Abstract
To explore the specific effects and potential mechanisms of Aitongxiao Formula (ATXF) in treating HCC. Network pharmacology was first used to predict the potential targets and pathways of ATXF against liver cancer. In vitro and in vivo experiments were conducted to evaluate the effects of ATXF. The CCK8 assay and other experiments were used to assess the inhibitory effect of ATXF on cells, and the Transwell assay was used to verify the impact on cell migration and invasion. Xenograft models of liver cancer were successfully established by subcutaneously inoculating liver cancer cells, and the actual effects of different concentrations of ATXF in vivo were evaluated. Advanced technologies such as PCR and IHC were used to detect changes in core targets and other key targets in the pathways. ATXF inhibited the proliferation and metastasis behavior of liver cancer cells. It also suppressed the growth of liver cancer tumors in mice. ATXF regulated pathological changes in tumor tissues, including proliferation marker Ki67 and apoptosis marker caspase3. Network pharmacology techniques was predicted 13 effective components and 119 potential targets of ATXF. The main active components were quercetin, sitosterol, luteolin, and eugenol. These components may precisely targeted key molecules such as IL6, TP53, AKT1, TNF, IL1B, and EGFR. By examining the tumor tissues in mice, it was found that ATXF can regulate the changes in these six indicators and modulate the main proteins of the MAPK/ERK signaling pathway, thus exerting its therapeutic effect. ATXF effectively inhibits the development of hepatocellular carcinoma both in vitro and in vivo. The unique complex network of multi-component, multi-target, and multi-pathway interactions provides valuable clues and new insights for further revealing the mechanisms of action of this formula.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the most common pathological type of primary liver cancer.The current main clinical treatments include surgical resection, radiotherapy, chemotherapy, traditional Chinese medicine treatment, and liver transplantation, among others. Although these treatments can inhibit tumor growth in the short term, we still face issues such as a high risk of recurrence, metastasis, and low long-term survival rates for patients. The specific pathogenesis has not been fully elucidated. Therefore, there is an urgent need to find new targets, explore related molecular mechanisms, and provide new insights and directions for the prevention and treatment of liver cancer1[,2.
Traditional Chinese Medicine (TCM), as an important part of complementary and alternative medicine, has attracted attention due to its multi-target effects, lower side effects, and significant therapeutic effects. TCM not only adds value to traditional and modern medicine but may also continue to play a role in the future. ATXF contains 12 types of Chinese herbs. Hedyotis Diffusae and Scutellaria Barbata are the sovereign herbs; Radix Paeoniae Rubra, Corydalis Rhizoma, and Cyperi Rhizoma are the ministerial herbs; Linderae Radix, Hedysarum Multijugum Maxim, Gynostemmae Pentaphylli Herba, Sparganii Rhizoma, and Curcumae Rhizoma are the adjuvant herbs, which can regulate the effects of other herbs. It is reported that Hedyotis Diffusae Herba has anti-inflammatory, antioxidant, anti-cancer, and detoxifying effects, and therefore it has been used in the treatment of various inflammatory diseases and cancers3,4. Scutellaria barbata, as a herb in the family Labiaceae, is widely distributed in northeast Asia. According to research, the plant shows a variety of potential medicinal value, and is widely used in the treatment of a variety of inflammatory diseases, and shows the inhibitory effect on the growth of cancer cells, which can induce the apoptosis of cancer cells5–9. Radix Paeoniae Rubra, is widely used in various classic prescriptions, playing a crucial role. Its main effects include nourishing the blood, alleviating pain, regulating emotions to reduce irritability, and assisting in the treatment of liver diseases and cancers. Scientific research indicates that the extracts of Radix Paeoniae Rubra exhibit the ability to inhibit the growth of liver cancer cells, further verifying its potential and value in modern medical applications5,10,11. Corydalis is rich in various chemical components, among which alkaloids are the main active ingredients, exhibiting analgesic, sedative, and hypnotic effects12.The root extract of Cyperi Rhizoma has various biological activities such as anti-inflammatory, antioxidant, analgesic, anti-tumor, and antibacterial properties13. Linderae Radix, Hedysarum Multijugum Maxim, Gynostemmae Pentaphylli Herba, Sparganii Rhizoma, and Curcumae Rhizoma are used as adjuvant herbs, and are documented to have various effects in the Chinese Pharmacopoeia14–17. However, as a mixture of 12 kinds of traditional Chinese medicines, the chemical components of ATXF are highly complex. This is because it not only contains a large number of ineffective and unknown components but may also contain toxic substances, which have adversely affected its safety, stability, and efficacy. Therefore, to gain a more comprehensive understanding of the therapeutic effects of this formula in treating liver cancer, it is necessary to further explore scientific and technological methods. Network pharmacology, as a systematic approach that integrates laboratory research with clinical investigation serves as an effective means to deeply understand the complex relationships between Chinese herbs and diseases.
