Abstract
The emergence of methicillin-resistant Staphylococcus aureus (MRSA) as a major public health concern, particularly in hospital- and community-acquired infections, underscores the urgent need for novel antibiotic therapies. In response to this challenge, there has been renewed interest in exploring natural products derived from traditional plant sources as potential alternatives for combating multi-drug resistance. This study reveals the important mechanism by which the natural compound berberine blocks the WTA biosynthesis pathway by targeting and inhibiting the key enzymes TarO, TarS, and TarM for the synthesis of muramic acid (WTA) in MRSA.Specifically, tarO is the first key enzyme in the synthesis of WTA. tarS and tarM are responsible for the glycosylation of WTA. As a result, BBR significantly inhibits the activities of TarO and TarSM, leading to hindered WTA synthesis and causing structural defects in the cell wall. Notably, this effect can specifically restore the sensitivity of MRSA to β-lactam antibiotics (such as Penicillin and Cefazolin). Drug susceptibility tests indicate that tarO and tarSM mutant strains exhibit significantly enhanced sensitivity to oxacillin, methicillin, and cefotaxime. Additionally, the combination antimicrobial assay demonstrated that BBR synergistically enhanced the effects of oxacillin, methicillin, and cefotaxime on both wild-type and mutant strains, and recovered strains. Further experiments constructing deletion and complementation strains confirmed that the sensitizing effect of BBR directly relies on its inhibition of WTA synthesis. In conclusion, this study not only clarifies a new target for BBR to overcome β-lactam antibiotic resistance but also provides a theoretical basis for developing synergistic antimicrobial strategies based on WTA pathway inhibitors.
Similar content being viewed by others
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a leading clinical pathogen, commonly linked to respiratory infections, bloodstream infections, and skin and soft tissue infections, resulting in considerable morbidity and mortality1. MRSA produces a unique penicillin-binding protein, PBP2a, encoded by the mecA resistance gene. This protein reduces the affinity for β-lactam antibiotics while enabling peptidoglycan synthesis, conferring resistance2. Consequently, the development of new antimicrobial agents remains an essential and challenging area of research.
Bacterial cell wall synthesis is essential for bacterial survival and growth, and several clinically used antibiotics target this pathway to induce bacterial death. The bacterial cell wall comprises peptidoglycan (PG), lipoteichoic acid (LTA), and wall teichoic acid (WTA)3. The latest research demonstrates that the synthetic pathway of WTA is intricately linked to the resistance mechanism of MRSA against β-lactam antibiotics. When WTA synthesis is inhibited through targeted interventions, MRSA strains that have previously developed resistance to β-lactam antibiotics undergo structural remodeling of their cell walls and restore sensitivity to these antibiotics4. This finding establishes a critical theoretical foundation for the development of innovative antibacterial strategies aimed at targeting WTA synthesis, thereby addressing the clinical challenges associated with MRSA treatment5,6,7,8,9,10.WTA synthesis begins with the enzyme TarO11,12 and is further polymerized and glycosylated by key enzymes such as TarA, TarL, TarS, and TarM, with export across the cell membrane facilitated by TarGH13,14. TarO plays a critical role in the early stages of WTA synthesis in MRSA, and its inhibition reduces the expression of bacterial virulence genes, restoring susceptibility to β-lactam antibiotics15. In the β-lactam resistance mechanism of MRSA, glycosylated-modified WTA plays a pivotal role. Specifically, the TarM enzyme catalyzes the α-O-GlcNAcylation reaction, whereas the TarS enzyme is responsible for the β-O-GlcNAcylation process16. The synergistic action of these two enzymes markedly strengthens the drug resistance barrier in MRSA. Moreover, WTA is intricately involved in various bacterial physiological processes, such as the development of antibiotic resistance, biofilm matrix formation, and cell division regulation. These features render WTA a critical target for elucidating bacterial life activity mechanisms and designing novel antibacterial strategies17.
