Abstract
Autoimmune encephalitis (AE) has been described as a severe neurological complication of coronavirus disease 2019 (COVID-19). Following the adjustment of COVID-19 prevention strategies on December 7, 2022, the virus spread rapidly and extensively across China. This study aimed to explore the changing characteristics of AE pre- and post- COVID-19 epidemic in Guangxi, China. A total of 169 patients who were first diagnosed with AE and admitted to the First Affiliated Hospital of Guangxi Medical University from November 1, 2021 to December 31, 2023 were enrolled in this case-control study. Patients with the onset of AE before or after December 7, 2022, were respectively classified into the pre- and post- COVID-19 epidemic groups. There were 78 AE patients in the pre-COVID-19 epidemic group and 91 patients in the post-COVID-19 epidemic group. Compared to the AE patients pre-COVID-19 epidemic group, AE patients in the post-COVID-19 group had higher rates of abnormal movements (p = 0.013), autonomic dysfunction (p = 0.003), higher CASE scores (p = 0.041), and higher probabilities of complications such as pneumonia (p = 0.025) and other autoimmune diseases (p = 0.014). A higher proportion of AE patients in the post-COVID-19 pandemic period received rituximab treatment compared to those in the pre-COVID-19 (16.48% vs. 6.41%, p = 0.043). Among the AE patients infected with COVID-19, those who has a relapse of AE also had a higher risk of complications with tumors, autoimmune diseases, cranial magnetic resonance imaging (MRI) abnormalities, and higher baseline modified Rankin Scale (mRS) (median [IQR]:4[4,5] vs. 3[2.75,4.25], p = 0.029). AE patients in the post-COVID-19 epidemic group suffer from more severe clinical symptoms and higher rates of other immune diseases. Rituximab is commonly used in the post-COVID-19 epidemic period. Relapsed AE patients with COVID-19 had a higher risk of complications with tumors, autoimmune diseases, abnormal MRIs, and higher baseline mRS.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) is an acute disease that results from infection with the severe acute respiratory syndrome coronavirus 2 (SARS CoV-2)1. After the acute phase of infection, some people develop long-lasting symptoms, known as long COVID-192,3. Autoimmune encephalitis (AE) comprises a group of non-infectious immune-mediated inflammatory disorders of the brain parenchyma often involving the cortical or deep grey matter4. Previous studies have suggested that AE may exhibit immune cross-reactivity with SARS CoV-25. Patients with COVID-19 have been reported to have an increased risk of new-onset autoimmune diseases following the acute phase of infection6. Previous studies have reported that SARS CoV-2 might be related to systemic autoimmune diseases, such as autoimmune hemolytic anemia or Guillain-Barre syndrome7,8. COVID-19 has been linked to encephalopathy and AE, with multiple case reports highlighting this association9,10. Notably, AE cases have shown a concerning rise since the onset of the COVID-19 pandemic11. On December 7, 2022, the COVID-19 isolation policies in China were adjusted in some regions, including Guangxi. It was clearly stated that a negative COVID-19 nucleic acid test result was not required to enter public places. Prior to this, strict isolation policies were implemented for individuals who tested positive for the nucleic acid test. Following this modification of prevention measures, COVID-19 infection became widely prevalent in China, so the onset of AE before or after December 7, 2022 was classified as pre- or post-COVID-19 epidemic group. This study aimed to explore the changing characteristics of AE pre- and post- COVID-19 epidemic in Guangxi, China.
Patients and methods
Study population
In this study, patients who were first diagnosed with autoimmune encephalitis at Guangxi Medical University from November 1, 2021 to December 31, 2023, were enrolled to investigate the clinical characteristics, prognosis, and risk factors of AE patients before and after the epidemic of COVID-19 in Guangxi, China. A total of 226 AE patients from October 1, 2021 to December 31, 2023, of which 52 patients with previously diagnosed AE and 5 patients who were lost to follow-up were excluded. Thus, a total of 169 patients with new-onset AE were included in this case-control study. The inclusion criteria for patients diagnosed with AE was defined according to the diagnostic criteria of autoimmune encephalitis in 202112. Among these patients, 78 AE patients’ onset was between November 1, 2021 and December 7, 2022 (defined as the pre-COVID-19 epidemic group). 91 AE patients’ onset was between December 8, 2022, and December 31, 2023 (defined as the post-COVID-19 epidemic group). All of the enrolled patients were followed up for at every 3 months (Fig. 1).
