Abstract
Heterogeneous catalysis is considered as a suitable alternative to conventional organic synthesis for the selective production of industrially significant fine chemicals. The development of supported catalysts by dispersing minimal quantities of active component can reduce production costs and enhance energy efficiency. The current work reports the development of Deep eutectic solvent (DES) modified multiwalled carbon nanotube (MWCNT) system and its activity in the Knoevenagel condensation reaction. The catalytic system was developed by grinding a very low concentration (0.83mM) of DES with desired amount of MWCNT. Various interactions of the three component DES with MWCNT were analysed by X-ray photoelectron spectroscopy (XPS). The reaction favoured a novel compound selectively with yield around 92% in solvent free medium. Anti-cancerous studies of the synthesized compound demonstrated a strong IC50 value of 15.62 µg/ml and a statistically calculated IC50 value of 9.8 µg/ml. Acridine orange/ Ethidium Bromide (AO/EB) dual fluorescence staining studies revealed that the test ligand with lowest concentration of 7.8 µg/ml was capable to induce apoptosis in 100% of MCF-7 cells. It is evident from the studies that the synthesized compound is a strong anticancer agent with potential to be investigated further.
Similar content being viewed by others
Introduction
Heterogeneous catalysis is relevant as it can facilitate the synthesis of industrially important chemicals in a sustainable manner. Development of efficient novel catalytic systems and the novel chemical synthesis mediated by them is indispensable to advance the energy and environmental fields. The dispersion of minimal quantity of novel active component on high surface area supports is the recent trend in catalysis to facilitate fine chemical synthesis selectively1,2. In a green chemistry perspective, organic transformations for the synthesis of industrially and pharmaceutically important fine chemicals must be carried out under ambient conditions by using some cost effective, stable, sustainable, and selective catalysts prepared by dispersing novel active components on the stable high surface area supports3,4,5,6. Metal catalysed organic transformations are well known and recently, palladium pincer complexes have been applied successfully for the organic transformation via direct arylation route7. Organosulfur compounds are active components of drugs and these compounds have been synthesized effectively by rhodium-based catalysts8.
Among the various methodologies developed, acid catalysed organic transformations are very common as it is more reliable, selective, environmentally benign, economical and the usage of toxic mineral acids can be curtailed. Acidic sites on the catalytic surface can facilitate the fine chemical synthesis highly feasible in a solvent free environment. Especially, zeolite and its modified forms were used extensively for isomerization, alkylation, and aromatization in the synthesis of valuable petrochemicals. Recently, hydro conversion of n-octane has been performed effectively over Ni modified ZSM-5 zeolites9,10,11,12. Mesoporous silica materials, especially, bare, and modified MCM-41 systems were widely studied for various catalytic applications13. The world without fine chemicals is unimaginable due to its widespread applications in diverse areas, however the green and sustainable synthesis of them through simple, cost effective and benign routes are inevitable to advance the field and safeguard the environment14. Single atom catalysts are another category in which good number of well-defined mononuclear active sites can facilitate chemical synthesis and organic transformations effectively without violating the green protocols15. According to “United Nations sustainable development goals (SDGs) 2030”, the use of toxic organic solvents and harmful chemicals, generation of huge quantity of secondary wastes and chemical processes with large energy consumption are not favourable16. The possibility of using ionic liquids and its modified forms in the design and development of novel and efficient heterogenous systems for organic transformation is growing rapidly17. “Supported ionic liquid phase catalysts (SILPC),” and “solid catalysts” modified with ionic liquids are extensively studied systems (SCILL)”. One of the major advantages is the use of minimal amount of ionic liquids for the catalyst development. This approach also avoids the drawbacks of applying ionic liquids in homogeneous medium, such as high cost, high viscosity and inefficient separation from the reaction mixture18.
Deep eutectic systems (DESs) are categorized alongside ionic liquids (ILs) due to their similar physical properties. However, DESs and ILs are not directly interchangeable because they differ in their chemical properties. DESs consist of “mixtures of Lewis or Brønsted acids and bases, which yield various types of anionic and/or cationic species”19. DESs have dual role as it can act as solvent as well as catalysts in chemical synthesis. Most of the chemical industries make use of huge quantities of organic solvents for the synthesis of various chemicals, which is detrimental. The use of nontoxic and eco-friendly inorganic solvents has got much attention nowadays due to its green characteristics20. Generally, DESs are considered as superior green solvent with intrinsic properties of low cost, less toxicity, less volatility, non-flammability and biodegradability21,22. DESs have been applied successfully as a solvent and catalyst for carbon-sulfur bond formation via thia-Michael addition reaction23,24. The triple role of DESs as a solvent, reagent and catalyst was investigated by applying it for the synthesis of bisamides23,25. A current study reported the synthesis of α-diazocarbonyl compounds via Aldol type coupling26.
By considering the green protocols and SDGs, a novel DES modified MWCNT system was developed by mixing small quantity of DES with desired quantity of MWCNT. The synthesized DES exhibits acidic nature, which promotes the condensation reaction in a solvent free environment, and the minimal usage of the active component significantly enhances the value of the work. The synthesized system was well characterized and evaluated its activity in the synthesis of 5-(benzo thiophene 2-ylmethylene) pyrimidine-2,4,6-trione (BTMPT). The catalytic system demonstrated high selectivity to the condensation product and maintained its activity effectively up to five cycles without any appreciable loss in performance. In the literature, no prior report has described the synthesis of a compound using a singly grafted DES supported catalysts. Additionally, this is the first work explains the use of a ternary DES in the development of a feasible heterogeneous catalyst. The highly selective synthesis of a novel bio active compound greatly enhances the significance of this work.