Berberine, an isoquinoline alkaloid found in plants such as17 such as Coptis and Phellodendron bark18, has garnered increasing attention for its therapeutic effects on cancer, diabetes, hyperlipidemia, cardiovascular disease, and central nervous system disorders19. In traditional Chinese medicine, berberine (BBR) has been used to treat bacterial diarrhea and gastroenteritis. Recent studies suggest that BBR can effectively reduce methicillin resistance in MRSA by downregulating the fni, sarA, and mecA genes20. Additionally, BBR enhances bacterial cell membrane permeability and disrupts the proton motive force, exerting inhibitory effects on MRSA21. Despite the known anti-MRSA activity of BBR19,22,23 and its derivatives24,25, the underlying mechanisms remain poorly understood. Previous research has shown that BBR has antibacterial activity against MRSA, damaging cell wall integrity26, and inhibiting MRSA by modulating bacterial cell wall hydrolysis, leukotoxin expression, and other virulence factors27.While the academic community has hypothesized that BBR may interact with the WTA synthesis pathway, there is currently no direct evidence to support the notion that it specifically binds to critical enzymes involved in this process, such as TarO, TarS, and TarM. The precise mechanism by which BBR may influence enzyme function remains uncertain; it is not yet established whether BBR exerts its effects by directly targeting the active sites of these enzymes to inhibit the initiation and glycosylation stages of WTA synthesis, or whether it operates through indirect mechanisms, such as modulating cell membrane permeability. Building on these findings, we constructed double mutant and recovery strains of key genes of the WTA synthesis pathway, tarO, tarS, tarM, and tarSM, to investigate how BBR affects WTA synthesis and MRSA resistance to β-lactam antibiotics by regulating these genes.
Results
The effect of bbr on key enzymes in the WTA biosynthesis process
Previous studies found that BBR compromises the integrity of the MRSA cell wall. To further explore the destructive impact of BBR on cell wall integrity, molecular docking simulations were conducted using AutoDock Vina to analyze the interactions between BBR and key proteins involved in WTA synthesis: TarS, TarO, TarA, TarB, TarM, TarL, TarH, and TarG. The binding energy values provide insight into the stability of these interactions, with lower values indicating stronger binding. Binding energies were classified as strong (<−5.0 kcal/mol), good (−4.25 to −5.0 kcal/mol), and weak (> −4.25 kcal/mol). The results revealed that BBR showed strong binding affinity with proteins TarA, TarS, TarO, TarM, TarL, and TarB, with optimal binding energies of −9.9, −9.0, −8.5, −8.1, −8.1, and − 8.0 kcal/mol, respectively. The binding energies for TarH and TarG were − 6.2 and − 6.6 kcal/mol, respectively(Fig. 1A). Hydrogen-bonding interactions between the amino acid residues of target proteins and BBR were analyzed using PyMOL v2.5.4 (Table 1 and Table S1), and 3D interaction diagrams were generated (Fig. 1B).
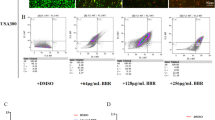
qPCR analysis of BBR on key genes of WTA synthesis pathway tarO, tarS, tarM
Molecular docking revealed that BBR strongly binds to the key enzymes in the WTA synthesis pathway, namely TarO, TarS, TarM, and TarA. TarO is a pivotal gene in the initial step of WTA synthesis, while TarS and TarM are glycosylation enzymes essential for β-lactam resistance. To investigate the effects of BBR on these genes, qRT-PCR was used to assess the mRNA expression levels in the USA300 strain at various BBR concentrations. Additionally, deletion and recovery strains were constructed to verify the inhibitory effects of BBR on tarO, tarS, and tarM. At a concentration of 32 µg/mL of BBR, the expression levels of tarO, tarS, and tarM in both the wild and recovered strains remained largely unchanged. However, at 64 µg/mL of BBR, the mRNA of these genes were altered, with tarM showing the most substantial reduction, up to 80%. These results suggest that BBR impedes the expression of tarO, tarS, and tarM during WTA biosynthesis, potentially hindering WTA production and exerting a bacteriostatic effect (Fig. 2).
A quantitative polymerase chain reaction (qPCR) analysis was conducted to investigate the impact of BBR on the critical genes involved in the WTA synthesis pathway:tarO>, tarS, and tarM. (A) mRNA expression level of tarO; (B) mRNA expression level of tarS; (C) mRNA expression level of tarM. Note: *P<0.05,**P<0.01.
