Abstract
A set of mixed metal oxide catalysts, MOx-Co3O4 (M: Fe, Mn, Cu, Ni, Cr, and Zn) enveloped in polymer nanofilms was fabricated using the solid-phase synthesis method. These catalysts were subsequently studied for their catalytic performance in the low-temperature oxidation of carbon monoxide. Characterization of the catalysts was accomplished using various techniques, including X-ray diffraction (XRD), N2 adsorption-desorption, temperature-programmed reduction (H2-TPR), temperature-programmed desorption of oxygen (O2-TPD), Fourier transform infrared spectroscopy (FTIR), thermogravimetric and differential thermal analyses (TGA and DTA) and scanning electron microscopy (FESEM). The outcomes of these investigations indicated that the composite oxides possess different characteristic features. The catalytic activity of these catalysts exhibited a decreasing trend as follows: Fe-Co > Mn-Co > Cu-Co > Ni-Co > Zn-Co > Cr-Co. Among the catalysts prepared, Fe-Co nanoparticles revealed the greatest specific surface area (138 m2.g− 1) and the largest pore volume (0.45 cm3.g− 1), resulting in the most superior catalytic activity, achieving total CO conversion at 72 °C. Additionally, the Fe-Co catalyst demonstrated exceptional long-term stability at low temperature (60 °C). Furthermore, the study investigated the impact of various parameters such as calcination temperature, CO content, gas hourly space velocity (GHSV), and pretreatment conditions.
Similar content being viewed by others
Introduction
Carbon monoxide (CO), originating from the incomplete combustion of carbon compounds, poses a hazardous ambient threat1,2,3. CO is a colorless, odorless, and non-irritating gas that possesses toxicity and flammability4,5. The elimination of these emissions is essential for human health and the environment. It is broadly recognized that the conversion of CO into non-toxic CO2 through catalytic oxidation is a viable solution for addressing this issue6,7,8. Nevertheless, the suitable catalysts for this oxidation reaction primarily comprise precious and transition metal oxides. Precious metals such as platinum (Pt), palladium (Pd), ruthenium (Ru), and gold (Au) supported on CeO2, TiO2, SiO2, and ZrO2 have shown significant efficacy in CO oxidation9,10,11,12,13. Despite their general resistance to moisture-induced deactivation, sintering, and particle agglomeration, their scarcity and elevated costs impose restrictions on their utilization14,15,16. Consequently, researchers have been actively exploring non-noble metal oxides as potential substitutes for CO oxidation in recent decades. In this regard, transition metal oxides with partially filled d-type orbitals, such as Mn2O317, CeO218, Co3O419, Fe2O320, CuO21 and NiO22 have been the subject of research investigations. These compounds have attracted significant attention in various oxidation processes due to their multiple oxidation states and the formation of redox cycles between high and low oxidation states23. In line with previous reports, cobalt and cerium oxides have emerged as strong contenders for catalyst materials24,25,26,27. This can be attributed to their unique fluorite structure, the presence of defects created by Ce3+ ions, and the comparatively lower energy of the Co-O bond28. However, the presence of cobalt in the catalyst formulation could enhance the electronic structure and oxygen mobility29. For instance, Jin Sa et al.30 conducted a study on a range of Co3O4 catalysts and reported that these catalysts achieved complete CO conversion at room temperature. Additionally, the nanostructured form of Co3O4 demonstrated outstanding performance, showing remarkable activity and stability in the catalytic process of CO oxidation. The findings indicated that using bimetallic catalysts could improve both the structural properties and catalytic efficiency in CO oxidation31,32. CeO2-ZrO2 mixed oxide catalysts were produced via the sol-gel method, and the resulting samples were employed in the catalytic oxidation of CO33. The results demonstrated that the catalytic efficiency is closely associated with the Zr: Ce molar ratio due to the difference in the degree of reducibility. The descending trend in CO conversion was observed by raising the Zr: Ce ratio. However, adding transition metals to cobalt could improve the reduction behavior and enhance the oxygen storage capacity of the catalyst system34. There are few reports available to evaluate the performance of cobalt-containing mixed oxide catalysts in the CO oxidation process. For instance, Biabani and Rezaei35 investigated iron-cobalt mixed oxide catalysts with various molar ratios of iron and cobalt in the context of CO oxidation. Their findings revealed that the pure cobalt sample had a uniform hexagonal closed-pack structure. Additionally, the activity analysis revealed that the inclusion of cobalt in iron oxide had a significant impact on enhancing CO conversion at low temperatures. One of the most important challenges in this process is the existence of water in the reaction environment. In fact, water molecules occupying the active Co3+ sites can impede the adsorption of both CO and oxygen, which ultimately causes catalyst deactivation. Furthermore, the dissociative adsorption of water at the Co3+ site can lead to the depletion of the lattice oxygen present on the catalyst surface. Moreover, carbonates that result from CO2 adsorption can readily transform into bicarbonates, which exhibit greater stability when exposed to water36. In an effort to prevent the deactivation of catalysts derived from transition metal oxide at lower temperatures and in more humid conditions, scientists have investigated the idea of applying hydrophobic coatings to the catalyst surfaces. Nevertheless, the practicality of this approach has been constrained by technical challenges in achieving the necessary level of hydrophobicity and impermeability to shield the catalytic active sites from water molecules36,37. Nonetheless, the pursuit of catalyzing CO oxidation using affordable transition metal oxides in conditions with high moisture levels, below the boiling point of water at normal pressure (100 °C), has remained a formidable challenge. One solution to this challenge involves employing a polydimethylsiloxane coating on Co3O4, which capable of efficiently preventing deactivation when exposed to water. Nevertheless, this polymer layer also substantially hinders the diffusion of reactants to the active sites36. In a study, Shen et al.38 introduced an innovative technique for creating a polymer nanofilm-coated Fe3Co16Ox catalyst with water-resistant properties. This was achieved through a method employing solid-phase core-shell conformation. Notably, this catalyst displayed prolonged and effective catalytic activity in the complete oxidation of CO, lasting for over 720 h at 90 °C under a humid environment containing 3.1% water vapor.
