Abstract
The use of CO₂ or electrical stunning to render pigs unconscious and insensible before exsanguination is a common practice. Although both methods are widely implemented, they differ fundamentally in their underlying mechanisms, and studies have produced conflicting results regarding their influence on meat quality. In the case of CO₂ stunning, impaired pulmonary gas exchange causes a rapid onset of systemic hypoxia, accompanied by hypercapnia, which in turn lowers blood pH and triggers additional physiological stress responses. Electrical stunning, by contrast, induces a generalized epileptic seizure by depolarizing neuronal cell membranes, markedly increasing oxygen consumption in both the brain and peripheral muscles. Coupled with apnea-induced cessation of respiration, this leads to rapid systemic hypoxia and associated metabolic disturbances. With this study, we aim to generate comprehensive metabolic data as a foundation for deeper understanding of the animal’s physiological responses depending on the stunning method used. We identified differences in metabolite pathways associated with the stunning method and evaluated potential influence on early post mortem processes relevant to meat quality development. After CO2 stunning, there was a marked increase in purine degradation into inosine (p < 0.0001) and hypoxanthine (p < 0.0001), along with increased levels of C4 intermediates (succinate, fumarate and malate, all p < 0.0001) in the tricarboxylic acid (TCA) cycle. In contrast, electrical stunning showed a higher rate of glycolysis, as indicated by reduced levels of C6 sugars (e.g. glucose p < 0.0001), and elevated levels of TCA cycle entry metabolites such as citrate (p = 0.0053) and aconitate (p = 0.0009). Our findings suggest that purinergic signaling acts as a rapid emergency response mechanism during gas stunning, reflected by pronounced purine catabolism. The distinct metabolite patterns likely result from different physiological stress responses, such as CO2-induced acidosis and variable oxygen availability. In addition, differences in cellular redox balance (NAD⁺/NADH) between stunning methods may further modulate glycolytic flux and TCA cycle activity. These divergent metabolic states at the time of death may, in turn, influence subsequent post mortem biochemical processes and ultimately influence meat quality development.
Similar content being viewed by others
Introduction
The stunning process at slaughter is intended to induce unconsciousness and insensibility prior to exsanguination. In commercial European slaughterhouses, pigs are typically stunned either electrically or by exposure to a high CO2 concentration. During electrical stunning, a defined current (minimum of 1.3 A for at least 4 s) spans the brain1, causing strong depolarization of nervous cell membranes. This results in excessive, uncoordinated activity resembling an epileptic seizure2,3. Additional cardiac perfusion induces irreversible ventricular fibrillation. The excessive increase in activity during these epileptic-like seizures leads to rapid shifts in energy and oxygen demand. Combined with respiratory arrest and tachycardia, a hypoxic environment is established throughout the body. As aerobic metabolism decreases under these conditions, glycolysis is accelerated, leading to increased lactic acid production. In addition to the rapid metabolic changes, blood pressure increases, which can cause rupture of blood vessels and hemorrhages. Nass et al.4 reported similar phenomena in epileptic patients, noting increased lactic acid, creatinine and ammonia levels during tonic–clonic seizures.
In contrast, gas stunning with CO₂ relies on the narcotic and respiratory-stimulating effects of carbon dioxide. Typically, a concentration of > 80% in a deep pit is used. Following absorption into the bloodstream, CO₂ crosses the blood–brain barrier and diffuses into the cerebrospinal fluid, leading to cerebral acidosis. This altered brain environment impairs central nervous system function and subsequent loss of consciousness. Notably, blood pH decreases significantly from 7.3–7.5 to 6.6–6.9 during CO₂ stunning, as reported by Martoft6 and Hognestad5. Additionally, Martoft et al.6 also observed an intracellular pH decrease in the brain to pH 6.7 within 60 s exposure to 90% CO2. In comparison, electrical stunning causes a less pronounced pH reduction, with Becerril-Herrera7 documenting a decrease to pH 7.14. The duration of exposure in the CO₂ pit ranges between 100 and 180 s, which is longer than the process of electrical stunning. During the initial 10–40 s, animals often exhibit aversive reactions such as increased activity and vocalizations, indicating a period of distress8,9.
While CO₂ stunning enables group handling of pigs and is associated with advantages in terms of throughput and operator safety, it remains controversial due to persistent animal welfare concerns. In contrast, electrical stunning is considered to induce unconsciousness more rapidly, but requires individual animal restraint and precise electrode placement, which can itself be stressful for the animals. Animal welfare during stunning is a major focus of current research and public debate. However, in addition to minimizing animal suffering, stunning methods must also preserve meat quality. It is well established that stress experienced by pigs shortly before or during slaughter can negatively impact meat quality10,11,12,13. This is closely linked to disruptions in energy metabolism, as a balanced energy status is a prerequisite for optimal meat characteristics such as pH, water-holding capacity, and color. Acute stress leads to imbalances in energy metabolism, which in turn can compromise the conversion of muscle to meat. Therefore, understanding how different stunning methods influence metabolic pathways—particularly those associated with energy metabolism—is essential. Such knowledge can contribute to the development of slaughter practices that are both ethically sound and capable of maintaining high product quality. This study was based on the hypothesis that CO₂ and electrical stunning induce distinct metabolic responses in pigs, resulting in divergent metabolite profiles, particularly in pathways linked to energy metabolism and meat quality. To elucidate these fundamental metabolic differences and connect them to relevant biochemical pathways, a nontargeted metabolite profiling approach was applied, complemented by targeted quantification of purines and TCA cycle metabolites in sticking blood.
Methods
The study aimed at the investigation of metabolic pathway differences upon two stunning techniques in pigs—CO2 and electrical stunning. Therefore, metabolites were analysed by nontargeted two-dimensional gas chromatography coupled mass spectrometry (GCxGC-qMS) profiling, and quantification of purines and tricarboxylic acid cycle (TCA) metabolites.
Animals and sample acquisition
Blood samples were randomly collected at 1 day from 35 finishing pigs (gilts and surgically castrated males at a ratio 1:1, slaughter weight 110–120 kg) at a commercial slaughterhouse. No ethical approval was required for this study because the blood samples were collected during the routine slaughter process at a commercial slaughterhouse. All animals were stunned using standard procedures (either electrical or CO₂ stunning) prior to bleeding, in accordance with applicable animal welfare regulations. The sampling did not involve any additional handling or interventions beyond the conventional slaughter process. The animals originated from a single herd, ensuring uniformity in feeding and age. Prior to exsanguination, the pigs were alternately subjected to either electrical stunning or carbon dioxide (CO₂) stunning, in accordance with standard commercial slaughter practices. For CO2 stunning, a concentration of 95% CO2 in a dip-lift system was used at a time interval of 100 s. The time between CO2 stunning and exsanguination was ca. 20–30 s so that all the observed metabolic shifts were induced within 120 s. Electrical stunning was carried out using a hand-held stunning device (Haas, Neuler, Germany) by head stunning for 6 s followed by cardiac arrest stunning for 4 s, each at 230 V and 1.6 Ampere. The time interval between stunning and bleeding was again about 20–30 s, resulting in an overall time from the start of stunning to bleeding of approximately 50 s. Blood samples were collected during exsanguination, immediately frozen in liquid nitrogen, and stored at − 80 °C until further use.