BBR has been demonstrated to possess a substantial bactericidal effect on USA300
Flow cytometry assay results revealed that in the absence of BBR, the natural mortality rate of the mutant strains was higher than that of the wild strain, particularly in the ▲tarO and ▲tarSM double mutant strains. Upon treatment with 64 µg/mL of BBR, the mortality rates of both wild and mutant strains increased, with the ▲tarO and ▲tarSM strains exhibiting the highest die-off, reaching approximately 97%. The ▲tarS and ▲tarM mutant strains showed a slightly lower mortality rate of around 85%, while the wild USA300 strain exhibited 76% die-off. The bactericidal effect of BBR was significantly stronger on the mutant strains than the wild strain (Fig. 3).The bacteria were labelled with a fluorescent dye and examined for MRSA inhibition using confocal laser microscopy (CLSM). As shown in Fig. 4A, the entire surface of the bacteria in each control group showed green fluorescence and little to no red fluorescence, indicating a high number of viable bacteria. Treatment with 64 µg/mL of berberine resulted in a reduction in green fluorescence and an increase in red fluorescence, suggesting an obvious antimicrobial effect of berberine on MRSA, which is consistent with the TEM results(Fig. 4B).
The impact of BBR on the cell wall structure of USA300
The present study investigates the effects of BBR on the cell wall structure of wild and mutant strains (i.e. USA300, ▲tarS, ▲tarO, ▲tarM, ▲tarSM) were observed using transmission electron microscopy (TEM). The study revealed that different strains exhibited varied morphological and structural alterations in response to berberine.The untreated ▲tarS, ▲tarM mutants, and wild-type (WT) strains showed no significant morphological changes, and the cell wall structure remained intact and substantial, exhibiting only a modest degree of cytoplasmic crumpling.However, the ▲tarO, ▲tarSM mutants, and WT strains exhibited clear morphological alterations, including incomplete cell wall structure and cytoplasmic crumpling. After treatment with BBR, the ▲tarO and ▲tarSM strains exhibited heightened sensitivity and were particularly susceptible to damage. When 128 µg/mL of BBR was applied, a decrease in colony count was observed for both the mutant strains. The cell wall structure became loose and discontinuous, indicating signs of degradation and partial lysis, with irregular division processes and abnormal cytoplasmic characteristics, such as crumpling. This ultimately led to the separation of the cell membrane from the cell wall and the formation of enlarged interstitial spaces.At a higher concentration of 512 µg/mL BBR, a significant thinning of the cell walls of the ▲tarO and ▲tarSM strains were observed, accompanied by the appearance of a substantial number of degradation gaps in the cell wall. The cell wall boundaries appeared indistinct, and there was a significant release of bacterial contents. The bacteria appeared visibly deformed, with considerable loss of internal content. In contrast, 512 µg/mL BBR caused cytoplasmic solidification in the ▲tarM and ▲tarS strains, resulting in an uneven bacterial surface and abnormal protrusions (Fig. 5A-C).
Inhibition of USA300 WTA synthesis by BBR
WTA-PAGE electrophoresis showed that the ▲tarO and ▲tarSM mutants lacked WTA expression, while all other strains exhibited normal WTA synthesis when not treated with berberine (Fig. 6A). TarO is a critical gene in the initial step of WTA biosynthesis and is essential for WTA production. Deletion of tarO directly leads to the absence of WTA expression. TarM is responsible for α-O-glcNAcylation, while TarS catalyzes β-O-glcNAcylation in S. aureus. The ▲tarSM double mutation impairs WTA glycosylation, resulting in no WTA expression (Fig. 6A). Consistent with the results in Fig. 4, TEM showed that the untreated tarO and tarSM mutants treated with berberine exhibited obvious morphological changes with incomplete cell wall structure. Furthermore, WTA synthesis was significantly reduced in all strains following treatment with 64 µg/mL BBR (Fig. 6B and C). Furthermore, it was found that WTA migration was faster after berberine treatment than in the untreated group.These results confirm that BBR inhibits WTA synthesis in USA300.
