infections were associated with higher Tcm levels, which potentially reflect a conserved immune memory. Additionally, pTfh responses at D90 were strongly correlated with Omicron-specific antibody levels, highlighting the role of pTfh cells in B cell-mediated antibody production. This coordinated response between T and B cells, despite varying exposure histories, emphasizes the importance of the two-pronged immune response in defending against SARS-CoV-2 infection.
In vaccinated individuals, antibody levels after Omicron infection were initially similar, irrespective of prior exposure. However, by 90 days, those with prior exposure exhibited higher binding and ACE-2 inhibition rates across most variants, excluding Omicron. This suggests that prior infection and vaccination enhance long-term immune durability. This finding is consistent with studies showing stronger immune responses in vaccinated individuals with previous infections32,35,36. In the present study, vaccinated secondary Omicron infected individuals exhibited broader cross-reactive antibody responses to pre-Omicron variants, whereas unvaccinated primary Omicron infected individuals displayed a more restricted antibody response. These findings suggest that prior exposure via infection or vaccination induces immune imprinting in subsequent Omicron infection. While this could reflect immune memory, the nature of the response, characterized by stronger recognition of conserved spike regions rather than novel variant-specific epitopes suggests the role of immune imprinting. The implications of antigen imprinting where the immune system preferentially recalls responses to previously encountered antigens may limit the development of robust, variant-specific antibody responses during subsequent exposures to newer variants like Omicron37,38. This selective recall may reduce the diversity and magnitude of neutralizing antibodies to novel epitopes, potentially compromising protection against reinfection. Over time, repeated exposures to antigenically distinct variants may reinforce this imprinting, influencing the durability and breadth of long-term humoral immunity.
No significant differences were detected in T-cell responses between previously exposed and unexposed vaccinated individuals. Notably, previously exposed individuals demonstrated stronger IFN-γ responses to the WT variant, which could be attributed to anamnestic responses, whereas unexposed individuals exhibited a higher IL-2 response, which may facilitate memory cell generation and bolster primary and secondary CD8 T cell responses, enhancing the overall immune response39. Cross-reactive T cell responses to WT and Delta variants were observed in both vaccinated groups, reflecting conserved T cell immunity. In contrast to unvaccinated individuals who exhibited a decline in polyfunctional CD4 T cell responses following D90, vaccinated individuals maintained robust triple-cytokine + CD4 T cell responses. The previously exposed-vaccinated individuals, in particular, showed elevated IFN-γ + IL-2 + CD4 T cell levels for Delta and Omicron at D90, highlighting their role in memory generation and sustained immunity through the convalescent phase. These findings highlight the long-term persistence of polyfunctional CD4 T cell levels elicited by vaccination, even without prior exposure. Regarding memory phenotypes, previously exposed individuals showed a balanced Tcm–Tem CD4 phenotype, whereas unexposed individuals predominantly exhibited Tem cells. CD8 memory responses were largely associated with Tem and Temra cells in both groups, consistent with previous findings40,41,42,43. Memory B cell responses, including RBD-specific ASCs, were comparable regardless of exposure. This is consistent with the findings of Goel et al.43, who showed that vaccination primes robust memory B-cell responses to variants in subsequent exposures.
Collectively, our findings indicated that this south Indian community has been exposed to multiple waves of SARS-CoV-2 variants and vaccines, with high seropositivity, although antibody levels were higher in individuals with prior exposure. The antibody kinetics analysis provided strong evidence for antigen imprinting following various exposures; however, further investigation is needed to comprehensively assess both individual- and community-level consequences. Robust T-cell responses to SARS-CoV-2 variants following Omicron infection after different exposures may counterbalance the decreasing neutralizing capacity of antibodies and prevent severe SARS-CoV-2 infection. This study has a few limitations. We did not analyze antibody and T-cell responses based on specific vaccine types, although vaccination combined with Omicron infection elicited robust polyfunctional CD4 T-cell responses. We did not assess immunological responses in individuals who received either a single vaccine dose or a complete three-dose regimen, which could have provided insights into dose-dependent immunity variations. While the majority of infections were likely caused by Omicron variants during the Omicron period, confirmation was possible only for a subset of samples by WGS, although the possibility of co-circulation with other variants remains minimal. The study did not evaluate the durability of immune responses over time, limiting our understanding of long-term immunity. To address the durability, we are following the same cohort with two subsequent blood draws that would help to assess the cellular and humoral immune responses as COVID-19 transits from a pandemic to an endemic phase.
Data availability
Data sets generated during the current study are available from the corresponding author on reasonable request.
References
Karim, S. S. A. & Karim, Q. A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet 398, 2126–2128. https://doi.org/10.1016/S0140-6736(21)02758-6 (2021).
Hu, J. et al. Increased immune escape of the new SARS-CoV-2 variant of concern Omicron. Cell. Mol. Immunol. 19, 293–295. https://doi.org/10.1038/s41423-021-00836-z (2022).
Abdullah, F. et al. Decreased severity of disease during the first global Omicron variant Covid-19 outbreak in a large hospital in tshwane, South Africa. Int. J. Infect. Dis. 116, 38–42. https://doi.org/10.1016/j.ijid.2021.12.357 (2022).