Results and discussions
Fourier transform infrared spectroscopy analysis
The FTIR spectra of bare CNT, bare DES and DES modified CNT are shown in Fig. 1. The broad band which is observed in the DES system around 3290 cm−1 is attributed to the stretching vibrational mode of ‘OH’ group present in lactic acid27,28. The band at 1073 cm−1 is ascribed to the stretching of C-N bond of the choline chloride29. The band at 1199 cm−1 is indexed to the C-O stretching of glyoxal moiety30. The bands around 1482 cm−1 and 953 cm−1 are allocated to the asymmetric deformation and in plane rocking of methyl groups. The sharp and intense band at 1738 cm−1 is mainly due to the acidic group out of plane stretching of lactic acid31. The slight variations of C = O stretching of lactic acid and C-O stretching of glyoxal moieties from the original values may be due to their hydrogen bonding interaction with choline chloride. The results unmistakably confirmed the successful incorporation of three components to form the DES. The band observed in pure MWCNT at 3350 cm−1 is mainly due to the asymmetric stretching of the hydroxyl group of adsorbed water and the weak band at 1450 cm−1 is attributed to the ‘OH’ bending vibration. C-H stretching modes of MWCNT was observed at around 2900 cm−1 and 2500 cm−132. The band appeared at 1216 cm−1 can be attributed to the C-O stretching of carbon attached to the hydroxyl group33,34. In the DES/MWCNT system, the band for OH stretching was found to be shifted to 3220 cm−1 and the C-H vibrational mode also was found to be shifted to a lower value of 2350 cm−1. The C-N stretching frequency of the choline chloride moiety shifted from 1073 to 1067 cm⁻¹, while the C-O stretching frequency of the glyoxal moiety was also shifted to 1150 cm⁻¹. The bands due to asymmetric deformation and in plane rocking of methyl groups found intact in the modified system. The intensity of C = O stretching vibration band was reduced to a greater extend in the modified system. The shifts of vibrational frequencies for most of the functionalities in the DES/MWCNT system unambiguously confirmed the strong interaction between MWCNT and DES.
Powder X-ray diffraction analysis (p-XRD)
Figure 2 displays the X-ray diffraction (XRD) patterns of the prepared catalysts. The diffraction peaks for MWCNT are observed around 26.02° and 43.17°, which correspond to the (002) and (100) crystal planes of graphitic carbon35. In contrast, incorporating DES into MWCNT led to a decrease in the intensity of the graphitic carbon peak at 25.48°, and the peak at 42.42° was also reduced. This reduction is likely due to the interference with the diffraction patterns caused by DES masking the crystal planes of the CNT. The lower peak intensities in the grafted system indicate successful integration of DES, consistent with previous observations36. The DES grafted MWCNT exhibited peaks at 21.11°, 25.48°, 42.42° and 47.82°, indicating the interaction between the MWCNT and choline chloride based DES system. The appearance of peaks at 2θ = 21.11° and 42.42° reveal the presence of choline chloride on the surface of MWCNTs37. The reduction in intensity and shift of certain peaks indicate the effective incorporation of DES onto MWCNT system.
Transmission Electron microscopy (TEM)
Figure 3 shows the TEM micrographs of MWCNTs modified with DES. The MWCNT was found to be intact with good aspect ratio after the modification, which shows the stability of the support. Normally, carbon nanotubes exhibit high degree of bundling, but the modification reduces the bundling to a lesser extent. Roughness at certain regions of MWCNT is attributed to the surface adsorption of organic moiety38. The presence of protrusions and deformations at certain region on the micrograph indicates the adsorption of organic species. The distribution of the active component is non uniform due to its less concentration. The extent of bundling of MWCNT and the agglomeration of the modifier at certain regions are more, which might have occurred during the mechanical grinding process. The modifier has a dual role as it could reduce the degree of bundling of CNT by acting like a capping agent and enhance the dispersion of CNT in certain solvents38,39. The selective area electron diffraction (SAED) pattern exhibited the polycrystalline nature of the final system with an ordered arrangement. The TEM results unmistakably confirm the incorporation of active component onto the scaffold and the high stability of the final system.
X-ray photoelectron spectroscopy (XPS)
XPS analysis was carried out to assess the electronic structure of the developed system, with the resulting spectra presented in Fig. 4. Table 1 summarizes the elemental concentrations of DES/MWCNT system. Deconvolution of the C1s peak for MWCNT reveals that the primary peak at 284.77eV corresponds to the graphitic carbon in the carbon nanotube structure40,41. Elevated binding energies observed in the functionalized MWCNT suggest the presence of carbon atoms linked to various other functional groups42. The high-resolution C1s peaks at 284.77eV and 286.52eV correspond to the C-C, C-O, and C-H environments in the system43. Carbon atoms bonded to nitrogen (C–N) and hydroxyl groups (C–OH) appear at 286.46 eV and 286.52 eV, respectively44. Additionally, a peak at approximately 291.15eV is attributed to the C = O group45,46. In the high-resolution scan over N1s, peaks at 399.48eV and 400.7eV are attributed to the C–N and C = N/O–N environments, respectively. The broad N1s core level peaks centred at 399.9eV and 402.92eV can be assigned to the unprotonated and protonated amine species present in the DES molecules44,47,48.
In the O1s spectra of DES/MWCNT system, three distinct peaks observed at 531.2eV, 532.78eV, and 534.4eV, correspond to the C = O, C–OH and O-C-O groups which are the functionalities of the three components present in the DES system, respectively44,45. The XPS fitting data confirmed that DES was chemically functionalized or grafted onto the surface of MWCNTs. Cl2p XPS spectrum displays peaks at binding energies around 197.84 and 199.37eV, which could be assigned to the Cl2p3/2 and Cl2p1/2 spin orbital splitting components, respectively49,50. A slight chemical shift in the binding energy values from their original positions is attributed to changes in the chemical environment caused by intramolecular hydrogen bonding between lactic acid and glyoxal in the final DES system.
Pyridine adsorption infrared spectroscopy analysis
Pyridine FTIR is a useful method to study the acidity of the catalyst surface (Fig. 5). It was carried to find out the presence and strength of acidic sites on the prepared catalytic system. The infrared spectrum, displaying strong peaks in the range of 1400 to 1700cm−1 is indicative of significant acidity in acidic solids. The bands at 1575cm−1, 1667.6cm−1, 1471.5cm−1 and the very intense band at 1430cm−1 indicate different acidic properties of the prepared acid catalyst51,52. Typically, the band at 1430cm⁻¹ is associated with “strongly Lewis-bound pyridine”, while the band at 1575cm⁻¹ corresponds to “weakly Lewis-bound pyridine”. The band near 1667.6cm⁻¹ is attributed to the vibration of the pyridinium ion ring, resulting from pyridine molecules attached to “Brønsted acid sites.” Additionally, the band at 1471.5cm⁻¹ is linked to pyridine interacting with both “Brønsted and Lewis sites”52,53. The result unambiguously confirmed the strong acidic nature of the prepared catalyst.
