Abstract
Mannitol is widely used for treating brain edema caused by various diseases, but it has been reported to cause acute kidney injury. However, the prognosis for patients with non-traumatic intracerebral hemorrhage who also have acute kidney injury and continue to receive mannitol has not yet been documented. This study presents a retrospective cohort analysis utilizing the MIMIC-IV (medical information mart for intensive care-IV) database. The study population comprised adult patients diagnosed with non-traumatic intracerebral hemorrhage (ICH) and concurrent acute kidney injury (AKI). Mannitol administration during the intensive care unit (ICU) stay was considered the exposure variable. The primary endpoint for analysis was 28-day all-cause mortality. To account for potential confounding factors, multivariable analytical methods were employed. The 28-day mortality rate within the total cohort was 25%. In the mannitol group, the 28-day mortality rate was 50.4% (58/115), compared to 21.9% (203/927) in the control group. Mannitol use was associated with a significantly higher 28-day all-cause mortality in both the multivariable analysis (HR 2.42; 95% CI 1.80–3.25; p < 0.001) and the univariable analysis (HR 2.31; 95% CI 1.67–3.19; p < 0.001). Other variables independently associated with mortality included higher heart rate, mean arterial pressure, respiratory rate, platelet count, sodium, chloride, lactate, urea nitrogen, creatinine, SAPSII, SOFA, GCS, and Charlson Index. The in-hospital mortality rate was 47.8% (55 out of 115) in the mannitol treatment group and 16.2% (150 out of 927) in the non-treatment group. Mannitol use was associated with higher 28-day all-cause mortality in patients with non-traumatic ICH and AKI. However, given the methodological limitations and incomplete confounder adjustment of this study, this finding should be interpreted with caution. Further research is needed to confirm this relationship and explore the underlying mechanisms.
Similar content being viewed by others
Introduction
Non-traumatic intracerebral hemorrhage (ICH) is a serious medical condition characterized by the sudden bleeding into the brain tissue without any history of trauma1. It represents a significant cause of morbidity and mortality worldwide. According to the Global Burden of Disease Study 20,192, among all stroke cases globally, intracerebral haemorrhage constituted 27.9% (3.41 million [2.97–3.91]) and subarachnoid haemorrhage constituted 9.7% (1.18 million [1.01–1.39])1,2,3,4. ICH is associated with a high risk of adverse outcomes, including neurological deficits, disability, and death3.In patients with non-traumatic ICH, the occurrence of acute kidney injury (AKI) is a common complication that further exacerbates the clinical course and prognosis. Studies have shown that up to 23% of patients with ICH develop AKI during their hospitalization, and this condition is associated with increased mortality and prolonged hospital stay5,6,7,8. The pathophysiology of AKI in the setting of ICH is multifactorial and may include hypoperfusion, systemic inflammation, and nephrotoxic medication use6.Treatment options for patients with non-traumatic ICH and AKI are limited, and the use of osmotic agents such as mannitol has been a cornerstone of management to reduce intracranial pressure and improve cerebral perfusion. Mannitol is an osmotic diuretic that works by drawing water out of the brain tissue, leading to a decrease in intracranial pressure and improved cerebral blood flow9. However, the use of mannitol is not without potential risks, including electrolyte imbalances, dehydration, and renal dysfunction. Previous studies have shown that mannitol can lead to renal dysfunction through several potential mechanisms. For instance, Lin et al.10 found that mannitol infusion was associated with an increased risk of AKI in patients with acute stroke, potentially due to its osmotic effects on the renal tubules, leading to cellular damage and inflammation. Fang et al.11 reported that mannitol was an independent risk factor for AKI following cerebral trauma. The current literature has established that mannitol can be effective in reducing intracranial pressure and improving cerebral perfusion in patients with non - traumatic ICH. However, the impact of mannitol therapy on the prognosis of patients with non - traumatic ICH who also develop AKI remains unclear. Most existing studies have focused on the efficacy of mannitol in reducing intracranial pressure or its potential nephrotoxic effects in isolation, but there is limited research exploring how mannitol use influences clinical outcomes in patients with the combined conditions of non - traumatic ICH and AKI. This knowledge gap makes it challenging for clinicians to make informed decisions about the use of mannitol in this specific patient population. In this context, the present study aims to investigate the effect of mannitol use on the clinical outcomes of patients with non - traumatic ICH and AKI, using a retrospective cohort design, in order to fill this gap and provide more evidence - based guidance for clinical practice. We hypothesize that the use of mannitol may be associated with worse clinical outcomes in patients with non - traumatic ICH and AKI, potentially due to its nephrotoxic effects. This study aimed to evaluate the association between mannitol use and 28 - day mortality among patients with non - traumatic ICH and AKI. Secondary outcomes measures included ICU length of stay and the need for dialysis.