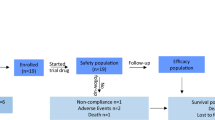
In this study, we employed techniques such as network pharmacology and molecular docking analysis to reveal the potential therapeutic targets of ATXF in the treatment of HCC. The effects of ATXF on the growth and metastasis of liver cancer were explored through in vitro and in vivo cell experiments. Furthermore, we confirmed the mechanism of the MAPK/ERK signaling pathway. In summary, this study not only provides valuable experimental data for the further development and utilization of the traditional Chinese medicine compound ATXF but also opens up new perspectives and methods for our understanding of the complex relationship between traditional Chinese medicine and diseases (Fig.1).
Research flow chart of the effects of ATXF in hepatocellular carcinoma. Chinese herbal medicine pictures from website: https://www.gigaomics.com and https://zhongyibaike.com.
Materials and methods
Network pharmacology section
Obtain the common targets of HCC and ATXF
We searched for compounds and their corresponding targets in the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) database (https://old.tcmsp-e.com/tcmsp.php) using the Chinese Pinyin names “Baihuasheshecao,” “banzhilian,” “chishao,” “yanhusuo,” “xiangfu,” “wuyao,” “huangqi,” “jiaogulan,” “sanleng,” “ezhu,” “honghua,” and “gancao.” The filtering criteria were set to oral bioavailability greater than or equal to 30% and drug-likeness greater than or equal to 0.18. Subsequently, we retrieved the UniProt IDs of the targets from the UniProt database (https://www.uniprot.org), specifying “Homo sapiens” as the species. All gene names were then assigned official gene symbols, and targets that did not meet the filtering criteria were eliminated.
Constructed a network of compounds and their targets using Cytoscape 3.9.1 (http://www.cytoscape.org). In this network, nodes represented targets, genes, molecules, or proteins, and connections between nodes indicated interactions between them. The “degree” value of a node reflected the number of connections it had in the network; a higher value indicated a greater likelihood of being a key target of the compound.
Searched for the keyword “hepatocellular carcinoma” in the OMIM (http://www.omim.org), GeneCards (https://www.genecards.org), and Genebank databases (https://www.ncbi.nlm.nih.gov/genbank) to obtain disease targets. We combined the disease targets from these three databases, removed duplicates, and obtained the final list of disease-related targets.
Construction and analysis of PPI Networks
Used bioinformatics and evolutionary genomics (https://bioinformatics.psb.ugent.be/webtools/Venn/) to intersect the potential targets of the retrieved compounds with disease targets, selected the overlapping targets, and imported them into the STRING database (https://string-db.org) to obtain protein interaction relationships. Imported the results into Cytoscape 3.9.1 software to construct and analyze the interaction network.
Screening core targets and key targets
Used the Cytoscape plugin MCODE for clustering analysis, seted the filtering conditions as degree cutoff: 2, k-core: 2, and selected the core clusters with the closest relationships in the network. Applied the CytoHubba plugin to analyze the PPI network and core clusters to obtain network topological parameters. Selected targets with a higher degree of sharing between the PPI network and core clusters as key targets, and searched for the proteins of these key targets in the DisGeNET database (http://www.disgenet.org).
Gene ontology and pathway enrichment analysis
The core targets were imported into KEGG database (https://www.genome.jp/kegg/) for KEGG and GO analysis including biological process (BP), cellular component (CC) and molecular function (MF) analysis. The pathways with P values < 0.05 and targets enriched in these pathways were selected in the KEGG analysis to obtain important pathways and key targets related to ATXF in HCC. Finally, the target-pathway network of ATXF on treating HCC was established and visualized using Cytoscape 3.9.1 software.
Molecular docking validation
Utilized Autodock software to perform molecular docking on the first 13 active ingredients and 6 key targets. The structures of all compounds were downloaded from the PubChem database (https://pubchem.ncbi.nlm). The three-dimensional structures of key targets were downloaded from the Protein Data Bank (PDB) (http://www.rcsb.org/pdb/home/home.do). The higher the absolute value of the docking score, the stronger the binding ability of the small molecule to the protease target was. The top six compound pairs with higher binding energy were screened according to the binding energy obtained from the docking.
Experimental verification section
ATXF preparation
The formulation consists of Hedyotis Diffusae Herba (30 g), Scutellaria Barbata (15 g), Paeonia Veitchii (30 g), Corydalis Rhizoma (20 g), Cyperi Rhizoma (10 g), Linderae Radix (10 g), Hedysarum Multijugum Maxim (20 g), Gynostemmae Pentaphylli Herba (12 g), Sparganii Rhizoma (10 g), Curcumae Rhizoma (10 g), Carthami Flos (6 g), and Glycyrrhizae Radix et Rhizoma (10 g). The preparations were compounded by the First Affiliated Hospital of Guangxi University of Chinese Medicine. The resulting aqueous solution was concentrated to 13.4 g/mL and stored at −20 °C for future using. ATXF was diluted with complete culture medium to the final working concentration for subsequent cell experiments.
Cell culture and treatment
The liver cancer cell lines HCCLM3 (cat: ZQ0023) and MHCC97L (cat: ZQ0019) were purchased from Shanghai Zhongqiao Xinzhou Biotechnology Co., Ltd., and the liver cancer cell line HepG2 was purchased from Wuhan Servicebio Technology Co., Ltd. HCCLM3\HepG2 and MHCC97L cells were cultured using DMEM basal medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin antibiotics, under conditions of 37 °C and 5% CO2. In subsequent experiments, ATXF was be dissolved in DMEM to attain the desired concentration.