Effect of β-lactam antibiotics on the sensitivity of standard strains
The sensitivity of standard, mutant, and restored strains to β-lactam antibiotics was evaluated utilizing the microbroth dilution method. The results demonstrated a marked decrease in the sensitivity of the ▲tarO and ▲tarSM strains to all three tested β-lactam antibiotics. Specifically, the minimum inhibitory concentration (MIC) for the ▲tarO strain was 0.016 µg/mL for oxacillin, 0.032 µg/mL for methicillin, and 2 µg/mL for cefotaxime. In contrast, the ▲tarSM strain exhibited an MIC of 4 µg/mL for oxacillin and methicillin and 4 µg/mL for cefotaxime. The sensitivity of the ▲tarS and ▲tarM strains to these β-lactams was also reduced, although to a lesser extent than in the ▲tarO and ▲tarSM strains. Both of these strains exhibited an MIC of 16 µg/mL for oxacillin and methicillin, and 32 µg/mL for cefotaxime. Notably, the MIC values for recovered strains were comparable to those of wild-type strains. Further details are provided in Table 2.
Synergistic effects of BBR with β-lactams
Related studies have shown that inhibitors of early peptidoglycan synthesis, such as ticlopidine and tunicamycin, can enhance the efficacy of β-lactams targeting PBP2 in MRSA strains (16, 17, 20). To determine whether BBR similarly synergizes with β-lactam drugs, the MICs of oxacillin, methicillin, and cefotaxime were assessed in the presence of BBR in MRSA strains. As anticipated, co-treatment with BBR increased the susceptibility of all strains to these β-lactams, with MICs decreasing by 2-8-fold. Detailed results are provided in Table 3.
BBR down-regulates the expression level of drug resistance gene mecA
The primary mechanism of resistance in MRSA is mediated by the mecA gene. qPCR analysis revealed that BBR significantly down-regulated the expression of mecA. Notably, the ▲tarO and ▲tarSM strains exhibited the greatest reduction in mecA expression, with a decrease of approximately 80%. Detailed results are provided in Fig. 7.
BBR is non-toxic to L929 cells
The cytotoxicity of BBR was assessed using the CCK-8 assay on L929 mouse fibroblasts. No cytotoxic effects were observed after exposing the cells to BBR for 24 h, even at high concentrations (512 µg/mL). Approximately 90% of the cells remained viable after 24 h (Fig. 8). These results confirm that BBR is non-toxic to L929 cells, suggesting that the inhibitory concentrations used are safe for mouse fibroblasts.
Discussion
In the present study, BBR demonstrated a significant antibacterial effect on MRSA. Previous research has shown that BBR increases MRSA cell membrane permeability, disrupts the cell wall structure, and induces bacterial killing26. Additionally, BBR regulates the hydrolysis function of the bacterial cell wall, leukotoxin expression, and other virulence factors, contributing to its antibacterial effects against MRSA17. This study further investigates the regulatory role of BBR on WTA, a key component of the MRSA cell wall.
S. aureus WTA has been demonstrated to regulate the spatial and temporal localization of autolysin Atl5 and PBP4, which are essential for peptidoglycan hydrolysis and cross-linking6,7. Deletion of WTA impairs peptidoglycan cross-linking, thereby restoring MRSA’s susceptibility to β-lactam antibiotics. As a result, the inhibition of WTA biosynthesis has emerged as a promising approach for combating MRSA infections.