Metabolite profiling
Chemicals
All the chemicals used were commercially obtained via Merck (Darmstadt, Germany), Altmann Analytics (Munich, Germany) or VWR (Darmstadt, Germany) and are listed in Supplementary Table S1.
GCxGC-qMS metabolite analyses
Sample preparation for GCxGC-qMS metabolite profiling was performed according to Wagner et al.14. In brief, frozen blood samples were lyophilized and homogenized to a fine powder. A total of 20 mg of the powder was extracted with 600 µl of ice-cold methanol:water (8:2, v/v) containing an internal standard mixture (Supplementary Table S1) using a bead mill homogenizer (Bead Ruptor 24 Elite, Omni International, USA). After centrifugation, the pellet was re-extracted with 600 µl of ice-cold methanol:chloroform (2:1 v/v) following the same procedure as the first extraction. The combined supernatants were transferred into 2 ml glass vials containing 200 µl glass inserts, then dried in a vacuum centrifuge (Christ Speedvac RVC 2–18 CD plus, Germany). The dried extracts were stored under a protective argon atmosphere at − 80 °C until analysis. Quality control (QC) and blank samples were included in the analysis alongside the biological samples. The QC samples were prepared by pooling aliquots from multiple blood samples. The blank samples contained no biological matrix. Prior to GC–MS measurement, the samples were derivatized. First, methoximation was carried out using a 20 mg/ml solution of methoxyamine hydrochloride (MAH) in pyridine incubated at 50 °C for 1 h with shaking. In the second step, 70 µl of N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) containing 1% trimethylchlorosilane (TMCS) was added, and the samples were shaken at 70 °C for 1 h. The measurements were carried out using a Shimdazu GCMS QP2020 instrument (Shimadzu, Duisburg, Germany). Instrumentation details and parameter setting are provided in Supplementary Table S2. Full-scan data were acquired in a mass range of 60–550 m/z. For nontargeted GCxGX-qMS measurements, total ion chromatogram (TIC) integration was performed using the GCMS Postrun Analysis Module within the instrument software GCMSsolution (Version 4.45, Shimadzu, Duisburg, Germany). Data processing steps included peak filtering, peak alignment, signal intensity drift correction and quality assessment, as described by Egert et al.15 and Weinert et al.16. Missing values (corresponding to signals that were not detected) were imputed with random values between half the limit of detection (LOD) and LOD. The LOD was estimated as the smallest detected peak area across the entire data set. Compound annotation was performed using the NIST 14 library database, as well as an in-house created library, both of which were implemented in the GCMS Postrun Analysis Module within the instrument software GCMSsolution. Visualization of two-dimensional (2D) chromatograms was done using the GC Image Software (Version 2.7, GC Image, Lincoln, Nebraska). A series of n-alkanes (C7-C30) was used as a retention time standard.
For the analysis of citrate cycle metabolites, the first extraction step was identical to that of nontargeted metabolite extraction. Re-extraction was performed with methanol:water (8:2, v/v). The combined extracts were then divided into two portions: one for direct dry in a measurement vial using a vacuum centrifuge, and the other for a reduction step using borodeuteride. For the reduction, 500 µl of the extract were mixed with 5 µl of 2 N NaOH and 25 µl of freshly prepared 10 mg/ml NaBD4 in 50 mM NaOH. The mixture was incubated for 1 h at room temperature with shaking. After incubation, the pH was adjusted to pH 4 with a 3.5 N HCl solution. A 100 µl aliquot was then transferred into a measurement vial and dried in a vacuum centrifuge. A standard curve was prepared in the same manner, incorporating 5 mg of blood matrix to account for matrix effects during MS measurement. The standards and concentrations used are listed in Supplementary Table S1. Prior to GC‒MS measurements, the samples were derivatized, at first using 30 µl of a MAH solution for 1 h at 50 °C, and subsequently with 50 µl of MTBSTFA/TBDMCS for 1 h at 70 °C. The protocol and corresponding calculations were adapted from Mamer et al.17. The peak areas for quantifying and qualifying ions were integrated via the implemented Postrun Analysis Module within the instrument software GCMSsolution (Version 4.45, Shimadzu, Duisburg, Germany).
Purine quantification by LC-ESI-QToF MS
The blood powder was extracted in two steps using a methanol:water (8:2, v/v) solution, with a final sample-to-volume ratio of 1:8 (w/v). To clean up the raw extract, solid-phase extraction (SPE) was performed using a Waters HLB Prime cartridge (1 cc, 30 mg; Waters, Eschborn, Germany). The flow-through was used for LC-ESI‒QToF MS measurements. For quantification, a calibration curve was prepared and the calibrants were spiked with a quality control sample extract to account for matrix effects. Standards and concentrations used are listed in Supplementary Table S1, and instrumentation details and parameter settings are provided in Supplementary Table S2. Peak areas of the corresponding ions were integrated using the Data Analysis software (V 4.2, Waters, Eschborn, Germany). The ion extraction accuracy was set to ± 0.005 m/z. Additional information on compound integration can be found in Supplementary Table S1.
Analysis of heat shock protein (HSP) gene expression
Total RNA was isolated from 400 µl EDTA stabilized blood using 1200 µl of Lysis Blood buffer (Roboklon, Germany) following the manufacturer’s instructions. 5 µl of each sample were converted into cDNA using the NG dART RT KIT (Roboklon, Germany) according to the manufacturer’s instructions, and stored at 4 °C until further use. The qPCR was performed on a Bio-Rad qPCR Cycler CFX 96 (Biorad, Germany). For each reaction, 10 µl cDNA was mixed with 2 × SG qPCR Master Mix (Roboklon, Germany), primers (10 pmol/µl of downstream and upstream primer, respectively), and RNA free water to a final volume of 25 µl. The thermal profile was set according to the manufacturer’s guidelines: an initial enzyme activation step at 95 °C for 10 min, followed by 41 cycles of denaturation at 95 °C for 15 s, annealing at 59 °C for 30 s, and elongation at 72 °C for 30 s. A final inactivation step was included at 95 °C for 15 s. Each qPCR run included a negative control (without DNA) alongside the experimental samples. The primers used are described in Zimmermann and Brüggemann18. Delta Ct -values were calculated by subtracting the animal-specific Ct values of the housekeeping genes (GAPDH, ß-Actin) from the Ct values of the heat shock proteins. These delta Ct values were used for all further analyses.