This study was approved by the ethics committee of the First Affiliated Hospital of Guangxi Medical University (2024-E233-01). We complied with the Declaration of Helsinki Ethical Principles for medical research involving human subjects. Written informed consents was obtained from all the patients enrolled in this study.
Data collection
The clinical data of the patients with AE were obtained from hospitalization records and outpatient neurology clinic notes. Clinical data included demographics, premonitory symptoms, major clinical manifestations, complications and comorbidities, AE classification, antibodies, cerebrospinal fluid (CSF) findings, serum and CSF antibody titers (by cell-based assay, CBA), electroencephalogram (EEG), magnetic resonance imaging (MRI) and immunotherapy treatment. The Clinical Assessment Scale in Autoimmune Encephalitis (CASE) score was used to assess severity of symptoms. The Anti-NMDAR Encephalitis One-Year Functional Status (NEOS) score was used to predict patients’ functional status one year after diagnosis. A higher NEOS score indicates a poorer prognosis. The CSF findings included in the chart review were cell counts, protein levels, glucose levels, chloride concentration, and IgG levels. Abnormal values were defined as CSF pleocytosis ≥ 5 × 106 cells/L, CSF protein level > 450 mg/L, and CSF-IgG level>40 mg/L.
Immunotherapy included intravenous glucocorticoids, intravenous immunoglobulin (IVIG), plasma exchange (PE), and immunosuppressants (rituximab, cyclophosphamide, mycophenolate mofetil and azathioprine). Tumor screening was performed in patients with AE. The modified Rankin scale (mRS) score was used for prognostic evaluation and was assessed at the first admission, after treatment, and at follow-up visits. COVID-19 vaccine. Infection of COVID-19 was confirmed by a positive nasopharyngeal swab SARS-CoV-2 polymerase chain reaction (PCR) testing, positive nasopharyngeal swab SARS-CoV-2 antigen testing, or the development of typical clinical symptoms after close contact with COVID-19 patients.
Statistical analysis
Statistical analysis was performed using IBM SPSS 25.0 and figures were generated with using GraphPad Prism 9.5. Normally distributed data were described as mean ± standard deviation, whereas non-normally distributed data were described as medians (interquartile range, IQR). Categorical variables were presented as n (%) and compared using the χ2 test and Fisher’s exact test. Mann–Whitney U-test was used for continuous variables. Two-tailed P < 0.05 was considered statistically significant.
Results
The baseline characteristics of AE patients in pre- and post-COVID-19 epidemic
There was no statistically significant difference in demographics between AE patients pre- and post- COVID-19 epidemic (Table 1). Among the 169 AE patients, there were 78 (46.15%) AE patients with an onset before the COVID-19 epidemic and 91 (53.85%) AE patients with an onset after the COVID-19 epidemic. The median age at onset was 21.5 (IQR:12.75, 43.50) years old in the pre-COVID-19 epidemic group, and 24 (IQR:12, 52) years old in the post-COVID-19 epidemic group. There were 126 AE patients infected with COVID-19, 108 of them were diagnosed by nasopharyngeal swab SARS-CoV-2 PCR and antigen testing, while the remaining 18 were diagnosed based on typical clinical symptoms following close contact with confirmed COVID-19 cases. The length of hospital stay was similar between the two groups (p = 0.843).