Based on these findings, the present work aimed to synthesize a range of nanofilm-coated composite oxides containing MOx-Co3O4 (M = Fe, Mn, Cu, Ni, and Zn), and to evaluate their performance in the CO oxidation process. The objective was to identify an enhanced Co-based catalyst capable of efficiently converting CO at lower temperatures. We conducted comprehensive characterization to investigate the structure and surface characteristics of the nanofilm-coated materials, offering valuable insights into the relationships between structure and catalytic activity.
Experimental
Materials
Manganese (II) nitrate tetrahydrate (Mn(NO3)2.4H2O), cobalt (II) nitrate hexahydrate (Co(NO3)2·6H2O), iron (III) nitrate nonahydrate (Fe(NO3)3·9H2O), copper (II) nitrate trihydrate (Cu(NO3)2·3H2O), zinc (II) nitrate hexahydrate (Zn(NO3)2·6H2O), chromium (III) nitrate nonahydrate (Cr(NO3)3·9H2O), and nickel (II) nitrate hexahydrate (Ni(NO3)2·6H2O) were employed as precursor materials without any purification. Ethylene glycol and oxalic acid were used as the polymer precursors. All materials were purchased from Merck Company.
Powder Preparation procedure
The MOx-Co3O4 catalysts were developed using the modified solid-phase method36. To enhance the uniformity and interaction among the constituents, aqueous solutions of metal nitrates were employed in place of their solid counterparts. A calculated amount of oxalic acid (OA, ≥ 99.0%) and ethylene glycol (EG, ≥ 99.0%) was pre-mixed. Initially, solid Co(NO3)2·6H2O (≥ 99.0%) was dissolved in the aqueous solution of other metal nitrates (50 wt%). This solution was then combined with the OA and EG in a calculated molar ratio of EG/OA = 1:1. The resulting mixture was ground in an agate mortar for 30 min. The sample was subjected to overnight drying at 110 °C and subsequently subjected to calcined at 230 °C with a temperature ramp of 3 °C/min for a duration of 4 h.
Physicochemical analysis techniques
The identification of crystalline phases and measurement of crystallite sizes were conducted using X-ray diffraction (XRD). The surface characteristics of the catalysts were assessed with an automated analytical instrument (BELSORP-mini II). Prior to measurements, the samples were treated with degassed at 200 °C for 2 h. Hydrogen temperature-programmed reduction (H2-TPR) analysis was performed employing a dedicated chemisorption apparatus equipped with a thermal conductivity detector (TCD). For this procedure, 30 mg of the fresh catalyst underwent pretreatment under a N2 atmosphere at 200 °C for 1 h, afterward, and was then cooled to ambient temperature. The sample was then subjected to a flow of reducing gas (5% H2 in Ar, 20 ml/min) and the temperature was ramped from ambient to 700 °C with a rate of 10 °C/min. Furthermore, oxygen temperature-programmed desorption (O2-TPD) was conducted with an instrument equipped with a TCD. Similarly, 30 mg of the fresh catalyst was subjected to degassing under vacuum at a temperature of 200 ℃ for 1 h and then cooled to ambient temperature. The sample was exposed to a gas stream of 5% O2 in Ar (20 ml/min) for 45 min at 40 ℃ to achieve sufficient oxygen adsorption. Then, the sample was flushed with He stream (20 ml/min) for 30 min and subsequently, heated to 700 ℃ at a heating rate of 10 ℃ min-1 under pure He at a flow rate of 20 mL min- 1. The surface characteristics of the formulated catalysts were analyzed using field emission scanning electron microscopy (FESEM) with a MIRA3 TESCAN instrument. Thermogravimetric (TG) and differential thermal analysis (DTA) were carried out with a Bahr STA 504 system under an air atmosphere at a heating rate of 10 °C/min. Fourier transform infrared (FTIR) spectroscopy was conducted within 450–4000 cm- 1 utilizing potassium bromide (KBr) pellets comprising 1 wt% of the sample (Spectrum 100 (PerkinElmer USA)).
Catalytic activity measurements
To conduct the catalytic evaluation, 100 mg of catalyst sample was pressed, comminuted, and sieved to particles with a mesh size of 30–50. Subsequently, the resulting catalyst particles were loaded into the center of a vertically oriented quartz reactor with an inner radius of 3.5 mm and a length of 500 mm, which was positioned within an electric furnace. To monitor the reaction temperature, a flexible K-type thermocouple was introduced at the base of the catalyst bed. Flow velocities of the reactant gases (Ar, O2, and CO) were regulated and monitored using digital mass flow controllers. Preceding the reaction, the samples underwent pre-oxidation at 200 °C for 1 h under a mixed flow of O2 diluted in Ar (20% O2 in Ar). The gas stream composition for the reaction included CO: O2:Ar = 1:5:19, with gas hourly space velocity (GHSV) of 60,000 ml/(g·h). To examine the impact of water on the catalytic activity, the reaction gas mixture was passed through a water bubbling apparatus (∼0 °C) and 3% H2O was obtained. The resulting gas flow was analyzed employing an online gas chromatograph (M600D, Younglin). The CO conversion was determined by the following equation (Eq. 1.):
.
Results and discussion
Textural characteristics of the as-synthesized samples
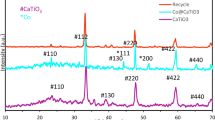
The crystalline texture of the freshly prepared catalysts was examined through XRD analysis, and the resulting patterns are illustrated in Fig. 1. The profiles illustrated that the Co3O4 diffraction patterns were observed at 2θ = 31.12°, 36.6°, 39.16°, 44.52°, 58.92°, and 64.88°39,40. However, various patterns were detected for the investigated samples. For the Fe-Co catalysts, one prominent diffraction peak was observed at 2θ = 36.6°, indicating the presence of Co3O4 or Fe2O341.
The overlap between the crystalline phases of iron oxide and cobalt oxide makes it extremely difficult to distinguish them precisely. Among the samples studied, the Mn-Co catalyst exhibited the lowest degree of crystallinity, possibly due to the small particle size or poor ordering of the metal. Based on the previous reports, the CuO diffraction peaks were formed at 2θ = 36.6°, 39.16°, and 58.92°42. Also, the detected diffraction peaks at 2θ = 36.6°, 44.52°, and 64.88° over the Ni-Co catalyst was belonged to the crystalline phases of NiO or Co3O4 44,45. The outcomes demonstrated that the addition of zinc oxide into the catalyst composition improved the crystallinity of the catalyst, and the diffraction peaks associated with cobalt oxide became more intense. Only one diffraction peak at 2θ = 36.6° was seen for the Cr-Co catalyst, relating to the crystalline phases of Cr2O3 or Co3O420,46.