Catalytic activity study
The prepared system was found to be active for aldol condensation between Benzaldehyde and Cyclohexanone which was reported in the previous work54. Hence, it was decided to check its activity in the synthesis of a novel compound through Knoevenagel condensation of Barbutiric acid (BA) and Benzothiophene carbaxaldehyde (BTC) in a solvent free environment. The catalysts with different concentrations of deep eutectic solvent on MWCNT were evaluated to understand the best feasible system for the synthesis. The catalyst with 0.83mM DES modified system exhibited more efficiency compared to the other systems and the high activity of the system was finalized via trial-and-error method. Different concentrations of the reactants were tried, and the maximum yield and short duration was observed with 2:1 (BA to BTC) molar ratio. As control, the reaction was performed using bare MWCNT system and the product yield was found to be marginal. The acid catalysed condensation favoured the product selectively with yield higher than 92%. The reaction progress was monitored by thin layer chromatography using ethyl acetate and n- hexane (1:10) as eluents and the product was obtained through recrystallization in DMSO. A mechanistic pathway is proposed to substantiate the condensation reaction on catalyst surface. Various parameters were optimized to find out the best feasible conditions to drive the reaction. The structure of the crystallized aldol product was confirmed by 1HNMR, 13CNMR and LC-MS analyses. The details are available in supplementary file.
Effect of molar ratio
The molar ratio of the reactants was varied as 1:1, 1:2 and 2:1, with 2:1 ratio was determined to be optimal for achieving maximum yield. Thus, this ratio finalized was used for all other optimisations. Table 2. Shows the effect of molar ratio on the product yield.
Effect of concentration of DES
MWCNT with different concentrations of DES was prepared by varying the concentrations from 0.4mM to 1.1mM and the reaction was performed with 0.02g of each of the catalyst systems for 1h at 80°C. The bare carbon nanotube did not show any activity under the said conditions. Among the systems, 0.83mM DES incorporated system exhibited the highest yield compared to other systems. The catalytic activity was found to be increased with increase in concentration of DES, then reached an optimum and reduced thereafter. Table 3. shows the effect of DES concentration on the yield of the reaction. The result clearly indicates the influence of minimal quantity of DES in obtaining the product selectively with high yield.
Effect of time
The reaction was carried out at different time durations varying from 20 min to 80 min and the calculated maximum yield was 89.45% with a duration of 1h. After an hour, the catalyst activity was declined due to the blockage of active sites with some impurities formed along with the major product. The results are shown in Table 4.
Effect of temperature
The temperature of the reaction was also optimized by varying it from room temperature to 100°C. The activity was found to be increased with increase in temperature. The product yield was calculated to be around 92.76% with high degree of selectivity at 80°C. Further increase in temperature did not give an appreciable yield. Table 5. represents the effect of temperature on the reaction.
Recyclability studies
The 0.83mM DES/MWCNT catalyst was successfully recovered from the reaction mixture with minimal loss and reused for subsequent reaction cycles. The modified system maintained comparable activity up to the fourth cycle. After each reaction cycle, the recovered catalyst was analysed using FTIR to confirm its stability. The results are tabulated in Table 6. A decline in catalyst activity was observed after the fourth cycle, which is attributed to a slight loss of the active component resulting from its weakened interaction with the support during repeated reaction cycles. Additionally, there was a slight reduction in the amount of recovered catalyst because of its finely powdered nature. The decrease in activity could also be attributed to the blockage of surface-active sites by impurities and small amounts of polymeric or oily by-products formed along with the major compound.
FTIR analysis of the recovered systems
FTIR spectra of the used systems are shown in the Fig. 6. The catalyst was recovered almost completely after every cycle and exhibited good activity till fourth cycle. The retention of almost all absorption bands in the recovered system undeniably confirms its high stability. The excellent stability of the support and its strong adherence to the active component contributed to the high stability of the final system.
Thermogravimetric analysis of DES/MWCNT
Figure 7 shows the thermogravimetric curves for bare DES/MWCNT and the system recovered after the fourth cycle. The thermal behaviour of bare DES/MWCNTs was thoroughly evaluated and compared with that of DES/MWCNTs after the fourth catalytic cycle. The analysis was performed under a nitrogen atmosphere by heating the samples from room temperature to 950 °C at a constant rate of 10 °C min⁻¹55. The TGA profiles of both the systems exhibited an initial weight loss attributed to the evaporation of moisture and residual solvent molecules, followed by a subsequent mass loss corresponding to the decomposition of the DES components incorporated onto the nanotubes, and finally, a degradation-related mass loss at elevated temperatures. Both the systems demonstrated remarkable thermal stability up to 500 °C. A weight loss of approximately 8.45% was observed for both the samples within the temperature range of 100 °C to 200 °C, which is attributed to the removal of adsorbed as well as lattice-bound water molecules56. Furthermore, a significant weight loss of 13.37% for bare DES/MWCNTs and 14.16% for the system after the fourth cycle was observed between 300 °C and 400 °C, likely due to the removal of impurities or amorphous carbon content57. A minor weight loss of about 1.62% was observed in the temperature range of 265 °C to 272 °C, which may be attributed to the slight decomposition of choline chloride. However, beyond this, the system remained thermally stable up to 500 °C58. Therefore, based on the previously reported weight losses, the developed catalysts appear to be stabilized by the successful grafting of DES onto MWCNTs59. The results further confirm the excellent stability of 0.83mM DES/MWCNT catalyst, both before and after the reaction, with negligible weight loss. The catalyst was successfully recovered after each cycle and maintained good catalytic performance up to the fourth cycle. The superior stability of the MWCNT support and its moderately strong interaction with the active components significantly contributed to the overall stability of the system.