Nyberg, T. et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 Omicron (B.1.1.529) and delta (B.1.617.2) variants in england: a cohort study. Lancet 399, 1303–1312. https://doi.org/10.1016/S0140-6736(22)00462-7 (2022).
Thiruvengadam, R. et al. Clinical and experimental evidence suggest Omicron variant of SARS-CoV-2 is inherently less pathogenic than delta variant independent of previous immunity. Eur. J. Med. Res. 28, 421. https://doi.org/10.1186/s40001-023-01373-3 (2023).
Carreño, J. M. et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature 602, 682–688. https://doi.org/10.1038/s41586-022-04399-5 (2022).
Cele, S. et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 602, 654–656. https://doi.org/10.1038/s41586-021-04387-1 (2022).
Planas, D. et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 602, 671–675. https://doi.org/10.1038/s41586-021-04389-z (2022).
Xiang, T., Wang, J. & Zheng, X. The humoral and cellular immune evasion of SARS-CoV-2 Omicron and sub-lineages. Virol. Sin. 37, 786–795. https://doi.org/10.1016/j.virs.2022.11.007 (2022).
Khan, K. et al. Omicron BA.4/BA.5 escape neutralizing immunity elicited by BA.1 infection. Nat. Commun. 13, 4686 (2022).
Cao, Y. et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 608, 593–602. https://doi.org/10.1038/s41586-022-04980-y (2022).
Pouwels, K. B. et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat. Med. 27, 2127–2135. https://doi.org/10.1038/s41591-021-01548-7 (2021).
Earle, K. A. et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 39, 4423–4428. https://doi.org/10.1016/j.vaccine.2021.05.063 (2021).
Dejnirattisai, W. et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 185, 467–484. https://doi.org/10.1016/j.cell.2021.12.046 (2022). e15 e415.
Yu, J. et al. Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 variants. N Engl. J. Med. 386, 1579–1580. https://doi.org/10.1056/NEJMc2201849 (2022).
Zens, K. D. et al. Longitudinal humoral and cell-mediated immune responses in a population-based cohort in zurich, Switzerland between March and June 2022 – evidence for protection against Omicron SARS-CoV-2 infection by neutralizing antibodies and spike-specific T-cell responses. Int. J. Infect. Dis. 133, 18–26. https://doi.org/10.1016/j.ijid.2023.04.407 (2023).
Yang, Y. et al. Viral and antibody dynamics of acute infection with SARS-CoV-2 Omicron variant (B.1.1.529): a prospective cohort study from shenzhen, China. Lancet Microbe. 4, e632–e641. https://doi.org/10.1016/S2666-5247(23)00139-8 (2023).
Zaballa, M. E. et al. Seroprevalence of anti-SARS-CoV-2 antibodies and cross-variant neutralization capacity after the Omicron BA.2 wave in geneva, switzerland: a population-based study. Lancet Reg. Health Eur. 24, 100547. https://doi.org/10.0547 (2023).
Simanavičius, M. et al. Prevalence of SARS-CoV-2-specific antibodies in a sample of the Lithuanian population-based study in spring 2023. Heliyon 10, e29343. https://doi.org/10.1016/j.heliyon.2024.e29343 (2024).
Sun, K. et al. Rapidly shifting Immunologic landscape and severity of SARS-CoV-2 in the Omicron era in South Africa. Nat. Commun. 14, 246. https://doi.org/10.1038/s41467-022-35652-0 (2023).
Yadav, P. D. et al. Broadly reactive SARS-CoV-2-Specific T-cell response and participation of memory B and T cells in patients with Omicron COVID-19 infection. J. Immunol. Res. 2023, 8846953. https://doi.org/10.1155/2023/8846953 (2023).
Dhalaria, P., Arora, H., Singh, A. K., Mathur, M. & Ajith Kumar, S. COVID-19 vaccine hesitancy and vaccination coverage in india: an exploratory analysis. Vaccines (Basel). 10 (5), 739. https://doi.org/10.3390/vaccines10050739 (2022).
Gupta, R. & Misra, A. COVID19 in South asians/asian indians: heterogeneity of data and implications for pathophysiology and research. Diabetes Res. Clin. Pract. 165, 108267. https://doi.org/10.1016/j.diabres.2020.108267 (2020).
Madhavan, R. et al. Risk of COVID-19 re-infection and its predictors (CORES): protocol for a community-based longitudinal cohort study in vellore, India. BMJ Open. 12, e059869. https://doi.org/10.1136/bmjopen-2021-059869 (2022).
Rouers, A., Tay, M. Z., Ng, L. F. P. & Renia, L. B-cell elispot assay to analyze human memory B cell and plasmablast responses specific to SARS-CoV-2 receptor-binding domain. Star. Protoc. 4 (1), 102130 (2023).
Solastie, A. et al. Changes in SARS-CoV-2 Seroprevalence and population immunity in finland, 2020–2022. Emerg. Microbes Infect. 12, 2222849. https://doi.org/10.1080/22221751.2023.2222849 (2023).