CCK-8 assay
Used the Cell Counting Kit 8 (CCK-8, UElandy, shanghai) method, the cell viability of four types of liver cancer cells was detected. In a 96-well plate, each well contained approximately 5 × 10^3 cells. After 24, 48, and 72 h of cell culture treatment, CCK 8 was first mixed with the base medium (at a concentration of 10%) and then added to each well for incubation for 1–4 h. The optical density at 450 nm was measured for each well using a spectrophotometer (TECAN, Switzerland).
Plate cloning formation experiment
Upon seeding 700 cells per well in a 6-well plate, the cells were observed to adhere and form a distinct cell colony. Subsequently, varying concentrations of drugs (50 mg/ml and 100 mg/ml) were introduced, and the culture medium was exchanged every three days. Following a period of 14 days, the medium was removed. After fixing cells with 4% paraformaldehyde, they were stained with crystal violet. After washing with PBS, photographs of the entire plate and individual wells were captured for subsequent data analysis.
Group grouping and treatment of the animals
All experimental protocols were approved by the Experimental Animal Welfare and Ethics Committee of Guangxi Medical University, and all methods were carried out in accordance with institutional guidelines and regulations(License No. 202408012). Female nude mice (6–8 weeks of age) were purchased from the Animal Center of Guangxi Medical University. After 1 week of adaptation, we injected the cultured HCCLM3 tumor cells subcutaneously into the right axilla of the mice (2 × 106 cells/vehicle). After the injection, the mice were fed a standard diet. After tumor formation, the tumor volume is measured every two days. The calculation formula was: tumor volume = length×width2 × 0.5 and the tumor growth curve was drawn. The tumor volume reached 100mm3. Mice were randomly divided into 4 groups: (1) normal control group (n = 6), (2) L-ATXF group (24 mg/kg/d, n = 6), (3) H-ATXF group (72 mg/kg/d, n = 6), and (4) lenvatinib group (10 mg/kg/d, n = 6). Mices from each treated group were gavaged for 8 consecutive days, and normal control mice were given equal amounts of saline. After 8 d of gavage, the mice were euthanized by cervical dislocation, and tissue samples of tumor, spleen, liver, heart, lung, and kidney were collected. All experiments were performed in accordance with relevant guidelines and regulations (ARRIVE guidelines).
Pathological examination
HE staining
After fixation with 4% paraformaldehyde, mouse tumor tissues were dehydrated, paraffin-embedded, sectioned, dewaxed, stained with hematoxylin, and sealed with neutral glue. Histopathological changes were observed by light microscopy.
Immunohistochemical staining
The expression level of caspase 3 and Ki67 in mouse tumor tissues was determined by immunohistochemical staining.Under 200×magnification, 3 random fields were recorded, and the percentage of total positive cells was calculated. Ki67 staining shows proliferating cells with brown granules in the nucleus. For caspase3 staining, cells with brown granules are positive expression cells.
Capillary-based Immunoassay
Protein separation and detection were performed using an automated capillary electrophoresis system (Simple Western System and Compass Software; ProteinSimple). Protein separation was carried out using the JESS separation capillary cartridge (12–230 kDa). Antibodies against the following proteins were used; phospho ERK1/2(HuaBio, Hangzhou), MEK(HuaBio, Hangzhou), phospho MEK(HuaBio, Hangzhou), and Vinculin(ZEN BIO, Chengdu). Signals were detected with an enzyme-labeled secondary anti-rabbit antibody and visualized using ProteinSimple software.
Real-time polymerase chain reaction (real-time PCR)
Total RNA was extracted from different phenotypes macrophages using TRIzol reagent (Thermo Fisher, USA). Reverse transcription was performed using a MigntyScript First chain cDNA Master Mix kit (Shenggong, Shanghai). The cDNAs were subjected with SGExcel FastSYBR qPCR (Shenggong, Shanghai). The relative expression of mRNA was normalized to GAPDH. The detection was analyzed with ABI Q5 software version 2.1 (Applied Biosystems, USA). Primer information is shown in (Table 1).
Data analysis
Data were expressed as the means ± standard deviation(SD) or standard error of mean (SEM). Statistical analysis was performed using the statistical software package SPSS v.18.0. One-way analysis of variance (ANOVA) and student’s unpaired t-test was used to analyze statistical differences. Significance values were set at *p<0.05, **p<0.01,***p<0.001 and ****p< 0.0001.
Results and analysis
Acquisition of the main protein targets and disease-related targets of the traditional Chinese medicine Formula ATXF
Through the TCMSP database, 12 traditional Chinese medicine monomers were screened for their main components. According to the screening criteria, 189 main components and 323 targets were obtained (Table 2). Using “hepatocellular carcinoma” as a keyword, after removing duplicates, a total of 11,414 HCC disease targets were found in the GeneCards database. In the OMIM database, 511 targets were obtained, and the TTD database yielded 52 disease-related targets. The GeneCards database was supplemented. All targets obtained were merged and duplicate genes were removed, resulting in 5,706 HCC disease-related targets for subsequent data analysis. The intersection of potential targets of traditional Chinese medicine compound with disease targets yielded 217 potential HCC treatment targets (Fig. 2A).
