Abstract
IgG-based therapeutic antibodies are increasingly adopted for diverse human diseases, such as cancer and autoimmune disorders displaying remarkable therapeutic performance. A key factor in their success lies in the extended half-life of IgG molecules, which is regulated by the pH-dependent interaction between IgG and neonatal Fc receptor (FcRn). This interaction prevents lysosomal degradation of IgG. Despite the frequent use of humanized rodent models expressing human FcRn (hFcRn) in preclinical studies, these models often fail to accurately replicate human antibody pharmacokinetics (PK) due to the use of non-native promoters that influence FcRn expression. To overcome this limitation, we developed an innovative humanized FcRn knock-in (hiFcRn) mouse model using CRISPR/Cas9 technology. This model integrates hFcRn cDNA into the endogenous locus of the mouse Fcgrt gene, completely replacing native mouse FcRn (mFcRn) expression. The hiFcRn mouse model offers a more human-relevant platform for the preclinical evaluation of therapeutic antibodies and Fc-fusion proteins.
Similar content being viewed by others
Introduction
Over the past four decades, more than 140 IgG-based therapeutics have received clinical approval, revolutionizing the treatment of various human diseases, including cancer, infectious diseases, and autoimmune disorders1,2. The therapeutic landscape has expanded beyond monoclonal antibodies to include antibody fragments, bispecific antibodies, and antibody–drug conjugates (ADCs), reflecting the increasing versatility and innovation in this field1,2. A critical factor underlying the success of IgG-based therapeutics is their prolonged serum persistence, typically around three weeks in humans (except for the IgG3 subclass with a one-week half-life)3,4. This extended circulation depends predominantly on the neonatal Fc receptor (FcRn), a member of the major histocompatibility complex (MHC) class I family.
FcRn is a heterodimeric membrane protein composed of an α chain and β2-microglobulin5. It is expressed in a variety of cell types, including parenchymal cells (such as endothelial and epithelial cells) and hematopoietic cells, and is predominantly localized in intracellular acidified endosomes6,7,8. FcRn binds to the Fc region of IgG in a pH-dependent manner with a 2:1 stoichiometry9, interacting at endosomal pH (5.5–6.5) and releasing IgG at serum pH (7.0–7.5)7,8,9,10,11,12. Circulating IgGs in the bloodstream are internalized via pinocytosis, where they encounter FcRn within early endosomes and form FcRn–IgG complexes. These complexes protect IgG from lysosomal degradation and recycle it to the cell surface, releasing it back into the bloodstream7,11,13,14. Engineered Fc variants that enhance this pH-dependent FcRn binding have demonstrated the potential to extend IgG serum half-life, reduce dosing frequency, and improve both patient convenience and therapeutic efficacy15,16. Therefore, accurately modeling pH-dependent FcRn interactions is crucial for predicting the pharmacokinetics and pharmacodynamics of IgG-based therapeutics in humans.
Rodent models are among the most frequently utilized platforms in preclinical research on IgG-based therapeutics, owing to their accessibility, affordability, and the wealth of existing data supporting their use. However, the inherent differences in the pH-dependent binding profiles of human IgG to human FcRn (hFcRn) versus mouse FcRn (mFcRn) present significant challenges. These discrepancies limit the predictive accuracy of rodent models for the pharmacokinetics of human IgG-based therapeutics, especially for engineered Fc variants designed to enhance serum persistence17,18,19,20. To address these limitations, several transgenic mouse models expressing hFcRn (e.g., the widely used Jackson Laboratory Tg32 and Tg276 lines) have been developed. These models provide a more physiologically relevant platform for studying the pharmacokinetics of human IgG-based therapeutics12,16, offering critical insights for the optimization of Fc engineering and translational success.
Nevertheless, current hFcRn transgenic mouse models rely on non-native promoters, such as the synthetic CAG or human FcRn promoter, which may not fully replicate the complex tissue-specific expression of FcRn in humans. Moreover, random integration of transgenes can lead to tandem repeats and positional effects, potentially resulting in irregular or non-physiological FcRn expression21,22. Additionally, the usage of exogenous promoters, such as the synthetic CAG promoter or the human endogenous FcRn promoter, introduces further challenges in achieving physiologically relevant expression12,23. The CAG promoter—a synthetic construct composed of the cytomegalovirus (CMV) immediate-early enhancer, chicken beta-actin promoter, and rabbit beta-globin polyadenylation signal—may not fully replicate the tissue-specific expression patterns and regulatory controls of FcRn in humans. Similarly, while the human endogenous FcRn promoter has been employed in rodent models, species-specific differences in transcriptional regulation can result in deviations in expression levels and kinetics. Consequently, there is a continuing need for animal models that express hFcRn under endogenous regulatory control, faithfully mimicking the spatiotemporal expression patterns and functional dynamics of FcRn in humans. Such models would provide a robust platform for studying the pharmacokinetics of IgG-based therapeutics and optimizing Fc engineering for clinical translation.
Here, we describe a novel humanized FcRn knock-in (hiFcRn) mouse model. In this system, hFcRn cDNA is precisely integrated into the endogenous locus of the murine Fcgrt gene, positioned between the promoter region and the native mFcRn coding sequence while keeping endogenous murine signal peptide. A stop codon in the human cDNA ablates murine FcRn (mFcRn) expression, while allowing hFcRn to be expressed under the control of the native mFcRn promoter. This design preserves the tissue-specific and temporal expression patterns of FcRn more closely than exogenous-promoter-based approaches.