Mechanism for the reaction
The proposed mechanism for the reaction is shown in Fig. 8. The mentioned possible mechanism of the DES-catalysed Knoevenagel condensation60 involves the activation of the aldehyde by the acidic three-component deep eutectic solvent (DES)61, which forms hydrogen bonds, increasing electrophilicity. Simultaneously, DES facilitates enolate ion formation from the active methylene compound which is barbuteric acid62. This enolate nucleophilically attacks the carbonyl carbon, generating a β-hydroxy intermediate63. The acidic nature of DES promotes dehydration of this intermediate, yielding the α,β-unsaturated product. The DES not only stabilizes intermediates and transition states but also enhances proton transfer, making the process efficient and environmentally friendly.
Cytotoxicity studies
Statistical evaluation
IC50 value
The half maximal inhibitory concentration (IC50) is a metric used to understand how well a compound inhibits a biological or biochemical function (Table 7). It reflects the concentration of a substance needed to decrease the activity of a particular biological target such as an enzyme, cell, receptor, or microorganism by 50% (Fig. 9)64.
The IC50 of a drug is obtained by plotting a dose-response curve and evaluating how different concentrations of the antagonist influence the inhibition of agonist activity. IC50 values are calculated by identifying the concentration of the antagonist needed to achieve a 50% reduction in the maximum biological effect of the agonist65. IC50 values for cytotoxicity tests were determined through “nonlinear regression analysis” (curve fitting) based on the “sigmoid dose-response curve” and calculated accordingly66.
The control untreated cells demonstrated a healthy cell morphology, that is flat and adhering to the culture dish surface, however, the treated cells demonstrated spherical cell morphology that is characteristic of apoptotic process. The cell morphology of (A) untreated healthy cells, (B) treated with 7.8µg/ml of ligand, (C) treated with 15.6µg/ml of ligand, (D) treated with 31.25µg/ml of ligand are shown in Fig. 10. The percentage of cells that demonstrate apoptotic morphology is directly proportional to the concentration of the treated ligand. The test ligand demonstrated an observed IC50 value of 15.62µg/ml and a statistically calculated IC50 value of 9.8µg/ml. The value obtained is comparable to the standard anticancer drugs such as Doxorubicin with an IC50 value of 8.306µM; Cisplatin with an IC50 value of 13.3µM; and Vinblastine with an IC50 value of 67.12µM on MCF-7 Cells67,68,69. The remarkable IC50 value of the test ligand, along with its comparable activity to the established anticancer drugs, highlights its potential for further investigation in anticancer applications70.
Apoptosis investigation (AO/EB fluorescence staining)
To explore the apoptotic potential of the test ligand, fluorescence staining was carried out on MCF-7 cells that had been exposed to the test ligand for 24h. Dual staining with Acridine Orange (AO) and Ethidium Bromide (EB) was used to detect apoptosis in the MCF-7 cells. The fluorescence resulting from this staining method was captured using a fluorescence microscope. Figure 11 displays the fluorescence images of MCF-7 cells treated with different concentrations of the test ligand, followed by dual staining. The control MCF-7 cells fluorescence is shown in Fig. 11(A), and the cells treated with 7.8µg/ml, 15.62µg/ml, and 31.25µg/ml are shown in Fig. 11(B), Fig. 11(C), and Fig. 11(D), respectively. All the control cells demonstrate green color fluorescence, suggesting its healthy and live nature. It was observed that the cells under all the three different tested concentrations demonstrated only red and yellow fluorescence, with no cells showing green fluorescence. This strongly suggests that, even at the lowest concentration (7.8µg/ml) of test ligand, the compound was able to induce apoptosis in 100% of cells. AO/EB dual fluorescence staining studies clearly indicate that the test ligand is a potent anticancer agent, effectively inducing apoptosis in MCF-7 cells at all three tested concentrations.
Experimental section
Materials and methods
Benzothiophene carboxaldehyde (> 99%), and Barbituric acid (99%), were purchased from Merck, India Ltd. Choline chloride (Assay on dry material 98-100.5%), Glyoxal (40 wt% in H2O) and Lactic acid (≥ 85%) were purchased from SDFCL limited, India. All the chemicals were used without any further purification. The double-distilled water procured from Merck; India Ltd. was used in the preparation of the catalysts. Powder X-ray Diffraction (XRD) analysis was used to evaluate the structure, crystalline nature, and phase purity of the developed catalytic system. A Bruker AXS D8 Advance diffractometer operating at room temperature and with a scan rate of 0.05° per second was used to perform the XRD measurements. A Si (Li) PSD detector was used to gather data for the analysis, which covered a range of 10–80° and used Ni-filtered Cu Kα radiation (λ = 1.54 Å)71.
Morphology and average particle size was evaluated by Transmission electron microscopy analysis, which was performed using ultra-high-resolution analytical electron microscope Jeol/JEM 3010 (Source: LaB6, Voltage: 200 kV, Point to point resolution: 0.23 nm, and Magnification: 2000 X–1500000 X). Complete electronic structure was studied by X-ray photoelectron spectroscopy using an Omicron Nanotechnology XPS system with a monochromatic Al Kα radiation (hυ = 1,486.6 eV) of source voltage 15 kV and emission current of 20 mA. The obtained XPS spectra were deconvoluted using the Casa XPS program, (Casa Software Ltd, UK), in which the background was simulated using the Shirley function72. Mass data of the synthesized product was analysed by Mass Spectrometer with LC-MS/MS Model: Waters; Synapt G2 High detection Mass spectrometry. Thermo NICOLET FT-IR instrument was used to evaluate the vibrational modes of various functional groups in the final system. The surface acidity was analysed by pyridine adsorption followed by IR analysis. NMR data of the synthesized compound was analysed using FT NMR Spectrometer System-400 MHz, Make: Agilent, USA Model: 400MR DD2. The thermal stability of the system was assessed by thermogravimetric analysis (TGA) using PerkinElmer STA 6000 simultaneous thermal analyser with 5°C/min heating rate from 30° to 950°, to determine the onset weight losses.