Using Cytoscape 3.9.1, we constructed the network relationships between compounds and predicted targets (Fig. 2B). The resulting network includes 525 nodes and 3675 interaction edges. Then, we obtained the degree values of compounds in the compound-target network. Based on the compound-component-target-pathway network, we screened the main components: The top 13 compound components were screened according to Degree values, which are Quercetin, β-sitosterol, Luteolin, Jaranol, Kaempferol, Stigmasterol, Isorhamnetin, Formononetin, Baicalein, Hyndarin, Chryseriol, Calycosin, and 7-Methoxy-2-methyl isoflavone (Table 3).
ATXF treatment of HCC PPI network and key target analyse
Using the common targets of ATXF and HCC, a PPI network was constructed using the string database (https://cn.string-db.org), consisting of 217 nodes and 5077 interaction edges (Fig. 3A). The Centiscape2.2 plugin was used to analyze the PPI network, and core clusters and key targets were selected based on Degree, closeness, and betweenness values. The key targets were adjusted using cytoscape3.7.2 (Fig. 3B).
GO and KEGG analysis related to the targets of ATXF
Conducted GO functional annotation and KEGG pathway analysis on the 201 targets of the PPI network. Then visualized the top 10 as a bubble chart. In BP, ATXF has a significant impact on positive regulation of pri-miRNA transcription from RNA polymerase II promoter, response to xenobiotic stimulus, and positive regulation of gene expression. In CC, it is related to the RNA polymerase II transcription factor complex, macromolecular complex, and centrosome. In MF, RNA polymerase II sequence-specific DNA binding transcription factor binding is associated with enzyme binding, protein kinase binding (Fig.4A). Through KEGG pathway analysis, a total of 182 enrichment results were obtained. The top 20 pathways were screened based on adjusted p < 0.05, mainly involving Pathways in cancer, Lipid and atherosclerosis, Human cytomegalovirus infection, Hepatocellular carcinoma, and microRNA in cancer signaling pathways. This suggests that the effective components of ATXF may treat hepatocellular carcinoma by acting on these pathways (Fig. 4B).
Verification of molecular docking results
Explored the potential mechanisms of ATXF in the treatment of HCC, this study utilized molecular docking technology to analyze the interactions between the bioactive components of ATXF and key targets. Through this method, we were able to predict the binding affinity between these active components and target proteins, and accordingly generated a heatmap to visually represent the intensity of their interactions (Fig. 5A).
In the docking analysis that was conducted, the active ingredients we had screened out in ATXF were able to exhibit good binding capabilities with key targets, suggesting the great potential of the formula in treating HCC through these key targets. Specifically, Stigmasterol showed strong binding energy with AKT1 (docking score = −6.97) and TNF (docking score = −7.48); kaempferol also demonstrated significant binding capabilities with TNF (docking score = −6.95) and EGFR (docking score = −7.15); baicalein had strong binding affinity with IL1B (docking score = −6.98); and 7-Methoxy-2-methyl isoflavone showed good binding affinity with AKT1 (docking score = −6.96) (Fig. 5B).
These findings elucidated the interactions between the active components of ATXF and key targets, offering molecular-level evidence to understand its role in the treatment of hepatocellular carcinoma. Through this molecular docking analysis, we could predict and explain the potential efficacy of ATXF in treating HCC, thereby providing a significant theoretical foundation for subsequent experimental research and clinical application.
ATXF inhibited the proliferation, migration and invasion of Hepatocellular Carcinoma Cells
To study the inhibitory effect of ATXF on HCC, we treated three different cells HCCLM3, MHCC97L, and HepG2 with varying concentrations of ATXF and detected at multiple time points. The experimental results showed that when cells were exposed to different concentrations of ATXF, cell viability exhibited a significant dose-dependent decline. Specifically, as the concentration of ATXF increased, the survival rate of liver cancer cells significantly decreased, and the drug effect was enhanced over time (Fig. 6A). This suggests that ATXF has potential anti-HCC activity and can effectively inhibit the growth and proliferation of liver cancer cells. Based on these results, we subsequently used two concentrations of 50 mg/ml and 100 mg/ml for further experiments.
To investigate the impact of ATXF on cell proliferation, this study had employed a plate cloning formation experiment. As shown in Fig. 6D, when compared to the control group, a notable reduction was observed in both the number and size of clones of the three cells that were treated with ATXF.
Transwell assays indicated that the 10% FBS DMEM and two concentrations ATXF signifcantly decreased the migration and invasion of three cells (Fig. 6E). Taking into account these experimental findings, it can be unequivocally confirmed that ATXF had indeed inflicted damage on the proliferation, migration, and invasion capabilities of hepatocellular carcinoma cells.