Our data show that the hiFcRn mouse fully replaces mFcRn expression with hFcRn at both the mRNA and protein levels. Pharmacokinetic profiling using antibodies with different pH-dependent FcRn binding reveals that the hiFcRn mouse recapitulates key aspects of human IgG–FcRn interactions. In addition to its potential utility for pharmacokinetic analyses, this model provides an endogenously regulated framework for studying other FcRn-related functions, such as immunogenicity and Fc-dependent recycling. We anticipate that the hiFcRn mouse will be a useful complement to existing transgenic lines in bridging preclinical and clinical outcomes for IgG-based therapeutics.
Result
Generation and validation of human FcRn knock-in (hiFcRn) mouse model
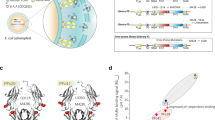
To overcome the limitation of existing models, we synthesized 3,174 bp donor sequence which includes right and left homologous arms and the hFcRn gene. This sequence was inserted into the mFcRn coding region via Clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein 9 (Cas9) genome editing system (Fig. 1A)24. To preserve the native translocation of protein, we fused the endogenous signal peptide sequence from the murine Fcgrt (comprising of 21-amino-acids) to the 5’ of hFcRn cDNA. Additionally, a late polyadenylation (LPA) signal sequence derived from Simian vacuolating virus (SV40) was synthesized at 3’ region of hFcRn cDNA to ensure efficient termination of transcript and enhanced mRNA stability. The stop codon introduced by hFcRn cDNA effectively knocked out the native mouse Fcgrt gene, a feature that differentiates our model from existing ones established on the mFcRn-null background.
Generation and validation of human FcRn knock-in (hiFcRn) mouse model. (A) Schematic diagram illustrating the knock-in strategy for integrating human FcRn (FCGRT) cDNA into the murine Fcgrt locus. (B) mRNA expression levels of Fcgrt (mouse) and FCGRT (human) in the liver, lung, heart and kidney tissues of 10-week-old wild-type (+/+), heterozygous knock-in (KI/+), and homozygous knock-in (KI/KI) mice. RT-qPCR results were normalized to Gapdh, and all groups were of the C57BL/6N genetic background (n = 3/group). (C,D) ELISA results showing hFcRn levels in liver (C) and lung (D) tissue extracts from wild-type (+/+), heterozygous knock-in (KI/+), homozygous knock-in (KI/KI) and Tg32 (homozygous, Jackson Laboratory) mice (n = 3/group). (E) Quantification of hFcRn in tissue extracts from the lungs and liver of heterozygous knock-in (KI/+), homozygous knock-in (KI/KI), and Tg32 (homozygous) mice, performed using soluble hFcRn titrated in parallel (data not shown). (F) Western blot analysis human and mouse FcRn protein expression from various murine tissue extracts. Data are presented as mean ± s.d. from one experiment performed in duplicates.
In homozygous hiFcRn mice, FCGRT fully replaced Fcgrt expression (Fig. 1B and C). Levels of FCGRT mRNA in these mice were comparable to those of mFcRn in wild-type controls. In heterozygous mice, both Fcgrt and FCGRT were expressed at intermediate levels, further confirming that our hiFcRn knock-in strategy effectively preserves native regulation of FcRn expression.
To evaluate and quantify hFcRn expression in hiFcRn mouse tissues and to compare it with existing hFcRn transgenic mouse models, we performed ELISA and Western blot (WB) analysis on protein extracts from the multiple tissues―liver, lung, heart, brain, kidney and spleen―of homozygous hiFcRn, heterozygous hiFcRn, wild-type (C57BL/6), and Jackson’s Tg32 (homozygous) mice. For ELISA, protein extracts were applied to plates coated with rozanolixizumab, an anti-FcRn antibody with a reported KD of 34 pM for hFcRn at neutral pH25. Subsequently, hFcRn binding was analyzed using the ADM31 clone, an hFcRn-selective antibody that does not bind mFcRn26. Robust hFcRn binding signals were detected in protein extracts from the lung and liver tissues of homozygous hiFcRn, heterozygous hiFcRn, and homozygous Tg32 mice, whereas the signals from C57BL/6 mice were negligible and comparable to the blank control (Fig. 1D-E). Quantification of hFcRn in the protein extracts, using soluble hFcRn as a standard (data not shown), revealed distinct expression levels across tissue types and genotypes. In the lung tissues of homozygous and heterozygous hiFcRn mice, hFcRn expression levels were quantified as 10.69 ± 0.81 and 25.11 ± 2.03 pmol/g of tissue, respectively, and in liver tissues, 34.78 ± 2.32 and 80.87 ± 3.50 pmol/g of tissue. Consistent with mRNA expression analysis, these results demonstrate that hFcRn expression levels are approximately two-fold higher in homozygous hiFcRn mice compared to heterozygous counterparts. Notably, the hFcRn expression levels in the lung and liver tissues of homozygous Tg32 mice were higher than those in homozygous hiFcRn mice, measuring 49.24 ± 2.31 and 142.11 ± 4.96 pmol/g of tissue, respectively. Furthermore, tissue proteins detected by an antibody which has cross-reactivity on human and mouse FcRn showed comparable protein expression over tissue types and genotypes (Fig. 1F). Together, these findings confirm that homozygous hiFcRn mice express hFcRn at both the mRNA and protein levels, effectively replacing mFcRn. Furthermore, the hFcRn expression patterns in hiFcRn mice differ from those in Jackson’s Tg32 mice, indicating distinct overall expression levels between the two models.
Generation of human IgG Fc variants with distinct pH-dependent binding profiles to hFcRn and mFcRn
To determine whether the hiFcRn mouse model expresses functional hFcRn replacing mFcRn and to evaluate its utility for predicting the pharmacokinetic profiles of human IgG therapeutic antibodies, we utilized three trastuzumab-Fc variants—WT (wild-type Fc), IHH, and PFc29—each with distinct pH-dependent binding characteristics to hFcRn.