Anti-cancerous activity studies
The monolayer cell culture was trypsinized and the cell count was adjusted to 1.0 × 105 cells/ml using respective media containing 10% FBS (Fetal bovine serum). To each well of the 96 well microtiter plate, 100µl of the diluted cell suspension (50,000cells/well) was added. After 24 h, when a partial monolayer was formed, the supernatant was flicked off, washed the monolayer once with medium and 100µl of different test concentrations of test drugs were added on to the partial monolayer in microtiter plates. The plates were then incubated at 37oC for 24h in 5% CO2 atmosphere. After incubation, the test solutions in the wells were discarded and 100µl of MTT (3-(4,5-Dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide) (5 mg/10 ml of MTT in (phosphate buffered saline) was added to each well. The plates were incubated for 4h at 37oC in 5% CO2 atmosphere. The supernatant was removed and 100µl of DMSO was added and the plates were gently shaken to solubilize the formed formazan. The absorbance was measured using a microplate reader at a wavelength of 590nm. The percentage growth inhibition was calculated using the following formula and concentration of test drug needed to inhibit cell growth by 50% (IC50) values is generated from the dose-response curves for each cell line73.
Calculation of Inhibition is as follows:
Where OD is optical density.
Fluorescence studies
The apoptotic potential of the test ligand was found by fluorescence staining on MCF-7 cells with the exposure time 24h. Acridine Orange (AO) and Ethidium Bromide (EB) were used for staining. The result was captured using a fluorescence microscope.
Materials preparation
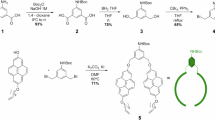
MWCNTs utilized as catalytic support were prepared by highly sophisticated catalytic chemical vapor deposition technique74,75. The synthesis procedure of DES required for the catalyst development is reported elsewhere61. The preparation was carried out by mixing equimolar ratio of Choline Chloride with Lactic Acid and Glyoxal in a glass container maintained at 80 °C for 30min. DES modified MWCNT was synthesized through an efficient and inexpensive mechanical grinding route. 0.5g of pristine MWCNTs was mechanically grinded for an hour with definite quantity of prepared DES in a mortar to get DES modified MWCNTs. The time duration was optimized to get final system and the obtained catalyst was dried in a hot air oven at 150 °C for about 12h54. The DES grafted MWCNTs are designated as DES/MWCNT for convenience. Figure 12 shows the schematic representation of the preparation of DES/MWCNT.
Conclusion
A heterogeneous catalyst was developed by dispersing a small quantity of three component acidic DES onto MWCNTs. The developed DES/MWCNT system was efficiently employed in the synthesis of a novel organic compound in a solvent free medium. FTIR, TEM and XPS analyses confirmed the effective incorporation of DES onto MWCNTs. The optimized condition for the reaction was achieved using 0.02g of 0.83mM DES/MWCNT at 80°C, resulting in a yield of 92%. The novel compound formed was tested for its anticancer activity and it demonstrated an observed IC50 value of 15.62 µg/ml and theoretically calculated IC50 value of 9.8µg/ml. Fluorescence studies further supported the findings, revealing that even a minute concentration of the synthesized compound has significant potential to induce 100% apoptosis in MCF-7 cells.
Data availability
All data generated or analysed during this study are included in this published article. The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Azizi, N. & Edrisi, M. Deep eutectic solvent immobilized on SBA-15 as a novel separable catalyst for one-pot three-component Mannich reaction. Microporous Mesoporous Mater. 240, 130–136. https://doi.org/10.1016/j.micromeso.2016.11.009 (Mar. 2017).
Clark, J. H. & Rhodes, C. N. Clean Synthesis Using Porous Inorganic Solid Catalysts and Supported Reagents (Royal Society of Chemistry, 2000). https://doi.org/10.1039/9781847550569
Azizi, N., Edrisi, M. & Abbasi, F. Mesoporous silica SBA-15 functionalized with acidic deep eutectic solvent: A highly active heterogeneous N-formylation catalyst under solvent-free conditions. Appl. Organomet. Chem. 32 (1), e3901. https://doi.org/10.1002/aoc.3901 (2018).
Anastas, P. T. & Warner, J. C. Green Chemistry: Theory and Practice (Oxford University Press, 2000).
Tanwar, B. et al. An ‘all-water’ strategy for regiocontrolled synthesis of 2-aryl quinoxalines. RSC Adv. 5 (16), 11873–11883. https://doi.org/10.1039/c4ra16568c (2015).
Kommi, D. N., Kumar, D., Seth, K. & Chakraborti, A. K. Protecting group-free concise synthesis of (RS)/(S)-lubeluzole. Org. Lett. 15 (6), 1158–1161 (2013).
Urgoitia, G., Herrero, M. T., Churruca, F., Conde, N. & Sanmartin, R. Direct arylation in the presence of palladium pincer complexes. Molecules 26 (14), 4385. https://doi.org/10.3390/molecules26144385 (2021).
Arisawa, M. & Yamaguchi, M. Rhodium-catalyzed synthesis of organosulfur compounds involving S-S bond cleavage of disulfides and sulfur. Molecules 25 (16), 3595. https://doi.org/10.3390/molecules25163595 (2020).
Wei, Q. et al. Synthesis of Ni-Modified ZSM-5 zeolites and their catalytic performance in n-Octane hydroconversion. Front. Chem. 8, 586445. https://doi.org/10.3389/fchem.2020.586445 (2020).
Chen, L. et al. Hydroconversion of n-octane over nanoscale HZSM-5 zeolites promoted by 12-molybdophosphoric acid and Ni. Catal. Commun. 8 (3), 416–423. https://doi.org/10.1016/j.catcom.2006.06.034 (2007).
Sun, Y. et al. The influence of zoned al distribution of ZSM-5 zeolite on the reactivity of hexane cracking. Mol. Catal. 484, 110770. https://doi.org/10.1016/j.mcat.2020.110770 (2020).
Yamaguchi, A. et al. Deactivation of ZSM-5 zeolite during catalytic steam cracking of n-hexane. Fuel Process. Technol. 126, 343–349. https://doi.org/10.1016/j.fuproc.2014.05.013 (2014).