A total of 152 AE patients received one or more doses of COVID-19 vaccines. Among them, 105 patients were administered inactivated vaccines, 26 received adenovirus vector vaccines, and 21 were given recombinant protein vaccines. The remaining 17 patients did not receive vaccination due to various reasons. More AE patients in the post-COVID-19 epidemic group were vaccinated than those in the pre-COVID-19 epidemic group (94.51% vs. 84.62%, p = 0.033). In addition, more AE patients in the post-COVID-19 epidemic group were infected with COVID-19, compared to AE patients in the pre-COVID-19 epidemic group (89.01% vs. 57.69%, p < 0.001).
The clinical characteristics of AE in pre- and post- COVID-19 epidemic
The clinical manifestations of the two groups were compared as shown in (Table 2). Significant differences were observed in autonomic dysfunction (20.51% vs. 41.76%, p = 0.003), abnormal movements (35.90% vs. 54.95%, p = 0.013), and seizures (39.74% vs. 54.95%, p = 0.049) between the AE pre- and post-COVID-19 groups. Compared to the AE patients with onset before the COVID-19 epidemic, AE patients with onset after the COVID-19 epidemic were likely to have complications such as pneumonia (40.66% vs. 24.36%, p = 0.025) other immune diseases (23.08% VS 8.97%, p = 0.014). Among AE patients with onset after the COVID-19 epidemic, the most common immune disease was thyroid dysfunction (7.69%, 7/91), followed by systemic lupus erythematosus (2.20%, 2/91) and Sjogren’s syndrome (2.20%, 2/91). There was no difference in the risk of association with tumors between AE patients with onset before or after the COVID-19 epidemic (p > 0.05). The median CASE score was higher in the post-COVID-19 epidemic group (median 8, IQR:4–11) than in the pre-COVID-19 epidemic group (median 5, IQR:3–9; p = 0.041). There were no significant differences in the rates of ICU admission (11.54% vs. 15.38%), mechanical ventilation (8.97% vs. 8.79%), and the median length of ICU stay (18, IQR:5.25,25 vs. 11.5, IQR:5,18.25) between the pre- and post-COVID-19 epidemic groups (p>0.05).
The auxiliary examination results showed that there were no significant differences in the elevated opening pressure, CSF pleocytosis, elevated CSF IgG, elevated CSF protein levels or abnormal of cranial MRI findings between the two groups. Abnormal EEG (n=74, 81.3%) was more common in the post-COVID-19 epidemic group. Specifically, epileptic discharges appeared more commonly in the post-COVID-19 epidemic group (28.57%) than in the pre-COVID-19 epidemic group (12.82%, p = 0.013).
All patients with AE in the hospital received immunotherapy, along with symptomatic and supportive treatments, of which approximately 64.10% (50/78) AE patients in the pre-COVID-19 epidemic group and 48.35% (44/91) AE patients in the post-COVID-19 epidemic group were treated with IVIG (p = 0.040). AE patients with onset after the COVID-19 epidemic were more likely to receive rituximab (16.48%, 15/91) compared to those with onset before the COVID-19 epidemic (6.41%, 5/78, P = 0.043). No statistical differences were observed in the proportion of patients with AE receiving steroids, PE, cyclophosphamide, or other immunotherapies. There were no significant differences between the mRS score and mortality at discharge in the before and post-COVID-19 epidemic groups (p > 0.05).
In the cohort, 9 (9.89%) AE patients in the post-COVID-19 epidemic group and 4 (5.13%) AE patients in the pre-COVID-19 epidemic group suffered from relapse (p > 0.05). Among the relapsed AE patients, 12 (92.31%, 12/13) of them were infected with COVID-19.