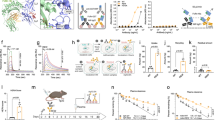
To generate these three trastuzumab-Fc variants for this study, we performed transient expression in Expi293F cells, followed by purification using Protein A affinity chromatography and size exclusion chromatography. This approach successfully yielded trastuzumab-Fc variants with high purity (> 99.3%) (Fig. 2A–D). The binding profiles of these purified trastuzumab-Fc variants to hFcRn and mFcRn were analyzed under endosomal and serum pH conditions using ELISA (Fig. 2E and F). As expected, trastuzumab with WT exhibited higher binding to mFcRn than to hFcRn at endosomal pH, aligning with prior reports18,27. Trastuzumab-IHH which is a mutated variant carrying the I253A, H310A, and H435A triple substitution (collectively referred to as ‘IHH’ mutations) abrogates FcRn binding on its mutant IgG region. As expected, this showed binding on neither hFcRn nor mFcRn under both endosomal and serum pH conditions. Trastuzumab-PFc29 demonstrated significantly increased binding to hFcRn at endosomal pH compared to trastuzumab. Interestingly, trastuzumab-PFc29 also showed enhanced binding to mFcRn at endosomal pH and markedly higher binding to mFcRn at serum pH. These results confirm the successful generation of three model antibody-Fc variants with distinct and unique pH-dependent binding profiles, establishing them as valuable tools for validating the hiFcRn mouse model’s utility in pharmacokinetic evaluation of IgG antibodies bearing WT or engineered Fc variants.
Generation of human IgG Fc variants with distinct pH-dependent binding profiles to hFcRn and mFcRn. (A–C) Size-exclusion chromatography (SEC) profiles of purified trastuzumab (A), trastuzumab-IHH (B), and trastuzumab-PFc29 (C) obtained using a Superdex® Increase 200 column using an NGC chromatography system. (D) SDS-PAGE analysis of the trastuzumab-Fc variants under non-reduced conditions following SEC. (E,F) Binding of trastuzumab-Fc variants to hFcRn and mFcRn was assesed at endosomal pH (pH 6.0) (E) and serum pH (pH 7.4) (F) was assessed using ELISA.
hiFcRn mouse as a robust model for predicting human pharmacokinetics of IgG antibodies mediated by the hFcRn binding and recycling mechanism
To assess whether the hiFcRn mouse model accurately reflects the pH-dependent binding profiles between human IgG Fc and hFcRn, and to evaluate its potential as a predictor of pharmacokinetics influenced by hFcRn-mediated intracellular trafficking and recycling in humans, pharmacokinetic analyses were conducted using the three trastuzumab-Fc variants with distinct pH-dependent FcRn binding profiles. Each trastuzumab-Fc variant was intravenously administered to C57BL/6 and hiFcRn mice, with serum concentrations monitored over time to calculate pharmacokinetic parameters (Fig. 3A and Table 1).
hiFcRn mouse as a robust model for predicting human pharmacokinetics of IgG antibodies mediated by the hFcRn binding and recycling mechanism. (A) Schematic diagram illustrating pharmacokinetics evaluation strategy in wild-type and hiFcRn mice. (B,C) Serum concentration vs. time profiles for the trastuzumab-Fc variants (WT, IHH and PFc29) in wild-type (B) and hiFcRn (C) mice. Each drug was intravenously administered at 5 mg/kg to 10-week-old mice (5/group), and serum concentrations were measured over time.
The serum half-life (t1/2) of trastuzumab with WT was 13.10 ± 4.59 days in C57BL/6 mice and 8.3 ± 1.41 days in hiFcRn mice (Fig. 3B, C, and Table 1). This extended half-life in C57BL/6 mice is attributed to the higher binding affinity of WT for mFcRn compared to hFcRn at endosomal pH, consistent with previously reported findings27 (Fig. 2E and F). In contrast, trastuzumab-IHH, which lacks binding to both hFcRn and mFcRn, exhibited a rapid decline in serum concentration in both C57BL/6 and hiFcRn mice. This result confirms that both C57BL/6 and hiFcRn mice express functional FcRn. Notably, trastuzumab-PFc29 demonstrated an extended serum half-life of 18.93 ± 3.46 days in hiFcRn mice, significantly longer than trastuzumab. This result is consistent with findings observed in cynomolgus monkeys16, highlighting the enhanced pH-dependent binding of PFc29 to hFcRn as the key determinant of its superior pharmacokinetic profile (Fig. 2E and F). In C57BL/6 mice, however, trastuzumab-PFc29 showed a shorter half-life (10.47 ± 1.35 days) compared to trastuzumab. This finding is consistent with reports that pH-independent enhancements in FcRn binding under both endosomal and serum pH conditions can reduce serum persistence compared to selective pH-dependent FcRn binding improvements28. To further compare the pharmacokinetic profiles of human IgG in transgenic mouse models with different hFcRn expression profiles, we performed pharmacokinetic analysis of trastuzumab using Jackson’s homozygous Tg32 mice under identical conditions (Supplementary Fig. 1 and Supplementary Table 1). In line with the observed differences in tissue hFcRn expression, the serum half-life of trastuzumab in homozygous Tg32 mice was 11.8 ± 2.5 days, exceeding that observed in hiFcRn mice. In summary, the hiFcRn mouse model expresses functional hFcRn and accurately reflects the pH-dependent binding profiles observed for human IgG Fc variants. This model serves as an exceptional platform for predicting the pharmacokinetic profiles of human IgG antibodies, including WT and engineered Fc variants, by reflecting hFcRn binding-mediated intracellular trafficking and recycling. These findings establish the hiFcRn mouse as a critical tool for accelerating the preclinical development of therapeutic IgG antibodies.