The N2 adsorption-desorption isotherms for the samples can be seen in Fig. 2a. The isotherms for all samples including Fe-Co, Cu-Co, Cr-Co, Mn-Co, Ni-Co and Zn-Co fall into the type IV classification, and they are marked by an H3-type hysteresis loop, which suggests the formation of a complex mesoporous structure. This distinctive hysteresis pattern indicates the presence of pores with narrow openings, resembling “bottle-neck” structures. The presence of the H3 hysteresis loop at relatively high pressures (P/P₀ > 0.8) suggests that the materials possess significant interparticle porosity or large mesopores formed by non-uniform particle packing46,47.
Moreover, in the Ni-Co catalyst, there is a notable and rapid increase in nitrogen adsorption when the relative pressure (P/P0) ranges from 0.8 to 1.0, suggesting enhanced accessibility to mesopores, possibly reflecting partial uniformity in pore structure. Figure 2b illustrates the pore size distributions of the catalysts. All samples display a multimodal pore structure that includes both meso- and macro-sized pores. On the other hand, the broader pore distributions in the macropore region were detected for the Ni-Co sample.
The textural properties of the catalysts are presented in Table 1. As demonstrated, the Fe-Co catalyst displays the most substantial surface area (SBET = 138 m2/g) and total pore volume (0.45 cm3/g) among all the samples. In contrast, the Cu-Co catalyst shows the highest mean pore diameter (15.58 nm), whereas the Mn-Co catalyst exhibits the lowest mean pore diameter (11.48 nm).
The reduction behavior of the samples was assessed through H2-TPR analysis, and the outcomes are depicted in Fig. 3a. The catalysts exhibited distinct reduction patterns, reflecting variations in metal–oxygen bond strength and metal dispersion. In the Fe-Co catalyst, two significant reduction peaks were observed at approximately 300 °C and above 400 °C. The lower reduction peak was ascribed to the oxygen depletion of Fe2O3 and formation of Fe3O4 and the phase change of Co3+ to Co2+. On the other hand, the higher reduction peak primarily resulted from the reduction of Co2+ to metallic cobalt (Co⁰)48,49. Three reduction peaks were observed in the sample containing manganese oxide. The reduction of MnOx is seen to take place in two distinct temperature ranges: A low-temperature reduction area (150–300 °C) and a high-temperature reduction region (300–450 °C)50. Previous studies have suggested that the minor peak observed below 300 °C is linked to the reduction of surface Mn4+49. In contrast, due to the inability of MnO to undergo reduction within the experimental temperature range, a weak reduction peak is observed for the Mn4+/Mn3+ transformation on the catalyst surface at lower temperatures. However, a distinct reduction peak, occurring at approximately 400 °C, is detected in the manganese oxide sample. This peak can be attributed to the simultaneous reduction of MnO2 to MnO, with Mn2O3 and Mn3O4 acting as intermediate components in this process51,52. In the case of the Cu-Co catalyst, a distinct reduction peak was observed below 400 ℃, signifying the reduction characteristics of the copper bulk oxide53. Furthermore, the outcomes revealed that the inclusion of Cu in the sample formulation caused the reduction peak to shift to lower temperature regions, attributed to the strong capability of Cu to dissociate hydrogen at lower temperatures54.
In the Ni-Co catalyst, the reduction peak identified below 300 °C can be attributed to the reduction of free NiO, while the reduction peak occurring at higher temperature regions is associated with the reduction of nickel oxide strongly interacting with cobalt oxide39,55. However, in the Zn-Co catalyst, the observed reduction peaks result from the reduction of cobalt oxide with varying oxidation states. The relatively weak reduction peak detected at approximately 280 °C in the sample containing chromium oxide is linked to the partial reduction of chromium oxide, involving a change from Cr6+ to Cr+ 3 or Cr5+ to Cr3+56,57. Additionally, two other reduction peaks in this sample are ascribed to the reduction of cobalt oxide. The findings also demonstrate that the presence of Fe, Mn, and Cu enhances the degree of reducibility, causing a partial displacement of the maximum reduction peak to lower temperature regions.
Figure 3b displays the O2-TPD profiles of the as-calcined samples. This analysis revealed a three-step process of oxygen desorption. Initially, there was a release of physically adsorbed oxygen at temperatures below 300 ℃. In the temperature range between 300 ℃ and 500 ℃, as well as above 500 ℃, the removal of chemisorbed oxygen species (O− and O2−) and the release of bulk lattice oxygen were occurred during the heating operation58. In our current investigation, we identified two distinct desorption peaks with high intensity within the temperature range of 100–300 ℃ and 800–950 ℃ for the Fe-Co sample. However, a single desorption peak was appeared in the sample containing oxides of manganese, copper, and nickel at high temperatures, which is affiliated with the release of bulk lattice oxygen. When examining the Zn-Co catalyst, we detected two desorption peaks with moderate intensity below 300 ℃ and within the 300–600 ℃ range. Furthermore, our results demonstrated that a higher amount of oxygen is released at temperatures exceeding 900 ℃ in this sample. Notably, there was no clear desorption peak observed in the chromium oxide-promoted sample, indicating a limited oxygen storage capacity. It is essential to emphasize that mobility of oxygen displays a crucial role in enhancing catalytic activity in the oxidation process, and the high capacity of oxygen mobility in the Fe-Co catalyst contributes to its superior performance in CO oxidation.
Figure 4 displays the TG-DTG and DTA profiles of the FeCo catalyst, revealing two distinct stages of pyrolysis. The endothermic peak observed in the range of 180 to 280 °C on the DTA curve, along with the initial weight loss on the TG profile, was attributed to the evaporation of water that is physically adsorbed and the elimination of hydroxyl groups and residual moisture59. A rapid weight loss occurs in the range of approximately 280–400 °C, corresponding to the decomposition of reagents into NOx and organic phases, as well as the formation of metal oxide phases. This process is accompanied by an intense heat-release peak at 300 °C on the DTA graph. The observed weight loss is credited to the formation of crystallized FeCo inorganic phase58,60.