Methods
Subjects
This study utilized data from the MIMIC-IV (Medical Information Mart for Intensive Care IV) version 3.0 database. MIMIC-IV 3.0 contains comprehensive data from multiple ICUs within the Beth Israel Deaconess Medical Center, a large tertiary care hospital in Boston, USA. The hospital’s critical care department includes several specialized ICUs, such as Medical ICU (MICU), Cardiac ICU (CCU), Neurological ICU (NICU), and Surgical ICU (SICU). These ICUs admit a diverse range of patients. The dataset encompasses approximately 220,000 distinct hospital admissions of over 120,000 adult and pediatric patients admitted to the ICU between 2008 and 2022, covering a wide array of data types, such as demographics, vital signs, laboratory results, and medications12.The author(WC) joined and completed online the course on protecting human research participants proposed by the US National Institutes of Health’s, and therefore obtained permission to access the dataset. The certificate number is 10,311,970. The use of the database for research was then authorized by the review committee of MIT and Beth Israel Deaconess Medical Center, and a waiver of informed consent was also granted to us. We followed the STROBE guidelines for observational research13.We enrolled ICH patients on the basis of the International Classification of Diseases (ICD)-9/10 guidelines, including ICD-9 code 431 and ICD-10 codes I610–I619 and I62.9 for ICH. Acute kidney injury is defined according to KDIGO criteria14. Specifically, it is based on an increase in serum creatinine level of at least 0.3 mg/dL within 48 h compared to baseline, or urinary output below 0.5 mL/kg/h for 6 h. Baseline serum creatinine was determined as follows: for patients with prior outpatient creatinine measurements, the most recent value within the past 365 days was used as the baseline. For patients without prior lab results, the lowest creatinine level measured during the first 7 days of hospitalization was considered the baseline creatinine. This approach aligns with KDIGO guidelines and ensures accurate identification of AKI. To ensure the study’s integrity and the robustness of its findings, we adhered to stringent exclusion criteria. (1) Individuals under the age of 18 at the time of admission were excluded. (2) For patients with multiple ICU admissions, only the first admission was considered. (3) Patients with end-stage renal disease were not included. (4) Those with ICU stays of less than 24 h were excluded due to often incomplete records and insufficient time to assess the impact of mannitol therapy. (5) patients missing critical admission-day data, such as demographic information, vital signs, laboratory test results (e.g., serum creatinine levels for AKI diagnosis), and treatment details (e.g., mannitol usage), were not included. These variables are essential for accurately evaluating the study outcomes and the effects of mannitol therapy.
Data extraction
In our study, we utilized Structured Query Language (SQL) scripts obtained from the GitHub repository (https://github.com/MIT-LCP/mimic-iv) to extract data from the MIMIC-IV database. The specific scripts used for data extraction were the standard SQL scripts provided by MIMIC-IV on GitHub. No modifications were made to these scripts.We collected comprehensive patient data, including characteristics like age, sex, and BMI. Our focus included vital signs within the first 24 h of ICU admission, such as heart rate (HR), mean arterial pressure (MAP), SpO2, temperature, and respiration rate (RR), as well as laboratory tests including hemoglobin, platelet count, white blood cell (WBC) count, sodium, potassium, calcium, blood urea nitrogen (BUN), creatinine, glucose, and blood gas analysis. We also assessed neurological status using the Glasgow Coma Scale (GCS), disease severity scores like the Simplified Acute Physiology Score (SAPS) II and the Sequential Organ Failure Assessment (SOFA) score, and whether the patient had sepsis. Additionally, we recorded details about the patient’s ICU stay, specifically noting if they required mechanical ventilation or underwent surgical procedures. Comorbidities such as hypertension, coronary heart disease, congestive heart failure, diabetes mellitus, and liver disease were also extracted to provide a complete picture of the patient’s health status.
Mannitol exposure
Mannitol exposure is defined as all prescriptions containing mannitol after admission to the ICU, including details such as dosage, frequency, duration of treatment, and the time relative to ICU admission.