The biosynthesis of WTA relies on the participation of key proteins such as TarO, TarS, and TarM. Molecular docking simulations were performed to study the interaction between BBR and these key proteins involved in the cell wall synthesis pathway. The results revealed that BBR exhibited strong binding affinity for these proteins, particularly with TarO, TarS, and TarM (Fig. 1). Further validation through qRT-PCR confirmed that BBR downregulated the expression of tarO, tarS, and tarM in the WTA biosynthesis process, as evidenced in the ▲tarO, ▲tarM, ▲tarS mutant strains, and their recovery strains constructed by homologous recombination (Fig. 2). Additionally, PAGE electrophoresis demonstrated that BBR inhibited the expression of tarO, tarS, and tarM, exerting a bacteriostatic effect. TarO is an enzyme involved in the synthesis of WTA. Tunicamycin inhibits the function of TarO, which leads to impaired function of the penicillin-binding protein PBP2 and high activation of the two-component signal transduction system VraRS27. This, in turn, results in dramatic down-regulation of the expression levels of genes encoding important virulence factors, such as phenol-soluble modulatory protein (PSM) and staphylococcal protein A (SpA), thereby compromising the integrity of the MRSA cell wall.TarS and TarM act as glycosyltransferases, catalysing the glycosylation modification of WTA16. TarS plays a key role in cell wall synthesis and maintenance in MRSA. This glycosylation modification affects the structure and function of WTA, influencing a variety of physiological processes in the bacterium.TarM and its encoded proteins have been studied only to a limited extent. There is no definitive study reporting the specific effects of tarM deficiency on growth and resistance to antibiotics or pathogenicity of MRSA28. However, it is hypothesised that TarM may indirectly regulate MRSA by catalysing the linkage of specific glycans. It is hypothesised that TarM may indirectly regulate MRSA cell wall integrity or host interactions by catalysing the attachment of specific sugar groups.In the double mutant strains lacking tarS and tarM, no glycosylated WTA was synthesized, leading to impaired peptidoglycan synthesis and, consequently, a disrupted bacterial cell wall structure. Therefore, BBR exerts its inhibitory effect by repressing the expression of tarO, tarS, and tarM, thereby hindering WTA biosynthesis.
In addition, the significant in vitro inhibitory effect of BBR on MRSA was observed through flow cytometry. The results showed that the number of dead bacteria in USA300 reached 76.30% when treated with 64 µg/mL BBR, while the number of dead bacteria in the ▲tarO and ▲tarSM mutant strains was higher than in the wild-type strain (Fig. 1). TEM further revealed the effect of BBR on the ultrastructure of MRSA. BBR caused obvious damage to the USA300 cell wall, with even more pronounced destructive effects observed in the ▲tarO and ▲tarSM mutants (Fig. 2). BBR inhibited the expression of the tarO, tarS, and tarM genes, which in turn suppressed the synthesis of WTA. This inhibition compromised the structural integrity of the bacterial cell wall, making it more vulnerable to the damaging effects of BBR.
To further investigate the impact of blocked WTA synthesis on β-lactam antibiotic resistance, pharmacological sensitivity tests revealed that the mutant strains lacking the tarO, tarS, and tarM genes exhibited significantly reduced resistance to oxacillin, methicillin, and cefotaxime (Table 2). WTA has been identified as a crucial antibacterial target directly associated with β-lactam resistance29,30. Notably, the ▲tarO and ▲tarSM mutant strains were significantly more susceptible to these antibiotics. The ▲tarO strain was up to 2,000 times more susceptible than the USA300 wild strain (Fig. 5A). Related studies have found that TarO is involved in the initial stage of WTA synthesis. Inhibiting TarO activity reduces the lowest inhibitory concentration of β-lactams by over 50-fold, restoring the clinical susceptibility of 75-80% of clinical isolates. This suggests that TarO is closely related to MRSA’s resistance to β-lactams, which is consistent with the results of the present study.The tarO gene is essential for the initial stages of WTA biosynthesis, facilitating the production and processing of lipid-linked sugars that subsequently bind to peptidoglycan, thus reinforcing the cell wall31. Depoxidil is essential for both WTA and PG synthesis. Abnormal WTA synthesis leads to an accumulation of depoxidil, which disrupts PG biosynthesis and compromises the bacterial structure. As a result, bacterial susceptibility to PG-inhibiting antibiotics, such as β-lactams, is enhanced (Fig. 6A)32. The ▲tarO strain, which lacks functional WTA synthesis, exhibited significantly increased sensitivity to all three β-lactam antibiotics.The glycosylation of WTA by the tarS and tarM genes is strongly associated with MRSA’s resistance to β-lactam antibiotics. Glycosylated WTA also serves as a scaffold for peptidoglycan synthase.Glycosylated WTA serves as a scaffold for PG synthase15,31,32.The absence of TarS and TarM impairs WTA glycosylation, altering the structure and composition of the cell wall. This affects the binding of antibiotics to cell wall targets and increases the sensitivity of MRSA to β-lactam antibiotics. Therefore, TarS and TarM are potential drug targets, and inhibiting them may re-sensitise MRSA to β-lactam antibiotics. Inhibiting TarS and TarM could provide a new strategy for the clinical treatment of MRSA infections by re-sensitising MRSA to β-lactam antibiotics33.