Clinical characteristics of anti-NMDAR encephalitis in pre- and post- COVID-19 epidemic
Of the 169 cases of AE, there were 58 cases of anti-NMDAR encephalitis included in the analysis. 26 anti-NMDAR encephalitis patients had an onset of AE before the COVID-19 epidemic and 32 anti-NMDAR encephalitis patients had an onset after the COVID-19 epidemic. There were no statistical differences in gender, age, or COVID-19 vaccination status between the anti-NMDAR encephalitis patients pre- and post-COVID-19 epidemic (Table 3). The most common clinical manifestations in both the pre- and post-COVID-19 groups were abnormal mental status (80.77% vs. 84.38%), epilepsy (69.23% vs. 78.13%), and disturbance of consciousness (50.00% vs. 59.38%). CASE scores and NEOS scores were obtained at the time of first admission for patients with anti-NMDAR encephalitis, no statistical difference was observed in CASE scores and NEOS scores between the two groups (p > 0.05). No significant differences were found in the rate of anti-NMDAR encephalitis with or without ovarian teratomas, pneumonia, or other immune diseases. All anti-NMDAR encephalitis cases were confirmed by positive CSF NMDAR antibody titers. However, there was no significant difference in cerebrospinal fluid NMDAR antibody titers between the patients with anti-NMDAR encephalitis in the pre-and post-COVID-19 epidemic groups. All patients with anti-NMDAR encephalitis received intravenous methylprednisolone, except for 2 patients with gastrointestinal bleeding. Approximately 43.75% (14/32) of the anti-NMDAR patients were treated with rituximab in the after group, compared to only 15.38% (4/26) in the before group (p = 0.025). No significant differences were observed in the administration rates of first-line immunotherapy or other immunomodulatory therapies between the pre- and post-COVID-19 groups.
Features of relapsed AE in AE patients infected with COVID-19
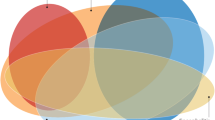
The relapse of AE occurred in 12 (9.52%, 12/126) patients infected with COVID-19 and 1 (2.33%, 1/43) patient without COVID-19. Among the AE patients infected with COVID-19, patients with or without relapse were compared. The features of relapsed AE in patients infected with COVID-19 are presented in Table 4. There was no significant difference in demographics between patients with or without relapse in AE patients infected with COVID-19. Among these relapsed AE patients, approximately 42% (5/12) were anti-NMDAR encephalitis and 17% (2/12) were anti-myelin oligodendrocyte glycoprotein (MOG) encephalitis. Unknown antibody types constituted approximately 17% (2/12) (Fig. 2). Abnormal cranial MRI (100%) occurred in all AE patients infected with COVID-19 and 3 (25.00%) were associated tumors. 58.33% of patients with AE relapse and COVID-19 had comorbidities with other immune diseases, which was higher than patients without AE relapse and COVID-19 (p<0.05). The median baseline mRS scores in patients with relapsed AE and COVID-19 were 4 (IQR 4,5) and 4 (IQR 2.75,4.25) in patients without AE relapse and COVID-19 (p = 0.029). After treatment, no significant difference between the median mRS scores between the two groups (p > 0.05).
Discussion
COVID-19 has profoundly affected global socio-economic structures and public health over the past four years and remains an ongoing pandemic due to the emergence of new variants. In some countries, individuals may experience up to three or four waves of infections annually13. A wide spectrum of neurological and neuropsychiatric symptoms are commonly observed during the acute phase of COVID-19 14,15. These symptoms can persist into the post-acute phase, referred to as long COVID16. Several case reports and systematic reviews have described the neurological manifestations of COVID-19, including acute disseminated encephalomyelitis and autoimmune encephalitis17,18. However, there is a lack of cohort studies examining patients with autoimmune encephalitis who are infected with COVID-19. Therefore, this study provides a cohort study that focuses on the characteristics in AE patients in China, diagnosed before and after the adjustment of COVID-19 prevention strategies since the beginning of December 2022.