The surface chemical structure of the FeCo catalyst, both before and after calcination, was examined using Fourier transform infrared (FTIR) spectroscopy. Figure 5. presents the FTIR spectra for the as-prepared and calcined FeCo catalysts at 230 °C. An adsorption band centered at 3374 cm− 1, indicative of water chemisorbed on the outer surface of the catalyst, is observed, resulting in changes in the spectrum59. This intensity of peak decreases after calcination. The adsorption peak at 2344 cm− 1 linked to the alkyl tail group, whereas the adsorption peak near 1621 cm− 1 is attributed to the deformation vibration of H2O. Peaks identified at 1360 cm− 1 are linked to the deformation vibration of -CH2 or -CH3 groups, with the band at 1321 cm− 1 possibly attributed to NO3− ions, which are absent after calcination. Peaks at 1321 cm− 1 may originate from the adsorption of bidentate carbonate species. Generally, adsorption peaks around 1621, 1360, and 1321 cm− 1 may arise from carbonate species. The broad peak at 1087 cm− 1 results from the C-O vibration of the polymer (-(CH2CH2-O-COCOO-)n) in the catalyst. Additionally, the band at 817 cm− 1 can be ascribed to symmetric bending of Fe–O or Fe–O–Fe stretching vibration of Fe–O–Fe. Furthermore, adsorption peaks at 545 cm− 1 and 482 cm− 1 suggest the presence of metal-oxygen bonds in the complex and could be linked to the stretching vibrations of Fe-O-Co. In the calcined sample, two absorption peaks at 661 cm− 1 and 567 cm− 1 are detected, corresponding to Co3+-O and Co2+-O stretching, respectively, and can be assigned to Fe-Co stretching modes58,60,61.
Assessment of stability and the influence of process parameters on the catalytic performance
We conducted the CO oxidation reaction using the catalysts under investigation. The mechanism of carbon monoxide (CO) oxidation over cobalt-based oxide catalysts (such as Co₃O₄ or mixed spinels like MCo₂O₄) typically follows a redox pathway known as the Mars–van Krevelen mechanism. In this mechanism, the adsorbed CO undergoes a direct reaction with the lattice oxygen present on the catalyst surface, resulting in the formation of CO₂ and the creation of an oxygen vacancy (OV); this vacancy is later filled by molecular O₂ from the gas phase, thus regenerating the active surface of the catalyst62. The Mars–van Krevelen mechanism is illustrated in the diagram shown in Fig. 6.
As observed in the Fig. 7, the catalytic performance improved as the reaction temperature increased from ambient to 180 ℃. Notably, for all calcined catalysts (except for the Cr-Co catalyst), complete oxidation of CO to CO2 was achieved at around 100 ℃, underscoring the remarkable CO conversion capabilities of the catalysts were examined. However, some minor differences were observed in the initial CO conversion at lower temperatures in the sample containing Fe, Mn, Cu, and Ni. The results highlighted that the Fe-Co catalyst exhibited the highest catalytic efficiency, reaching 91.5% CO conversion at 50 °C, which can be ascribed to its superior oxygen storage and mobility properties. In contrast, the catalysts promoted by Zn and Cr displayed the weakest performance. In the case of the Cr-Co catalyst, the light-off temperature was approximately 125 ℃, which was 75 ℃ higher than that of the Fe-Co catalyst, indicative of its lower oxygen storage capacity. Table 2 compares examples of noble metal catalysts with the results of the present study.
To assess the catalytic stability of the Fe-Co catalyst, it was exposed to CO oxidation at 60 °C for 10 h under dry conditions (with 20% O2 and 4% CO). The findings from this experiment revealed that there was no notable reduction in CO conversion throughout the testing period, as depicted in Fig. 8.
As mentioned earlier, water poisoning is an important issue for all oxidation catalysts based on transition metal oxide operating at low temperatures. As shown in Fig. 8, in the wet condition at 60 °C and 3% H2O, the conversion rate remained unchanged in the first 4 h, and it slightly declined to ∼85% until the end of 10 h operation. The loss of conversion efficiency can be clarified by the blockage of the catalyst’s surface-active sites by H2O, which inhibiting the adsorption of CO and O2 onto these active centers. Additionally, hydroxyl groups are produced from surface lattice O, which causes the reduction in conversion, due to the inert nature of surface hydroxyl groups toward oxidation of CO at low temperature70.
Figure 9. illustrates the FE-SEM images of the fresh and used Fe-Co catalysts after stability tests under dry and wet conditions. As demonstrated in these figures, the particle size is assessed to be less than 50 nm. After the stability test under dry conditions, the morphology of the fresh catalyst remained unchanged, which validates the high morphological stability of this catalyst under the conditions of the aforementioned reaction. However, slight agglomeration was observed in the catalytic structure. After the stability test under wet conditions, the particles also showed signs of agglomeration. This structural change is attributed to the inclusion of water in the feed. Chemical adsorption of water molecules occurs, followed by their dissociation on the catalyst surface. The generated hydrogen atoms interact with oxygen atoms on the surface, forming hydroxides that are either physically adsorbed on oxygen vacancies or chemically adsorbed on the surface, creating OH groups. As a result, the adsorption and transformation of gaseous oxygen into highly active lattice oxygen is hindered, leading to a reduction in the catalyst’s oxidation activity60.
X-ray elemental mapping and energy dispersive X-ray (EDX) analysis are illustrated in Figs. 10 and 11. The X-ray mapping results affirmed that the elements are evidently distributed on the catalyst surface.
In Fig. 11, the EDX experiment displays the elemental composition found on the catalyst surface after the catalysts were used. The results obtained further execute the being of Fe, Co, and C in the catalyst composition, with no impurities detected. The higher peak intensity in the used catalysts can also be due to agglomeration of particles.
The textural parameters of the spent catalysts are summarized in Table 3. The spent catalyst exhibited almost identical surface characteristics in comparison with the fresh catalysts. As shown in Fig. 12a, the N2 adsorption-desorption isotherms of the used catalysts did not change significantly after the reaction. Moreover, as observed in Fig. 12b, the pore size distributions has shifted slightly towards the micropore region.