Statistical data analysis
Multivariate and univariate statistical analyses were using the JMP software (17.1.0, SAS Institute Inc., Cary/NC, USA). We applied principal component analysis (PCA) for an overall observation of variations among sample groups. For direct comparison of individual signals, we first conducted a distribution analysis by applying the Anderson Darling test, followed by ANOVA and Wilcoxon tests. The Volcano Plot was generated using R (4.5.0., The R-Project for Statistical Computing), highlighting metabolites with significant fold changes and adjusted p-values (Welch test).
Results
Blood metabolite profiles differ as a consequence of the stunning method
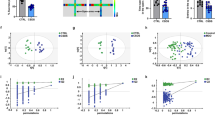
A comprehensive GCxGC-qMS approach was employed to investigate metabolite pathway differences resulting from the stunning method. A total of 513 molecular features were analyzed for statistical evaluation. The nontargeted principal component analysis (PCA), considering all 513 signals, revealed notable metabolic variation both among individual pigs and between stunning techniques (Fig. 1 A). Several variables contributed strongly to this separation, as reflected by their log₂ fold changes shown in Fig. 1B. Principal components 1 and 2 differed significantly between the two stunning methods (Fig. 1C), underscoring the distinct metabolic effects of the treatments.
Principal component analysis from nontargeted GCxGC-qMS metabolite profiling of sticking blood from 35 pigs undergoing different stunning methods. (A) PCA score plot (B) Volcano plot highlighting most significant molecular features (C) ANOVA of principle components 1 and 2 indicating a significant difference of metabolite profiles between CO2 and electrical stunning. Orange—electrical stunning and turquoise—CO2 stunning.
We applied univariate statistical tests to identify discriminating variables between both stunning techniques. The ANOVA/Wilcoxon tests (p ≤ 0.05) revealed 117 variables as significantly different (Supplementary Table S3). For 54 of the diversifying signals, the database search revealed annotations to particular metabolites (level 2) or metabolite groups (level 3) according to the Metabolomics Standards Initiative19. The results for the annotated and significant features presented in Table 1 indicate large differences in central metabolite pathways between the stunning techniques.
Differential energy demand and TCA cycle alterations in CO₂- versus electrically-stunned pigs
Under CO₂ stunning, the most notable findings were the substantial accumulation of ATP degradation products, including inosine and hypoxanthine, as well as uracil, a breakdown product of UDP-sugars (Fig. 2, Table 1). These results were accompanied by differences in the energy-producing pathways of glycolysis and the TCA cycle.
In the energy consuming part of glycolysis, up to the cleavage into glyceraldehyde-3-phosphate (GA3P), metabolite levels were elevated under CO2 conditions compared to electrical stunning. However, no significant differences were observed in the energy-generating part of glycolysis, nor in lactic acid levels, between the two stunning techniques. Upon entry into the TCA cycle, a shift between the two stunning methods was again observed. Citrate and aconitate concentrations were significantly lower under CO₂ stunning, while following decarboxylation from C6 to C4 acids, such as succinate, fumarate, and malate, the concentrations were higher under CO₂ conditions (Fig. 2).
Further analysis of purines by LC–MS was conducted to assess the concentrations of key energy substrates (Table 2). No significant difference in ATP levels was observed, but notable differences were found in other adenylates. ADP levels were slightly higher, while AMP and adenosine concentrations were significantly elevated in blood from electrically stunned pigs. In contrast, adenine levels were significantly elevated in blood samples from CO₂-stunned pigs, whereas no differences were detected for the degradation product IMP. However, further breakdown into inosine and hypoxanthine revealed substantial differences between the two stunning methods, with significantly greater accumulation observed following CO₂ stunning. These findings were consistent with the patterns of inosine and hypoxanthine detected in GCxGC-qMS profiling. From guanylate-derived purines, guanosine and guanine were detected. Guanosine was only detected in blood from CO2-stunned pigs, while guanine showed a slight difference between the two techniques. Overall, the total blood purine concentration was markedly increased following CO2 stunning.
Cellular and oxidative stress markers following CO₂ and electrical stunning
Notably, the ascorbate degradation product threonate was more abundant in blood from CO2-stunned pigs compared to electrically stunned pigs. Among the glutathione precursors, only cysteine showed a significant difference, with higher levels observed after electrical stunning (Table 1).
To draw direct conclusions about stress response induction under such acute physiological conditions, we analyzed the mRNA expression of heat shock proteins HSP27, HSP70, HSP90, and CRYAB. However, no differences in expression were detected between the two stunning methods.
Limited impact on proteinogenic amino acids
The profile of proteinogenic amino acids was generally only slightly affected by the stunning method. Significant differences were observed for alanine, serine, aspartate and glutamine, which showed slightly higher levels after CO2-stunning, whereas cysteine was more abundant in blood from electrically stunned pigs (Table 1). Cysteamine, an intermediate in taurine biosynthesis from cysteine and a degradation product of Coenzyme-A, was found at significantly higher levels under CO2 conditions. 2-Hydroxy-3-methylbutyric acid, a conversion product of branched-chain amino acids, slightly increased under hypoxia, as did aminoadipate, a degradation product of lysine. In contrast, aminomalonate, which can arise from perturbed serine or threonine metabolism, accumulated more after electrical stunning (Table 1).
Effects on lipid metabolism
Several blood lipids exhibited differences between the two stunning techniques. Among these, the monoacylglycerides 1-monopalmitin, 1-monolinolein and 2-oleoylglycerol were significantly more abundant following CO2 treatment (Table 1). Additionally, the phospholipid precursors O-phosphorylanolamine and ethanolamine displayed the same trend. In contrast, medium-chain fatty acids, specifically octanoic, nonanoic, and decanoic acids, as well as the long-chain fatty acid dodecanoic acid, were more abundant in blood after electrical stunning (Table 1).
Discussion
This study investigated how different stunning methods affect blood metabolite profiles, with the aim of identifying associated physiological pathways and their relevance to post mortem development. Both stunning methods cause significant physiological stress and place a heavy burden on energy metabolism due to the procedures involved in inducing unconsciousness prior to exsanguination. Electrical stunning triggers generalized seizures through neuronal depolarization, increasing muscular activity and oxygen demand. In contrast, exposure to high levels of CO₂ causes respiratory acidosis due to hypercapnia, accompanied by hypoxia. The present study investigated whether, given their different modes of action, different metabolic responses are elicited.
Distinct metabolic profiles induced by the stunning method
Indeed, the different metabolic responses of the pigs to the two stunning techniques were clearly reflected in their overall metabolite profiles (Fig. 1). Despite considerable individual variability—likely due to physiological and behavioral differences—a clear separation between the two groups was evident in the PCA of all detected metabolic features (Fig. 1), as well as in targeted analyses of purines and TCA cycle metabolites (Figs. 2 and 3). The pronounced effects of the stunning methods on blood metabolites indicate that specific interventions can significantly alter metabolic patterns within seconds.