Main results
The primary endpoint of this study is the 28-day all-cause mortality rate after admission to ICU. Secondary outcomes include in-hospital mortality.
Statistical analysis
This retrospective analysis was conducted without a predefined statistical analysis plan, given the nature of the study design. A power calculation was not deemed necessary due to the observational nature of the research, and the sample size was determined by the extent of data availability extracted from the existing database.
The study cohort was divided into two groups: those who received mannitol therapy (mannitol group) and those who did not (non-mannitol group). To handle missing data while preserving the dataset’s integrity and statistical power, we used a multiple imputation strategy. Variables with a missingness rate exceeding 50% were excluded from analysis (see Table S1 for details). For variables with a missingness rate of less than 50%, we applied the chained equations (MICE) method for multiple imputation. This covered vital signs (HR, MAP, SpO₂, temperature, RR) and lab tests (hemoglobin, platelet count, WBC count, sodium, potassium, calcium, BUN, creatinine, glucose, blood gas analysis). We created five imputed datasets and pooled results using Rubin’s rules to ensure analysis reliability and validity, accounting for the uncertainty from missing data. To safeguard against multicollinearity among the predictive variables, which could compromise the validity of the regression models, the variance inflation factor (VIF) was calculated for each variable included in the analysis. A VIF threshold of less than five for each variable served as evidence that multicollinearity was not a concern in the dataset, ensuring that the relationships between variables were appropriately modeled without distortion caused by redundant predictors.
Numbers (percentages) were utilized to present categorized data, while mean ± standard deviation or median (interquartile range) were used for continuous data. The Shapiro-Wilk test was used to assess the normality of continuous variables. Based on the results, parametric tests (analysis of variance test) were used for normally distributed data, whereas non-parametric tests (rank sum test) were applied for non-normally distributed data. The characteristics of the subjects in the outcome group were compared using the Chi-square test or Fisher’s exact test for categorical variables. KM survival curves were plotted for various subgroups to display survival rates. These curves were compared using the log-rank test. Univariate Cox regression analyses were performed to preliminarily explore the associations of mannitol use with 28-day all-cause mortality rate after admission to ICU and in-hospital mortality in ICH patients with AKI. Variables with statistical significance (P < 0.05) in the univariate analyses were subsequently included in the multivariate Cox regression models. The outcomes presented hazard ratios (HRs) along with 95% confidence intervals (CIs). The multivariate Cox regression was adjusted for potential confounders, including age, gender, Heart Rate (HR), Mean Arterial Pressure (MAP), Respiratory Rate (RR), Platelet count, Sodium, Calcium total, Chloride, PH, Lactate, Urea nitrogen, Creatinine, The Sequential Organ Failure Assessment (SOFA), Simplified Acute Physiology Score II (SAPS II), Glasgow Coma Score (GCS), Charlson Index, Ventilation. Additionally, subgroup analyses, stratified by relevant covariates, were conducted to further reduce survival bias and enhance the robustness of the findings. Subgroup analyses were stratified according to relevant covariates to reduce the impact of survival bias. All analyses were performed using Free statistics software version 1.6 and the statistical package R 4.1.1. A two-tailed test indicating P < 0.05 is considered statistically significant15.
Subgroup analyses
Subgroup analyses in the cohort were based on age (< 65 vs. ≥ 65 year), sex (female vs. male), GCS(< 9 vs. ≥ 9)16,AKI stage(1,2,3)14,Charlson Comorbidity Index (< 6 vs. ≥ 6)17, and Simplified Acute Physiology Score II (< 40 vs. ≥ 40)18.
Results
Patient selection
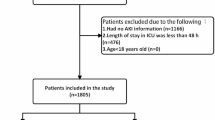
In the MIMIC-IV (version 3.0) database, a total of 1,685 patients were admitted to the ICU due to ICH with AKI. After excluding patients younger than 18 years old, those with non-first ICU admissions, ICU stays shorter than 24 h, and patients with uremia, the final cohort consisted of 1,042 ICH patients with AKI. Among these, 115 patients (11.0%) were exposed to mannitol, while 927 (89.0%) were not (Fig. 1).