Discussion
A fundamental factor contributing to the therapeutic efficacy of IgG-based therapeutics is their prolonged serum persistence, which is critically dependent on FcRn-mediated intracellular recycling. Consequently, pharmacokinetic analysis of IgG-based therapeutics is essential for predicting their therapeutic efficacy. However, human IgG exhibits species-specific differences in FcRn binding profiles, making it challenging to use animal models to accurately predict pharmacokinetics in humans.
In the study of antibody dynamics using animal models, Tg32 and Tg276 rodent models are among the extensively used and widely accepted experimental platforms. Although Tg32 model is originally designed as a homozygous, hemizygous Tg32 mice—despite their semi-humanized genetic background—have been widely adopted and validated as a practical tool for modeling human IgG pharmacokinetics, particularly due to their moderate FcRn expression and potentially more physiological IgG half-life. These models have made a profound impact on translation research by enabling a more human-relevant framework for evaluating IgG pharmacokinetics and guiding Fc engineering strategies. However, the Tg32 model employs the human FCGRT promoter to regulate hFcRn expression. This promoter was intended to drive the expression of the hFcRn transgene and closely mimic human expression patterns, which may not naturally occur or function identically in mice. While this model has proven highly valuable in numerous studies and remains a popular platform, it may face certain limitations. FcRn undergoes transcriptional regulation through transcription factors such as SP1, and species-specific differences in these regulatory pathways must be carefully considered in addition to promoter selection29. Furthermore, interspecies variations in signaling pathways such as TNF-α and NF-κB, which directly or indirectly influence FcRn expression, can introduce additional complexities30,31. Although these pathways are highly conserved in eukaryotes, additional components must be optimized to achieve a more human-like physiological relevance in rodents.
Meanwhile, Tg276 model utilizes the powerful exogenous CAG promoter to achieve high levels of hFcRn expression. This design can yield robust FcRn levels in vivo but may introduce non-physiological expression patterns due to the absence of native regulatory elements. Despite these conditions, both Tg32 and Tg276 have provided invaluable insights into FcRn-IgG interactions for many therapeutic antibody programs. However, this model does not accurately replicate tissue-specific expression patterns and physiological responsiveness, as it lacks regulatory mechanisms responding to external stimuli. Consequently, this model deviates from the human physiological environment, limiting its utility for accurately modeling FcRn dynamics in vivo.
Moreover, their reliance on random transgene integration introduces significant challenges. Random integration can lead to positional effects, resulting in unpredictable expression patterns. Moreover, tandem repeats of the transgene often occur, causing gene dosage effects and potential silencing through epigenetic modifications, which reduce expression stability and fidelity. These limitations put an emphasis on the need for a model that aligns transgene expression with endogenous regulatory elements. Consequently, the tissue expression patterns of hFcRn in Tg32 and Tg276 mice differ from those observed in humans, underscoring the limitations of these models23.
Beyond IgG recycling, FcRn has broader physiological and immunological roles. FcRn binds not only IgG but also albumin in a pH-dependent manner, contributing to the prolonged circulation and biodistribution of both molecules via transcytosis32,33,34. Furthermore, FcRn transports IgG across the placenta, providing passive immunity to the fetus, and mediates the binding of immune complexes (ICs) to facilitate antigen presentation and T-cell activation35,36,37,38,39. These diverse functions underscore the importance of accurately replicating FcRn expression and regulation to predict not only the pharmacokinetics but also the pharmacodynamics and immunological roles of IgG-based therapeutics in humans.
To address these limitations, we developed the hiFcRn mouse model, which integrates hFcRn cDNA into the endogenous Fcgrt locus. A similar approach has been reported recently by others, underscoring the growing interest in more precise genome editing strategies for FcRn humanization40. Like our hiFcRn mice, that approach also employed CRISPR/Cas9 to integrate the human FcRn coding sequence at murine Fcgrt locus. However, in our model, precise genomic integration preserves not only the native murine promoter but also the signal peptide, ensuring proper protein processing and expression regulation, and we directly validated that our hiFcRn line expresses human FcRn mRNA and protein at physiologically relevant levels across multiple tissues. By maintaining the native murine promoter, the hiFcRn model ensures tissue-specific and temporally regulated expression of human FcRn in a manner more physiologically consistent with endogenous mouse FcRn dynamics. This approach eliminates the variability associated with random transgene integration and exogenous promoters, providing a more consistent and predictive biological response.
One potential difference from fully humanized systems is that our hiFcRn mice still retain mouse β2-microglobulin (β2m). However, previous publications have shown that human FcRn can pair functionally with mouse β2m in transgenic settings, resulting in minimal differences in IgG half-lives relative to mice expressing both human FcRn and human β2m41. Thus, we anticipate that our hiFcRn mice accurately recapitulate key pharmacokinetic behaviors of human IgG, despite the presence of murine β2m.
Pharmacokinetic analysis validates the utility of the hiFcRn model. The serum half-life of WT Fc was shorter in hiFcRn mice compared to C57BL/6 mice, reflecting the higher binding affinity of WT human IgG to mFcRn at endosomal pH. In contrast, the PFc29 variant, engineered for enhanced FcRn binding at acidic pH, exhibited a significantly prolonged serum half-life in the hiFcRn model, consistent with pharmacokinetic data obtained in cynomolgus monkeys16. These findings demonstrate the hiFcRn model’s ability to predict the pharmacokinetic improvements conferred by Fc engineering strategies. Notably, while the Tg32 model exhibited higher FcRn expression levels and a longer serum half-life for WT Fc, this overexpression may amplify the FcRn-mediated recycling beyond physiological levels, limiting its predictive accuracy for human pharmacokinetics. In contrast, the physiologically regulated FcRn expression in the hiFcRn model offers a biologically relevant platform for studying FcRn-mediated pharmacokinetics of human IgG antibodies.