In the current study, the number of hospitalized new onset AE patients in our hospital increased after the adjustment of prevention strategies. This suggests that AE may be a neurological complication of COVID-19. This is consistent with previous studies9,19 which had found that encephalitis is a complication of COVID-19, especially in severely ill COVID-19 patients. Pardis et al. had reported a sharp rise in AE cases during the COVID-19 pandemic11. COVID-19 may invade the central nervous system through the following four pathways: olfactory nerve pathway, synaptic connection pathway of neurotropic viruses, infected lymphocyte hematogenous migration pathway and vascular endothelial cell infection pathway20,21. The spike protein of SARS-CoV-2 alters the blood–brain barrier (BBB) function, which provides an additional mechanism of potential CNS entry22. Also, as more neurological complications are reported after the COVID − 19 pandemic, clinicians are more aware of screening for AE, which may raise detection rates. Previous study had showed that vaccination is a protective factor for COVID-1923. However, in our study, within the post COVID-19 epidemic group, more AE patients had been vaccinated against COVID-19. Moreover, this group also had a higher number of patients contracted COVID-19. In the early stages of the epidemic, there was a shortage of COVID-19 vaccines. Additionally, some individuals hesitated to get vaccinated due to limited awareness of the vaccine’s importance24. Moreover, AE is an autoimmune disease, and patients diagnosed with AE prior to the COVID-19 epidemic often did not receive the newly developed vaccine after their diagnosis.
This study also showed that AE patients in the post-COVID-19 epidemic group had higher rates of developing more severe clinical manifestations of seizures, abnormal movements, and autonomic dysfunction. The median CASE score, which was used to assess the severity of autoimmune encephalitis in the AE patients, was higher in the post-COVID-19 epidemic group. This suggests that AE patients in the post-COVID-19 epidemic group may experience more severe symptoms. Pneumonia was common in AE after COVID-19 infection and the rate of antibiotic use was much higher. This may be related to the fact that more AE patients in the post-COVID-19 epidemic group were infected with COVID-19, which led to pneumonia. There were no statistically significant differences in the rate of ICU admission, ventilator utilization, the length of ICU stays, or the mortality of AE. This is in contrast to previous studies25,26 which suggested that AE patients with COVID-19 would have higher ICU admissions, ventilator utilization rates, and mortality rates. A probable cause of this phenomenon is an ongoing viral mutation of the virus. In the most recent variant, SARS-CoV-2 is less pathogenic and less symptomatic than previous variants27,28.
AE patients in the post-COVID-19 epidemic group were more likely to have comorbidities with other autoimmune diseases. This suggests that COVID-19 infection could act as a trigger for autoimmune conditions, potentially leading to immune system dysregulation18. Additionally, this study found that IVIG was used less frequently in AE patients in the post-COVID-19 epidemic group, which appears to contradict the perception of immunoglobulins being effective in treating COVID-1929. This may be attributed to a shortage of immunoglobulin supplies following the widespread COVID-19 epidemic. However, it is also important to consider that both COVID-19 infection and IVIG therapy have been associated with coagulation disorders, leading to clot formation and pulmonary embolism with detrimental effects on patient recovery and survival30,31.
In the post-COVID-19 group, a higher number of AE patients were treated with the second-line immunosuppressive drug rituximab, likely due to concerns about encephalitis relapse. This indicates that the widespread prevalence of COVID-19, along with AE patients contracting the virus, may have influenced clinicians to favor second-line immunosuppressive therapies to reduce the risk of encephalitis relapse.
Despite the higher CASE scores and more severe clinical presentations in AE patients in the post-COVID-19 epidemic group, the mRS scores of AE patients improved significantly after immunotherapy. No statistical difference was observed in the mRS scores between the pre-or post-COVID-19 epidemic groups. This suggests that AE patients were responsive to immunotherapy pre- and post- COVID-19 epidemic. Our results are consistent with a previous study32which had found that IVIG and corticosteroids were helpful in the treatment of COVID-19 patients with encephalitis.
In this study, anti-NMDAR encephalitis accounted for 34.23% (58/169) of AE, of which 26 anti-NMDAR encephalitis patients had an onset in pre-COVID-19 epidemic and 32 patients had an onset post-COVID-19 epidemic. Mental and behavioral disorders were the most common neurological manifestations of anti-NMDAR encephalitis in both groups, followed by seizures. Our results are consistent with those of a systematic review by Ahmad33which showed that altered mental status was the most prevalent neurological manifestation among the anti-NMDAR encephalitis patients with COVID-19, followed by focal or generalized seizures.