Cohort characteristics
Table 1 presents the patients’ baseline characteristics. In the entire cohort, patients who received mannitol were generally younger and more likely to be female. They also had a higher Charlson comorbidity index, lower WBC counts and platelet counts, and higher BUN levels. Mean arterial pressure was significantly higher in the mannitol group, whereas oxygen saturation was significantly lower. Additionally, patients in the mannitol group had significantly higher serum calcium levels and were more likely to require mechanical ventilation, with longer ventilation durations. In contrast, patients not receiving mannitol had significantly higher Glasgow scores and serum sodium levels and included more patients with hypertension. Regarding mannitol usage in the mannitol group, the median (IQR) time from ICU admission to first mannitol dose was 0.7 (0.3, 1.9) days. The total median (IQR) dose of mannitol administered was 100.0 (75.0, 200.0) grams. The median (IQR) daily medication frequency was 1.0 (1.0, 3.5) doses per day.
Univariate Cox regression analysis
Table 2 illustrates the findings of a univariate Cox regression analysis, which identified several significant variables associated with increased 28-day mortality risk in patients with ICH and AKI post-ICU admission, as compared to the non-mannitol user reference group. These variables include elevated heart rate, mean arterial pressure, respiratory rate, sodium and chloride levels, pH values, lactate levels, urea nitrogen, creatinine levels, and higher SAPS II and SOFA scores, as well as the utilization of ventilation.
Outcome
The total cohort exhibited a 28-day mortality rate of 25%. Notably, the mannitol group reported a significantly higher mortality rate of 50.4% (58 out of 115), compared to 21.9% (203 out of 927) in the control group. Figure 2 provides a graphical representation of the Kaplan-Meier survival curve for 28-day all-cause mortality, stratified by mannitol use. The association between mannitol use and elevated 28-day all-cause mortality was confirmed through both multivariable analysis (HR 1.94, 95% CI 1.38–2.72, p < 0.001) and univariable analysis (HR 2.42, 95% CI 1.80–3.25, p < 0.001). Furthermore, the proportional hazards assumption was tested using Schoenfeld residuals. The global test was significant (p = 0.004), suggesting a possible violation of the proportional hazards assumption. This finding should be interpreted cautiously and considered in the context of the model’s limitations. Detailed findings are tabulated in Table S2 and Table 3.
Subgroup analyses
Table 4 displays the findings from analyses examining the 28-day mortality rates across various subgroups within our study cohort. These analyses accounted for a range of factors, such as age, gender, HR, MAP, RR, platelet count, total calcium, chloride, pH, lactate, urea nitrogen, creatinine, SOFA, SAPS II, GCS, Charlson Index, and ventilation, to mitigate potential confounding influences. The outcomes are categorized based on pre-defined subgroups, including age, gender, GCS, AKI stage, SAPSII, SOFA, and Charlson index. The interaction analysis summaries are provided in Table S3. The data indicates that the use of mannitol is associated with increased mortality rates in specific subgroups. Particularly, patients with KDIGO stage 1 AKI who received mannitol had a significantly elevated mortality risk (HR = 2.52; 95% CI: 1.18–5.39; p < 0.05). A similar pattern was observed in patients with KDIGO stage 2 AKI, where mannitol use was associated with higher mortality rates (HR = 1.89; 95% CI: 1.17–3.05; p < 0.05). Conversely, among patients with KDIGO stage 3 AKI, mannitol use tended towards lower mortality rates, albeit not significantly (HR = 0.76; 95% CI: 0.30–1.87; p = 0.74). Notably, the interaction analysis revealed a statistically significant difference in the impact of mannitol across different AKI stages (P for interaction = 0.035), suggesting that the relationship between mannitol use and mortality varies considerably depending on the AKI stage.
Furthermore, a significant interaction was identified regarding the SOFA score (P for interaction = 0.009). Patients with a SOFA score < 5 experienced a significantly increased mortality risk with mannitol use (HR = 2.96; 95% CI: 1.91–4.58; p < 0.001), whereas those with a SOFA score ≥ 5 exhibited a non-significant increase in mortality risk (HR = 1.32; 95% CI: 0.75–2.32; p = 0.32).
For other subgroups, such as age, gender, GCS, SAPSII, and Charlson index, the P-values for interaction did not reach statistical significance (all P > 0.05), indicating that the effect of mannitol does not significantly vary across these subgroups after accounting for multiple covariates.
Sensitivity analyses
To account for survival bias, a sensitivity analysis excluding patients who died within 48 h of ICU admission was conducted. Mannitol use remained independently associated with increased 28-day mortality (adjusted HR 1.89, 95% CI 1.35–2.65; p < 0.001), after adjusting for age, gender, HR, MAP, RR, platelet count, calcium, chloride, pH, lactate, urea, creatinine, SOFA, SAPS II, GCS, Charlson Index, and ventilation.(Table 5).