The stunning technique has a decisive influence on the processes of energy metabolism
Several aspects of central metabolism were differentially impacted during the brief period of stunning and subsequent bleeding. Most notably, the stunning techniques resulted in pronounced differences of energy metabolites. Stunning imposes an enormous burden for energy metabolism per se. The significant differences in inosine and hypoxanthine clearly indicated a strong impact of CO2 stunning on adenosine phosphate degradation and regeneration (Fig. 3). This can be attributed to the sudden drop in oxygen and pH as well as the rise in pCO2 under gas stunning, which disrupts ATP production via the mitochondrial electron transport chain (ETC)20,21,22,23,24. As ATP turnover far exceeds its cellular concentration, supply and demand must be tightly balanced25. Thus, stabilization of the ATP supply for energy-consuming processes is existential, and different metabolic strategies exist to overcome acute ATP shortages: phosphocreatine utilization (phosphagen system), anaerobic glycolysis, interconversion of purine phosphates and substrate-level phosphorylation. Among these, the phosphagen system provides the most rapidly accessible energy source. The critical role of phosphocreatine in cell survival under anoxic or severely hypoxic conditions has been highlighted in several studies26,27. In this direction, Braasch28 firstly reported significant depletion of PCr and ATP within just 30 s of coronary occlusion in the ischemic area. Similarly, Norberg29 observed a marked decline in PCr levels in the cerebral cortex within 10–20 s of hypoxia. Our study suggests a similar scenario in the blood of CO2-stunned pigs where elevated creatinine levels indicated a quick shift from ATP production via the ETC to phosphocreatine utilization (Fig. 2). This is also consistent with Wilken26, who reported an almost complete depletion of PCr and a 54% reduction in ATP levels after 30 min of anoxia in mouse pups. Similarly, Tsuji et al.30 documented a significant drop in PCr in the brain within just 3–4 min of exposure to only 4% oxygen. Although the cited studies reflect only hypoxic or ischemic conditions and not the specific effects of hypercapnia and acidosis, the rapid depletion of PCr seems to represent a shared metabolic response to impaired oxidative phosphorylation.
ATP can also be regenerated via anaerobic glycolysis, which is strongly upregulated under oxygen-limited conditions. This is reflected in rapid glycogen depletion during ischemia31 and increased lactic acid production during intense physical activity32 or epileptic seizures4,33,34. However, the metabolite levels associated with anaerobic glycolysis (e.g., pyruvate, lactic acid) did not differ between the two stunning techniques. This is likely due to the fact that both mechanisms independently promote lactate formation.
CO₂ stunning triggers elevated purine catabolites compared to electrical stunning
While immediate energy demands are met by the phosphagen system, additional energy supply can be facilitated through increased activity of the adenylate kinase (AK), which catalyzes the reaction 2ADP—> ATP + AMP. AMP is subsequently deaminated to IMP to further favor the AK reaction, and IMP might in a following step be degraded to inosine and hypoxanthine35. Under hypoxic conditions, either caused by ischemia or elevated muscle activity, increased AK activity along with higher AMP levels has been observed, and related to cellular metabolic adaptations36,37,38. The lower AMP amounts observed under CO₂ stunning conditions might be explained by its rapid degradation, as indicated by the substantial accumulation of inosine and hypoxanthine in comparison to electrical stunning. Such rapid accumulation of purine degradation products in blood serum has been reported under hypoxic conditions following various clinical syndromes31,39,40 and suggested as an indicator for ATP depletion41. As a consequence, hypoxanthine and inosine have been proposed as biomarkers for cardiac ischemia42.
However, most medical studies have focused on time frames spanning several minutes–hours. In contrast, our findings suggest a very rapid accumulation of inosine and hypoxanthine upon extreme conditions—hypercapnia and severe hypoxia occurring within just a few seconds. An interesting assumption was presented in a study by Miller et al.43, who analyzed the metabolic responses of rat dentate granule neurons during acute electrical stimulation: An increase in exogeneous inosine has been linked to fuel glycolysis via the nonoxidative pentose phosphate pathway (PPP) by using the ribose from inosine as a substrate for generating G6P. While this hypothesis is not directly associated with CO2 stunning, the elevated levels of inosine, hypoxanthine and hexose phosphate (Fig. 3) observed in our study could also suggest a similar mechanism for nonoxidative energy generation. In addition, increased purine metabolites levels have been observed in mouse blood following seizures, and were correlated with seizure severity and brain damage44. In our findings, the total purine concentration after CO2 stunning exceeded the level after electrical stunning (Table 2), potentially indicating a more severe reaction following anaerobia and acidosis.
In addition to their role as energy equivalents, extracellular ATP and its intermediates are important signaling molecules within the purinergic signaling cascade45. A range of selective receptors have been identified that mediate tissue-specific adaptive responses to maintain purine-dependent homeostasis46,47,48,49. In response to acute hypoxia, ATP is rapidly exported and inactivated to adenosine in the vasculature, which is further inactivated through deamination to inosine and hypoxanthine. It is therefore tempting to speculate that the substantial differences in inosine and hypoxanthine levels detected herein—markedly higher after CO2 stunning—may be a consequence of both extracellular signaling, where adenosine is degraded, and energy production and sensing through the orchestrated activities of AK, AMPD and AMPK, and further IMP degradation35,36,50. The large difference in total purine concentration in blood suggests that, responses upon CO2 stunning conditions occur much faster than under electrical stunning. The rapid excretion of purines into the bloodstream may serve as a fast alarm signal for energy imbalance and adaption.
Impaired glycolytic flux during CO₂ stunning
The rate of glycolysis is strongly influenced by pH changes, primarily due to the sensitivity of phosphofructokinase (PFK), a major regulatory enzyme, to acidification. Under CO₂ stunning, the associated rapid drop in pH likely impairs key enzymatic steps, with inhibition of PFK contributing to the accumulation of upstream glycolytic intermediates. Foldbergrova51 demonstrated in rat brain tissue that rising CO₂ concentrations (6–40%) led to increased glucose and glucose-6-phosphate levels, consistent with impaired glycolytic flux due to intracellular acidification. LaMonte et al.52 similarly emphasized the high sensitivity of glucose metabolism to pH changes. Spriet et al.53 demonstrated in vitro that acidosis significantly reduces PFK activity during short-term muscle contractions, highlighting the direct inhibitory effect of low pH on glycolysis. However, they caution that in vivo conditions are more complex, and the extrapolation of in vitro findings to intact muscle metabolism requires careful consideration of additional regulatory factors. Accordingly, the elevated glucose and hexose phosphate levels observed in CO₂-stunned pigs likely reflect this complex, pH-dependent inhibition.
