Abstract
Saccharomyces cerevisiae is the most used yeast for wine production around the world. Several characteristics make this yeast the wine yeast of excellence; among them is a great tolerance to higher concentrations of sugar and alcohol. Nevertheless, some compounds could have detrimental effects on its development like pesticides. Tebuconazole is one of the most common fungicides used in agriculture, belonging to the largest group of fungicides, the triazoles chemical group, that act on sterol biosynthesis. Yeasts have different responses to compensate for stress, and changes in their cell wall are one of the main ones. This work aimed to obtain new variants of S. cerevisiae through adaptive laboratory evolution (ALE) using Tebuconazole as selection pressure and to evaluate changes in yeast cell wall structure, composition, and fermentative behavior. Three new variants of S. cerevisiae were obtained. Analysis of the relative expression of genes associated with cell wall components showed that the third variant obtained YCPUC209C, had overexpression of genes FKS1, FKS3, CHS3, and SED1 in comparison with the original strain, also morphological analysis through TEM microscopy showed that YCPUC209C had an increase of 22.2% of cell wall thickness and 19% increase in the amount of glucan in comparison to the original strain. These cell wall changes were accompanied by hypersensitive to β-1,3-glucanase activity. Increased tolerance to pesticides Buprofezin and Spirotetramat presence during alcoholic fermentation was achieved by YCPUC209C, improving fermentative efficiency parameter. Changes in cell wall structure and composition reported in this work open new lines of analysis like the evaluation of yeast pesticide dissipation capacity since it is reported that components, such as glucan and chitin, can bond to these contaminants, reducing their residues in the wine.
Similar content being viewed by others
Introduction
Saccharomyces cerevisiae is the most used yeast in today’s food and alcoholic beverage industries for all kinds of products. S. cerevisiae plays an essential role in the wine industry. During alcoholic fermentation, yeast ferments sugar to ethanol with the resulting liberation of CO2 and other compounds. When alcoholic fermentation is concluded, cell constituents such as polysaccharides or proteins are released from yeast cells1.
An important problem for wine commercialization is the presence of pesticide residues. The pesticide residues present in the wine result from field applications, in which residues find their way through the vinification process to the bottle. It is well known that pesticide reduces their concentration during the alcoholic fermentation process2,3,4,5,6,7,8,9,10,11. Pesticide dissipation is mainly due to wine yeast, which can degrade and adsorb the residues at various extents without altering their fermentative activities12. It has been described that the removal by adsorption would probably be the main one8. Also, related to the pesticide’s physicochemical properties, lipophilicity would be a significant factor in pesticide dissipation in the winemaking process9. The underlying mechanisms could be due to chemical bounding with the components of yeast cell walls, such as chitin and glucan13. The same authors evaluated cell wall capacity to adsorb pesticides, finding that chitin and glucan had an adsorption capacity due to their functional groups capable of xenobiotic binding. In addition, specific mannoproteins secreted or passively released by S. cerevisiae during or after fermentation can reduce the formation of haze14,15,16. Since basic monomers of proteins have similar structures with some pesticides, like Glyphosate with glycine, it can be theorized that the same interaction could be found with pesticides present in wine.
Different attempts have been reported to improve the yeast’s capacity to adsorb wine components. Gonzalez-Ramos et al.17 worked with a recombinant S. cerevisiae strain overproducing mannoproteins, a consequence of deleting the gene KNR4, to stabilize wine against protein haze. Nevertheless, genetically modified organisms have low acceptance by consumers and could add a new problem instead of a solution.
Adaptive Laboratory Evolution (ALE) is a strategy based on using a stressor agent that acts as a selection pressure, forcing an adaptive response of yeast and obtaining variants with phenotypes of interest. ALE has been proven to be an effective tool for improving different characteristics of yeasts. For instance, Cadiere et al.18,19 improved the enological properties of S.cerevisiae EC1118 using ALE strategy and gluconate as selection pressure. Dhar et al.20 using S. cerevisiae BY4741 obtained populations that developed asymmetric cross-protection, where oxidative stress protects against salt stress. Also, some studies have reported using ALE to improve ethanol resistance in S. cerevisiae21,22,23,24. Non-Saccharomyces yeasts have also been subjected to ALE experiments. da Silveira et al.25 improved the ethanol tolerance of Kluyveromyces marxianus CCT 7735. Hemansi et al.26 used K.marxianus JKH5 and improved fermentative parameters such as specific growth rate, lag phase, and fermentation efficiency, using different inhibitors as selection pressure (furfural, acetic acid, and vanillin).
Fungicides with different modes of action are currently used to control fungal infections in Vitis vinifera, such as Keto Reductase Inhibitors (Fenhexamid), Dicarboxamides acting on MAP/Histidine-Kinase in signal transduction (Iprodione), or DeMethylation inhibitors (Tebuconazole). However, the use of fungicides is linked to hazardous effects on human health27. Tebuconazole has been reported as a contaminant of fermentations in several publications9,28,29 with diverse effects on yeast and wine quality. Tebuconazole belongs to the Triazole family, one of the most used fungicides around the world30 which affects the biosynthesis of ergosterol in fungi, causing membrane disruption and affecting the migration of cell wall components from the inner of the cell to the cell wall, and is used widely in wine grape production, for this reason, is commonly detected in wine. Some reports have shown the detrimental effect of some fungicides on wine yeast, such as producing stuck and sluggish fermentations31 affecting wine aroma3 and altering yeast growth and metabolism during alcoholic fermentation32.
In this work, we used the ALE strategy on S. cerevisiae yeast, using Tebuconazole fungicide as a selection pressure, and analyzed the obtained variants to identify changes in the expression of select genes of cell wall composition that could influence pesticide adsorption, cell wall morphology, and composition. Improvement in tolerance and response of the obtained variants to the presence of three different pesticides during alcoholic fermentation was evaluated through different fermentation parameters.
Materials and methods
Yeast strain and growth conditions
The S. cerevisiae wine yeast strain YCPUC209 from the Yeast Microbiology and Genetics Laboratory collection, belonging to the Pontificia Universidad Católica de Chile was initially used for growth assays and then used as a parental strain for ALE. The yeast was propagated in YPD medium (20 g/L glucose, 5 g/L peptone, and 5 g/L yeast extract).
Pesticide effect on yeast growth
The effect of three different pesticides, one fungicide, and two insecticides, on the growth of S. cerevisiae yeast YCPUC209, was analyzed in a growth assay. The selection of pesticides was determined according to their nature of usage, physicochemical properties, and the existence of known degradation metabolites. The preculture of the selected yeast was grown in a YPD liquid medium (20 g/L glucose, 5 g/L peptone, and 5 g/L yeast extract). The resulting population was then used for a microplate growth assay, where yeast was inoculated at a concentration of 1 × 105 cell/mL, using YPD liquid medium, and supplemented with different concentrations of 1 and 2 mg/L of Tebuconazole (TE), 1 and 2 mg/L of Buprofezin (BU), and 2 and 4 mg/L of Spirotetramat (SP), additionally, a control without pesticides was prepared. A 96-well microplate was used, and the experiments were done in triplicate. Optical density at 600 nm (OD600) was measured in a spectrophotometer 800-TS microplate reader coupled with the Gen5™ software (BioTek, Vermont, USA) using 1 h intervals for 235 total hours. Data were processed with Graphpad software, and estimation of the specific growth rate (µmax) was determined according to literature33 Lag phase was calculated mathematically according to Buchanan and Cygnarowicz34and generation time was also estimated.