Secondary outcomes
The in-hospital mortality rate was 47.8% (55 out of 115) in the mannitol treatment group and 16.2% (150 out of 927) in the non-treatment group. Univariate analysis revealed that mannitol use was associated with a higher in-hospital mortality rate (HR = 2.97; 95% CI: 2.17–4.07; P < 0.001). Multivariate analysis further confirmed this association, with an adjusted hazard ratio of 2.69 (95% CI: 1.90–3.80; P < 0.001) (Table 2).
Discussion
The current study findings indicate that the use of mannitol is associated with an increased 28-day all-cause mortality in patients with non-traumatic intracerebral hemorrhage (ICH) and acute kidney injury (AKI). Specifically, the 28-day mortality rate in the mannitol treatment group was 50.4% (58/115), whereas it was 21.9% (203/927) in the group without mannitol. Univariate analysis showed that mannitol use was associated with higher mortality (HR = 2.42; 95% CI: 1.80–3.25; P < 0.001), and multivariate analysis further confirmed this result (HR = 2.31; 95% CI: 1.67–3.19; P < 0.001). In subgroup analyses, mannitol was associated with increased mortality in specific subgroups, particularly in patients with KDIGO stage 1 and 2 AKI. However, in KDIGO stage 3 AKI patients, mannitol use showed a non-significant trend towards reduced mortality, although this result was not statistically significant (HR = 0.76; 95% CI: 0.30–1.87; P = 0.74). Additionally, in patients with a SOFA score < 5, mannitol use was associated with a significantly increased mortality risk (HR = 2.96; 95% CI: 1.91–4.58; P < 0.001), whereas in patients with a SOFA score ≥ 5, the trend of increased mortality with mannitol was not significant (HR = 1.32; 95% CI: 0.75–2.32; P = 0.32). For other subgroups (including age, gender, GCS, SAPSII, and Charlson index), the P-values for interaction were not statistically significant (all P > 0.05), indicating no significant difference in the effect of mannitol across these subgroups after adjusting for multiple covariates.
In the multivariate analysis, we adjusted for a series of potential confounding factors, including age, gender, heart rate (HR), mean arterial pressure (MAP), respiratory rate (RR), platelet count, total calcium, chloride, pH, lactate, urea nitrogen, creatinine, SOFA score, SAPS II score, GCS score, Charlson index, and ventilation status. The selection of these covariates was based on factors previously identified in the literature as being related to the prognosis of ICH and AKI, as well as variables that showed association with mortality in univariate analysis (P < 0.05). Additionally, we considered key confounding factors such as GCS score, ventilation status, pH, and lactate levels, which have significant impacts on prognosis in ICH and AKI patients. Through stepwise selection and multicollinearity testing (variance inflation factor VIF < 5), we ensured the rationality and independence of covariates in the final model. We also conducted a proportional hazards assumption test using Schoenfeld residuals. The global test was significant (p = 0.004), suggesting a potential violation of the proportional hazards assumption. This finding should be considered when interpreting the results of the Cox model.
For the inconsistent results in subgroup analyses, such as the non-significant trend towards reduced mortality with mannitol in KDIGO stage 3 AKI patients, we speculate that this may be due to: (1) insufficient statistical power due to the small sample size; (2) KDIGO stage 3 AKI patients might have received more aggressive renal replacement therapy (RRT), which could have obscured the potential adverse effects of mannitol; (3) in patients with severe renal insufficiency, the metabolism and excretion of mannitol may be significantly affected, altering its pharmacodynamic properties. Additionally, patients with a SOFA score < 5 generally represent those with milder organ dysfunction, and mannitol use may have a more pronounced negative impact on these patients, whereas in patients with a SOFA score ≥ 5, the effect of mannitol may be obscured by other factors due to the severity of underlying diseases.
Over the past 50 years, mannitol has been widely recognized as an effective treatment for brain edema, capable of reducing intracranial pressure (ICP) in various conditions19,20. It reduces the total water content in brain tissue and cerebrospinal fluid, decreases blood volume by inducing vasoconstriction21,22and improves cerebral perfusion by reducing viscosity or altering red blood cell rheology23. Furthermore, mannitol may offer protective effects against biochemical losses in stroke patients as a free radical scavenger24.While mannitol is effective in treating brain edema caused by various conditions, it is associated with several common side effects, including impaired kidney function25,26alterations in serum electrolytes and osmolality27blood volume overload, and rebound edema28. Most existing research has focused on the risk of AKI associated with mannitol use in patients with stroke or traumatic brain injury, along with the potential mechanisms underlying these effects10,11. However, the prognosis of patients with non-traumatic intracerebral hemorrhage (ICH) and AKI who receive mannitol has received less attention.