Compared to CO₂ stunning, electrical stunning showed a greater consumption of C6 sugars. This difference abruptly stopped between the oxidation and reduction reactions from glycerinealdehyde-3-phsohate (GA3P) to 3-phosphoglycerate (3PG), suggesting a metabolic bottleneck at this NAD⁺-dependent step. Apart from red blood cells, the abrupt disruption of the mitochondrial ETC during CO₂ stunning prevents NAD⁺ regeneration, reducing the NAD⁺/NADH + H⁺ ratio and potentially explaining the observed GA3P accumulation. Elevated hexose phosphate levels may also result from metabolic tailback or feedback inhibition. Additionally, the higher glucose levels in CO₂-stunned pigs could also result from imbalances in adenosine phosphate pools, as two ATP molecules are consumed in the initial steps of glycolysis. Limited ATP availability may therefore further impair glucose phosphorylation and utilization.
Disrupted TCA cycle dynamics and C4 acid accumulation under CO₂ stunning
In the initial steps of the TCA cycle, electrically stunned pigs showed higher levels of citrate and aconitate, while similar amounts of 2-ketoglutarate were detected in both groups. In contrast, after the second decarboxylation step, CO2-stunned pigs exhibited a significantly higher abundance of C4 organic acids. This pattern suggests a reduction in TCA cycle flux under CO2 conditions, consistent with known adaptive responses to hypoxia54,55. Norberg56, by contrast, reported a time-dependent increase in the TCA metabolite pool over 1–30 min at moderately reduced oxygen levels (6–8%). However, most of these studies focus on slower responses to hypoxia and do not reflect the acute and extreme conditions during stunning—particularly the combined effects of hypercapnia and a rapid pH drop. The precise metabolic consequences of this acidification remain poorly understood.
The elevated C4 acids may result from disrupted NADH and FADH₂ oxidation due to rapid electron transport chain (ETC) failure. Limited NAD⁺ availability likely impairs mitochondrial malate dehydrogenase, leading to malate accumulation and potentially driving the reverse conversion of oxaloacetate to malate57, thereby supporting NAD⁺ regeneration. This enables continued function of the α-ketoglutarate dehydrogenase complex (KGDHC), which drives substrate-level phosphorylation via the conversion of succinyl-CoA to succinate, coupled with GDP to GTP conversion and ATP generation. This bypass pathway has been reported to be upregulated under hypoxic conditions57,58,59 and is regulated by ADP, ATP, pH, and Ca2⁺59,60. Reduced oxaloacetate availability for condensation with acetyl-CoA may contribute to the observed decreases in citrate and aconitate levels61. Additionally, ETC dysfunction may impair the malate-aspartate shuttle, which requires NAD+ for the oxidation of malate to oxaloacetate in the mitochondrial matrix. As NAD⁺ becomes limiting, malate may accumulate further. The observed increase in fumarate levels could reflect a backflux reaction, shifting the succinate-malate equilibrium in response to rising malate concentrations.
Amino acids related to the TCA cycle and oxidative stress regulation
The central amino acids glutamine (Gln), aspartic acid (Asp) and alanine (Ala) were slightly more abundant in the blood of CO2-stunned pigs. The observed higher levels of Ala and Asp might be due to their role as products of transaminase reactions yielding ɑ-ketoglutarate (ɑKG) for substrate-level phosphorylation provision. This assumption is supported by data from hypoxia studies56,57,62. Levels of Gln were higher in CO2-stunned pigs than in electrically stunned pigs, indicating that CO2-induced hypoxia and hypercapnia during pig stunning may trigger additional reactions —likely pH-dependent, compared to the hypoxic conditions applied during medical studies. In this context, LaMonte52 detected a stronger glutamate labeling under acidosis in their 13C glucose tracer flux measurements.
Amino acids cysteine, cysteamine, serine and aminomalonate were also differentially affected. Aminomalonate, which is involved in calcium-binding activity of proteins and has been identified as a toxicity marker in previous studies63, was more accumulated after electrical stunning compared to CO2 stunning. The origin of aminomalonate is not fully understood, and Copley64 suggested that it might arise as a constituent of proteins originating either from misincorporation during protein synthesis or as a result of posttranslational modification from another amino acid. It has also been hypothesized that it might be generated by the oxidation of serine via enzymatic or radical-mediated processes.
Electric shock and acidosis have been related to the generation of ROS52,65. A greater turnover of ROS-scavenging compounds can be suspected in electrically stunned pigs. Cysteine accumulation in these animals may result from glutathione (GSH) degradation by membrane-bound gamma-glutamyl transpeptidase (GGT) and dipeptidases66,67. In addition, GSH de novo synthesis has been found to be reduced under acidosis52. Further evidence of elevated ROS levels in electrically stunned pigs comes from higher threonate levels—a degradation product of ascorbate and dehydro-l-ascorbic acid (DHA)68. These findings collectively suggest that electrical stunning induces slightly higher oxidative stress compared to CO2 stunning.
On the other hand, cysteamine, a product of the constitutive degradation of coenzyme A, also functions as an antioxidant. However, due to its high reactivity and broader role in sulfur metabolism, including taurine production, the higher levels observed after CO2 stunning cannot be definitively linked to counteracting oxidative stress.
Fatty acid chain length differences
The higher abundance of medium-chain fatty acids observed after electrical stunning is in accordance with the finding that fatty acid oxidation depends on O2 availability. Achten et al.69 reported a strong correlation between the maximal rate of fat oxidation and VO2 max. Comparing the acute anoxia during CO2 stunning with the presumably less severe O2 shortage during electrical stunning it can be concluded that ß-oxidation of fatty acids is utilized to fill the acetyl-CoA pool and fuel the TCA cycle. This interpretation aligns with the higher citrate levels observed after electrical stunning. At the moment of death, these ongoing oxidation processes were interrupted by the bleeding of the animals. Furthermore, lower amounts of MAGs in electrically stunned pigs may also reflect enhanced fat hydrolysis, which supplies free fatty acids for oxidation under these conditions.
Associations to early post mortem processes
Both stunning techniques resulted in distinct metabolic conditions at the time of death and are therefore likely to influence post mortem biochemical processes. Previous studies on carcass and meat quality have indicated better carcass characteristics of CO2-stunned pigs70,71. However, regarding meat quality, particularly color, water-holding capacity, and tenderness, the literature presents inconsistent findings10,71,72,73. Given that glycolysis and ATP degradation play central roles in early post mortem muscle metabolism, the observed differences in energy-related metabolites in this study are likely to affect resulting meat quality. Interestingly, the faster degradation of purines observed after CO₂ stunning could be interpreted as a sign of greater metabolic stress, suggesting a potential advantage for electrical stunning. Conversely, the higher levels of glycolytic intermediates detected under CO₂ conditions may support a more favorable energy status, which in turn could benefit meat quality. This apparent contradiction mirrors the divergent findings in the literature and underscores the need for a comprehensive evaluation of all slaughter-related factors. As meat quality depends also on pre-slaughter stress, a holistic approach that accounts for the entire pre- and perimortem context is essential to reliably assess the impact of stunning methods on meat quality.