Yang, Y. et al. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nat. Microbiol. 7, 423–433. https://doi.org/10.1038/s41564-021-01051-2 (2022).
Suryawanshi, R. K. et al. Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. Nature 607, 351–355. https://doi.org/10.1038/s41586-022-04865-0 (2022).
Medić, S. et al. Risk and severity of SARS-CoV-2 reinfections during 2020–2022 in vojvodina, serbia: A population-level observational study. Lancet Reg. Health Eur. 20, 100453. https://doi.org/10.1016/j.lanepe.2022.100453 (2022).
Mazzoni, A. et al. SARS-CoV-2 spike-specific CD4 + T cell response is conserved against variants of concern, including Omicron. Front. Immunol. 13, 801431. https://doi.org/10.3389/fimmu.2022.801431 (2022).
Keeton, R. et al. T cell responses to SARS-CoV-2 Spike cross-recognize Omicron. Nature 603, 488–492. https://doi.org/10.1038/s41586-022-04460-3 (2022).
Sette, A., Sidney, J. & Crotty, S. T cell responses to SARS-CoV-2. Annu. Rev. Immunol. 41, 343–373. https://doi.org/10.1146/annurev-immunol-101721-061120 (2023).
Le Bert, N. et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J. Exp. Med. 218. https://doi.org/10.1084/jem.20202617 (2021).
Kim, S. H. et al. Omicron BA.2 breakthrough infection elicits CD8 + T cell responses recognizing the Spike of later Omicron subvariants. Sci. Immunol. 9, eade6132. https://doi.org/10.1126/sciimmunol.ade6132 (2024).
Tarke, A. et al. Early and polyantigenic CD4 T cell responses correlate with mild disease in acute COVID-19 donors. Int. J. Mol. Sci. 23 (13), 7155. https://doi.org/10.3390/ijms23137155 (2022).
Graça, D. et al. Both humoral and cellular immune responses to SARS-CoV-2 are essential to prevent infection: a prospective study in a working vaccinated population from Southern France. J. Clin. Immunol. 43, 1724–1739. https://doi.org/10.1007/s10875-023-01558-9 (2023).
Martinuzzi, E. et al. Escape of SARS-CoV-2 variant Omicron to mucosal immunity in vaccinated subjects. Open. Forum Infect. Dis. 9, ofac362. https://doi.org/10.1093/ofid/ofac362 (2022).
King, S. M., Bryan, S. P., Hilchey, S. P., Wang, J. & Zand, M. S. First impressions matter: immune imprinting and antibody Cross-Reactivity in influenza and SARS-CoV-2. Pathogens 12 (2), 169. https://doi.org/10.3390/pathogens12020169 (2023).
Maltseva, M., Keeshan, A., Cooper, C. & Langlois, M. A. Immune imprinting: the persisting influence of the first antigenic encounter with rapidly evolving viruses. Hum. Vaccin Immunother 20(1):2384192. https://doi.org/10.1080/21645515.2024.2384192
Boyman, O., Cho, J. H. & Sprent, J. The role of interleukin-2 in memory CD8 cell differentiation. Adv. Exp. Med. Biol. 684, 28–41. https://doi.org/10.1007/978-1-4419-6451-9_3 (2010).
Dan, J. M. et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 371 https://doi.org/10.1126/science.abf4063 (2021).
Mateus, J. et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 374, eabj9853. https://doi.org/10.1126/science.abj9853 (2021).
Zhang, Z. et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 185, 2434–2451e17. https://doi.org/10.1016/j.cell.2022.05.022 (2022).
Goel, R. R. et al. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell 185, 1875–1887e8. https://doi.org/10.1016/j.cell.2022.04.009 (2022).
Acknowledgements
We wish to thank all the study participants for their important contribution; without them, it would have been impossible to generate the data. This work was supported by the Bill and Melinda Gates Foundation, Seattle, WA (INV-024915). The funders had no role in the study design, data collection, analysis, and interpretation of the data.
Funding
This work was supported by the Bill and Melinda Gates Foundation, Seattle, WA (Grant number INV-024915). The funders had no role in the study design, data collection, analysis, and interpretation of the data.
Author information
Authors and Affiliations
Contributions
G. K. and J. J. conceived the trial and contributed to the original protocol. J. S. P., R. M., and D. K. contributed to the project administration and data entry. S.B., S.I., V.G., performed the serology testing. R.M., D.G., C.S., P.S., A.K., P.D., L.R., S.G., performed the cellular immunology testing for SARS-CoV-2. R. M., A.E., I.T., performed the statistical analysis. A. G. is a part of the scientific advisory committee for the CORES study. R. M. drafted the initial report. G. K. and J. J. finalized the report. All authors reviewed and approved the final report.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study has been approved by the Institutional Review Board of Christian Medical College, Vellore (IRB min no: 13585). We certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate
Written informed consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Madhavan, R., Gupta, D., Paul, J.S. et al. Monitoring of antibodies against SARS-CoV-2 over 2 years and characterization of immune responses following Omicron infection in a South Indian community cohort. Sci Rep 15, 24756 (2025). https://doi.org/10.1038/s41598-025-10447-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10447-7