Martínez-Edo, G., Balmori, A., Pontón, I., Del Rio, A. M. & Sánchez-García, D. Functionalized ordered mesoporous silicas (MCM-41): synthesis and applications in catalysis. Catalysts 8 (12), 617. https://doi.org/10.3390/catal8120617 (2018).
Zhang, L., Ren, Y., Liu, W., Wang, A. & Zhang, T. Single-atom catalyst: A rising star for green synthesis of fine chemicals. Natl. Sci. Rev. 5 (5), 653–672. https://doi.org/10.1093/nsr/nwy077 (2018).
Morales, O. M. & Gonzalez Sustainable green chemistry, vol. 6, no. 4. Walter de Gruyter GmbH & Co KG, (2017). https://doi.org/10.1515/gps-2017-0089
Arcuri, A. & Partiti, E. SDG 12: ensure sustainable consumption and production patterns. Camb. Handb. Sustain. Dev. Goals Int. Law. 304–327. https://doi.org/10.1017/9781108769631.014 (2022).
Wolny, A. & Chrobok, A. Ionic liquids for development of heterogeneous catalysts based on nanomaterials for biocatalysis, Nanomaterials, vol. 11, no. 8, p. 2021. (2030).
Bartlewicz, O., Dąbek, I., Szymańska, A. & Maciejewski, H. Heterogeneous catalysis with the participation of ionic liquids. Catalysts 10 (11), 1–16. https://doi.org/10.3390/catal10111227 (2020).
Smith, E. L., Abbott, A. P. & Ryder, K. S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 114 (21), 11060–11082. https://doi.org/10.1021/cr300162p (2014).
Ünlü, A. E., Arlkaya, A. & Takaç, S. Use of deep eutectic solvents as catalyst: A mini-review. Green. Process. Synth. 8 (1), 355–372. https://doi.org/10.1515/gps-2019-0003 (2019).
Abbott, A. P., Capper, G., McKenzie, K. J. & Ryder, K. S. Voltammetric and impedance studies of the electropolishing of type 316 stainless steel in a choline chloride based ionic liquid. Electrochim. Acta. 51 (21), 4420–4425. https://doi.org/10.1016/j.electacta.2005.12.030 (2006).
Wu, M. et al. Deep eutectic solvents: green solvents and catalysts for the Preparation of pyrazine derivatives by Self-Condensation of d -Glucosamine. ACS Sustain. Chem. Eng. 6 (7), 9434–9441. https://doi.org/10.1021/acssuschemeng.8b01788 (2018).
Azizi, N., Yadollahy, Z. & Rahimzadeh-Oskooee, A. An atom-economic and odorless thia-Michael addition in a deep eutectic solvent. Tetrahedron Lett. 55 (10), 1722–1725. https://doi.org/10.1016/j.tetlet.2014.01.104 (2014).
Hartwig, D. et al. Deep eutectic solvents: an alternative medium for the Preparation of organosulfur compounds. Curr. Green. Chem. 7 (2), 179–200. https://doi.org/10.2174/2213346107999200616110434 (2020).
Azizi, N. & Alipour, M. Eco-efficiency and scalable synthesis of bisamides in deep eutectic solvent. J. Mol. Liq. 206, 268–271. https://doi.org/10.1016/j.molliq.2015.02.033 (2015).
Miraki, M. K., Mehraban, J. A., Yazdani, E. & Heydari, A. Deep eutectic solvent (DES) as dual solvent/catalyst for synthesis of α-diazocarbonyl compounds using aldol-type coupling. J. Mol. Liq. 234, 129–132. https://doi.org/10.1016/j.molliq.2017.03.065 (2017).
Zhuravlev, Y. et al. Structural, electronic, and vibrational properties of choline halides. Mater. Chem. Phys. 246, 122787. https://doi.org/10.1016/j.matchemphys.2020.122787 (2020).
Pawlukojć, A. & Hetmańczyk, I. N. S. DFT and temperature dependent IR studies on dynamical properties of acetylcholine chloride. Vib. Spectrosc. 82, 37–43. https://doi.org/10.1016/j.vibspec.2015.11.008 (2016).
Pourhossein, M., Heravizadeh, O. R., Omidi, F., Khadem, M. & Shahtaheri, S. J. Ultrasound-Assisted emulsified Microextraction based on deep eutectic solvent for trace residue analysis of Metribuzin in urine samples. Methods Objects Chem. Anal. 16 (3), 153–161. https://doi.org/10.17721/moca.2021.153-161 (2021).
Dey, A., Bera, B., Bera, R. & Chakrabarty, D. Influence of diethylene glycol as a porogen in a Glyoxal crosslinked Polyvinyl alcohol hydrogel. RSC Adv. 4 (80), 42260–42270. https://doi.org/10.1039/c4ra04742g (2014).
Cassanas, G., Morssli, M., Fabrègue, E. & Bardet, L. Vibrational spectra of lactic acid and lactates. J. Raman Spectrosc. 22 (7), 409–413. https://doi.org/10.1002/jrs.1250220709 (1991).
Elashmawi, I. S., Abdelrazek, E. M., Hezma, A. M. & Elzayat, A. M. Multi-walled carbon nanotubes (MWCNTs) filler effects on some physical properties of PCL/PMMA blend films (2022).
Gautam, R., Kumar, N. & Lynam, J. G. Theoretical and experimental study of choline chloride-carboxylic acid deep eutectic solvents and their hydrogen bonds. J. Mol. Struct. 1222, 128849. https://doi.org/10.1016/j.molstruc.2020.128849 (2020).
Muniyappan, M. & Iyandurai, N. Structural Analysis of Aa2024 Interaction Reinforced With Carbon Nanotubes and Silicon Nanocomposites Studied By Fourier Transform Infrared Spectroscopy, in IOP Conference Series: Materials Science and Engineering, vol. 1219, no. 1, p. 012046. (2022). https://doi.org/10.1088/1757-899x/1219/1/012046
Nie, P. et al. Preparation and tribological properties of polyimide/carboxyl-functionalized multi-walled carbon nanotube nanocomposite films under seawater lubrication. Tribol Lett. 58 (1), 1–12. https://doi.org/10.1007/s11249-015-0476-7 (2015).