Conclusions
Metabolite profiling has proven to be a valuable tool for assessing physiological responses associated with different stunning methods. In this study, both CO₂ and electrical stunning resulted in profound metabolic alterations in blood at death, albeit via distinct biochemical pathways. CO₂ stunning led to strong purine degradation, with significantly increased inosine and hypoxanthine levels (both p < 0.0001), and accumulation of TCA cycle intermediates (succinate, fumarate, malate; all p < 0.0001), suggesting mitochondrial dysfunction and purinergic emergency signaling. In contrast, electrical stunning showed relatively higher levels of glycolytic turnover, reflected by reduced levels of glucose (p < 0.0001) and elevated TCA cycle entry metabolites including citrate (p = 0.0053) and aconitate (p = 0.0009). These differences likely reflect distinct physiological stress responses: CO2-induced acidosis may impair glycolysis, while electrical stunning causes less severe hypoxia. Furthermore, differences in NAD⁺/NADH + H⁺ redox balances between stunning methods may affect both glycolysis and TCA cycle activity, contributing to the observed metabolite patterns.
Given the substantial impact of energy metabolism on meat quality development and its dependence on the stunning method, correlating blood metabolite profiles directly with meat quality appears promising. However, it is well known that preslaughter processes also significantly affect meat quality. Therefore, future studies should integrate not only post mortem analyses but also data from preslaughter handling and prestunning blood profiles. Since gas stunning remains the most widely used method in high-throughput slaughterhouses, further studies should investigate how variations in gas composition and pre-slaughter animal handling affect both biochemical processes and stress responses.
Data availability
All data generated and analysed during this study are included in this published article and its supplementary information files. The raw datasets (in the vendors file format) are available from the corresponding author on reasonable request.
Abbreviations
- AMP:
-
Adenosine 5′-monophosphate
- ADP:
-
Adenosine-5′-diphsphate
- ATP:
-
Adenosine-5′-triphsphate
- GMP:
-
Guanosine-5-monophosphate
- CO2 :
-
Carbon dioxide
- TCA:
-
Tricarboxylic acid cycle
- ROS:
-
Reactive oxygen species
- HIF:
-
Hypoxia-inducible factor
- GC:
-
Gas chromatograph/gas chromatography
- qMS:
-
Quadrupol mass spectrometer
- QC:
-
Quality check
- MSTFA:
-
N-methyl-N-(trimethylsilyl)trifluoroacetamide
- TMCS:
-
Trimethylchlorosilane
- MAH:
-
O-methoxylamine hydrochloride
- NaOH:
-
Natrium hydroxide
- MTBSTFA:
-
N-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide
- TBDMCS:
-
Tert-butyl dimethyl chlorsilan
- NaBD4:
-
Natrium borodeuteride
- HCl:
-
Hydrogen chloride
- LC:
-
Liquid chromatography
- ESI:
-
Electrospray ionization
- QToF:
-
Quadrupol time of flight mass analyzer
- MS:
-
Mass spectrometer/mass spectrometry
- PCA:
-
Principal component analysis
- OPLS-DA:
-
Orthogonal projection to latent structures discriminant analysis
- ANOVA:
-
Analysis of variance
- VIP:
-
Variable importance projection
- UDP:
-
Uridine diphosphate
- GA3P:
-
Glyceraldehyde 3-phosphate
- C4, C6:
-
Four and six carbon bodies
- mRNA:
-
Messenger ribonucleic acid
- HSP:
-
Heat shock protein
- CRYAB:
-
Crystallin alpha B
- ETC:
-
Electron transport chain
- PCr:
-
Phosphocreatin/creatine phosphate
- O2:
-
Oxygen
- NAD+:
-
Nicotinamide adenine dinucleotide (oxidized form)
- AK:
-
Adenylate kinase
- AMPK:
-
Adenosine monophosphate activated protein kinase
- PPP:
-
Pentose phosphate pathway
- AMPD:
-
Adenosine monophosphate deaminase
- 3PG:
-
3-Phosphoglycerate
- NADH:
-
Nicotinamide adenine dinucleotide (reduced form)
- OAA:
-
Oxalo acetic acid
- FADH2:
-
Flavin adenine dinukleotide (reduced form)
- FAD :
-
Flavin adenine dinukleotide (oxidized form)
- KGDHC:
-
Alpha-ketoglutarate dehydrogenase complex
- Ca:
-
Calcium
- GGT:
-
Gamma-glutamyl transpeptidase
- GSH:
-
Glutathione
- DHA:
-
Dehydro-l-ascorbic acid
- MAG:
-
Monoacylglycerol
References
Verordnung zum Schutz von Tieren im Zusammenhang mit der Schlachtung oder Tötung und zur Durchführung der Verordnung (EG) Nr. 1099/2009 des Rates (Tierschutz-Schlachtverordnung - TierSchlV); 20.12.2012 (BGBl. 2012 I 2982), BGBl. 2012 I 2982 (2012).
Hoenderken, R. Electrical Stunning of Slaughter Pigs (University of Utrecht, 1978).
Maennl, M. Betaeubung und Entblutung von Schwein und Rind. Schlachten von Schwein und Rind. 13. Kulmbach: Bundesanstalt fuer Fleischforschung 62–83 (1994).
Nass, R. D., Zur, B., Elger, C. E., Holdenrieder, S. & Surges, R. Acute metabolic effects of tonic-clonic seizures. Epilepsia Open 4(4), 599–608 (2019).
Hognestad, B. W., Digranes, N., Opsund, V. G., Espenes, A. & Haga, H. A. CO(2) stunning in pigs: Physiological deviations at onset of excitatory behaviour. Animals (Basel) 13(14), 2387 (2023).
Martoft, L., Stødkilde-Jørgensen, H., Forslid, A., Pedersen, H. D. & Jørgensen, P. F. CO2 induced acute respiratory acidosis and brain tissue intracellular pH: A 31P NMR study in swine. Lab. Anim. 37(3), 241–248 (2003).
Becerril-Herrera, M. et al. CO(2) stunning may compromise swine welfare compared with electrical stunning. Meat Sci. 81(1), 233–237 (2009).
Raj, A. B. M. & Gregory, N. G. Welfare implications of the gas stunning of pigs 1. Determination of aversion to the initial inhalation of carbon dioxide or argon. Anim. Welf. 4(4), 273–280 (1995).
Rodríguez, P. et al. Assessment of unconsciousness during carbon dioxide stunning in pigs. Anim. Welf. 17(4), 341–349 (2008).
Channon, H. A., Payne, A. M. & Warner, R. D. Halothane genotype, pre-slaughter handling and stunning method all influence pork quality. Meat Sci. 56(3), 291–299 (2000).
D’Souza, D. N., Dunshea, F. R., Warner, R. D. & Leury, B. J. The effect of handling pre-slaughter and carcass processing rate post-slaughter on pork quality. Meat Sci. 50(4), 429–437 (1998).