Adaptative laboratory evolution
ALE assay was carried out using a S. cerevisiae strain YCPUC209 from the Yeast Microbiology and Genetics Laboratory collection, belonging to the Pontificia Universidad Católica de Chile. The fungicide TE was selected as selection pressure since, among the three different pesticides used in this investigation, it was the only one with fungicide aptitude, having the potential to affect yeast development. Also, the results of the growth assay showed that it was the most detrimental for yeast growth among the pesticides used. At the start of the experiment, S.cerevisiae YCPUC209 cells from a single colony were inoculated in 5 mL YPD medium and incubated overnight at 28 °C in an orbital shaker at 250 rpm. Then, 1 × 105 cells/mL were subcultured into a new flask containing fresh YDP medium supplemented with 10 mg/L of TE and incubated at 28 °C in an orbital shaker at 250 rpm. When this population reached OD600 of 1, 1 × 105 cells/mL were subcultured into a new flask containing fresh YPD medium supplemented with 20 mg/L of TE and cultivated as described above. This process was repeated using 40 and 60 mg/L of TE. During this adaptive evolution process, samples of the evolving populations were taken and maintained in a glycerol stock (70% glycerol) at − 80 °C for phenotypic analysis.
Pesticide effect on alcoholic fermentation of original strain and variant evolved
After the ALE experiments, S. cerevisiae YCPUC209 and YCPUC209C wine yeast were used for fermentation studies with natural must from Sauvignon Blanc grapes obtained from a vineyard in Casablanca Valley, Valparaíso Region of Chile (Lat. 33°21’51.63"S; Long. 71°19’3.77"O). Grapes were sprayed with 30 mg/L SO2 and refrigerated at 4 °C. Then, they were crushed and pressed to obtain the must. The must values for °Brix, pH, and density at the beginning of fermentation were 22.6, 3.5, and 1089 respectively. Fermentation assays were conducted in a 250 mL shake flask in triplicate, containing 100 mL of must each. Fresh cultures of the yeasts were prepared in liquid YPD on a rotary shaker at 28 °C and 250 rpm. The preculture was used to inoculate at a concentration of 1 × 105 cells/mL. Analytical standard grades of TE (Sigma-Aldrich USA), BU (Sigma-Aldrich USA), and SP (Dr. Ehrenstorfer GmbH Germany) were added to cultures to evaluate the effect on fermentation parameters such as fermentative efficiency measured as the area under the curve and carbon dioxide production measured as final weight loss. Initial concentrations of 1 mg/L of TE, 1 mg/L of BU, and 2 mg/L of SP were used. Carbon dioxide production was evaluated by measuring daily weight loss. The temperature during the study was stable, with an average of 17,1 ± 0.29 °C. The final density of the media was measured to evaluate the completion of the fermentation. Data were processed using GraphPad software, and theoretical maximum CO2 release (tCO2max) was determined according to Peltier et al.35. Control of the yeast without any pesticide was set, for a total of eight treatments.
Quantification of cell wall carbohydrates
Yeast cells were grown in YPD medium at 30 °C for 24 h, then triturated in fast prep with mixed bed resin. Isolation of cell wall components and subsequent determination of the amount of sugar monomers, after extraction with acid hydrolysis, was performed using an HPLC with a Dionex Bio-LC system, as described Mora-Montes et al.36.
Sensitivity to cell wall perturbing agents
To evaluate the sensitivity strains to cell wall stressing agents, Calco Fluor white (CFW) and Congo Red (CR), each strain was initially grown on YPD. Cells were collected and washed with sterile water and suspensions were adjusted OD600nm = 0.6. A series of 10-fold dilutions were prepared and spotted on YPD agar containing either CFW (25 µg/mL or 50 µg/mL) or CR (25 µg/mL or 50 µg/mL)37. Petri dishes were incubated for 24 h at 28 °C.
Sensitivity to Zymolyase was determined using a modified version of a previously described assay38. Cells suspensions were adjusted to an OD600nm = 1 value of 0.6 (2.5 × 105 cells/mL) in 10 mM Tris/HCl, pH 7.5, containing 200 µg/mL of Zymolyase 20T (USBiological, USA). Zymolyase activity was monitored by following the decrease of OD600nm during 8 h. Data is represented as survival percentage (percentage of cells that survive after Zymolyase treatment).
Fluorescence microscopy imaging
Yeast strains were grown overnight at 24 °C in YPD. The culture was diluted to 3 × 106 cells/mL, incubated at 24 °C in YPD for 2 h, and then maintained at 24–38 °C for three additional hours. After this time, cells were processed, 1 mL of cells was collected, washed with PBS, and stained with Calcofluor White (Fluorescent brightener 28, Sigma-Aldrich, St Louis MO, final concentration 30 µg/mL) for 10 min39. Stained yeasts were then analyzed by fluorescence microscopy (Eclipse E-200). Digital images were processed according to Nakamura et al.40 with image J (freely downloadable from the ImageJ website, https://imagej.net/ij/download.html) and expressed as Integrated Density (IntDen) or its percent.
Transmission electron microscopy (TEM) analysis
Yeast strains were grown overnight at 24 °C in YPD. The samples were fixed with glutaraldehyde 2.5% in sodium cacodylate 0.1 M pH 7.2 buffer for 18 h, then washed for three minutes with distilled water and repeated three times. A post-fixation with 1% aqueous osmium tetroxide for 90 min. Samples were washed for 10 min in distilled water and repeated three times, and then En bloc stained with 2% aqueous uranyl acetate for 60 min. Then, samples were dehydrated with a 50, 70, 95, and 100% acetone battery for 20 min each. It was pre-embedded in 1:1 Epon: acetone overnight and then left in neat epon in the air for 4 h. It was included in fresh resin in silicone molds and polymerized in an oven at 60 °C for 48 h. Thin sections were obtained on a Leica Ultracut R ultramicrotome, stained with 4% uranyl acetate in methanol for 1 min and lead citrate for 4 min. The analysis was made in a Talos F200C TEM (Thermo Scientific) transmission electron microscope at 200 kV with support from the Unidad de Microscopía Avanzada, Pontificia Universidad Católica of Chile.