Veigas, L. M., Sunaja Devi, K. R., Chundattu, S. J. & Mohan, M. K. Synergistic Co-grafting of multiwalled carbon nanotubes using SO3H and choline chloride-urea in fabricating uniform thin films with enhanced visible light transparency and reduced sheet resistance. Opt. Mater. (Amst). 151, 115260. https://doi.org/10.1016/j.optmat.2024.115260 (2024).
Balaji, R. & Ilangeswaran, D. Choline chloride – Urea deep eutectic solvent an efficient media for the Preparation of metal nanoparticles. J. Indian Chem. Soc. 99 (5), 100446. https://doi.org/10.1016/j.jics.2022.100446 (2022).
Bhagavathi Kandy, S. et al. Effect of organic modification on multiwalled carbon nanotube dispersions in highly concentrated emulsions. ACS Omega. 4 (4), 6647–6659. https://doi.org/10.1021/acsomega.8b03179 (2019).
McQueen, E. W. & Goldsmith, J. I. Electrochemical analysis of single-walled carbon nanotubes functionalized with pyrene-pendant transition metal complexes. J. Am. Chem. Soc. 131 (48), 17554–17556. https://doi.org/10.1021/ja907294q (2009).
Khan, M. W. et al. Engineering N-reduced graphene oxide wrapped Co3O4@f-MWCNT hybrid for enhance performance dye-sensitized solar cells. J. Electroanal. Chem. 844, 142–154. https://doi.org/10.1016/j.jelechem.2019.05.008 (2019).
Yu, D. et al. Metal-organic framework derived co@nc/cnt hybrid as a multifunctional electrocatalyst for hydrogen and oxygen evolution reaction and oxygen reduction reaction. Int. J. Hydrogen Energy. 44 (60), 32054–32065. https://doi.org/10.1016/j.ijhydene.2019.10.149 (2019).
Okpalugo, T. I. T., Papakonstantinou, P., Murphy, H., McLaughlin, J. & Brown, N. M. D. High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon N Y. 43 (1), 153–161. https://doi.org/10.1016/j.carbon.2004.08.033 (2005).
Beamson, G. High resolution XPS of organic polymers. Sci ESCA 300 Database (1992).
Das, S. K., Dickinson, C., Lafir, F., Brougham, D. F. & Marsili, E. Synthesis, characterization and catalytic activity of gold nanoparticles biosynthesized with rhizopus oryzae protein extract. Green. Chem. 14 (5), 1322–1334. https://doi.org/10.1039/c2gc16676c (2012).
Wadekar, P. H., Khose, R. V., Pethsangave, D. A. & Some, S. One-Pot synthesis of sulfur and nitrogen Co-Functionalized graphene material using deep eutectic solvents for supercapacitors. ChemSusChem 12, 3326–3335. https://doi.org/10.1002/cssc.201900953 (2019).
Yu, T., Jiang, N. & Li, Y. Functionalized multi-walled carbon nanotube for improving the flame retardancy of ramie/poly(lactic acid) composite. Compos. Sci. Technol. 104, 26–33. https://doi.org/10.1016/j.compscitech.2014.08.021 (2014).
Yan, X. et al. Preparation and characterization of electrochemically deposited carbon nitride films on silicon substrate. J. Phys. D Appl. Phys. 37 (6), 907–913. https://doi.org/10.1088/0022-3727/37/6/015 (2004).
Bouayad, K. et al. Imidazo[4,5-b]pyridines as a new class of corrosion inhibitors for mild steel: experimental and DFT approach. Moroccan J. Chem. 6 (1), 22–34 (2018).
Geng, H. et al. Porous Fe3O4 Hollow spheres with chlorine-doped-carbon coating as superior anode materials for lithium ion batteries. RSC Adv. 5 (65), 52993–52997. https://doi.org/10.1039/c5ra09915c (2015).
Pełech, I., Narkiewicz, U., Moszyński, D. & Pełech, R. Simultaneous purification and functionalization of carbon nanotubes using chlorination. J. Mater. Res. 27 (18), 2368–2374. https://doi.org/10.1557/jmr.2012.243 (2012).
Parry, E. P. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J. Catal. 2 (5), 371–379. https://doi.org/10.1016/0021-9517(63)90102-7 (1963).
Du, P. et al. Synthesis of a novel micro/mesoporous composite material Beta-FDU-12 and its hydro-upgrading performance for FCC gasoline. RSC Adv. 6 (2), 1018–1026. https://doi.org/10.1039/c5ra19731g (2016).
Barzetti, T., Selli, E., Moscotti, D. & Forni, L. Pyridine and ammonia as probes for FTIR analysis of solid acid catalysts. J. Chem. Soc. - Faraday Trans. 92 (8), 1401–1407. https://doi.org/10.1039/ft9969201401 (1996).
Veigas, L. M. et al. In-Vitro Investigation of the α-Amylase Inhibition Activity of Bare Bis-Benzylidene-Cyclohexanone Synthesized by a Highly Selective Solvent-Free Route,ChemistrySelect, 8, 47, e202301807, 2023, doi: https://doi.org/10.1002/slct.202301807.
Naveen, C. & Premalatha, M. Thermo gravimetric and kinetic studies on dried solid waste of post-methanated distillery effluent under oxygen and nitrogen atmosphere. Bioresour Technol. 174, 126–133. https://doi.org/10.1016/j.biortech.2014.10.013 (2014).
Nicholson, P. Thermogravimetric analysis. Dict. Archaeol. 1, 576. https://doi.org/10.3139/9781569906446.010 (2008).
Srikanth, I. et al. Effect of high-temperature heat treatment duration on the purity and microstructure of MWCNTs. Bull. Mater. Sci. 39 (1), 41–46. https://doi.org/10.1007/s12034-015-0891-2 (2016).
Nguyen, D. et al. Deep eutectic solvent based on choline chloride and phenol as electrolyte additives in dye-sensitized solar cells: a comparison with 4-tert-butylpyridine. J. Aust Ceram. Soc. 58 (3), 913–921. https://doi.org/10.1007/s41779-022-00745-y (2022).