Gade, P. B. & Christensen, L. Effect of different stocking densities during transport on welfare and meat quality in Danish slaughter pigs. Meat Sci. 48(3), 237–247 (1998).
van der Wal, P. G., Engel, B. & Reimert, H. G. M. The effect of stress, applied immediately before stunning, on pork quality. Meat Sci. 53(2), 101–106 (1999).
Wagner, L. et al. Comparison of targeted (HPLC) and nontargeted (GC-MS and NMR) approaches for the detection of undeclared addition of protein hydrolysates in Turkey breast muscle. Foods 9(8), 1084 (2020).
Egert, B., Weinert, C. H. & Kulling, S. E. A peaklet-based generic strategy for the untargeted analysis of comprehensive two-dimensional gas chromatography mass spectrometry data sets. J. Chromatogr. A 1405, 168–177 (2015).
Weinert, C. H., Egert, B. & Kulling, S. E. On the applicability of comprehensive two-dimensional gas chromatography combined with a fast-scanning quadrupole mass spectrometer for untargeted large-scale metabolomics. J. Chromatogr. A 1405, 156–167 (2015).
Mamer, O. et al. The complete targeted profile of the organic acid intermediates of the citric acid cycle using a single stable isotope dilution analysis, sodium borodeuteride reduction and selected ion monitoring GC/MS. Metabolomics 9(5), 1019–1030 (2013).
Zimmermann, S., Brüggemann, D. A. Science tICoM, Technology, Potsdam/Berlin G, .08.04-09. Variation in the expression of different heat shock proteins mRNA in EDTA stabilized blood of pigs exposed to different stunning methods. in ICoMST 2019: Meat for Diversifying Markets—65th International Congress of Meat Science and Technology: Book of Abstracts. International Congress of Meat Science and Technology 107–8 (2019).
Sumner, L. W. et al. Proposed minimum reporting standards for chemical analysis chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 3(3), 211–221 (2007).
Fergie, N. et al. Hypercapnic acidosis induces mitochondrial dysfunction and impairs the ability of mesenchymal stem cells to promote distal lung epithelial repair. Faseb. J. 33(4), 5585–5598 (2019).
Greenbaum, N. L. & Wilson, D. F. Role of intramitochondrial pH in the energetics and regulation of mitochondrial oxidative phosphorylation. Biochim. Biophys. Acta 1058(2), 113–120 (1991).
Jubrias, S. A., Crowther, G. J., Shankland, E. G., Gronka, R. K. & Conley, K. E. Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J. Physiol. 553(Pt 2), 589–599 (2003).
Milliken, A. S., Kulkarni, C. A. & Brookes, P. S. Acid enhancement of ROS generation by complex-I reverse electron transport is balanced by acid inhibition of complex-II: Relevance for tissue reperfusion injury. Redox Biol. 37, 101733 (2020).
Wilson, D. F., Harrison, D. K. & Vinogradov, S. A. Oxygen, pH, and mitochondrial oxidative phosphorylation. J. Appl. Physiol. (1985) 113(12), 1838–1845 (2012).
Hochachka, P. W. & McClelland, G. B. Cellular metabolic homeostasis during large-scale change in Atp turnover rates in muscles. J. Exp. Biol. 200(2), 381–386 (1997).
Wilken, B., Ramirez, J. M., Probst, I., Richter, D. W. & Hanefeld, F. Anoxic ATP depletion in neonatal mice brainstem is prevented by creatine supplementation. Arch. Dis. Child-Fetal. 82(3), F224–F227 (2000).
Yoneda, K. et al. Effects of accumulation of phosphocreatine on utilization and restoration of high-energy phosphates during anoxia and recovery in thin hippocampal slices from the Guinea-Pig. Exp. Neurol. 82(1), 215–222 (1983).
Braasch, W., Gudbjarnason, S., Puri, P. S., Ravens, K. G. & Bing, R. J. Early changes in energy metabolism in the myocardium following acute coronary artery occlusion in anesthetized dogs. Circ. Res. 23(3), 429–438 (1968).
Norberg, K., Quistorff, B. & Siesjö, B. K. Effects of hypoxia of 10–45 seconds duration on energy metabolism in the cerebral cortex of unanesthetized and anesthetized rats. Acta Physiol. Scand. 95(3), 301–310 (1975).
Tsuji, M., Allred, E., Jensen, F. & Holtzman, D. Phosphocreatine and Atp regulation in the hypoxic developing rat-brain. Dev. Brain Res. 85(2), 192–200 (1995).
Cargnoni, A. et al. Relation between energy metabolism, glycolysis, noradrenaline release and duration of ischemia. Mol. Cell Biochem. 161, 187–194 (1996).
Sutton, J. R., Toews, C. J., Ward, G. R. & Fox, I. H. Purine metabolism during strenuous muscular exercise in man. Metabolism 29(3), 254–260 (1980).
Dale, N. & Frenguelli, B. G. Release of adenosine and ATP during ischemia and epilepsy. Curr. Neuropharmacol. 7(3), 160–179 (2009).
Yang, H. et al. Glycolysis in energy metabolism during seizures. Neural Regen. Res. 8(14), 1316–1326 (2013).
Johnson, T. A., Jinnah, H. A. & Kamatani, N. Shortage of cellular ATP as a cause of diseases and strategies to enhance ATP. Front. Pharmacol. 10, 98 (2019).
Dzeja, P. & Terzic, A. Adenylate kinase and amp signaling networks: Metabolic monitoring, signal communication and body energy sensing. Int. J. Mol. Sci. 10(4), 1729–1772 (2009).
Nakatsu, K. & Drummond, G. I. Adenylate metabolism and adenosine formation in the heart. Am. J. Physiol.-Leg. Content. 223(5), 1119–1127 (1972).
Pucar, D. et al. Mapping hypoxia-induced bioenergetic rearrangements and metabolic signaling by 18O-assisted 31P NMR and 1H NMR spectroscopy. Mol. Cell. Biochem. 256–257(1–2), 281–289 (2004).
Jennings, R. B., Reimer, K. A., Hill, M. L. & Mayer, S. E. Total ischemia in dog hearts, Invitro 1. Comparison of high-energy phosphate production, utilization, and depletion, and of adenine-nucleotide catabolism in total ischemia in vitro versus severe ischemia in vivo. Circ. Res. 49(4), 892–900 (1981).
Saugstad, O. D. Hypoxanthine as a measurement of hypoxia. Pediatr. Res. 9(4), 158–161 (1975).
Harkness, R. A., Simmonds, R. J. & Coade, S. B. Purine transport and metabolism in man: The effect of exercise on concentrations of purine bases, nucleosides and nucleotides in plasma, urine, leucocytes and erythrocytes. Clin. Sci. 64(3), 333–340 (1983).