Influence of calcination temperature
To investigate the effect of varying calcination temperature on the selected sample, the Fe-Co catalyst was exposed to calcination at 230 °C, 330 °C, and 430 °C. Figure 13 depicts the XRD patterns of these samples. As observed in this figure, the diffraction patterns exclusively consist of the Co3O4 and Fe2O3 crystalline phases, with no emergence of new phases formed due to the elevated calcination temperature.
However, the samples with calcination temperatures of 330 and 430 ℃ displayed new diffraction peaks related to the iron oxide and cobalt oxide. Conversely, the findings indicated that the diffraction peaks became more pronounced in samples subjected to higher calcination temperatures. This observation can be assigned to the sintering of particles and the formation of larger particles occurring at calcination temperatures of 330 °C and 430 °C.
Figure 14a and b display the N2 sorption isotherms and pore characteristic profiles of the FeCo samples calcined at 230 °C, 330 °C and 430 °C. The findings revealed that the Fe-Co catalyst, regardless of the calcination temperature, exhibited IV type isotherm with a H3 type hysteresis loop. Furthermore, the results confirmed that changes in calcination temperature had no impact on the isotherm type or hysteresis loop, but they significantly altered the slope of the isotherm and the formation point of the hysteresis loop with a higher calcination temperature from 230 to 430 °C. These results imply that the formation of the hysteresis loop transferred to higher relative pressure (P/P0), and the pore characteristic profile, Fig. 14b, shifted to higher values in the samples calcined at 330 and 430 °C due to the collapse of mesoporous particles and the development of larger pores.
Table 4 provides a summary of the textural characteristics of the selected Fe-Co catalyst calcined at 230, 330, and 430 ℃. As anticipated, the surface features of the sample were remarkably influenced by the calcination temperature. The results manifested that the rise in the calcination temperature diminished the BET area and pore volume, and resulted in an increase the pore size of the studied sample. Agglomeration of the particles and formation of crystals with larger size were the main reasons for the mentioned issue. Furthermore, the Co3O4 crystallite size was characterized through the XRD data and Scherrer equation, Eq. 2, and the results were also reported in Table 3.
The obtained results presented that elevating the calcination temperature leads to an increase in increase in crystallite size owing to sintering of the particles at elevated calcination temperatures.
Figure 15(a–c) provides an overview of the morphological attributes of the Fe-Co catalyst after undergoing calcination at temperatures of 230 °C, 330 °C, and 430 °C. All the catalysts exhibited a nanocrystalline structure. Furthermore, an elevation in the calcination temperature resulted in the aggregation of particles. Consequently, this phenomenon resulted in a growth in the particle size of the synthesized samples at elevated calcination temperatures.
The influence of varying calcination temperatures on the structure of the synthesized samples was assessed through X-ray elemental mapping and energy dispersive X-ray (EDX) analysis, as illustrated in Figs. 16 and 17. The X-ray mapping results demonstrated that the Fe-Co catalyst, when calcined at 230 °C, showed the most extensive spread of metal. This value declined as the calcination temperature increased, which is associated with crystal growth and particle sintering at elevated temperatures. Consequently, the sample calcined at 430 °C exhibited the lowest distribution of the active metal on the surface.
In Fig. 17 (a–c), the EDX experiment displays the elemental composition found on the catalyst surface after calcination at 230, 330, and 430 °C. The results obtained further validate the presence of Fe, Co, and C in the catalyst composition. Additionally, the findings demonstrate that the material weight ratios align with the calculated values used throughout the synthesis process.
The CO oxidation reaction was conducted using the Fe-Co catalysts calcined at 230 °C, 330 °C and 430 °C, and the light-off graph is depicted in Fig. 18. The catalyst subjected to calcination at 430 °C exhibited the lowest CO conversion. The results indicate that 90% of CO conversion was attained at temperatures of 50 °C, 70 °C, and 100 °C for the samples calcined at 230 °C, 330 °C, and 430 °C, respectively. This decline in CO conversion can be attributed to the catalyst sintering process, which consequently reduced its surface characteristics and catalytic efficiency.
Influence of processing parameters
The effect of GHSV on the CO oxidation efficiency of the chosen catalyst was examined while maintaining a constant feed ratio (O2:CO = 5:1) across various temperatures, as illustrated in Fig. 19a. The findings indicated that modifying the GHSV quantity ranging from 60,000 to 120,000 ml·h− 1.g− 1cat did not significantly alter the curve shape or CO conversion. Nevertheless, a slight increase in the conversion rate was identified at higher GHSV values. This phenomenon can be associated with the substantial heat released throughout the reaction, as the conversion of CO is an exothermic process. The generated heat from the catalyst bed results in the combustion of CO71.
The content of CO in the catalytic process was changed to study the impact of the feed ratio on the CO conversion. In this survey, the GHSV value was constant at 60,000 ml.h− 1.g− 1cat, and the results are presented in Fig. 19b. The findings implied that an elevation in the content of CO resulted in a corresponding increase in CO conversion, primarily because of the greater heat released within the catalyst bed throughout the reaction. The carbon monoxide oxidation reaction releases a significant amount of heat (283 kJ.mol− 1), leading to the formation of localized hotspots owing to the overheating of the catalyst active centers. Conversely, an increase in the CO feed resulted in higher yields.
The pretreatment environment stands as a critical factor significantly impacting the efficiency of catalysts in a wide range of catalytic processes. In this study, three atmospheres (O2, N2, and H2) were used to evaluate the impact of pretreatment conditions on the light-off curve. As shown in Fig. 20, the type of pretreatment atmosphere had a considerable effect on the CO conversion.
For O2 pretreatment, a mixture containing 20% O2 diluted with argon was injected into the reactor. In the case of H2 pretreatment, a mixture consisting of 20% H2 in Ar was used, and for N2 pretreatment, a mixture of 20% O2 in N2 was introduced into the system.
As is evident, the catalytic efficiency of the studied catalyst in CO oxidation is significantly affected by the pretreatment conditions. The catalyst that underwent oxygen pretreatment exhibited the highest CO conversion. This can be associated with the alteration of surface oxygen atoms, leading to the adsorption of active surface oxygen species and enhanced catalytic performance71. Conversely, reductive pretreatment had a pronounced impact on conversion of CO, resulting from the incomplete reduction of Fe-Co oxide and the generation of compounds with reduced oxygen capacity. Furthermore, the application of H2 pretreatment conditions may alter the catalyst’s crystalline structure and lower its oxygen state35.