Although the use of the endogenous murine promoter ensures physiologically regulated FcRn expression within the mouse, it does not reproduce the human-specific spatiotemporal expression profile of FcRn. In particular, well known species-specific differences—such as high FcRn expression in the neonatal rodent gut—highlight the intrinsic limitations of using murine regulatory elements to fully humanize expression patterns. This limitation is not unique to our model but is a shared constraint across genetically engineered mouse models. Nonetheless, maintaining natural promoter-driven expression in the mouse remains critical for avoiding ectopic or supraphysiological expression, thereby providing a biologically consistent output for evaluating FcRn-dependent pharmacokinetics and Fc-engineering strategies. Future refinements in mouse models incorporating human regulatory elements or tissue-specific control systems will be essential to further close the gap toward complete physiological humanization.
In conclusion, the hiFcRn provides a new option for preclinical testing of therapeutic antibodies, capitalizing on an endogenous regulatory framework to achieve stable expression of hFcRn across tissues. While previous models have contributed significantly to shaping our understanding of human IgG pharmacokinetics, the hiFcRn model may offer another avenue for refining predictions of IgG half-life and immunological function, thus broadening the platform available to antibody researchers. Future comparisons among Tg32 hemizygotes, Tg276, and hiFcRn mice will be particularly informative for determining which model best suits a given therapeutic or research questions.
Material and methods
Animals
All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Yonsei University (Permission number: IACUC-202111–1361-01). We adhered to the ARRIVE guidelines and conducted procedures in accordance with the guidelines of the Korean Food and Drug Administration (KFDA) and IACUC, ensuring strict compliance with animal ethics as guided by the IACUC. Mice were maintained in the specific pathogen-free (SPF) facility of the Yonsei Laboratory Animal Research Center and housed at 22 °C–24 °C under a 12 h light/12 h dark cycle. Animals had access to water and food ad libitum. hiFcRn mice (B6-Fcgrtem1(FCGRT)1Gmcr) were obtained from Gemcro, Inc., Seoul, Korea. For the pharmacokinetic experiments, mice in control and experimental groups received intraperitoneal injections of the drug. The serum half-life of each drug was determined by analyzing samples collected from the tail vein over time. In a separate set of animals, to analyze basal FcRn expression, mice were euthanized using CO2, and liver and lung tissues were harvested and stored at −80 °C until use.
Expression and purification of monoclonal antibodies
To generate IgG antibodies, plasmids encoding the heavy chains (pMAZ-rozanolixizumab, pMAZ-trastuzumab, pMAZ-trastuzumab-IHH, pMAZ-trastuzumab-PFc29) were co-transfected with pMAZ-rozanolixizumab or pMAZ-trastuzumab light chain plasmid at a 1:1 ratio into Expi293F cells using a fourfold excess of PEI MAX® (Polyscience, Warrington, PA, USA). For the expression of human and mouse FcRn-GST, the plasmids pcDNA3-hFcRn-GST42 and pMAZ-mFcRn-GST were similarly transfected into Expi293F cells. HER2-His was also expressed by transfecting Expi293F cells with a fourfold excess of PEI MAX®. Cells were cultured in Gibco FreeStyle™ 293 medium at 37 °C with 8% CO2 for 7 days. Following the incubation period, the cultures were centrifuged at 2,000 × g for 10 min to collect the supernatant, which was subsequently filtered through a 0.2 μm bottle-top filter (Corning, Corning, NY, USA). The filtered supernatant was loaded onto a disposable polypropylene column (Thermo Fisher Scientific, Waltham, MA, USA) packed with Protein A resins (Amicogen, Jinju, South Korea), washed with 10 column volumes (CV) of 1 × PBS, and eluted using 100 mM glycine–HCl (pH 2.7). The eluate was immediately neutralized with 1 M Tris (pH 8.0). Each sample was then subjected to size-exclusion chromatography on a Superdex® Increase 200 column (Cytiva, Marlborough, MA, USA) using an NGC chromatography system (Bio-Rad, Hercules, CA, USA) at a flow rate of 1 ml/min with Endotoxin-Free Dulbecco’s PBS (MilliporeSigma, Burlington, MA, USA), resulting in trastuzumab-Fc variants with over 99.3% purity. For human and mouse FcRn-GST, the filtered supernatant was applied to a Glutathione resin (GenScript, Piscataway, NJ, USA), washed with 10 CV of 1 × PBS, and eluted with 50 mM Tris–HCl (pH 8.0) containing 10 mM GSH. Buffer exchange was performed using an Amicon Ultra-4 spin column (3 kDa cutoff; MilliporeSigma, Burlington, MA, USA) with 1 × PBS. Similarly, for HER2-His, the filtered supernatant was applied to Ni–NTA resin (Qiagen, Hilden, Germany), washed with 10 CV of 1 × PBS, followed by a 10 mM imidazole buffer in 1 × PBS, and eluted with 250 mM imidazole. The eluate was further processed through buffer exchange using an Amicon Ultra-4 spin column with 1 × PBS.