Genes expression analysis
RNA extraction
A single colony of each variant obtained was grown on liquid YPD. When growth reached an OD 1. The culture was centrifuged at 3500 rpm for 10 min. Then, pellet was resuspended in 200 µL of RNA Buffer (50 mM Tris-HCl pH 7.4; 100 mM NaCl; 10 mM EDTA), and 400 µL phenol acid and glass beads were added. It was vortexed for 1 min, then on ice for 1 min, repeated, and vortexed for 3 min. 200 µL of RNA Buffer and 40 µL of 10% SDS were added. It was stirred for 6 min at 65 °C. It was centrifuged at 16,000 g for 15 min at 4 °C, and the upper phase was collected. 400 µL of phenol acid and 40 µL of 3 M sodium acetate were added. It was centrifuged at 16,000 x g for 15 min at 4 °C, and the upper phase was collected. One mL of cold 96% ethanol was added and refrigerated for 2 h at -80 °C. It was centrifuged at 16,000 x g for 10 min at 4 °C. Finally, the RNA Clean & Concentrator™-5 protocol (Zymo Research, USA) was followed. RNA quantification was performed on the Epoch™ equipment (BioTek, USA).
Relative expression analysis
For the relative expression analyses, the RNA samples of YCPUC209 and YCPUC209C were used, where the expression of genes associated with cell wall structure SED1, CHS3, FKS1, FKS2, and FKS3 were analyzed using Real-Time PCR (Table 1). These genes were chosen because are related to the synthesis of main cell wall components such as chitin (CH3), β-glucan (FKS1,2 and 3) and mannoproteins (SED1).
The cDNA synthesis was made through the protocol of RQ1 RNase-Free DNase (Promega, USA). Three µg of RNA of each yeast and treated with 3 µL de RQ1 RNase-Free DNase (1U/µL), 3 µL of RQ1 DNase 10X Reaction buffer, and Nuclease-Free water to reach a volume of 30 µL. The reaction was incubated at 37 °C for 1 h. 3 µL of solution RQ1 DNase Stop was added and incubated at 65 °C for 10 min. 22 µL of the previous solution was taken (2 µg de RNA) to form the cDNA, and the remaining 11 µL (1 µg de RNA) were stored at -20 °C as a negative control (RT-) for subsequent q-PCR. For the cDNA synthesis, 2 µL of primer oligo(dT)15 (500 µg/mL) (Promega, USA) was added to the 22 µL previously mentioned and incubated at 70 °C for 5 min in a thermal cycler Applied Biosystems™ Veriti™ 96-Well Thermal Cycler (Thermo Fisher Scientific, USA). Then the protocol of M-MLV RT (Moloney Murine Leukemia Virus Reverse Transcriptase) was used (Promega, USA). Ten µL de M-MLV RT 5X Reaction Buffer, 2.5 µL dNTPs (10 mM dATP, 10 mM dGTP, 10 mM dCTP y 10 mM dTTP) were added (Promega, USA), 1,2 µL Recombinant RNasin® Ribonuclease Inhibitor (40 U/µL), 2 µL of enzyme M-MLV RT (200 U/µL) y 10.3 µL of Nuclease free water was added, with a final volume of 50 µL. Finally, the thermocycler program used was 5 min at 25 °C, 60 min at 42 °C, and 15 min at 70 °C. the obtained cDNA was brought to a concentration of 20 ng/µL.
For the real-time quantification, each reaction was made in triplicate for each candidate gene, and as housekeeping genes, ACT1, TUB2, and ADH1 were used (Table 1). A negative control without cDNA was used, and a control RT- according to the protocol of 5x HOT FIREpol EvaGreen Qpcr Mix Plus (ROX) (Solis Biodyne, Estonia).
The reaction was made in MicroAmp® Fast Reaction Tubes (Applied Biosystems, USA) in a StepOne Real-Time PCR System Thermal Cycling Block (Thermo Fisher Scientific, USA) coupled to a StepOne Software (v2.3) (Applied Biosystems, USA). The program used was: 15 min at 95 °C, 40 cycles of amplification of 15 s at 95 °C, 20 s at 60 °C and 20 s at 72 °C. Melting curves were carried out at the end of PCR cycles, with the following program; 15 s at 95 °C, 1 min at 60 °C, and a final step increasing temperature to 95 °C for 15 s.
The quantification of the relative expression of candidate genes was made using a mathematical method 2−ΔΔCt described by Livak and Schmittgen41. This method supposes an optimal efficiency of the qPCR reaction by the candidate gene as for the reference one, and the relative expression is expressed according to Eq. 1.
where;
ΔΔCt = ((Ct gene in experimental condition – Ct housekeeping gene experimental condition) – (Ct gene control condition – Ct housekeeping gene control condition)).
The list of genes analyzed in this study is presented in Table 1.
Results
Effect of Tebuconazole, Buprofezin and Spirotetramatt on yeast growth
The growth trial showed that the fungicide TE significantly changed the growth kinetic of YCPUC209. The presence of pesticides negatively affected the normal development of yeast growth curves compared with the control (Fig. 1).
In Fig. 2, values of specific growth rate, lag phase, and generational time are presented for the different treatments. The lowest lag phase values were when the yeast was exposed to the presence of TE in 2 mg/L, followed by the treatment with the same pesticide in 1 mg/L (1.96 and 6.90 h, respectively).
Growth kinetic parameters, specific growth rate (h-1), lag phase (h), and generational time (h) of S. cerevisiae YCPUC209, in the presence of pesticides, Buprofezin 1 and 2 mg/L, Spirotetramat 2 and 4 mg/L, Tebuconazole 1 and 2 mg/L, and control without pesticides. Different letters above the bars represent significant differences between treatments according to the Tukey test.
The presence of BU and SP seemed to have had a lower effect on the lag phase, with no significant differences with the control for this parameter, even so, the treatment with SP in 4 mg/L had the highest value (9.57 h). Specific growth rate values showed that the only pesticide that significantly affected this parameter was TE in both concentrations used 0.0143 and 0.0171 h− 1 for 2 and 1 mg/L concentrations. The highest specific growth rate was the treatment of BU 1 mg/L but as the same as with lag phase values, there were no significant differences between the control and treatments with BU and SP. Confirming what was observed in the other kinetic parameters evaluated, once more, TE was the pesticide that most affected the generational time, as the treatments with 1 and 2 mg/L had the highest values (40.5 and 48.5 h), the fastest generational time was obtained by the treatment of BU 1 mg/L, and with a value of 35.6 h.
Tebuconazole as a selective pressure for the ALE experiment
S. cerevisiae wine yeast YCPUC209 was subjected to an adaptive laboratory evolution technique through serial batch cultivation, using the fungicide TE as a selection pressure in increasing concentration from 10 mg/L to 40 mg/L to generate variants with changes in the cell wall composition and structure. A total of three new variants were obtained, named YCPUC209A, YCPUC209B, and YCPUC209C, for the first, second, and third variants, respectively. The first step lasts 15 days. Then, two more steps of evolution were conducted with concentrations of TE of 20 and 40 mg/L, maintaining the same conditions. The time for the second and third evolution steps was 12 and 42 days, respectively. The entire evolution process lasted 69 days and a total of 164 generations. A final increase in TE concentration was made, specifically with 60 mg/L, but after 270 days, no growth was achieved.
Fermentation capacity of the original strain and the evolved variants
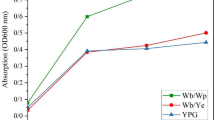
Fermentation curves showed that YCPUC209 behavior was very similar between treatments (Fig. 3). The highest value of tCO2max was 120.2 g/L by the treatment with BU, and the lowest value was from the control treatment (107.6 g/L), but no significant differences were found.