Conclusion
In summary, a range of MOx-Co3O4 catalysts were synthesized using a straightforward approach. Catalytic capabilities of these catalysts were explored for the efficient and low-temperature oxidation of carbon monoxide. The catalytic efficiency of the prepared catalysts diminished in the following sequence: Fe-Co > Mn-Co > Cu-Co > Ni-Co > Zn-Co > Cr-Co. The results revealed that the Fe-Co catalyst displayed the most impressive catalytic performance in CO oxidation, achieving nearly 100% CO conversion at 72 °C. Furthermore, the Fe-Co catalyst exhibited robust catalytic stability over time. The outcomes of the structural analysis indicated that the Fe-Co catalysts consist of well-dispersed mesoporous nanoparticles. Greater dispersion of Co3O4 is associated with an enhanced capacity for CO adsorption. The outstanding performance of this sample can be ascribed to its higher Co3+/Co2+ ratio. Additionally, the synergistic interplay between cobalt (Co) and iron (Fe) maximizes the dispersion of Co3O4 and promotes the creation of active sites (Co3+), ultimately leading to the highest CO adsorption capability. Furthermore, the introduction of iron (Fe) promotes the formation of a higher number of oxygen vacancies and enhances the mobility of surface lattice oxygen. These factors contribute to the outstanding catalytic efficiency of the Fe-Co catalyst.
The findings suggest that cost-effective iron-cobalt mixed metal oxide nanoparticles are promising, cost-effective catalysts for low-temperature CO oxidation. It was also observed that an elevation of the calcination temperature contributed to larger crystallite and particle sizes, along with a reduction in specific surface area, resulting in decreased catalytic efficiency of the prepared catalysts. Moreover, catalysts subjected to oxidative pretreatment (O2-pretreat) exhibited higher activity compared to those treated under reductive (H2-pretreat) and inert atmospheres (N2-pretreat). The results obtained from the GHSV tests indicated that the catalyst’s performance was at its highest when GHSV was set to 120,000 ml.h− 1.g− 1cat. Additionally, elevating the CO content in the feed resulted in improved performance.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Hussain, S. et al. Heterogeneous Co1/P1Mo12O40 single-atom catalyst for CO oxidation via termolecular Eley-Rideal (TER) mechanism. Mol. Catal. 550, 113539 (2023).
Ghiassee, M., Rezaei, M., Meshkani, F. & Mobini, S. Preparation and optimization of the MnCo2O4 powders for low temperature CO oxidation using the Taguchi method of experimental design. Res. Chem. Intermed.. https://doi.org/10.1007/s11164-019-03845-w (2019).
Zhou, H. & Qi, B. Investigation of flame spray synthesized La1-xSrxCoO3 perovskites with promotional catalytic performances on CO oxidation. J. Energy Inst. 93, 2381–2387 (2020).
Dey, S. & Dhal, G. C. A Review of Synthesis, Structure and Applications in Hopcalite Catalysts for Carbon Monoxide Oxidation. Aerosol Sci. Eng. vol. 3 Springer, (2019).
Bumajdad, A., Al-Ghareeb, S., Madkour, M., Sagheer, F. & Al Non-noble, efficient catalyst of unsupported α-Cr2O3 nanoparticles for low temperature CO oxidation. Sci. Rep. 7, 2–10 (2017).
Al Soubaihi, R. M. et al. Investigation of palladium catalysts in mesoporous silica support for CO oxidation and CO2 adsorption. Heliyon 9, e18354 (2023).
Shen, Z. et al. Unveiling the promoting mechanism of mo on the performance of CuCeOx catalyst for simultaneously NH3-SCR denitration and CO oxidation under oxygen-rich conditions. Sep. Purif. Technol. 355, 129561 (2024).
Wei, Z. Z., Li, D. C., Pang, X. Y., Lv, C. Q. & Wang, G. C. The mechanism of Low-Temperature CO oxidation on IB group metals and metal oxides. Chem. Cat. Chem. 4, 100–111 (2012).
Feng, C., Liu, X., Zhu, T. & Tian, M. Catalytic oxidation of CO on noble metal-based catalysts. Environ. Sci. Pollut Res. 28, 24847–24871 (2021).
Kim, H. J., Jang, M. G., Shin, D. & Han, J. W. Design of ceria catalysts for Low-Temperature CO oxidation. Chem. Cat. Chem. 12, 11–26 (2020).
Burange, A. S., Reddy, K. P., Gopinath, C. S., Shukla, R. & Tyagi, A. K. Role of palladium crystallite size on CO oxidation over CeZrO4-∆ supported Pd catalysts. Mol. Catal. 455, 1–5 (2018).
chu, B. et al. Insight into the effect of oxygen vacancies and OH groups on anatase TiO2 for CO oxidation: A combined FT-IR and density functional theory study. Mol. Catal. 511, 111755 (2021).
Martynova, Y., Shaikhutdinov, S. & Freund, H. J. CO oxidation on metal-supported ultrathin oxide films: What makes them active? Chem. Cat. Chem. 5, 2162–2166 (2013).
Wang, J., Chen, H., Hu, Z., Yao, M. & Li, Y. A review on the Pd-based three-way catalyst. Catal. Rev. Sci. Eng. 57, 79–144 (2015).
Huang, H., Xu, Y., Feng, Q. & Leung, D. Y. C. Low temperature catalytic oxidation of volatile organic compounds: a review. Catal. Sci. Technol. 5, 2649–2669 (2015).
Jeong, H. et al. Highly durable metal ensemble catalysts with full dispersion for automotive applications beyond single-atom catalysts. Nat. Catal. 3, 368–375 (2020).
Du, Y. et al. Recent advance of CuO-CeO2 catalysts for catalytic elimination of CO and NO. J. Environ. Chem. Eng. 9, 106372 (2021).
Mobini, S., Meshkani, F. & Rezaei, M. Supported Mn catalysts and the role of different supports in the catalytic oxidation of carbon monoxide. Chem. Eng. Sci. 197, 37–51 (2019).
Lin, H. Y. & Chen, Y. W. Low-temperature CO oxidation on au/fexoy catalysts. Ind. Eng. Chem. Res. 44, 4569–4576 (2005).