RNA extraction and qPCR analysis
Total RNA from homogenized tissue samples or cells was harvested using the TRIzol™ reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Total RNA (1 μg) was reverse-transcribed into complementary DNA (cDNA) using RevertAid First Strand cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA). Real-time qPCR was conducted on a CFX Connect™ real-time system (Bio-Rad Laboratories, Hercules, CA, USA) using the SYBR Green real-time PCR master mix (Life Technologies, Carlsbad, CA, USA). Gene expression analysis was performed using the 2-ΔΔCt method. Primers for human and mouse FcRn (FCGRT and Fcgrt) were designed on exon junction (Supplementary Table 2) and normalized by using mouse Actb as an internal reference gene.
Purification of tissue protein
Mouse liver and lung tissues were homogenized in a mild lysis buffer containing 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 0.5% Nonidet P-40, 0.1% CHAPS [3-((3-cholamidopropyl)dimethylammonio)−1-propanesulfonate], and a protease inhibitor cocktail. The homogenates were incubated on ice for 30 min, followed by centrifugation at 12,000 × g for 15 min at 4 °C. The resulting supernatants were collected, and protein concentrations were determined using the bicinchoninic acid (BCA) assay.
Western blot
Quantified protein samples from mouse tissues were mixed with 2 × SDS sample buffer. Equal amounts of protein (50 μg) were separated by SDS-PAGE on 12% polyacrylamide gels and transferred onto 0.2 μm nitrile-cellulose (NC) membranes (Genomed, Seoul, South Korea). Membranes were blocked with 5% skim milk or BSA in TBST (Tris-buffered saline with 0.1% Tween-20) for 30 min at room temperature, followed by incubation with primary antibodies against HSP90 (sc-13119, Santa Cruz Biotechnology) and human/mouse FcRn (MBS1488262, MyBioSource) overnight at 4 °C. After washing, membranes were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. Immunoreactive bands were visualized using enhanced chemiluminescence (ECL) reagents (prepared in-house) and imaged using a chemiluminescence detection system (ChemiDoc™, Bio-Rad).
ELISA
To quantify hFcRn in mouse tissues, 50 μL of rozanolixizumab (diluted to 10 μg/mL in 0.05 M Na₂CO₃, pH 9.6) was coated onto flat-bottom, high-binding, 96-well polystyrene microplates (Corning, Corning, NY, USA) and incubated for 16 h at 4 °C. After coating, the wells were blocked with 100 μL of 4% skim milk (GenomicBase, Namyangju, South Korea) diluted in 1 × PBS (pH 7.4) at room temperature for 2 h, followed by four washes with 150 μL of PBS-T (1 × PBS containing 0.05% Tween 20, pH 7.4). Serial dilutions of hFcRn (10–0.04 nM) and protein extracts (2–8 dilution factor) were added (50 μL/well) and incubated at room temperature for 1 h. Next, 50 μL of mouse anti-hFcRn (ADM31 clone, diluted to 1 μg/mL in 1 × PBS, pH 7.4)(Antibodies-Online, ABIN1774762) was added and incubated at room temperature for 1 h. The wells were washed with PBS-T, followed by the addition of 50 μL of anti-mouse IgG-HRP conjugate (Jackson ImmunoResearch, Philadelphia, PA, USA) diluted in 1% skim milk (pH 7.4), and incubated for 1 h. After washing the wells four times with PBS-T (pH 6.0 and pH 7.4), 50 μL of 1-Step™ Ultra TMB-ELISA substrate solution (Thermo Fisher Scientific, Waltham, MA, USA) was added for color development, and the reaction was stopped by adding 50 μL of 2 M H₂SO₄. Absorbance was measured at 450 nm using an Epoch microplate spectrophotometer (BioTek, Winooski, VT, USA).
To evaluate the pH-dependent binding characteristics of trastuzumab-Fc variants with human and mouse FcRn, 50 μL of HER2-His (diluted to 4 μg/mL) was coated onto wells, followed by blocking. For pH-dependent binding analysis, 50 μL of trastuzumab-Fc variants (diluted to 150 nM in 1% skim milk, pH 7.4) was added and incubated for 1 h. After washing with PBS-T (pH 6.0 and pH 7.4), 50 μL of serially diluted human or mouse FcRn-GST (10 nM initial concentration, 1:4 ratio) was added and incubated at room temperature for 1 h. The wells were then washed and incubated with 50 μL of anti-GST-HRP conjugate (Cytiva, Marlborough, MA, USA) diluted in 1% skim milk. Finally, after washing under pH 6.0 and pH 7.4 conditions, color development and absorbance were measured as described above.
For serum antibody quantification, mouse serum samples were added to the blocked wells, followed by incubation with 50 μL of goat anti-human IgG (H + L)-HRP conjugate (Jackson ImmunoResearch, Philadelphia, PA, USA) diluted in 1% skim milk. The wells were washed under pH 7.4 conditions, and color development and absorbance measurement were performed as previously described.
Pharmacokinetic profile analysis
The pharmacokinetic profiles of trastuzumab-Fc variants were analyzed using hiFcRn and C57BL/6 J, and Tg32 (B6.Cg-Fcgrttm1Dcr Tg(FCGRT)32Dcr/DcrJ homozygous) mice. The hiFcRn and C57BL/6 groups each consisted of five mice (three 10-week-old females and two males), while the Tg32 group consisted of three female mice. Trastuzumab-Fc variants were administered intravenously at a dose of 5 mg/kg. Blood samples (25 μL) were collected from the submandibular vein at 30 min, and on days 1, 7, 14, 21, 28, 35, 42, 49, and 56 post-administration. The collected samples were centrifuged at 1,000 × g for 15 min to separate the serum. The serum concentration of trastuzumab-Fc variants was quantified using ELISA, as described above. Pharmacokinetic parameters were calculated using PK-solver43.