Based on previous research and conclusions, we infer that the poor prognosis of patients with ICH and AKI who receive mannitol may be due to the following reasons: (1) Cardiovascular Risk: In patients with pre-existing cardiac dysfunction or increased cardiac load28mannitol’s effect on improving cerebral perfusion can increase the risk of cardiovascular events. This risk is further heightened in patients with impaired renal function, who are more likely to experience heart failure or exacerbated cardiac dysfunction29. (2) Renal Tubular Cell Injury: The hyperosmotic properties of mannitol can cause injury to renal tubular cells, especially in patients with pre-existing renal impairment. This damage can further deteriorate renal function10,11,29,30. (3) Electrolyte Disturbances: Mannitol can lead to electrolyte imbalances, such as hyponatremia and hypokalemia, which can adversely affect cardiac and neurological functions27,30,31. (4) Reduced Efficacy in AKI Patients: In patients with AKI, the effectiveness of mannitol may be limited due to impaired renal function affecting its metabolism and excretion21,22,32.
The current study aimed to evaluate the association between mannitol use and 28-day mortality among patients with non-traumatic ICH and AKI. Secondary outcomes measures included ICU length of stay and the need for dialysis. Our findings suggest that mannitol use may be associated with worse clinical outcomes in patients with non-traumatic ICH and AKI, potentially due to its nephrotoxic effects.
Study limitations
Our study has several limitations that should be acknowledged. First, the data is derived from a single center (MIMIC-IV), which may limit the generalizability of our findings to broader populations. Second, despite our efforts to adjust for potential confounders, residual confounding may still be present, potentially influencing the results. Third, we lack longitudinal data on creatinine changes and mannitol accumulation, which could have provided valuable insights into renal function dynamics over time. Furthermore, our study did not include detailed information on key clinical confounders such as electrolyte trends and volume status, which are important factors in patient management and outcome assessment. Additionally, the absence of data on nephrotoxic medications and contrast examinations is a significant limitation, as their use could have a substantial impact on renal function and subsequent patient outcomes. Particularly, regarding intracerebral hemorrhage (ICH), we were unable to provide specific details on its type, localization, and size. This limitation arises from the complexity of extracting such detailed information and the constraints inherent in the MIMIC-IV database. The lack of these ICH-related details prevents a more nuanced analysis of how these factors might influence patient outcomes, especially in the context of renal function and the use of therapies like mannitol. Moreover, we also acknowledge that the high missingness rate for some variables is another limitation, which may affect the robustness of our findings. We sincerely regret these shortcomings and recognize the importance of these factors in a comprehensive assessment of patient care and prognosis.
Conclusion
Given the methodological limitations and incomplete confounder adjustment, we’ve revised our conclusion to avoid misleading causal interpretations. Our findings suggest an association between mannitol use and increased mortality in patients with non-traumatic ICH and AKI, but further research is needed to confirm this relationship and explore the underlying mechanisms.
Data availability
we used the MIMIC-III database, accessible via the MIMIC Code Repository on GitHub (https://github.com/MIT-LCP/mimic-code). This repository is a central platform for sharing code to analyze the MIMIC database series. Researchers can use the code in the repository to build and analyze datasets.
References
GBD 2019 Stroke Collaborators. Global, regional, and National burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 20 (10), 795–820. https://doi.org/10.1016/S1474-4422(21)00252-0 (2021).
Fang, Y. et al. Programmed cell deaths and potential crosstalk with Blood-Brain barrier dysfunction after hemorrhagic stroke. Front. Cell. Neurosci. 14, 68. https://doi.org/10.3389/fncel.2020.00068 (2020).
An, S. J., Kim, T. J., Yoon, B. W. & Epidemiology Risk factors, and clinical features of intracerebral hemorrhage: an update. J. Stroke. 19 (1), 3–10. https://doi.org/10.5853/jos.2016.00864 (2017).