ATXF inhibited the proliferation, migration and invasion of HCC cells. Inhibition and survival of (A) HCCLM3 (B) MHCC97L (C) HepG2 at different time points after different concentrations of ATXF. (D) The colony formation was visualized by microscopy after ATXF treatments (14 days at 0, 50, and 100 mg/mL). (E) Transwell assay for three cells treated with different concentration of ATXF and relative quantification cell number. Data are presented as the mean ± SD (n = 3, ***p < 0.001). ****, p < 0.0001, ####, p < 0.0001).
ATXF promoted HCC cell apoptosis
To clarify whether the inhibitory effect of ATXF on HCC cell growth was related to the induction of apoptosis, we first quantified apoptosis rates using flow cytometry with V-FITC/PI double stainin. HCCLM3, MHCC97L, and HepG2 cells were treated with ATXF for 24 h and the results showed that compared with the control group, ATXF significantly increased both early apoptosis rates (Annexing V+/PI-) and late apoptosis rates (Annexing V-/PI+) in all three cell lines. The total apoptosis rate confirmed that ATXF markedly promote HCC cell apoptosis (Figure 7A,B). To further validate these findings at the morphological level, we performed DAPI fluorescence microscopy for detection. Consistent with the flow cytometry results, ATXF treated HCCLM3, MHCC97L and HepG2 cells displayed obvious apoptotic bodies (Fig. 7C). Taken together, these findings demonstrate that ATXF induced cell apoptosis.
ATXF treatment inhibited tumor growth in xenograft tumor from HCCLM3 in vivo
In this experiment, we used lenvatinib as the positive control. The traditional Chinese medicine groups were treated with doses of 1 and 3 times the human dose as the low and high dose groups, respectively. The process of establishing animal models was as shown in Fig. 8A. Similarly, after euthanizing the mice, we conducted a comparative analysis of tumor images (Figs. 8B-C) and tumor weight and values (Fig. 8D-E). The results showed that compared with the control group, the low-dose drug group exhibited an inhibitory effect on tumor volume and weight, but there was no significant difference in volume changes. The high-dose drug group showed a significant difference in tumor volume reduction. Therefore, after applying a certain dosage, ATXF can inhibited the growth of tumors.
ATXF suppressed liver cancer growth in mice. (A) The schedule of subcutaneous liver cancer model treatment and tumor measurement. (B) and (C) Inhibition of tumor growth in nude mice with HCCLM3 xenografts treated with ATXF and Lenvatinib. (D) and (E) were measured tumor growth and tumor weight. Data are presented as means ± SEM (* p < 0.05; ****p < 0.0001).
ATXF affected the pathological changes in the tumor tissue
To further explore the pathological changes in tumor tissues after the action of ATXF, we performed H&E, caspase3, and Ki67 staining. The results found that the H&E staining of ATXF group showed necrosis and nuclear condensation, caspase3 staining found a significant increase in positive cells in ATXF group, and Ki67 staining showed a decrease in positive cells (Fig. 9A). Similarly, the apoptotic index and proliferation index showed the same trend in ATXF group (Fig. 9B-C). The histological results indicated that ATXF had anticancer activity, consistent with previous findings. During the treatment period, the physiological activity status of the nude mice in each group was normal. Compared with the control group, there were almost no significant pathological changes in the heart, liver, spleen, lung, and kidney tissues of the high-dose drug group (Fig. 10), suggesting that the nude mice did not experience systemic toxicity related to the combination treatment.
ATXF exerted potent inhibitory effect on tumor growth in vivo. (A) H&E staining, Ki67 staining for proliferation and caspase3 staining for apoptosis (magnification = 200×). (B) and (C) Analysis of proliferation rate and apoptosis positivity rate in the ATXF versus control groups (** p < 0.01; *** p < 0.001; ****p < 0.0001).
ATXF regulated 6 targets changes of tumor tissue through network pharmacology
To verify the action of ATXF on the six key targets screened, we detected the expression levels of the corresponding targets in the tumor tissues of nude mice from different experimental groups. The results showed that the expression of IL-6 and TNF-α was significantly upregulated, while the expression of AKT1 was significantly decreased in the ATXF treatment group. In addition, the expression of EGFR also showed a clear trend of upregulation (Fig. 11). These results indicate that ATXF can regulate the expression of inflammatory factors, reduce the expression of AKT1, and upregulate the expression of TP53.
ATXF inhibited tumor progression through MAPK/ERK signaling pathway
Through KEGG pathway enrichment analysis, the MAPK pathway was ranked within the top 20, with MAPK/ERK playing a significant role in cancer progression. To further study the mechanism of ATXF in regulating liver cancer progression, we detected the expression of MEK, pMEK, and pERK proteins in the tumor tissues of four groups of mice. The results showed that compared with the NC group, the expression of these proteins decreased after treatment with ATXF (Fig. 12). These results confirm that ATXF inhibits liver cancer progression, possibly partly by regulating the MAPK/ERK signaling pathway to control cell proliferation and other processes.