Data availability
The results from the present investigation are available from the corresponding author upon reasonable request.
References
The Antibody Society. Therapeutic monoclonal antibodies approved or in regulatory review. (date accessed), <https://www.antibodysociety.org/antibody-therapeutics-product-data/> (2024).
Strohl, W. R. Structure and function of therapeutic antibodies approved by the US FDA in 2023. Antib Ther 7, 132–156. https://doi.org/10.1093/abt/tbae007 (2024).
Blaese, R. M., Strober, W., Levy, A. L. & Waldmann, T. A. Hypercatabolism of IgG, IgA, IgM, and albumin in the Wiskott-Aldrich syndrome. A unique disorder of serum protein metabolism. J. Clin. Invest. 50, 2331–2338. https://doi.org/10.1172/jci106731 (1971).
Morell, A., Terry, W. D. & Waldmann, T. A. Metabolic properties of IgG subclasses in man. J. Clin. Invest. 49, 673–680. https://doi.org/10.1172/jci106279 (1970).
Burmeister, W. P., Huber, A. H. & Bjorkman, P. J. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature 372, 379–383. https://doi.org/10.1038/372379a0 (1994).
Karlsson, M. et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 7, 1–9. https://doi.org/10.1126/sciadv.abh2169 (2021).
Ober, R. J., Martinez, C., Vaccaro, C., Zhou, J. & Ward, E. S. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor. FcRn. J. Immunol. 172, 2021–2029. https://doi.org/10.4049/jimmunol.172.4.2021 (2004).
Montoyo, H. P. et al. Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice. Proc. Natl. Acad. Sci. U. S. A. 106, 2788–2793. https://doi.org/10.1073/pnas.0810796106 (2009).
West, A. P. & Bjorkman, P. J. Crystal structure and immunoglobulin G binding properties of the human major histocompatibility complex-related Fc receptor(,). Biochemistry 39, 9698–9708. https://doi.org/10.1021/bi000749m (2000).
Rodewald, R. pH-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J. Cell Biol. 71, 666–669. https://doi.org/10.1083/jcb.71.2.666 (1976).
Ober, R. J., Martinez, C., Lai, X., Zhou, J. & Ward, E. S. Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level. Proc. Natl. Acad. Sci. U. S. A. 101, 11076–11081. https://doi.org/10.1073/pnas.0402970101 (2004).
Ko, S., Jo, M. & Jung, S. T. Recent achievements and challenges in prolonging the serum half-lives of therapeutic IgG antibodies through fc engineering. BioDrugs 35, 147–157. https://doi.org/10.1007/s40259-021-00471-0 (2021).
Ward, E. S. et al. From sorting endosomes to exocytosis: association of Rab4 and Rab11 GTPases with the Fc receptor, FcRn, during recycling. Mol. Biol. Cell 16, 2028–2038. https://doi.org/10.1091/mbc.e04-08-0735 (2005).
Devanaboyina, S. C. et al. The effect of pH dependence of antibody-antigen interactions on subcellular trafficking dynamics. MAbs 5, 851–859. https://doi.org/10.4161/mabs.26389 (2013).
Zalevsky, J. et al. Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 28, 157–159. https://doi.org/10.1038/nbt.1601 (2010).
Ko, S. et al. An Fc variant with two mutations confers prolonged serum half-life and enhanced effector functions on IgG antibodies. Exp. Mol. Med. 54, 1850–1861. https://doi.org/10.1038/s12276-022-00870-5 (2022).
Andersen, J. T., Daba, M. B., Berntzen, G., Michaelsen, T. E. & Sandlie, I. Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding. J. Biol. Chem. 285, 4826–4836. https://doi.org/10.1074/jbc.M109.081828 (2010).
Ober, R. J., Radu, C. G., Ghetie, V. & Ward, E. S. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int. Immunol. 13, 1551–1559. https://doi.org/10.1093/intimm/13.12.1551 (2001).
Dall’Acqua, W. F. et al. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J. Immunol. 169, 5171–5180. https://doi.org/10.4049/jimmunol.169.9.5171 (2002).
Vaccaro, C., Bawdon, R., Wanjie, S., Ober, R. J. & Ward, E. S. Divergent activities of an engineered antibody in murine and human systems have implications for therapeutic antibodies. Proc. Natl. Acad. Sci. U. S. A. 103, 18709–18714. https://doi.org/10.1073/pnas.0606304103 (2006).
Yan, B. W., Zhao, Y. F., Cao, W. G., Li, N. & Gou, K. M. Mechanism of random integration of foreign DNA in transgenic mice. Transgenic Res. 22, 983–992. https://doi.org/10.1007/s11248-013-9701-z (2013).
Smirnov, A. & Battulin, N. Concatenation of Transgenic DNA: Random or Orchestrated?. Genes (Basel) 12, 1969. https://doi.org/10.3390/genes12121969 (2021).
Latvala, S., Jacobsen, B., Otteneder, M. B., Herrmann, A. & Kronenberg, S. Distribution of FcRn Across Species and Tissues. J. Histochem. Cytochem. 65, 321–333. https://doi.org/10.1369/0022155417705095 (2017).
Jang, D. E. et al. Multiple sgRNAs with overlapping sequences enhance CRISPR/Cas9-mediated knock-in efficiency. Exp. Mol. Med. 50, 1–9. https://doi.org/10.1038/s12276-018-0037-x (2018).
Smith, B. et al. Generation and characterization of a high affinity anti-human FcRn antibody, rozanolixizumab, and the effects of different molecular formats on the reduction of plasma IgG concentration. MAbs 10, 1111–1130. https://doi.org/10.1080/19420862.2018.1505464 (2018).