Fermentative efficiency measured as the area under the curve (AUC) showed similar behavior to total weight loss, with the highest value of 1959.7 (AUC) for BU treatment and the lowest value of 1712.0 (AUC) for the control treatment, with no significant differences between them (Fig. 4). Interesting results were obtained on the fermentation of the evolved variant YCPUC209C, which showed very different results between pesticide treatments. Total weight loss values of fermentation supplemented with TE negatively affected the performance YCPUC209C evolved variant, with an average value of 99.1 g/L, 9%, lower than the theoretical CO2max release, 108.9 g/L. CO2max values for the treatments with BU and SP, were 132.7 and 126.2 respectively (Fig. 4). Fermentative efficiency values showed that the treatment with the presence of BU significantly increased this parameter in comparison to the control by 13.3%, SP treatment also increased the fermentative efficiency by 7%. On the contrary, the treatment with TE reduced this parameter by 15.4%, showing an increased negative effect over YCPUC209C.
Final weight loss (g/L), and Fermentative efficiency measured as the area under the curve (AUC) of S. cerevisiae YCPUC209 and YCPUC209C, in the presence of pesticides Buprofezin (1 mg/L), Spirotetramat (2 mg/L), Tebuconazole (1 mg/L), and control without pesticides. Different letters above the bars represent significant differences between treatments according to the Tukey test.
Overall, the evolved variant YCPUC209C, improved its fermentation capacity in most treatments, and total weight loss increased compared to the original strain in the treatments with BU and SP by 10.4 and 15.7 respectively. The latter results will show an improved tolerance to the presence of BU and SP. On the contrary, the treatment with TE reduced the total weight loss by 14.3%, showing increased sensibility to this pesticide in the medium.
Most of the treatments finished the fermentation without problems. When final density was evaluated, fermentations conducted with YCPUC209 strain showed higher values compared with those conducted using YCPUC209C evolved yeast, ranging from 994 (Tebuconazole) to 999 (control). Fermentations with evolved yeast YCPUC209C showed values between 992 and 1011, and fermentation with Tebuconazole showed the highest value (1011), results that agree with the other parameters evaluated.
Genes expression analysis
Relative expression analysis showed that most genes evaluated were over-expressed on the third evolved variant obtained (YCPUC209C) compared to YCPUC209 (Fig. 6), except for FKS2 gene, which had a value of 0.652. The gene CHS3 had the highest value of relative expression of 9.526 compared to the original strain.
Changes cell wall thickness of evolved variant YCPUC209C
Several images were obtained during the TEM analysis, the main objective of this was to evaluate changes in the cell wall thickness. The original strain YCPUC209 and the third evolved variant YCPUC209C were used. One hundred forty-four independent measurements were made of cells from each strain. The result showed a 22.2% increase in cell wall thickness for YCPUC209C, with an average thickness of 106.16 nm compared to the original strain YCPUC209 (88.35 nm) (Fig. 5).
Alterations in cell wall composition in the YCPUC209C strain
In order to confirm whether the cell wall genes expression have effect on the cell wall in YCPUC209C variant, the levels of the glucan, mannan, and chitin in cell walls were analyzed by measuring the levels of their respective monosaccharides (Glucose, Mannose and Glucosamine respectively) upon chemical acid hydrolysis using a Dionex HPAEC-PAD28. This analysis revealed that the YCPUC209C variant exhibits clear alterations in the cell wall composition with a reduction of 40.8% and 13% in chitin and mannan levels, respectively, and a 19% increase in the amount of glucan (Table 2 and Fig. S1A and B) (Fig. 6).
To evaluate the cell wall integrity in the YCPUC209C variant, the cells were analyzed for their sensitivity to antifungal drugs, including Calcofluor white (CFW) (alters the assembly of chitin fibrils in the cell wall) and Congo red (CR) (alters the assembly of microfibrils of β1,3-glucan). An increase in sensitivity to CFW was observed in the YCPUC209C compared to the YCPUC209 strain. A similar result was found when the YCPUC209C variant was grown in the presence of CR, confirming that the cell wall in this strain had been affected (Fig. 7). In addition, treatment with zymolyase (a mixture of cell wall-hydrolytic enzymes including endo-β-1,3-glucanase activity), showed that the survival of the YCPUC209C variant was 48.8% in compared to control strain that was 73% (Fig. 7). The YCPUC209C variant is clearly hypersensitive to zymolyase, which is consistent with the S. cerevisiae strain having an altered cell wall structure, for instance, an altered β-glucan content or enhance accessibility to degrading enzymes. In addition, the yeast cells in YCPUC209C variant appeared to be less aggregated than in WT (Fig. S1C), conceivably due to cell surface alterations.
Effects on the cell wall of S. cerevisiae YCPUC209C variant. (A) Sensitivity to CFW or CR YCPUC209 strain and YCPUC209C variant. Cells were incubated in malt medium in the presence or absence of 25–50 µg/mL CFW or CR for 72 h at 20 °C. (B) Zymolyase sensitivity. YCPUC209 strain and YCPUC209C variant were incubated with 200 µg/mL zymolyase 20T at 25 ºC, and the decrease in OD600 nm was monitored over time (survival %). (C) The YCPUC209C strain presented a reduction in budding cell number.
Discussion
We evaluated the effect of pesticides on yeast´s growth. The results showed that the pesticides evaluated in the present study had variable effects on the normal development of S. cerevisiae YCPUC209 (Fig. 1). Overall, there was a negative impact on yeast growth; however, strain YCPUC209 was the least affected. Its evaluated kinetic parameters did not show significant differences when comparing the control with the treatments with BU and SP present in the medium. As expected, due to its nature, TE was the pesticide that most affected the development of strain YCPUC209, significantly impacting the values of the specific growth rate and generation time (Fig. 2). The negative effect of TE on growth has been previously reported42. The variable results of the present study are similar to those reported by Sarris et al.43 in which the fungicide Quinoxyfen did not affect the specific growth rate of S. cerevisiae but did affect final ethanol production. Another study by Cus and Raspor6 showed that Pyrimethanil in high concentration (10 mg/L) affected the initial growth of S. cerevisiae. Đorđević and Đurović-Pejčev44 studied the effect of Bifenthrin present in the growth media of S. cerevisiae, showing that this pesticide had a 12% growth inhibition with the highest concentration of the pesticide (7.5 mg/L). On the contrary, there have been studies that showed that pesticides can have favorable effects on yeast growth. Terpou et at45. reported that yeast biomass was higher when Myclobutanil was present in the medium at a concentration of 0.1 mg/L. The previous suggests that the effect of pesticides on yeast growth is variable and not only dependent on the nature of the active ingredient (fungicide, insecticide, or herbicide).
In general terms, consistent data was obtained on the effect on yeast development in the presence of the different pesticides studied, showing as could be predicted that the fungicide Tebuconazole was the one with a major negative effect over yeast, probably due to its fungicide nature activity, in comparison with Buprofezin and Spirotetramat, both been insecticides.