Approximately 43.75% (14/32) anti-NMDAR encephalitis patients in the post-COVID-19 epidemic group were treated with rituximab, and only 15.38% (4/26) anti-NMDAR encephalitis patients used rituximab in the pre-COVID-19 epidemic group (p = 0.025). This suggests a preference for rituximab as a second-line immunotherapy in combination with conventional first-line immunotherapy after the COVID-19 epidemic. The probable reasons for this are consistent with the previous statement that clinicians were more concerned about the relapse of encephalitis in the after the COVID-19 epidemic period. It is important to note that this observed phenomenon might be subject to the influence of multiple factors. These include, but are not limited to, the accessibility of healthcare services, the diagnostic awareness of clinicians, and the continuously evolving treatment practices.
Patients with anti-NMDAR encephalitis achieved a favorable prognosis at follow-up (mRS ≤ 1) in both groups, indicating that patients with anti-NMDAR encephalitis responded well to immunotherapy pre- and post- COVID-19 epidemic. This finding is similar to previous studies, which showed that the majority (74%) of the anti-NMDAR encephalitis patients with COVID-19 had a good outcome33.
A subgroup analysis of relapsed AE patients was conducted in AE patients with or without COVID-19 infection to explore the relationship between AE relapse and COVID-19. Approximately 9.52% (12/126) AE patients with COVID-19 infection experienced relapse, and about 2.33% (1/43) AE patients without COVID-19 infection experienced relapse (p = 0.188). This indicates that COVID-19 infection in AE patients did not increase their risk of AE relapse. A case report showed that patients with autoimmune encephalitis experienced early clinical relapse of encephalitis after receiving a second dose of the COVID-19 vaccine34. However, there was no association observed between vaccination status and AE recurrence in our study. Therefore, our study proves to a certain extent, that COVID-19 vaccination has no long-term effect on AE relapse. A real-world cross-sectional survey in China had indicated an overall favorable safety profile of the inactivated COVID-19 vaccine for AE patients35.
In this study, 42% of AE patients who experienced relapse and had a COVID-19 infection were diagnosed with anti-NMDAR encephalitis, followed by anti-MOG encephalitis and AE with unidentified antibody types as the next major categories. The data showed that relapsed AE patients with COVID-19 infection had a higher rate of abnormal cranial MRIs and comorbidities including other autoimmune diseases and tumors. A possible mechanism is that potential tumor antigens can cause immune activation, proliferation of T and B lymphocytes, and the production of tumor-specific antibodies, ultimately leading to cross-reactivity with NMDAR36. Furthermore, the structure of the NMDAR GluN1 subunit is similar to the SARS-CoV-2 nonstructural protein 8, which may also explain the association between this infection and the relapse of anti-NMDAR encephalitis37. SARS-CoV-2 on CNS is through systemic and local inflammatory response causing cytokines storming and immune cells reactivation, which may increase immune dysregulation and lead to more relapses for these patients38. In this study, most AE patients with relapse and COVID-19 infection had a good prognosis at their latest follow-up.
Conclusion
This study showed that AE patients in the post-COVID-19 epidemic group had more severe symptoms, higher CASE scores, and a higher rate of complications such as pneumonia and other immune diseases. Rituximab as a second-line immunotherapy is commonly used in combination with conventional first-line immunotherapy in the post- COVID-19 epidemic. COVID-19 infection did not increase the risk of AE relapse in AE patients. AE patients with AE relapse and COVID-19 had a higher rate of complication with autoimmune diseases, tumors, abnormal cranial MRIs, and baseline mRS.