Sand, K. M. et al. Dissection of the neonatal Fc receptor (FcRn)-albumin interface using mutagenesis and anti-FcRn albumin-blocking antibodies. J. Biol. Chem. 289, 17228–17239. https://doi.org/10.1074/jbc.M113.522565 (2014).
Petkova, S. B. et al. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int. Immunol. 18, 1759–1769. https://doi.org/10.1093/intimm/dxl110 (2006).
Yeung, Y. A. et al. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J. Immunol. 182, 7663–7671. https://doi.org/10.4049/jimmunol.0804182 (2009).
Mikulska, J. E. Analysis of Response Elements Involved in the Regulation of the Human Neonatal Fc Receptor Gene (FCGRT). PLoS ONE 10, e0135141. https://doi.org/10.1371/journal.pone.0135141 (2015).
van Bilsen, K. et al. The neonatal Fc receptor is expressed by human retinal pigment epithelial cells and is downregulated by tumour necrosis factor-alpha. Br. J. Ophthalmol. 95, 864–868. https://doi.org/10.1136/bjo.2010.187930 (2011).
Liu, X. et al. NF-kappaB signaling regulates functional expression of the MHC class I-related neonatal Fc receptor for IgG via intronic binding sequences. J. Immunol. 179, 2999–3011. https://doi.org/10.4049/jimmunol.179.5.2999 (2007).
Chaudhury, C. et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J. Exp. Med. 197, 315–322. https://doi.org/10.1084/jem.20021829 (2003).
Prabhat, P. et al. Elucidation of intracellular recycling pathways leading to exocytosis of the Fc receptor, FcRn, by using multifocal plane microscopy. Proc. Natl. Acad. Sci. U. S. A. 104, 5889–5894. https://doi.org/10.1073/pnas.0700337104 (2007).
Bern, M. et al. An engineered human albumin enhances half-life and transmucosal delivery when fused to protein-based biologics. Sci. Transl. Med. 12, 1–13. https://doi.org/10.1126/scitranslmed.abb0580 (2020).
Brambell, F. W. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet 2, 1087–1093. https://doi.org/10.1016/s0140-6736(66)92190-8 (1966).
Baker, K. et al. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 108, 9927–9932. https://doi.org/10.1073/pnas.1019037108 (2011).
Qiao, S. W. et al. Dependence of antibody-mediated presentation of antigen on FcRn. Proc. Natl. Acad. Sci. U. S. A. 105, 9337–9342. https://doi.org/10.1073/pnas.0801717105 (2008).
Hubbard, J. J. et al. FcRn is a CD32a coreceptor that determines susceptibility to IgG immune complex-driven autoimmunity. J. Exp. Med. 217, 1–14. https://doi.org/10.1084/jem.20200359 (2020).
Blumberg, L. J. et al. Blocking FcRn in humans reduces circulating IgG levels and inhibits IgG immune complex-mediated immune responses. Sci. Adv. 5, eaax9586. https://doi.org/10.1126/sciadv.aax9586 (2019).
Conner, C. M. et al. A precisely humanized FCRN transgenic mouse for preclinical pharmacokinetics studies. Biochem. Pharmacol. 210, 115470. https://doi.org/10.1016/j.bcp.2023.115470 (2023).
Proetzel, G. & Roopenian, D. C. Humanized FcRn mouse models for evaluating pharmacokinetics of human IgG antibodies. Methods 65, 148–153. https://doi.org/10.1016/j.ymeth.2013.07.005 (2014).
Berntzen, G. et al. Prolonged and increased expression of soluble Fc receptors, IgG and a TCR-Ig fusion protein by transiently transfected adherent 293E cells. J. Immunol. Methods 298, 93–104. https://doi.org/10.1016/j.jim.2005.01.002 (2005).
Zhang, Y., Huo, M., Zhou, J. & Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Prog. Biomed. 99, 306–314. https://doi.org/10.1016/j.cmpb.2010.01.007 (2010).
Acknowledgements
This work was supported by the National Research Foundation of Republic of Korea (NRF) grants funded by the Korean government (MSIT; No. 2020M3F7A1094089, 2020R1A2C1004684, and No. 2017R1A5A2015369), Ministry of Science and ICT (RS-2023-00224201, RS-2023-00261905 and RS-2024-0359509), National Cancer Center (HA22C0147), the BK21 FOUR program of the NRF under Ministry of Education, and the Starting growth Technological R&D Program (TIPS Program, No. RS-202300261778 and RS-2023-00224201), funded by the Ministry of SMEs and Startups (MSS, Korea) in 2023.
Funding
National Research Foundation of Republic of Korea, 2020M3F7A1094089, 2020M3F7A1094089, 2020M3F7A1094089, 2020M3F7A1094089, 2020M3F7A1094089, Ministry of Science and ICT, South Korea, 2023-00224201, 2023-00224201, 2023-00224201, 2023-00224201, 2023-00224201
Author information
Authors and Affiliations
Contributions
S.B.L. and M.K. conducted experiments, analyzed the data, and drafted the original manuscript. S.K. and S.L. performed the experiments and acquired the data. M.Y.P. and S.H.P. technically supported. M.J. and J.H.L provided specialized materials and developed specialized protocols. H.W.-L and S.T.-J conceptualized, designed and supervised the study. Additionally, H.W.L and S.T.J secured funding, and wrote the finalized manuscript. All authors contributed to this study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, S., Kyung, M., Park, M. et al. Advanced human FcRn knock-in mice for pharmacokinetic profiling of therapeutic antibodies. Sci Rep 15, 27186 (2025). https://doi.org/10.1038/s41598-025-11168-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11168-7