Rezaei, P., Rezaei, M. & Sonochemistry, F. M. U. & undefined. Ultrasound-assisted hydrothermal method for the preparation of the M-Fe2O3-CuO (M: Mn, Ag, Co) mixed oxides nanocatalysts for low-temperature CO. Elsevier. (2019).
Reddy, B. M., Rao, K. N. & Bharali, P. Copper promoted Cobalt and nickel catalysts supported on ceria-alumina mixed oxide: structural characterization and CO oxidation activity. Ind. Eng. Chem. Res. 48, 8478–8486 (2009).
Dey, S. & Mehta, N. S. Oxidation of carbon monoxide over various nickel oxide catalysts in different conditions: A review. Chem Eng. J. Adv 1, (2020).
Zhou, L. et al. Transition-Metal doped ceria microspheres with nanoporous structures for CO oxidation. Sci. Rep. 6, 1–7 (2016).
Han, B. et al. A highly active Rh1/CeO2 single-atom catalyst for low-temperature CO oxidation. Chem. Commun. 56, 4870–4873 (2020).
Morooka, Y. & Ozaki, A. Regularities in catalytic properties of metal oxides in propylene oxidation. J. Catal. 5, 116–124 (1966).
Umegaki, T., Inoue, T. & Compounds, Y. K. J. of A. and & undefined. Fabrication of hollow spheres of Co3O4 for catalytic oxidation of carbon monoxide. Elsevier. (2016).
Umegaki, T., Inoue, T. & Kojima, Y. Fabrication of Hollow spheres of Co3O4 for catalytic oxidation of carbon monoxide. J. Alloys Compd. 663, 68–76 (2016).
Said, A. E. A. A., El-Wahab, A., Soliman, M. M. M., Goda, M. N. & S. A. & Synthesis and characterization of mesoporous Fe-Co mixed oxide nanocatalysts for low temperature CO oxidation. Process. Saf. Environ. Prot. 102, 370–384 (2016).
Sa, Y. J., Kwon, K., Cheon, J. Y., Kleitz, F. & Joo, S. H. Ordered mesoporous Co3O4 spinels as stable{,} bifunctional{,} noble metal-free oxygen electrocatalysts. J. Mater. Chem. A. 1, 9992–10001 (2013).
Ma, L., Seo, C. Y., Chen, X., Sun, K. & Schwank, J. W. Indium-doped Co3O4 nanorods for catalytic oxidation of CO and C3H6 towards diesel exhaust. Appl. Catal. B Environ. 222, 44–58 (2018).
Goda, M. N., Said, A. E. A. A. & El-Aal, M. A. The catalytic performance of ultrasonically prepared CuxCo3 – xO4 catalysts towards CO oxidation at relatively low temperature. Mol. Catal. 494, 111121 (2020).
Liu, S. et al. Transition metal-based catalysts for selective catalytic reduction of NO by CO: A state-of-the-art review. Chem Eng. J. 486, (2024).
Biabani-Ravandi, A. & Rezaei, M. Low temperature CO oxidation over Fe–Co mixed oxide nanocatalysts. Chem. Eng. J. 184, 141–146 (2012).
Du, Y. & Mesoporous Co-Fe -O nanocatalysts: preparation, characterization and catalytic carbon monoxide oxidation. J. Environ. Chem. Eng. 2, 477–483 (2014).
Biabani-Ravandi, A., Rezaei, M. & Fattah, Z. Low-temperature CO oxidation over nanosized Fe-Co mixed oxide catalysts: Effect of calcination temperature and operational conditions. Chem. Eng. Sci. vol. 94 237–244 at (2013). https://doi.org/10.1016/j.ces.2013.02.002
Shen, Y. et al. Polymer nanofilm-coated catalysis: an approach for enhancing water-resistance of Co-Fe oxide nano-catalysts under moisture-rich condition. J. Catal. 352, 466–479 (2017).
Xie, X., Li, Y., Liu, Z. Q., Haruta, M. & Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 458, 746–749 (2009).
Jadhav, C. H. et al. Electrochemical supercapacitive performance study of spray pyrolyzed cobalt oxide film. Mater. Today Proc. 43, 2742–2746 (2021).
Kazemi, S., Alavi, S. M., Rezaei, M. & Akbari, E. Fabrication and evaluation of the Mn-promoted Ni/FeAl2O4 catalysts in the thermocatalytic decomposition of methane: impact of various promoters. Fuel 342, 127797 (2023).
Mirshafiee, F. & Rezaei, M. Engineering of the ferrite-based support for enhanced performance of supported pt, pd, ru, and Rh catalysts in hydrogen generation from NaBH4 hydrolysis. Sci. Rep. 14, 1–14 (2024).
Zhou, Y. et al. Mn and Fe oxides co-effect on nanopolyhedron CeO2 catalyst for NH3-SCR of NO. J. Energy Inst. 99, 97–104 (2021).
Dong, F., Meng, Y., Han, W., Zhao, H. & Tang, Z. Morphology effects on surface chemical properties and lattice defects of Cu/CeO2 catalysts applied for low-temperature CO oxidation. Sci. Rep. 9, 1–14 (2019).
Pourdelan, H., Alavi, S. M., Rezaei, M. & Akbari, E. Production of Pure Hydrogen through Thermocatalytic Methane Decomposition Using NiO-MgO Catalysts Promoted by Chromium and Copper Prepared via Mechanochemical Method. Int. J. Energy Res. 5132640 (2023). (2023).
He, Y. et al. Insights into the structure-activity relationships of highly efficient CuCe oxides for the low temperature CO oxidation and CO-SCR. J. Energy Inst. 104, 142–155 (2022).
Guo, J., Xiao, J., Gui, R., Gao, Y. & Wang, Q. Efficient simultaneous removal of NOx and CO at low temperatures over integrated Mn2Co1Ox/iron mesh monolithic catalyst via NH3-SCR coupling with CO oxidation reactions. Chem Eng. J 465, (2023).
Jokar, F., Alavi, S. M. & Rezaei, M. Investigating the hydroisomerization of n-pentane using Pt supported on ZSM-5, desilicated ZSM-5, and modified ZSM-5/MCM-41. Fuel 324, 124511 (2022).