Kumar, L., Puneeth, S. V., Tabassum, S. & Govindaraju, S. Comprehensive study of the physicochemical properties of three-component deep eutectic solvents and their implications for microbial and anticancerous activity. J. Indian Chem. Soc. 101 (11), 101443. https://doi.org/10.1016/j.jics.2024.101443 (2024).
Haferkamp, S., Kraus, W. & Emmerling, F. Studies on the mechanochemical Knoevenagel condensation of fluorinated benzaldehyde derivates. J. Mater. Sci. 53, 13713–13718. https://doi.org/10.1007/s10853-018-2492-0 (2018).
Lokesh Kumar, S., Tabassum, S. & Govindaraju, S. Novel deep eutectic solvent catalysed Single-Pot open flask synthesis of Tetrasubstituted-1H-Pyrroles. J. Mol. Liq. 401, 124592. https://doi.org/10.1016/j.molliq.2024.124592 (2024).
Kumar, S. L., Tabassum, S. & Govindaraju, S. Environmentally conscious synthesis of novel pyrano[2,3-d]pyrimidines via ternary deep eutectic solvents. J. Mol. Liq. 417, 126651. https://doi.org/10.1016/j.molliq.2024.126651 (2025).
Trotzki, R., Hoffmann, M. M. & Ondruschka, B. The Knoevenagel condensation at room temperature. Green. Chem. 10, 873–887. https://doi.org/10.1039/b808265k (2008).
Ravi, L. et al. β-Sitosterol, a phytocompound from Parthenium hysterophorus, reveals anti-diabetic properties through α-Amylase inhibition: an in-silico and in-vitro analysis. J. Biomol. Struct. Dyn. 41 (24), 15033–15044. https://doi.org/10.1080/07391102.2023.2186703 (2023).
Ravi, L. & Krishnan, K. Cytotoxic potential of N-hexadecanoic acid extracted from Kigelia pinnata leaves. Asian J. Cell. Biol. 12 (1), 20–27. https://doi.org/10.3923/ajcb.2017.20.27 (2016).
Zaharieva, M. M. et al. New insights in routine procedure for mathematical evaluation of in vitro cytotoxicity data from cancer cell lines. Int. J. Bioautomation. 22 (2), 87–106. https://doi.org/10.7546/ijba.2018.22.2.87-106 (2018).
Oncul, S. & Ercan, A. Discrimination of the effects of doxorubicin on two different breast cancer cell lines on account of multidrug resistance and apoptosis. Indian J. Pharm. Sci. 79 (4), 599–607. https://doi.org/10.4172/pharmaceutical-sciences.1000268 (2017).
Zou, J. et al. Curcumin increases breast cancer cell sensitivity to cisplatin by decreasing FEN1 expression. Oncotarget 9 (13), 11268–11278. https://doi.org/10.18632/oncotarget.24109 (2018).
Zandi, M. Synergistic effect of taxol and vincristine against MCF-7 cell line. Gene Cell. Tissue. 10 (3). https://doi.org/10.5812/gct-126544 (2023).
Ravi, L., Sreenivas, B. K. A., Kumari, G. R. S. & Archana, O. Anticancer cytotoxicity and antifungal abilities of green-synthesized Cobalt hydroxide (Co(OH)2) nanoparticles using Lantana camara L. Beni-Suef Univ. J. Basic. Appl. Sci. 11 (1). https://doi.org/10.1186/s43088-022-00304-1 (2022).
Qin, S. Y., Pei, Y., Liu, X. J., Zhuo, R. X. & Zhang, X. Z. Hierarchical self-assembly of a β-amyloid peptide derivative. J. Mater. Chem. B. 1 (5), 668–675. https://doi.org/10.1039/c2tb00105e (2013).
Veigas, L. M., Chandran, N., Krishna, B. M., KR, S. D. & Mohan, M. K. Heterojunction engineered MWCNT/Ag3PO4 via organic acid and its natural light-assisted photocatalytic efficiency. Nano-Structures Nano-Objects. 34, 100975. https://doi.org/10.1016/j.nanoso.2023.100975 (2023).
Ravi, L. et al. Stearyl palmitate a multi-target inhibitor against breast cancer: in-silico, in-vitro & in-vivo approach. J. Biomol. Struct. Dyn. 1–18. https://doi.org/10.1080/07391102.2023.2255271 (2023).
Kathyayini, H., Willems, I., Fonseca, A., Nagy, J. B. & Nagaraju, N. Catalytic materials based on aluminium hydroxide, for the large scale production of bundles of multi-walled (MWNT) carbon nanotubes, Catal. Commun., vol. 7, no. 3, pp. 140–147, Mar. (2006). https://doi.org/10.1016/j.catcom.2005.05.010
Kathyayini, H., Nagaraju, N., Fonseca, A. & Nagy, J. B. Catalytic activity of Fe, Co and Fe/Co supported on Ca and Mg oxides, hydroxides and carbonates in the synthesis of carbon nanotubes, J. Mol. Catal. A Chem., vol. 223, no. 1–2, pp. 129–136, Dec. (2004). https://doi.org/10.1016/j.molcata.2004.02.029
Acknowledgements
The authors are thankful to the management of Christ University, Bengaluru, India, for the facilities, and support provided. We extend our thanks to Professor Dr. N. Nagaraju for providing MWCNTs for the current work. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
L M Veigas: Project design, execution of the work and writing.Dr. M K Mohan: Project design, guidance, and technical writing.Dr. L Ravi: Anti Cancerous activity studies and writing.Dr. P N Chandra: Data interpretation and technical writing Dr. S G: Scheme of the reaction and Contribution of DESL S Kumar: Spectral data interpretation and write up.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Veigas, L.M., Ravi, L., Santhosh, G. et al. One pot synthesis of a novel bioactive compound employing a deep eutectic solvent grafted MWCNT system in a solventless environment. Sci Rep 15, 26100 (2025). https://doi.org/10.1038/s41598-025-11153-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11153-0