Limitations
This study has certain limitations. As a retrospective analysis, the study did not conduct COVID-19 variant testing on patients in the after epidemic period, and the sample size may not be sufficiently large, and some bias might have occurred. While it suggests a correlation between AE and COVID-19, it does not clarify the underlying mechanisms between the two diseases. Nonetheless, this study offers a comprehensive analysis of the available data with valuable preliminary insights.
Data availability
Data supporting the findings of this study are accessible upon request from the corresponding author.
References
Velavan, T. P. & Meyer, C. G. The COVID-19 epidemic. Trop. Med. Int. Health. 25 (3), 278–280 (2020).
Davis, H. E. et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 38, 101019 (2021).
Nasserie, T., Hittle, M. & Goodman, S. N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: A systematic review. JAMA Netw. Open. 4 (5), e2111417 (2021).
Graus, F. et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 15 (4), 391–404 (2016).
Sanchez-Larsen, A. et al. Anti-NMDA-R encephalitis post-COVID-19: case report and proposed physiopathologic mechanism. Neurologia (Barcelona Spain) (2022).
Siow, I. et al. Encephalitis as a neurological complication of COVID-19: A systematic review and meta‐analysis of incidence, outcomes, and predictors. Eur. J. Neurol. 28, 3491–3502. https://doi.org/10.1111/ene.14913 (2021).
Lazarian, G. et al. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br. J. Haematol. 190, 29–31 (2020).
Zhao, H. et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: Causality or coincidence? Lancet Neurol. 19, 383 (2020).
Siow, I. et al. Encephalitis as a neurological complication of COVID-19: A systematic review and meta‐analysis of incidence, outcomes, and predictors. Eur. J. Neurol. 28, 3491–3502 (2021).
Garg, R. K., Paliwal, V. K. & Gupta, A. Encephalopathy in patients with COVID-19: A review. J. Med. Virol. 93 (1), 206–222 (2021).
Saffari, P. et al. A Sharp rise in autoimmune encephalitis in the COVID-19 era: A case series. Cureus 15, e34658. https://doi.org/10.7759/cureus.34658 (2023).
Abboud, H. et al. Autoimmune encephalitis: Proposed best practice recommendations for diagnosis and acute management. J. Neurol. Neurosurg. Psychiatry. 92, 757–768 (2021).
Callaway, E. COVID’s future: Mini-waves rather than seasonal surges. Nature. 617 (7960), 229–230. https://doi.org/10.1038/d41586-023-01437-8 (2023).
Helms, J. et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 382, 2268–2270 (2020).
Larvie, M., Lev, M. H. & Hess, C. P. More on neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med.. https://doi.org/10.1056/NEJMc2015132 (2020).
Monje, M. & Iwasaki, A. The neurobiology of long COVID. Neuron 110, 3484–3496 (2022).
Stoian, A. et al. The occurrence of acute disseminated encephalomyelitis in SARS-CoV-2 infection/vaccination: Our experience and a systematic review of the literature. Vaccines 11, 1225 (2023).
Stoian, A. et al. Autoimmune encephalitis in COVID-19 infection: Our experience and systematic review of the literature. Biomedicines 10, 774 (2022).
Abenza-Abildúa, M. J. et al. Neurological complications in critical patients with COVID-19. Neurología (English Edition). 35, 621–627 (2020).
Karnik, M. et al. A review on SARS-CoV-2-induced neuroinflammation, neurodevelopmental complications, and recent updates on the vaccine development. Mol. Neurobiol. 58, 4535 (2021).
Jha, N. K. et al. Evidence of coronavirus (CoV) pathogenesis and emerging pathogen SARS-CoV-2 in the nervous system: A review on neurological impairments and manifestations. J. Mol. Neurosci. 2192–2209 (2021).
Buzhdygan, T. P. et al. The SARS-CoV-2 Spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol. Dis. 146, 105131 (2020).
Vitiello, A., Ferrara, F., Troiano, V. & La Porta, R. COVID-19 vaccines and decreased transmission of SARS-CoV-2. Inflammopharmacology 29, 1357–1360. https://doi.org/10.1007/s10787-021-00847-2 (2021).