Co-pharmacological sensitization revealed that the antibacterial effect was significantly enhanced when BBR was combined with oxacillin, methicillin, and cefotaxime. Significant synergistic or additive inhibitory effects were observed with these combinations (Table 3). Previous studies have indicated that WTA deficiency can result in the mislocalization of PBP2a or PBP4 in USA300, resulting in the displacement of PBP2 across the cell membrane and affecting peptidoglycan cross-linking29,34. BBR has been shown to inhibit WTA biosynthesis, thereby disrupting the localization of PBPs, hindering peptidoglycan synthesis, and reducing cell wall resistance. Additionally, BBR interferes with bacterial cell wall synthesis, leading to bacterial inhibition. As a result, the combination of BBR with β-lactam antibiotics significantly enhances bacterial susceptibility.
Previous studies have shown that BBR disrupts MRSA cell structure, but the underlying mechanism remains unclear26. To further investigate the mechanism of action of BBR on the bacterial cell wall, the present study identifies that BBR inhibits key enzymes in the WTA biosynthetic pathway by targeting the genes tarO, tarS, and tarM. This inhibition reduces WTA expression, leading to impaired peptidoglycan synthesis, the main structural component of the cell wall, and ultimately causes the destruction of USA300 cell wall structure. The findings provide deeper insight into the molecular mechanisms by which BBR affects the MRSA cell wall.However, this study currently only involves in vitro research. In vivo research in animal models is needed to further investigate its pharmacokinetics, bioavailability and drug-drug interactions. This will lay the theoretical groundwork for clinical studies. Additionally, combining BBR with β-lactam antibiotics enhances bacterial susceptibility, resulting in synergistic antibacterial effects. The results suggest that BBR is a potent inhibitor when used in conjunction with existing β-lactam antibiotics, and has the potential to restore the efficacy of this critical class of antibiotics against MRSA. These findings open up new avenues for clinical strategies in anti-MRSA therapy.
Materials and methods
Bacterial strains and growth conditions
This study adhered to all relevant ethical guidelines. The bacterial strains and plasmids used are listed in Table S1. S. aureus strains were cultured in tryptic soy broth with shaking at 250 rpm or on TSA (Difco) at 37 °C. E. coli strains were cultured in LB broth at 37 °C with shaking at 250 rpm or on LB agar plates at 37 °C. Antibiotics were used for plasmid maintenance: erythromycin (80 µg/mL) for S. aureus USA300 LAC and derivatives, and carbenicillin (150 µg/mL) for E. coli. L929 mouse fibroblasts, used for cytotoxicity assessments, were cultured in RPMI-1640 medium with 10% FBS (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C with 5% CO2.
BBR and antibiotics
BBR was purchased from Beijing WoKe Biological Technology Co., Ltd. (Production batch number: XW20868312) and dissolved in dimethyl sulfoxide (DMSO). The following antibiotics were used: oxacillin, methicillin, and cefotaxime, all sourced from MedChemExpress.
Molecular docking
Molecular docking was employed to explore the potential binding mode of BBR with protein. The chemical structure of BBR was sourced from PubChem (https://pubchem.ncbi.nlm.nih.gov/), and the binding affinity was analyzed utilizing AutoDock Vina 1.2.5. Core targets for the TarO protein were identified through the NCBI database (https://www.ncbi.nlm.nih.gov/protein/). The amino acid sequence was used to predict the structure of the core target protein with AlphaFold, and the 3D model was downloaded in PDB format. PyMOL software was used to eliminate solvents and organics from the protein structures. AutoDock software (v1.5.6) was then applied for molecular docking, and the results were visualized with PyMOL software (v2.5.4).