Kim, J. Y. & Bae, H. J. Spontaneous intracerebral hemorrhage: management. J. Stroke. 19 (1), 28–39. https://doi.org/10.5853/jos.2016.01935 (2017).
Hoste, E. A. J. et al. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 14 (10), 607–625. https://doi.org/10.1038/s41581-018-0052-0 (2018).
Barea-Mendoza, J. A. et al. Traumatic brain injury and acute kidney injury-Outcomes and associated risk factors. J. Clin. Med. 11 (23), 7216. https://doi.org/10.3390/jcm11237216 (2022).
Yang, S. Y. et al. Nomenclature and diagnostic criteria for acute kidney injury – 2020 consensus of the Taiwan AKI-task force. J. Formos. Med. Assoc. Taiwan. Yi Zhi. 121 (4), 749–765. https://doi.org/10.1016/j.jfma.2021.08.005 (2022).
Fujarski, M. et al. Prediction of acute kidney injury in the intensive care unit: preliminary findings in a European open access database. Stud. Health Technol. Inf. 294, 139–140. https://doi.org/10.3233/SHTI220419 (2022).
Witherspoon, B. & Ashby, N. E. The use of mannitol and hypertonic saline therapies in patients with elevated intracranial pressure: A review of the evidence. Nurs. Clin. North. Am. 52 (2), 249–260. https://doi.org/10.1016/j.cnur.2017.01.002 (2017).
Lin, S. Y. et al. Incidence and risk factors for acute kidney injury following mannitol infusion in patients with acute stroke: A retrospective cohort study. Med. (Baltim). 94 (47), e2032. https://doi.org/10.1097/MD.0000000000002032 (2015).
Fang, L. et al. Mannitol is an independent risk factor of acute kidney injury after cerebral trauma: a case-control study. Ren. Fail. 32 (6), 673–679. https://doi.org/10.3109/0886022X.2010.486492 (2010).
Yang, Q. et al. Association between wait time of central venous pressure measurement and outcomes in critical patients with acute kidney injury: A retrospective cohort study. Front. Public. Health. 10, 893683. https://doi.org/10.3389/fpubh.2022.893683 (2022).
Vandenbroucke, J. P. et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 4 (10), e297. https://doi.org/10.1371/journal.pmed.0040297 (2007).
Ostermann, M. et al. Controversies in acute kidney injury: conclusions from a kidney disease: Improving global outcomes (KDIGO) conference. Kidney Int. 8 (2), 294–309 https://doi.org/10.1016/j.kint.2020.04.020 (2020).
Liu, Q. et al. Association between lactate-to-albumin ratio and 28-days all-cause mortality in patients with acute pancreatitis: A retrospective analysis of the MIMIC-IV database. Front. Immunol. 13, 1076121. https://doi.org/10.3389/fimmu.2022.1076121 (2022).
Bullock, M. R. et al. Surgical management of acute subdural hematomas. Neurosurgery 58 (3 Suppl), S16–24 (2006).
Zhang, T., Chen, R., Wen, D., Wang, X. & Ma, L. The prognostic value of the Charlson comorbidity index in aged patients with intracerebral hemorrhage. BMC Neurol. 22 (1), 443. https://doi.org/10.1186/s12883-022-02980-z (2022).
Huang, K. B. et al. The prediction of 30-day mortality in patients with primary Pontine hemorrhage: a scoring system comparison. Eur. J. Neurol. 19 (9), 1245–1250. https://doi.org/10.1111/j.1468-1331.2012.03724.x (2012).
James, H. E., Langfitt, T. W., Kumar, V. S. & Ghostine, S. Y. Treatment of intracranial hypertension. Analysis of 105 consecutive, continuous recordings of intracranial pressure. Acta Neurochir. (Wien). 36 (3–4), 189–200. https://doi.org/10.1007/BF01405391 (1977).
Bereczki, D., Fekete, I., Prado, G. F. & Liu, M. Mannitol for acute stroke. Cochrane Database Syst. Rev. 2007 (3), CD001153. https://doi.org/10.1002/14651858.CD001153.pub2 (2007).
Davis, M. & Lucatorto, M. Mannitol revisited. J. Neurosci. Nurs. J. Am. Assoc. Neurosci. Nurses. 26 (3), 170–174. https://doi.org/10.1097/01376517-199406000-00012 (1994).
Donato, T., Shapira, Y., Artru, A. & Powers, K. Effect of mannitol on cerebrospinal fluid dynamics and brain tissue edema. Anesth. Analg. 78 (1), 58–66. https://doi.org/10.1213/00000539-199401000-00011 (1994).