Brüssow, H. COVID-19: Vaccination problems. Environ. Microbiol. 23, 2878–2890. https://doi.org/10.1111/1462-2920.15549 (2021).
Baud, D. et al. Real estimates of mortality following COVID-19 infection. Lancet. Infect. Dis. 20 (7), 773 (2020).
Abenza-Abildúa, M. J. et al. Neurological complications in critical patients with COVID-19. Neurologia 35, 621–627. https://doi.org/10.1016/j.nrl.2020.07.014 (2020).
Suzuki, R. et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature 603, 700–705 (2022).
Fan, Y. et al. SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduct. Target. Ther 7 (1), 141 (2022).
Danieli, M. G. et al. Intravenous immunoglobulin as an important adjunct in the prevention and therapy of coronavirus 2019 disease. Scand. J. Immunol. 94, e13101. https://doi.org/10.1111/sji.13101 (2021).
Biswas, I. & Khan, G. A. Coagulation disorders in COVID-19: Role of toll-like receptors. J. Inflamm. Res. 13, 823–828. https://doi.org/10.2147/jir.S271768 (2020).
Guo, Y., Tian, X., Wang, X. & Xiao, Z. Adverse effects of Immunoglobulin therapy. Front. Immunol. 9, 1299. https://doi.org/10.3389/fimmu.2018.01299 (2018).
Paterson, R. W. et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain J. Neurol. 143, 3104–3120. https://doi.org/10.1093/brain/awaa240 (2020).
Sawalha, A., Alkilani, H. & Abdelaziz, R. The association between autoimmune encephalitis mediated by n-methyl--aspartate receptor autoantibodies and covid-19: A systematic review. Encephalitis 4, 3–10 (2023).
Vences, M. et al. Post-vaccinal encephalitis with early relapse after BNT162b2 (COMIRNATY) COVID-19 vaccine: A case report. Vaccines 10 https://doi.org/10.3390/vaccines10071065 (2022).
Liu, X. et al. Safety of inactivated COVID-19 vaccines in autoimmune encephalitis: A real-world cross-sectional survey. Mult Scler. Relat. Disord. 70, 104495. https://doi.org/10.1016/j.msard.2022.104495 (2023).
Camdessanché, J. P. et al. Brain immunohistopathological study in a patient with anti-NMDAR encephalitis. Eur. J. Neurol. 18, 929–931. https://doi.org/10.1111/j.1468-1331.2010.03180.x (2011).
Vasilevska, V. et al. Molecular mimicry of NMDA receptors May contribute to neuropsychiatric symptoms in severe COVID-19 cases. J. Neuroinflamm. 18, 245. https://doi.org/10.1186/s12974-021-02293-x (2021).
Steardo, L., Steardo Jr, L., Zorec, R. & Verkhratsky, A. Neuroinfection May contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. (Oxford England). 229, e13473 (2020).
Acknowledgements
The authors would like to thank the participants and their families.
Funding
This study was supported by the National Natural Science Foundation of China (82060236, 82460254) and the Natural Science Foundation of Guangxi Province (CN) (2023GXNSFAA026247).
Author information
Authors and Affiliations
Contributions
WH and XTQ conceived the study. XTQ, YW, MLL, YLC and ZQL drafted the manuscript. XTQ, ALJ, DLS, YZH, CL, YSW and SYZ performed the literature search and collected the data. XTQ ALJ, MLL analyzed and visualized the data. XTQ, YW and WH helped with the final revision of this manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and informed consent
This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (2024-E233-01). We had complied with the Declaration of Helsinki Ethical Principles for medical research involving human subjects. Written informed consents was obtained from all the patients enrolled in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Que, X., Wu, Y., Liang, M. et al. The characteristic change of autoimmune encephalitis after the COVID-19 epidemic in Guangxi, China. Sci Rep 15, 26400 (2025). https://doi.org/10.1038/s41598-025-10750-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10750-3