Three new variants were obtained from the evolution experiment named YCPUC209A, YCPUC209B, and YCPUC209C. The generations passed during the evolution experiment until the final variant, YCPUC209C, was 164. The number of generations depends on the selection pressure used and the phenotype to be obtained, among other46. For instance, 250–350 generations are usually passed to set an adaptive mutation on S. cerevisiae47,48,49,50. Nonetheless, some studies obtained new variants in a smaller number of generations. For instance, Ekberg et al.51 evolved a Saccharomyces pastorianus strain using ALE technique and obtained a new variant after 200 generations, and Hemansi et al.26 achieved new adapted strains after 60 generations.
The evolved variant YCPUC209C showed improved fermentation parameters like generational time, specific growth rate, fermentation efficiency, and total weight loss compared to the original strain. Regarding tCO2max, higher productions, above the theoretical maximum, were observed in those fermentations that were supplemented with pesticides (BU, SP) 126.4 to 132 g/L of CO2. Similar results have been reported by Chavez Lopez et al.52, who evaluated the effect of Quinoxyfen on S. cerevisiae fermentation. They observed that the maximum CO2 production in Sangiovese musts treated with pesticide was significantly higher than in untreated must. A similar trend is observed in Trebbiano must. Furthermore, Peltier et al.35who described the implementation of a small-scale fermentation methodology, observed variations similar to those described above. Concerning the model used for the calculation of tCO2max in this study, which was based on what was reported by Peltier et al.35da Silva et al.53 observed that the amount of CO2 calculated from the model underestimated or overestimated the amount of CO2 released, depending on the progress of fermentation. In addition, several reports in the literature describe pesticides present in the must, could be used as a carbon source by yeast, impacting CO2 produce. Funayama et al.54 studied the disappearance of [14 C]Buprofezin and the production of CO2 in flooded and non-flooded soils, and determined that 24% of the initially Buprofezin applied was recovered as 14CO2 trapped, and that when the soil was sterilized, the trapped 14CO2 amount was negligible, showing the effect of microorganisms over Buprofezin degradation. Maddela & Venkateswarlu55 found that Pseudomonas sp. grew better in MSM with 50 µg/mL of Buprofezin or Acephate, suggesting the bacteria could use these insecticides as a carbon source. Similarly, Li et al.56 reported that Rhodococcus sp. strain YL-1 degraded 92% of Buprofezin in 48 h, with cell growth linked directly to Buprofezin as the sole carbon and nitrogen source. Spirotetramat also showed biodegradability, with 4–42% of it mineralized to CO₂ in related studies57.
On the other hand, Tebuconazole presence in the must seems to increase the negative effect on YCPUC209C variant, suggesting that the fungicide might induce some modifications of yeast metabolism. In this regard, Gonzalez-Rodriguez et al.58 reported that the presence of tebuconazole in synthetic must at a concentration of 2 mg/L caused a decrease in the sugar consumption rate and lower growth and ethanol production. In addition, the maximum biomass reached was lower in the series incubated with tebuconazole, which demonstrates the toxic effect of this fungicide on yeast. These effects are related to the inhibitory effect of triazoles on the conversion of lanosterol to ergosterol in the cell membrane59. Sharma et al.60 reported that pesticides had a negative effect on yeast growth, slowing it down. However, this negative effect was variable and dependent on the active ingredient; this is how Hexaconazole, Propiconazole, and Endosulfan negatively affected S. cerevisiae, but Malathion, Deltamethrin, and Chlorpyriphos had no adverse effect on this yeast. This improvement is often observed in ALE studies, where strains develop tolerance to specific inhibitors. However, the effect of pesticides on yeast is complex and can vary depending on the specific compound. While ALE can lead to improved fermentation parameters, it’s important to note that it doesn’t always result in increased tolerance to all stressors. In this case, YCPUC209C became more susceptible to Tebuconazole. Indirect effects of ALE have been reported, Cadière et al.18 use ALE to increase the carbon flux through the PP pathway on S. cerevisiae EC1118, and evaluated the potential of this strategy on alcoholic fermentation studies using d-gluconolactone which is metabolized via the PP pathway to generate 6-phosphogluconate which usually is considered a weak carbon source. The evolved strain showed a 1.5-fold increase in the PP pathway flux. Additionally, it exhibited changes in amino acid synthesis, faster fermentation, and enhanced aroma production. This is consistent with the concept of acquired stress resistance, where exposure to a low-level stressor can confer tolerance to lethal dose of the same or a second stress61 This change often involves alterations to the cell wall or plasma membrane, crucial for maintaining cellular balance and responding to external factors like tebuconazole. This aligns with the increased expression of cell wall genes and changes in cell wall thickness observed in the YCPUC209C variant.
Transmission Electron Microscopy (TEM) analysis showed a significant increase in cell wall thickness for the evolved variant YCPUC209C. This change could be part of the adaptation of yeast to exposure to selective pressure, causing a reorganization of cell wall components or an increase in the concentration of some of them, resulting in a thickening of the cell wall42,62,63. Kang et al.42 investigated the effect of TE on Fusarium culmorum morphology, structure, and cell wall components. Through TEM Images, the authors observed changes in the cell wall thickness and reported considerable thickening of the hyphae cell of F. culmorum wall after exposure to TE. Cell wall anomalies due to sterol inhibitors like TE include general thickening of the cell wall, the occurrence of membranous cell wall inclusions, and localized areas of abnormal cell wall deposition63. When fungicides disrupt ergosterol biosynthesis, it leads to the accumulation of sterol intermediates and a depletion of functional sterols. This disrupts membrane function, activating chitin and β-1,3-glucan synthesis. It has been reported that mutants of S. cerevisiae lacking genes associated with the FKS family (cell wall components) impacted cell wall thickness by reducing it64.
It is well known that the presence of different stressor agents, such as chemicals like pesticides, can directly alter or indirectly affect the cell wall stability, inducing different responses by the cell65,66. One of the best characteristics in yeast compensatory response is variations in cell wall component content, with the increase in the chitin fraction being the most frequent67,68. The results of gene expression analysis showed that CHS3, FKS1, and FKS3 were 9, 1.3, and 1.5-fold over-expressed, respectively, in YCPUC209C compared to the original strain, which could be attributed to a compensatory mechanism due to exposure to the stressor agent (TE) of the evolved variant YCPUC209C. Even so, FKS2 gene results showed a small repression of its relative expression.
Chitin constitutes only 1–2% of the walls of yeasts of the Saccharomyces genus, and the CHS3 gene codes for chitin synthase CSIII catalytic subunit, which makes the majority of cell wall chitin, and the chitin ring during bud emergence65,69. A chs3 mutant presents no chitin at the bud site or lateral wall70. It has been reported that under conditions of cell wall stress, such as exposure to agents that perturb the cell surface, chitin levels in the cell wall increase dramatically in a Chs3-dependent manner, the latter being a component of the normal cell wall synthesis machinery that can be stress-activated71. This suggests that the overexpression of CHS3 gene observed in the evolved variant was in response to TE. However, other studies in insects have shown that tebuconazole significantly inhibited the expression of the chitin synthase gene72. Hence, the next experiments will be aimed at elucidating this issue.