Andrews, R. J., Bringas, J. R. & Muto, R. P. Effects of mannitol on cerebral blood flow, blood pressure, blood viscosity, hematocrit, sodium, and potassium. Surg. Neurol. 39 (3), 218–222. https://doi.org/10.1016/0090-3019(93)90186-5 (1993).
Mizoi, K., Suzuki, J., Imaizumi, S. & Yoshimoto, T. Development of new cerebral protective agents: the free radical scavengers. Neurol. Res. 8 (2), 75–80. https://doi.org/10.1080/01616412.1986.11739734 (1986).
De Vlieger, G. & Meyfroidt, G. Kidney dysfunction after traumatic brain injury: pathophysiology and general management. Neurocrit Care. 38 (2), 504–516. https://doi.org/10.1007/s12028-022-01630-z (2023).
Kim, Y. C. et al. A simple scoring system using the red blood cell distribution width, Delta neutrophil index, and platelet count to predict mortality in patients with severe Sepsis and septic shock. J. Intensive Care Med. 34 (2), 133–139. https://doi.org/10.1177/0885066618787448 (2019).
Manninen, P. H., Lam, A. M., Gelb, A. W. & Brown, S. C. The effect of high-dose mannitol on serum and urine electrolytes and osmolality in neurosurgical patients. Can. J. Anaesth. J. Can. Anesth. 34 (5), 442–446. https://doi.org/10.1007/BF03014345 (1987).
Fink, M. E. Osmotherapy for intracranial hypertension: mannitol versus hypertonic saline. Contin Minneap. Minn. 18 (3), 640–654. https://doi.org/10.1212/01.CON.0000415432.84147.1e (2012).
Gelman, S. Does mannitol save the kidney? Anesth. Analg. 82 (5), 899–901. https://doi.org/10.1097/00000539-199605000-00001 (1996).
Buckley, M. S., Leblanc, J. M. & Cawley, M. J. Electrolyte disturbances associated with commonly prescribed medications in the intensive care unit. Crit. Care Med. 38 (6 Suppl), S253–264. https://doi.org/10.1097/CCM.0b013e3181dda0be (2010).
Liamis, G. et al. Hyponatremia in acute stroke patients: pathophysiology, clinical significance, and management options. Eur. Neurol. 82 (1–3), 32–40. https://doi.org/10.1159/000504475 (2019).
Mathisen, O., Raeder, M. & Kiil, F. Mechanism of osmotic diuresis. Kidney Int. 19 (3), 431–437. https://doi.org/10.1038/ki.1981.36 (1981).
Acknowledgements
We are very grateful for the contributions of our colleagues in the Department of Critical Care of the Second Affiliated Hospital of Guangzhou Medical University. We are also very grateful to the Physionet website for providing the MIMIC-IV database, and to Beth Israel Deaconess Medical Center for providing patient consultation data as well as the MIT Computational Physiology Laboratory team for developing and maintaining the database.
Funding
This work was supported by grants from the Natural Science Foundation of Guangdong Province (2022A1515010397) to Dr. Zhenghui Zhang; grants from the National Natural Science Foundation of China to Dr. Weiyan Chen (82202371).
Author information
Authors and Affiliations
Contributions
ZH Zhang, DL Wen and WX, Chen were jointly responsible for the conception, design, data collection, data analysis, and editing of the manuscript. WX, Chen, QZ Chen and YL Wang performed the data processing and data code review. WX, Chen and QZ Chen drafted the manuscript. QZ Chen and YL Wang reviewed the statistical analysis and literature search. ZH Zhang, DL Wen, QL Yang,, WY, Chen, LL Tao, JZ Zheng and WX Chen revised the manuscript and double-checked the statistical analysis results. All authors made substantial contributions and approved the content of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The MIMIC-IV database has already received approval from the institutional review boards at Beth Israel Deaconess Medical Center and the Massachusetts Institute of Technology. Our research adhered to local legislation and institutional guidelines. Furthermore, the need for written informed consent was waived by the same ethics committee, given that the database does not contain protected health information.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, W., Chen, Q., Wang, Y. et al. Impact of mannitol on mortality in patients with non-traumatic intracerebral haemorrhage and acute kidney injury: a retrospective study. Sci Rep 15, 26612 (2025). https://doi.org/10.1038/s41598-025-11175-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11175-8