Effect of ATXF on MAPK/ERK signaling pathway in xenograft mice. (A) Simple western analysis of MEK, pMEK and pERK protein expression in tumor tissues. A was control group, B was low dose ATXF group, C was high dose group and D was Lenvatinib group. (B) and (C) Quantitative results of pMEK/MEK and pERK protein expression by Image J software. The graphs showed the mean ± SD (* p < 0.05; ** p < 0.01; *** p < 0.001).
Discussion
Currently, there is no comprehensive understanding of the potential mechanisms of liver cancer metastasis. In this study, our results show that ATXF can effectively inhibit the progression of liver cancer both in vivo and in vitro. With the advancement of modern medical technology, research into the treatment of cancer with Chinese medicine is also deepening. Many Chinese herbal ingredients have been found to inhibit the growth of tumor cells, induce apoptosis of tumor cells, and block the formation of tumor blood vessels, offering more treatment options and hope for cancer patients18–20. In recent years, although traditional Chinese medicine has achieved significant therapeutic effects in the field of cancer treatment, conclusive evidence that it can effectively inhibit the development of HCC still needs further accumulation and establishment. The traditional Chinese medicine compound ATXF studied in this article contains 12 kinds of Chinese herbs. Network pharmacology techniques were used to analyze its main components, including Quercetin, β-sitosterol, Luteolin, etc. Among them, Quercetin, as a natural bioactive compound, has various pharmacological effects including antioxidant, anti-inflammatory, and anti-tumor activities.β-sitosterol has been experimentally proven to possess various pharmacological properties, such as anti-inflammatory21, anti-atherosclerotic22, lipid-lowering and liver-protective23,immunomodulatory24. Luteolin has various anti-tumor effects, including inhibiting the growth of tumor cells, promoting apoptosis of cancer cells, sensitizing drug resistance, and reducing the metastasis of cancer cells25. Based on the comprehensive application of GEPIA and molecular docking technology, we identified six core targets: IL-6, IL-1β, TP53, AKT1, TNF, and EGFR, which exhibit significant network importance.
To further explore the effect of ATXF on hepatocellular carcinoma, we first investigated the effect of ATXF in vitro and found that it can effectively inhibit the proliferation of tumor cells and promote their apoptosis and also a certain inhibitory effect on the metastasis of cells. Furthermore, we constructed an in vivo xenograft tumor model to deeply explore the effect of ATXF on the progression of hepatocellular carcinoma in vivo. The main finding was that ATXF could significantly inhibit the growth of tumors. In this experiment, six important drug-disease common targets were screened out using network pharmacology. In the tumor microenvironment, IL-6 main sources are some cancer cells, TAMs, myeloid-derived suppressor cells (MDSC), Th2 cells, and CAFs. IL-6 is a major participant in chronic inflammatory diseases, autoimmune diseases, cancer, and tumor immunity. IL-6 binds to its receptor, and downstream signals are transmitted through the JAK-STAT, MAPK-ERK, and PI3K-Akt pathways, activating transcription26. IL-1β is an effective pro-inflammatory cytokine involved in regulating autoimmune inflammation, pro-inflammatory responses, cell proliferation, and cell differentiation. In the TME, IL-1β is upregulated in macrophages and tumor cells in various solid tumors, including breast cancer. It can control tumor invasion, upregulate the occurrence and development of primary tumors, increase the invasiveness of luminal breast cancer cells, and enhance the production of IL-6 through the NF-κB pathway, promoting tumor growth and invasiveness27. TNF-α is a major inflammatory cytokine that participates in the production of pro-inflammatory responses, as it can activate and upregulate more than 400 inflammatory genes and stimulate a variety of different cellular responses. TNFα and IL-1β are common pro-inflammatory cytokines found in the TME28. TP53, as a key tumor suppressor protein, strictly regulates the cell growth cycle by promoting cell apoptosis and DNA repair processes under specific conditions29. Therefore, the activation of TP53 is a key process in mediating apoptosis in liver cancer cells. AKT is a serine/threonine kinase with a molecular weight of about 60 kDa, also known as protein kinase B (PKB), which plays an important role in regulating various biological responses such as metabolism, cell survival, and angiogenesis. There are three subtypes of AKT: AKT1 (PKBα), AKT2 (PKBβ), AKT3 (PKBγ). The overactivation of AKT1 can promote the growth and survival of tumor cells, making it a target for many cancer treatment strategies27. We have found that ATXF can regulate changes in tumor tissues at the above targets, thus providing a basis for the exploration of drug mechanisms. The MAPK signaling pathway is an important intracellular signaling. Among them, the MAPK/ERK signaling pathway plays a crucial role in various biological processes such as cell proliferation30, differentiation31, and migration32[,33. We observed that after treatment with ATXF, the key proteins in the MAPK/ERK signaling pathway in hepatocellular carcinoma cells, such as pERK and pMEK, were significantly reduced. This indicates that ATXF may inhibit the proliferation and migration capabilities of liver cancer cells by suppressing the activity of the MAPK/ERK signaling pathway.
Although this study has initially revealed the role of ATXF from both extracellular and intracellular dimensions, its exact mechanism of action as a drug component has not yet been clearly validated. Therefore, future research still needs to further explore this field.