β-Glucan, one of the major cell wall components of S. cerevisiae, represents about 30–60% of the cell wall dry weight, and the FKS1 expression gives origin to the major β-1,3-Glucan synthase subunit and has been proven to be related to the cell wall integrity pathway and regulated through the transcription factor Rlm165,73,74,75. The previous would explain the upregulation of FKS1 and increase in the amount of β-glucan in 19% in the cell wall on YCPUC209C as a response to the evolutionary process. It has been reported that deletion of FKS1 would not be lethal, and inhibition or damage of β-1,3-Glucan results in an increased level synthesized by Chs3p67,76. However, the amount of chitin in the cell wall on YCPUC209C presented a reduction of 40.8%, which could be explained by the effect of Tebuconazole on the membrane or cell wall after a long time of exposure during the evolutionary step. This hypothesis could be explaining the sensitivity of the YCPUC209C variant to zymolyase or cell wall-perturbing agents CFW or CR, indicating evident cell wall alteration. It has been described that change in the cell wall induced by fungi is the mechanism to compensate for loss of cell wall integrity77.
Additionally, FKS2 gene expression was shown to be downregulated and had a relative expression value of 0.652. It has been reported that disruption of FKS2 differently from FKS1 showed to have a negligent effect on β-1,3-Glucan synthase or β-1,3-Glucan content on the cell wall but has an important role in the process of yeast sporulation64,78. In addition, it has been described that induction of FKS2 would compensate for the lack of FKS1 gene in terms of 1,3-β-D-glucan synthase activity79. Thus, our result would show similar results to those reported in the literature since FKS2 should not be upregulated during vegetative growth or when FKS1 shows no problems with its expression. FKS3 functions and activation have been less investigated and several efforts to elucidate these have been unsuccessful65,76,80. Nevertheless, it has been described that this gene would be related to sporulation and mating.
SED1 gene coding for Sed1 glycoprotein of the cell wall related to adhesion and formation of the cell wall of S. cerevisiae. It has been reported that SED1 is highly expressed during the stationary phase, 2.7-fold higher than in the log phase81. Ravishankar et al.82 studied the effect of Glyphosate-based herbicides on the growth of S. cerevisiae and developed an in-lab-evolution exposing the yeast to it. Among the genes analyzed involved in the cell wall, the reported SED1 was overexpressed in mutants that developed resistance to exposure to Glyphosate. Thus, the increase in tolerance in some of the pesticides studied in our work could be related to the augmented expression of SED1.
Our investigation proves that the ALE technique is a powerful tool for achieving changes in S. cerevisiae populations to improve tolerance of some contaminants like pesticides. Although TE tolerance was not increased, YCPUC209C showed an enhanced adaptation to medium with BU and SP. TE was shown to be a good stressor agent to achieve enhanced characteristics for evolved variants. Overexpression of the target genes evaluated in this investigation would be related to mechanisms developed by the evolved variant YCPUC209C, which could be good indicators of the presence of contaminants in the medium of growth. Regarding the cell wall composition, the evolved variant showed changes compared to the original strain, increasing the content of β-glucans and decreasing the content of chitin. These results open new lines of study related to cell wall components and the role of yeast in tolerating and dissipating pesticides during alcoholic fermentation.
Currently, our group is working on analyzing the genome and transcriptome of the evolved strain to identify changes at the genetic level that explain the phenotype obtained.
Data availability
The data presented in this study are available on reasonable request from the corresponding authors.
References
Fleet, G. H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 86, 11–22 (2003).
Sala, C. et al. Fate of some common pesticides during vinification process. J. Agric. Food Chem. 44, 3668–3671 (1996).
García, M. A. et al. Effect of fungicide residues on the aromatic composition of white wine inoculated with three Saccharomyces cerevisiae strains. J. Agric. Food Chem. 52, 1241–1247 (2004).
Angioni, A. et al. GC-ITMS determination and degradation of Captan during winemaking. J. Agric. Food Chem. 51, 6761–6766 (2003).
Torija, M. J., Rozès, N., Poblet, M., Guillamón, J. M. & Mas, A. Effects of fermentation temperature on the strain population of Saccharomyces cerevisiae. Int. J. Food Microbiol. 80, 47–53 (2003).
Cus, F. & Raspor, P. The effect of pyrimethanil on the growth of wine yeasts. Lett. Appl. Microbiol. 47, 54–59 (2008).
Čuš, F., Česnik, H. B., Bolta, Š. V. & Gregorčič, A. Pesticide residues in grapes and during vinification process. Food Control. 21, 1512–1518 (2010).
Bizaj, E., Cus, F. & Raspor, P. Removal of pyrimethanil and fenhexamid from Saccharomyces cerevisiae liquid cultures. Food Technol. Biotechnol. 49, 474–480 (2011).
Alister, C. et al. Effects of wine grape cultivar, application conditions and the winemaking process on the dissipation of six pesticides. Cienc. Investig Agrar. 41, 19–20 (2014).
Regueiro, J., López-Fernández, O., Rial-Otero, R. & Cancho-Grande, B. Simal-Gándara, J. A review on the fermentation of foods and the residues of pesticides-biotransformation of pesticides and effects on fermentation and food quality. Crit. Rev. Food Sci. Nutr. 55, 839–863 (2015).
Becerra, K., Ghosh, S. & Godoy, L. Pesticide and yeast interaction in alcoholic fermentation: A mini-review. Fermentation 9, 266 (2023).
Caboni, P. & Cabras, P. Pesticides’ influence on wine fermentation. Adv. Food Nutr. Res. 59, 43–62 (2010).
Cabras, P., Farris, G. A., Fiori, M. G. & Pusino, A. Interaction between fenhexamid and yeasts during the alcoholic fermentation of Saccharomyces cerevisiae. J. Agric. Food Chem. 51, 5012–5015 (2003).
Palmisano, G., Antonacci, D. & Larsen, M. R. Glycoproteomic profile in wine: a ‘sweet’ molecular renaissance. J. Proteome Res. 9, 6148–6159 (2010).
Brown, S. L. et al. Reducing haziness in white wine by overexpression of Saccharomyces cerevisiae genes YOL155c and YDR055w. Appl. Microbiol. Biotechnol. 73, 1363–1376 (2007).
Gonzalez-Ramos, D. & Gonzalez, R. Genetic determinants of the release of mannoproteins of enological interest by Saccharomyces cerevisiae. J. Agric. Food Chem. 54, 9411–9416 (2006).
Gonzalez-Ramos, D., Quirós, M. & Gonzalez, R. Three different targets for the genetic modification of wine yeast strains resulting in improved effectiveness of bentonite fining. J. Agric. Food Chem. 57, 8373–8378 (2009).
Cadière, A., Ortiz-Julien, A., Camarasa, C. & Dequin, S. Evolutionary engineered Saccharomyces cerevisiae wine yeast strains with increased in vivo flux through the Pentose phosphate pathway. Metab. Eng. 13, 263–271 (2011).
Cadière, A., Aguera, E., Caillé, S., Ortiz-Julien, A. & Dequin, S. Pilot-scale evaluation the enological traits of a novel, aromatic wine yeast strain obtained by adaptive evolution. Food Microbiol. 32, 332–337 (2012).