Farthing, D., Farthing, C. A. & Xi, L. Inosine and hypoxanthine as novel biomarkers for cardiac ischemia: From bench to point-of-care. Exp. Biol. Med. 240, 821–831 (2015).
Miller, A. et al. Spatially resolved metabolomics and isotope tracing reveal dynamic metabolic responses of dentate granule neurons with acute stimulation. Nat. Metab. 5(10), 1820 (2023).
Beamer, E. et al. Elevated blood purine levels as a biomarker of seizures and epilepsy. Epilepsia 62(3), 817–828 (2021).
Burnstock, G. Purinergic nerves. Pharmacol. Rev. 24(3), 509–581 (1972).
Ciruela, F., Fuxe, K., Illes, P., Ulrich, H. & Caciagli, F. Editorial: “purinergic signaling 2020—the state-of-the-art commented by the members of the Italian purine club”. Front Pharmacol. 12, 768923 (2021).
Huang, Z. et al. From purines to purinergic signalling: Molecular functions and human diseases. Signal Transduct. Tar. 6(1), 162 (2021).
Losenkova, K. et al. Endothelial cells cope with hypoxia-induced depletion of ATP via activation of cellular purine turnover and phosphotransfer networks. Bba-Mol. Basis Dis. 1864(5), 1804–1815 (2018).
Yegutkin, G. G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Bba-Mol. Cell Res. 1783(5), 673–694 (2008).
Camici, M., Allegrini, S. & Tozzi, M. G. Interplay between adenylate metabolizing enzymes and AMP-activated protein kinase. Febs. J. 285(18), 3337–3352 (2018).
Folbergrova, J., Siesjo, B. K. & Macmillan, V. Effect of hypercapnic acidosis upon some glycolytic and krebs cycle-associated intermediates in rat-brain. J. Neurochem. 19(11), 2507 (1972).
LaMonte, G. et al. Acidosis induces reprogramming of cellular metabolism to mitigate oxidative stress. Cancer Metab. 1(1), 23 (2013).
Spriet, L. L. Phosphofructokinase activity and acidosis during short-term tetanic contractions. Can. J. Physiol. Pharmacol. 69(2), 298–304 (1991).
Fuhrmann, D. C. & Brüne, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 12, 208–215 (2017).
Tang, K. et al. Hypoxia-reprogrammed tricarboxylic acid cycle promotes the growth of human breast tumorigenic cells. Oncogene 38(44), 6970–6984 (2019).
Norberg, K. & Siesjo, B. K. Cerebral metabolism in hypoxic hypoxia. 2. Citric-acid cycle intermediates and associated amino-acids. Brain Res. 86(1), 45–54 (1975).
Chinopoulos, C. Which way does the citric acid cycle turn during hypoxia? The critical role of α-ketoglutarate dehydrogenase complex. J. Neurosci. Res. 91(8), 1030–1043 (2013).
Phillips, D., Aponte, A. M., French, S. A., Chess, D. J. & Balaban, R. S. Succinyl-CoA synthetase is a phosphate target for the activation of mitochondrial metabolism. Biochemistry 48(30), 7140–7149 (2009).
Rodríguez-Zavala, J. S., Pardo, J. P. & Moreno-Sánchez, R. Modulation of 2-oxoglutarate dehydrogenase complex by inorganic phosphate, Mg, and other effectors. Arch. Biochem. Biophys. 379(1), 78–84 (2000).
Strumilo, S. Short-term regulation of the α-ketoglutarate dehydrogenase complex by energy-linked and some other effectors. Biochem.-Moscow+ 70(7), 726–729 (2005).
Brekke, E. et al. Direct measurement of backflux between oxaloacetate and fumarate following pyruvate carboxylation. Glia 60(1), 147–158 (2012).
Taegtmeyer, H. Metabolic responses to cardiac hypoxia. Increased production of succinate by rabbit papillary muscles. Circ. Res. 43(5), 808–815 (1978).
Xu, M. Y., Wang, P., Sun, Y. J., Yang, L. & Wu, Y. J. Identification of metabolite biomarkers in serum of rats exposed to chlorpyrifos and cadmium. Sci. Rep. 10(1), 4999 (2020).
Copley, S. D., Frank, E., Kirsch, W. M. & Koch, T. H. Detection and possible origins of aminomalonic acid in protein hydrolysates. Anal. Biochem. 201(1), 152–157 (1992).
Bodin, A. et al. Acute pathophysiological myocardial changes following intra-cardiac electrical shocks using a proteomic approach in a sheep model. Sci. Rep. 10(1), 20252 (2020).
Batsios, G. et al. In vivo detection of γ-glutamyl-transferase up-regulation in glioma using hyperpolarized γ-glutamyl-[1-(13)C]glycine. Sci. Rep. 10(1), 6244 (2020).
Griffith, O. W., Bridges, R. J. & Meister, A. Evidence that the gamma-glutamyl cycle functions in vivo using intracellular glutathione: Effects of amino acids and selective inhibition of enzymes. Proc. Natl. Acad. Sci. U. S. A. 75(11), 5405–5408 (1978).
Dewhirst, R. A. & Fry, S. C. The oxidation of dehydroascorbic acid and 2,3-diketogulonate by distinct reactive oxygen species. Biochem. J. 475, 3451–3470 (2018).
Achten, J. & Jeukendrup, A. E. Maximal fat oxidation during exercise in trained men. Int. J. Sports Med. 24(8), 603–608 (2003).
Marcon, A. V. et al. Pork quality after electrical or carbon dioxide stunning at slaughter. Meat Sci. 156, 93–97 (2019).
Zybert, A. Quantification of the effects of electrical and CO stunning on selected quality attributes of fresh pork: A meta-analysis. Anim.-Basel 12(14), 1811 (2022).
Stajković, S., Vasilev, D., Dimitrijević, M., Čobanović, N. & Karabasil, N. The effect of stunning methods on stress and meat quality parameters in pigs. Veterinarski Arhiv. 94(4), 297–304 (2024).
Terlouw, E. M. C., Deiss, V. & Astruc, T. Comparing gas and electrical stunning: Effects on meat quality of pigs when Pre-stunning physical activity is minimal. Foods 10(2), 319 (2021).
Acknowledgements
We thank Gabriele Schuessler for her highly valuable technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. Open Access funding enabled and organized by Projekt DEAL. The study was financially supported by the Federal Ministry of Food and Agriculture in Germany. Our institution belongs to the ministry, so there is no specific grant number.
Author information
Authors and Affiliations
Contributions
MP, SZ and DAB designed the study. MP, SZ and BE performed the analyses. MP and DAB interpreted the data. MP wrote the manuscript draft, and SZ, DAB and BE reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable. Sticking blood was taken during the standard slaughterhouse procedures.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peukert, M., Zimmermann, S., Egert, B. et al. CO2 and electrical stunning differentially affect energy metabolism in pigs. Sci Rep 15, 25979 (2025). https://doi.org/10.1038/s41598-025-10874-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-10874-6