Conclusion
This study has innovatively employed network pharmacology to analyse for the first time the significant role of ATXF and its active components in combating HCC. The network screening results were validated using both in vitro and in vivo experiments, and the effects of the drug were discussed. This discovery not only opens new avenues for in-depth exploration of ATXF in the treatment of HCC but also lays a solid theoretical foundation and provides valuable insights for its future clinical application.This study has several limitations. First, the drug components of ATXF should be deeply explored through mass spectrometry analysis techniques to ensure the accuracy of subsequent studies based on real drug components. Second, this study used nude mice as tumor models. Given that nude mice have certain immune deficiencies, the universality of the research results is somewhat limited. Despite this, the network pharmacology approach has shown significant advantages in studying multi-target drugs like traditional Chinese medicine compound prescriptions. This study provides a useful supplement to the methodology of traditional Chinese medicine compound research and lays a foundation for subsequent studies.
Data availability
The authors declare that all data supporting the findings of this study are available, including replicate experiments, and will be made available upon reasonable request to the corresponding author.
Change history
29 October 2025
The original online version of this Article was revised: The Funding section in the original version of this Article was omitted. It now reads: “This work was supported by the National Natural Science Foundation of China (Grant No. 82260867).”
References
Amini, M., Looha, M. A., Zarean, E. & Pourhoseingholi, M. A. Global pattern of trends in incidence, mortality, and mortality-to-incidence ratio rates related to liver cancer, 1990–2019: a longitudinal analysis based on the global burden of disease study. BMC Public. Health. 22, 604. https://doi.org/10.1186/s12889-022-12867-w (2022).
Rumgay, H. et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 77, 1598–1606. https://doi.org/10.1016/j.jhep.2022.08.021 (2022).
Zhang, R. et al. Isolation, purification, structural characteristics, Pharmacological activities, and combined action of Hedyotis diffusa polysaccharides: A review. Int. J. Biol. Macromol. 183, 119–131. https://doi.org/10.1016/j.ijbiomac.2021.04.139 (2021).
Han, X., Zhang, X., Wang, Q., Wang, L. & Yu, S. Antitumor potential of Hedyotis diffusa willd: A systematic review of bioactive constituents and underlying molecular mechanisms. Biomed. Pharmacother. 130, 110735. https://doi.org/10.1016/j.biopha.2020.110735 (2020).
Wang, L. et al. A review of the ethnopharmacology, phytochemistry, pharmacology, and quality control of Scutellaria Barbata D. Don. J. Ethnopharmacol. 254, 112260. https://doi.org/10.1016/j.jep.2019.112260 (2020).
Su, W. et al. Extraction optimization, structural characterization, and Anti-Hepatoma activity of acidic polysaccharides from Scutellaria Barbata D. Don. Front. Pharmacol. 13, 827782. https://doi.org/10.3389/fphar.2022.827782 (2022).
Fang, T. et al. Ethyl Acetate Fraction from Hedyotis diffusa plus Scutellaria barbata Suppresses Migration of Bone-Metastatic Breast Cancer Cells via OPN-FAK/ERK/NF-kappaB Axis. Evid Based Complement Alternat Med 3573240 (2020). (2020). https://doi.org/10.1155/2020/3573240
Yang, A. Y., Liu, H. L. & Yang, Y. F. Study on the mechanism of action of Scutellaria Barbata on hepatocellular carcinoma based on network Pharmacology and bioinformatics. Front. Pharmacol. 13, 1072547. https://doi.org/10.3389/fphar.2022.1072547 (2022).
Gong, B., Kao, Y., Zhang, C., Sun, F. & Zhao, H. Systematic investigation of scutellariae barbatae herba for treating hepatocellular carcinoma based on network Pharmacology. Evid. Based Complement. Alternat Med. 2018 (4365739). https://doi.org/10.1155/2018/4365739 (2018).
Lee, S. M. et al. Paeoniae radix, a Chinese herbal extract, inhibit hepatoma cells growth by inducing apoptosis in a p53 independent pathway. Life Sci. 71, 2267–2277. https://doi.org/10.1016/s0024-3205(02)01962-8 (2002).
Kwon, K. B. et al. Induction of apoptosis by extract through cytochrome c release and the activations of caspase-9 and caspase-3 in HL-60 cells. Biol. Pharm. Bull. 29, 1082–1086. https://doi.org/10.1248/bpb.29.1082 (2006).
Tan, C. N. et al. Potential target-related proteins in rabbit platelets treated with active monomers dehydrocorydaline and canadine from rhizoma corydalis. Phytomedicine 54, 231–239. https://doi.org/10.1016/j.phymed.2018.09.200 (2019).
Kuo, P. C. et al. Anti-inflammatory principles from. Bioorg Med Chem Lett 30 (2020). https://doi.org/10.1016/j.bmcl.2020.127224
Commission, C. P. Pharmacopoeia of the People’s Republic of China 315 (China Medical Science and Technology, 2015).
Fu, J. W. et al. H. Review of the botanical characteristics, phytochemistry, and Pharmacology of Astragalus Membranaceus (Huangqi). Phytother Res. 28, 1–13 (2014).