Li, C. et al. Promotional effect of tungsten modification on magnetic iron oxide catalyst for selective catalytic reduction of NO with NH3. J. Energy Inst. 93, 1809–1818 (2020).
Akbari, E., Alavi, S. M., Rezaei, M. & Larimi, A. Catalytic methane combustion on the hydrothermally synthesized MnO2 nanowire catalysts. Ind. Eng. Chem. Res. 60, 7572–7587 (2021).
Gao, Z. et al. Mesoporous SiO2-Encapsulated Nano-Co3O4 catalyst for efficient CO oxidation. Chem. Cat. Chem. 13, 4010–4018 (2021).
Ghiassee, M., Rezaei, M., Meshkani, F. & Mobini, S. Preparation of the Mn/Co mixed oxide catalysts for low-temperature CO oxidation reaction. Environ. Sci. Pollut. Res. 28, 379–388 (2020). (2020).
Fattah, Z., Rezaei, M., Biabani-Ravandi, A. & Irankhah, A. Preparation of Co–MgO mixed oxide nanocatalysts for low temperature CO oxidation: optimization of Preparation conditions. Process. Saf. Environ. Prot. 92, 948–956 (2014).
Zhang, Z. et al. Oxygen vacancies promote the activation of O2 in transition metal oxide doped ε-MnO2 for low-temperature CO oxidation. Sep. Purif. Technol. 352, 128109 (2025).
Wu, D. et al. Synergistic metal-support interaction promoting the formation of oxygen vacancies and their role in CO-PROX over CuO/NiO-CeO2 catalyst. J. Environ. Chem. Eng. 12, 111818 (2024).
Pourdelan, H., Alavi, S. M., Rezaei, M. & Akbari, E. Thermocatalytic decomposition of methane over NiO–MgO catalysts synthesized by the mechanochemical method. Catal. Lett. https://doi.org/10.1007/s10562-022-04175-0 (2022).
Zhang, Y. et al. CO oxidation on Ni-based single-atom alloys surfaces. Mol. Catal. 495, 111154 (2020).
Akbari, E., Alavi, S. M., Rezaei, M. & Montazeri, Z. AOx–MnOx (A = Ni, cu, fe, or Co) nanocatalysts fabricated by the mechanochemical Preparation method for lean methane catalytic combustion assisted by a DBD plasma reactor. ACS Appl. Nano Mater. 6, 16189–16200 (2023).
Song, W. et al. Mesoporous Co 3 O 4 with controlled porosity: inverse micelle synthesis and High-Performance catalytic CO oxidation at – 60°C. Chem. Mater. 26, 4629–4639 (2014).
Mobini, S., Meshkani, F. & chemical, M. R. J. of environmental & undefined. Surfactant-assisted hydrothermal synthesis of CuCr2O4 spinel catalyst and its application in CO oxidation process. Elsevier. (2017).
Varbar, M., Alavi-Amleshi, S. M., Rezaei, M. & Akbari, E. Catalytic oxidation of lean methane over Ni/MgAl2O4 synthesized by a novel and facile mechanochemical Preparation method. Combust. Sci. Technol. 195, 1819–1839 (2023).
Rezaei, P., Rezaei, M. & Meshkani, F. Low temperature CO oxidation over mesoporous iron and copper mixed oxides nanopowders synthesized by a simple one-pot solid-state method. Process. Saf. Environ. Prot. 119, 379–388 (2018).
Mirshafiee, F. & Rezaei, M. Enhancing hydrogen generation from sodium borohydride hydrolysis and the role of a Co/CuFe2O4 nanocatalyst in a continuous flow system. Sci. Rep. 14, 1–15 (2024).
Zhao, H., Wang, H. & Qu, Z. Synergistic effects in Mn-Co mixed oxide supported on cordierite honeycomb for catalytic deep oxidation of VOCs. J. Environ. Sci. (China). 112, 231–243 (2022).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Song, Y. et al. Constructing Pt/ZnO@SiO2 composite structures to enhance the thermal stability and CO oxidation activity of Pt-based catalysts. Particuology 100, 36–44 (2025).
Boronin, A., Slavinskaya, E., … A. F.-A. C. B. & 2021, undefined. CO oxidation activity of Pt/CeO2 catalysts below 0° C: platinum loading effects. Elsevier.
He, J. et al. Enhanced low-temperature CO oxidation activity through crystal facet engineering of Pd/CeO2 catalysts. Ceram. Int. 50, 36363–36374 (2024).
Comsup, N., Panpranot, J. & Praserthdam, P. The influence of Si-modified TiO2 on the activity of Ag/TiO2 in CO oxidation. J. Ind. Eng. Chem. 16, 703–707 (2010).
Du, M., Huang, J., Sun, D., Wang, D. & Li, Q. High catalytic stability for CO oxidation over Au/TiO2 catalysts by Cinnamomum camphora leaf extract. Ind. Eng. Chem. Res. 57, 14910–14914 (2018).
Farhang, Y. & Rezaei, M. Author ’ s Accepted Manuscript oxidation and Methane combustion. Ceramics International at (2018). https://doi.org/10.1016/j.ceramint.2018.08.211
Varbar, M., Alavi, S. M., Rezaei, M. & Akbari, E. Cobalt promoted Ni/MgAl2O4 catalyst in lean methane catalytic oxidation. Res. Chem. Intermed. 48, 1129–1150 (2022).
Akbari, E., Alavi, S. M., Rezaei, M. & Larimi, A. Barium promoted manganese oxide catalysts in low-temperature methane catalytic combustion. Int. J. Hydrogen Energy. 46, 5181–5196 (2021).
Acknowledgements
Giving access to the facilities from the Iran National Science Foundation (INSF) under the grant number of 4031093 to perform this project is kindly appreciated and acknowledged.
Author information
Authors and Affiliations
Contributions
ContributionsS.M.T.: Conceptualization, methodology, validation, investigation, writing—original draft. S.M.A.: Supervision, validation, funding, writing—review and editing. M.R.: Supervision, validation, funding, writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Teymoori, S.M., Alavi, S.M. & Rezaei, M. Catalytic oxidation of CO over the MOx – Co3O4 (M: fe, mn, cu, ni, cr, and Zn) mixed oxide nanocatalysts at low temperatures. Sci Rep 15, 25808 (2025). https://doi.org/10.1038/s41598-025-10737-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-10737-0