Dhar, R., Sägesser, R., Weikert, C. & Wagner, A. Yeast adapts to a changing stressful environment by evolving cross-protection and anticipatory gene regulation. Mol. Biol. Evol. 30, 573–588 (2013).
Ding, J. et al. Tolerance and stress response to ethanol in the yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 85, 253–263 (2009).
Stanley, D., Fraser, S., Chambers, P. J., Rogers, P. & Stanley, G. A. Generation and characterisation of stable ethanol-tolerant mutants of Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 37, 139–149 (2010).
Fiedurek, J., Skowronek, M. & Gromada, A. Selection and adaptation of Saccharomyces cerevisae to increased ethanol tolerance and production. Pol. J. Microbiol. 60, 51–58 (2011).
Chen, S. & Xu, Y. Adaptive evolution of Saccharomyces cerevisiae with enhanced ethanol tolerance for Chinese rice wine fermentation. Appl. Biochem. Biotechnol. 173, 1940–1954 (2014).
da Silveira, F. A. et al. Assessment of ethanol tolerance of Kluyveromyces Marxianus CCT 7735 selected by adaptive laboratory evolution. Appl. Microbiol. Biotechnol. 104, 7483–7494 (2020).
Hemansi, H., Patel, A. K., Saini, J. K. & Singhania, R. R. Development of multiple inhibitor tolerant yeast via adaptive laboratory evolution for sustainable bioethanol production. Bioresour Technol. 344, 126247 (2022).
Kast-Hutcheson, K., Rider, C. V. & LeBlanc, G. A. The fungicide Propiconazole interferes with embryonic development of the crustacean Daphnia Magna. Environ. Toxicol. Chem. 20, 502 (2001).
Gava, A., Emer, C. D., Ficagna, E., Fernandes de Andrade, S. & Fuentefria, A. M. Occurrence and impact of fungicides residues on fermentation during wine production- A review. Food Addit. Contam. Part. Chem. Anal. Control Expo Risk Assess. 38, 943–961 (2021).
Zhao, S. et al. Impact of chiral Tebuconazole on the flavor components and color attributes of Merlot and cabernet sauvignon wines at the enantiomeric level. Food Chem. 373, 131577 (2022).
Ku, T. et al. Tebuconazole induces liver injury coupled with ROS-mediated hepatic metabolism disorder. Ecotoxicol. Environ. Saf. 220, 112309 (2021).
Russo, P. et al. Pesticide residues and stuck fermentation in wine: new evidences indicate the urgent need of tailored regulations. Fermentation 5, 23 (2019).
Noguerol-Pato, R., Torrado-Agrasar, A., González-Barreiro, C., Cancho-Grande, B. & Simal-Gándara, J. Influence of new generation fungicides on Saccharomyces cerevisiae growth, grape must fermentation and aroma biosynthesis. Food Chem. 146, 234–241 (2014).
Barata, A. et al. The effect of sugar concentration and temperature on growth and volatile phenol production by Dekkera bruxellensis in wine. FEMS Yeast Res. 8, 1097–1102 (2008).
Buchanan, R. L. & Cygnarowicz, M. L. A mathematical approach toward defining and calculating the duration of the lag phase. Food Microbiol. 7, 237–240 (1990).
Peltier, E. et al. Wine yeast phenomics: A standardized fermentation method for assessing quantitative traits of Saccharomyces cerevisiae strains in enological conditions. PLoS One. 13, e0190094 (2018).
Mora-Montes, H. M. et al. Endoplasmic reticulum alpha-glycosidases of Candida albicans are required for N glycosylation, cell wall integrity, and normal host-fungus interaction. Eukaryot. Cell. 6, 2184–2193 (2007).
Yadav, J., Muend, S., Zhang, Y. & Rao, R. A phenomics approach in yeast links proton and calcium pump function in the golgi. Mol. Biol. Cell. 18, 1480–1489 (2007).
van der Vaart, J. M., Caro, L. H., Chapman, J. W., Klis, F. M. & Verrips, C. T. Identification of three mannoproteins in the cell wall of Saccharomyces cerevisiae. J. Bacteriol. 177, 3104–3110 (1995).
Cabib, E., Blanco, N., Grau, C., Rodríguez-Peña, J. M. & Arroyo, J. Crh1p and Crh2p are required for the cross-linking of Chitin to β(1‐6)glucan in the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 63, 921–935 (2007).
Nakamura, Y., Ishii, J. & Kondo, A. Bright fluorescence monitoring system utilizing Zoanthus sp. green fluorescent protein (ZsGreen) for human G-protein-coupled receptor signaling in microbial yeast cells. PLoS One. 8, e82237 (2013).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using Real- time quantitative PCR and the 2 Ϫ ⌬⌬ C T method. 408, 402–408 (2001).
Kang, Z., Huang, L., Krieg, U., Mauler-Machnik, A. & Buchenauer, H. Effects of Tebuconazole on morphology, structure, cell wall components and trichothecene production of fusarium culmorum in vitro. Pest Manag Sci. 57, 491–500 (2001).
Sarris, D., Kotseridis, Y., Linga, M., Galiotou-Panayotou, M. & Papanikolaou, S. Enhanced ethanol production, volatile compound biosynthesis and fungicide removal during growth of a newly isolated Saccharomyces Cerevisiae strain on enriched pasteurized grape musts. Eng. Life Sci. 9, 29–37 (2009).
Đorđević, T. M. & Đurović-Pejčev, R. D. Evaluation of Lactobacillus plantarum and Saccharomyces cerevisiae in the presence of bifenthrin. Curr. Microbiol. 72, 680–691 (2016).
Terpou, A. et al. Effect of myclobutanil pesticide on the physiological behavior of two newly isolated Saccharomyces cerevisiae strains during very-high-gravity alcoholic fermentation. Microorganisms 7, 666 (2019).
Olson-Manning, C. F., Wagner, M. R. & Mitchell-Olds, T. Adaptive evolution: evaluating empirical support for theoretical predictions. Nat. Rev. Genet. 13, 867–877 (2012).
McBryde, C., Gardner, J. M., de Barros Lopes, M. & Jiranek, V. Generation of novel wine yeast strains by adaptive evolution. Am. J. Enol. Vitic. 57, 423–430 (2006).
Novo, M. et al. Improved fermentation kinetics by wine yeast strains evolved under ethanol stress. Lebenson Wiss Technol. 58, 166–172 (2014).
Fletcher, E. et al. Evolutionary engineering reveals divergent paths when yeast is adapted to different acidic environments. Metab. Eng. 39, 19–28 (2017).
Catrileo, D., Acuña-Fontecilla, A. & Godoy, L. Adaptive laboratory evolution of native Torulaspora delbrueckii YCPUC10 with enhanced ethanol resistance and evaluation in co-inoculated fermentation. Front. Microbiol. 11, 595023 (2020).
Ekberg, J. et al. Adaptive evolution of the lager brewing yeast Saccharomyces Pastorianus for improved growth under hyperosmotic conditions and its influence on fermentation performance. FEMS Yeast Res. 13, 335–349 (2013).