Guo, Z. L., Kong, Y., Luo, M., Liu, Q. & Wu, Z. J. A Systematic review of phytochemistry, Pharmacology and pharmacokinetics on astragali radix: implications for astragali Radix as a personalized medicine. Int J. Mol. Sci. 1463–1486 (2019).
Lee, K. Y. & Jeon, Y. J. Macrophage activation by polysaccharide isolated from. Int. Immunopharmacol. 5, 1225–1233. https://doi.org/10.1016/j.intimp.2005.02.020 (2005).
Institute, T. Prevention and Treatment of Tumor (Commercial, 1971).
Ye, L. et al. Traditional Chinese medicine in the prevention and treatment of cancer and cancer metastasis. Oncol. Lett. 10, 1240–1250. https://doi.org/10.3892/ol.2015.3459 (2015).
Liao, Y. H. et al. Traditional Chinese medicine as adjunctive therapy improves the long-term survival of lung cancer patients. J. Cancer Res. Clin. 143, 2425–2435. https://doi.org/10.1007/s00432-017-2491-6 (2017).
Paniagua-Perez, R. et al. Evaluation of the Anti-Inflammatory capacity of Beta-Sitosterol in rodent assays. Afr. J. Tradit Complement. Altern. Med. 14, 123–130. https://doi.org/10.21010/ajtcam.v14i1.13 (2017).
Tada, H. et al. Sitosterolemia, hypercholesterolemia, and coronary artery disease. J. Atheroscler Thromb. 25, 783–789. https://doi.org/10.5551/jat.RV17024 (2018).
Yuan, C., Zhang, X., Long, X., Jin, J. & Jin, R. Effect of beta-sitosterol self-microemulsion and beta-sitosterol ester with Linoleic acid on lipid-lowering in hyperlipidemic mice. Lipids Health Dis. 18, 157. https://doi.org/10.1186/s12944-019-1096-2 (2019).
Efsa Panel on Nutrition. A combination of beta-sitosterol and beta-sitosterol glucoside and normal function of the immune system: evaluation of a health claim pursuant to Article 13(5) of regulation (EC) 1924/2006. EFSA J. 17, e05776. https://doi.org/10.2903/j.efsa.2019.5776 (2019).
Du, Y., Feng, J., Wang, R., Zhang, H. & Liu, J. Effects of flavonoids from Potamogeton crispus L. on proliferation, migration, and invasion of human ovarian Cancer cells. PLoS One. 10, e0130685. https://doi.org/10.1371/journal.pone.0130685 (2015).
Jones, S. A. & Jenkins, B. J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nature Reviews Immunology. 18(12), 773–789. https://doi.org/10.1038/s41577-018-0066-7 (2018).
Mantovani, A., Dinarello, C. A., Molgora, M. & Garlanda, C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity 50, 778–795. https://doi.org/10.1016/j.immuni.2019.03.012 (2019).
Liubomirski, Y. et al. Tumor-Stroma-Inflammation networks promote Pro-metastatic chemokines and aggressiveness characteristics in Triple-Negative breast Cancer. Front. Immunol. 10, 757. https://doi.org/10.3389/fimmu.2019.00757 (2019).
Kanapathipillai, M. Treating p53 mutant Aggregation-Associated Cancer. Cancers (Basel). 10. https://doi.org/10.3390/cancers10060154 (2018).
Zhang, H. H. et al. Ribosomal protein RPL5 regulates colon cancer cell proliferation and migration through MAPK/ERK signaling pathway. Bmc Mol Cell Biol 23 (2022). https://doi.org/10.1186/s12860-022-00448-z
Aure, M. H. et al. FGFR2 is essential for salivary gland duct homeostasis and MAPK-dependent seromucous acinar cell differentiation. Nature Communications 14 (2023). https://doi.org/10.1038/s41467-023-42243-0.
Liu, F. et al. Sonic Hedgehog signaling pathway mediates proliferation and migration of Fibroblast-Like synoviocytes in rheumatoid arthritis MAPK/ERK signaling pathway. Front. Immunol. 9 https://doi.org/10.3389/fimmu.2018.02847 (2018).
Wang, H. Z. et al. LncRNA UCA1 promotes pancreatic cancer cell migration by regulating mitochondrial dynamics via the MAPK pathway. Arch. Biochem. Biophys. 748 https://doi.org/10.1016/j.abb.2023.109783 (2023).
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82260867).
Author information
Authors and Affiliations
Contributions
X. Z.: Conceptualization, Investigation, Data Curation, Formal Analysis, Writing - Original Draft, Writing - Review & Editing; H.Z.: Investigation, Formal Analysis; M.X.: Methodology, Resources; R.W.: Supervision; J.T.: Supervision; Q. C.: Investigation; J.L. (Corresponding Author): Conceptualization, Funding Acquisition; Supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, X., Zhou, H., Xia, M. et al. Exploreing the potential mechanism of Aitongxiao formula inhibiting hepatocellular carcinoma in vitro and in vivo based on network Pharmacology. Sci Rep 15, 25783 (2025). https://doi.org/10.1038/s41598-025-11019-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11019-5