Chaves López, C. et al. Influence of quinoxyfen residues on Saccharomyces cerevisiae fermentation of grape musts. Food Technol. Biotechnol. 42, 89–97 (2004).
da Silva, T. et al. Hybridization within Saccharomyces genus results in homoeostasis and phenotypic novelty in winemaking conditions. PLoS One. 10, e0123834 (2015).
Funayama, S., Uchida, M., Kanno, H. & Tsuchiya, K. Degradation of buprofezin in flooded and upland soils under laboratory conditions. J. Pesticide Sci. 11, 605–610 (1986).
Maddela, N. R. & Venkateswarlu, K. Bacterial utilization of acephate and buprofezin. Insecticides – Soil. Microbiota Interactions 87–101 (2018).
Li, C. et al. Biodegradation of buprofezin by Rhodococcus sp. strain YL-1 isolated from rice field soil. J. Agric. Food Chem. 60, 2531–2537 (2012).
McGaw, A. Trade Advice Notice on Spirotetramat in the Product Movento 240 SC Insecticide. (2009).
González-Rodríguez, R. M., Cancho-Grande, B., Torrado-Agrasar, A. & Simal-Gándara, J. Mazaira-Pérez, J. Evolution of Tebuconazole residues through the winemaking process of Mencía grapes. Food Chem. 117, 529–537 (2009).
Ji, H. et al. A three-dimensional model of lanosterol 14alpha-demethylase of Candida albicans and its interaction with Azole antifungals. J. Med. Chem. 43, 2493–2505 (2000).
Sharma, J., Satya, S., Kumar, V. & Tewary, D. K. Dissipation of pesticides during bread-making. Chem. Health Saf. 12, 17–22 (2005).
Berry, D. B. & Gasch, A. P. Stress-activated genomic expression changes serve a preparative role for impending stress in yeast. Mol. Biol. Cell. 19, 4580–4587 (2008).
Bejaoui, H., Mathieu, F., Taillandier, P. & Lebrihi, A. Ochratoxin A removal in synthetic and natural grape juices by selected oenological Saccharomyces strains. J. Appl. Microbiol. 97, 1038–1044 (2004).
Fuller, M. S., Roberson, R. W. & Gisi, U. Effects of the sterol demethylase inhibitor, cyproconazole, on hyphal tip cells of sclerotium rolfsii. Pestic Biochem. Physiol. 36, 115–126 (1990).
Wang, J. et al. The FKS family genes cause changes in cell wall morphology resulted in regulation of anti-autolytic ability in Saccharomyces cerevisiae. Bioresour Technol. 249, 49–56 (2018).
Lesage, G. & Bussey, H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70, 317–343 (2006).
Sanz, A. B., García, R., Rodríguez-Peña, J. M., Nombela, C. & Arroyo, J. Slt2 MAPK association with chromatin is required for transcriptional activation of Rlm1 dependent genes upon cell wall stress. Biochim. Biophys. Acta Gene Regul. Mech. 1861, 1029–1039 (2018).
Popolo, L., Gualtieri, T. & Ragni, E. The yeast cell-wall salvage pathway. Med. Mycol. 39, 111–121 (2001).
Smits, G. J., Kapteyn, J. C., van den Ende, H. & Klis, F. M. Cell wall dynamics in yeast. Curr. Opin. Microbiol. 2, 348–352 (1999).
Chen, D. D. et al. Structure, catalysis, Chitin transport, and selective Inhibition of Chitin synthase. Nat. Commun. 14, 4776 (2023).
Shaw, J. A. et al. The function of Chitin synthases 2 and 3 in the Saccharomyces cerevisiae cell cycle. J. Cell. Biol. 114, 111–123 (1991).
Osmond, B. C., Specht, C. A. & Robbins, P. W. Chitin synthase III: synthetic lethal mutants and ‘stress related’ Chitin synthesis that bypasses the CSD3/CHS6 localization pathway. Proc. Natl. Acad. Sci. U S A. 96, 11206–11210 (1999).
Cai, Y. et al. The insecticidal activity and mechanism of Tebuconazole on Nilaparvata lugens (Stål). Pest Manag Sci. 79, 3141–3148 (2023).
Klis, F. M., Mol, P., Hellingwerf, K. & Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26, 239–256 (2002).
Križková, L., Ďuračková, Z., Šandula, J., Sasinková, V. & Krajčovič, J. Antioxidative and antimutagenic activity of yeast cell wall mannans in vitro. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 497, 213–222 (2001).
Montijn, R. C. et al. Localization of synthesis of beta1,6-glucan in Saccharomyces cerevisiae. J. Bacteriol. 181, 7414–7420 (1999).
García-Rodriguez, L. J. et al. Characterization of the Chitin biosynthesis process as a compensatory mechanism in the fks1 mutant of Saccharomyces cerevisiae. FEBS Lett. 478, 84–88 (2000).
Kapteyn, J. C. et al. Altered extent of cross-linking of beta1,6-glucosylated mannoproteins to Chitin in Saccharomyces cerevisiae mutants with reduced cell wall beta1,3-glucan content. J. Bacteriol. 179, 6279–6284 (1997).
Ishihara, S. et al. Homologous subunits of 1,3-beta-glucan synthase are important for spore wall assembly in Saccharomyces cerevisiae. Eukaryot. Cell. 6, 143–156 (2007).
Mazur, P. et al. Differential expression and function of two homologous subunits of yeast 1,3-beta-D-glucan synthase. Mol. Cell. Biol. 15, 5671–5681 (1995).
Dijkgraaf, G. J. P., Abe, M., Ohya, Y. & Bussey, H. Mutations in Fks1p affect the cell wall content of beta-1,3- and beta-1,6-glucan in Saccharomyces cerevisiae. Yeast 19, 671–690 (2002).
Shimoi, H., Kitagaki, H., Ohmori, H., Iimura, Y. & Ito, K. Sed1p is a major cell wall protein of Saccharomyces cerevisiae in the stationary phase and is involved in lytic enzyme resistance. J. Bacteriol. 180, 3381–3387 (1998).
Ravishankar, A., Pupo, A. & Gallagher, J. E. G. Resistance mechanisms of Saccharomyces cerevisiae to commercial formulations of glyphosate involve DNA damage repair, the cell cycle, and the cell wall structure. G3 (Bethesda). 10, 2043–2056 (2020).
Acknowledgements
We thank Dr. Hector Mora Monte from Universidad de Guanajuato (Mexico) for helping us with the cell wall composition analysis.
Author information
Authors and Affiliations
Contributions
L.G and K.B. conceived and designed the project. K.B. prepared and performed the evolved experiments, expression and microscopy assay. V.P and L.C performed the characterization cell wall experiments. K.B. and L.G performed the analysis. K.B, L.G. L.C wrote the paper. L.G and L.C supervised the work. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Becerra, K., Plaza, V., Castillo, L. et al. Cell wall modifications in Saccharomyces cerevisiae wine yeast through adaptive laboratory evolution with Tebuconazole. Sci Rep 15, 25438 (2025). https://doi.org/10.1038/s41598-025-11080-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-11080-0