MIC determination
Oxacillin, methicillin, and cefotaxime were serially diluted in Mueller-Hinton broth to final concentrations of 0.008, 0.016, 0.032, 0.064, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64, 128, and 256 µg/mL. In a 96-well plate, 100 µL of each antibiotic concentration was added to the respective wells. A bacterial suspension of 5 × 10⁵ CFU/mL was introduced to each well. The plate was incubated overnight at 37 °C, and the lowest concentration of drug that completely inhibited bacterial growth was recorded as the MIC. Negative controls (no bacterial suspension) and positive controls (no drug) were included in the experiment, which was repeated three times. The MIC values were determined according to the broth microdilution method following the guidelines of the Clinical and Laboratory Standards Institute (CLSI)35.
Synergistic antibacterial assay
In a 96-well plate, 50 µL of different concentrations of BBR and oxacillin, methicillin, or cefotaxime solutions were arranged in rows and columns. A bacterial suspension with a concentration of 5 × 10⁵ CFU/mL was added to each well. After overnight incubation at 37 °C, the culture was diluted tenfold, and 10 µL of the diluted bacterial suspension was added to each well. The plate was air-dried and incubated at 37 °C for an additional 24 h. The antibacterial effect of BBR combined with oxacillin, methicillin, or cefotaxime was assessed utilizing the FIC index as described by Kumarihamy et al.36. Synergy was defined as an FICI of ≤ 0.5; antagonism as an FICI > 2.0; additivity as an FICI > 0.5 but ≤ 1.0; and indifference as an FICI > 1.0 but ≤ 2.0. The experiment was repeated three times.
The FIC index was calculated as.
\({\text{FIC = [A*]/[A] + [B*]/[B]}}\), where [A*] denotes the MIC of compound A in the presence of compound B, [A] represents the MIC of compound A alone, [B*] indicates the MIC of compound B in the presence of compound A, and [B] indicates the MIC of compound B alone.
Bacterial viability assay
The AAT Bioquest MycoLight™ Bacterial Viability Assay Kit was used for a two-color fluorescence assay to assess bacterial viability in both gram-positive and gram-negative bacteria. In this assay, a combination of MycoLight™ Green and propidium iodide stains was employed. Live bacteria with intact cell membranes emit green fluorescence, whereas dead or dying bacteria with compromised cell membranes emit red fluorescence. The bacterial medium was diluted to a concentration of 10⁵ to 10⁸ cells/mL using 0.85% NaCl or an appropriate buffer. A sufficient volume of the suspension was prepared to provide at least 500 µL per test for flow cytometry (Beckman Coulter, USA). The two dyes were mixed in a 1:1 ratio, and 4 µL of the dye working solution (AAT Bioquest, USA) was added to each mL of bacterial suspension. The mixture was incubated at room temperature for 15 min in the dark. The stained bacterial cells were then analyzed by flow cytometry and confocal laser microscopy37.
TEM
A standard bacterial suspension was prepared at a concentration of 0.5 MIC units and incubated with BBR at 128 µg/mL (1 MIC) and 512 µg/mL (4 MIC) at 37 °C for 24 h. Subsequently, 10 mL of the bacterial suspension was centrifuged at 12,000 g for 10 min. The resulting pellet was collected, and the volume was reduced to 0.5 mL. The sediment was fixed with 2% glutaraldehyde in PBS for 2 h at 4 °C, followed by two washes with PBS. The sample was further fixed with 1% osmium in PBS for 2 h at 4 °C, then washed again with PBS. Dehydration was carried out stepwise with ethanol, and the sample was then replaced with propylene oxide. The sample was embedded in epoxy resin through immersion. Ultrathin sections were sliced utilizing an LKB V-type ultrathin microtome and stained with lead citratee. The samples were examined under a transmission electron microscope (H-7800, HITACHI, Japan) to observe the morphological changes in MRSA cells38.
WTA extraction and the polyacrylamide gel electrophoresis(PAGE) analysis
For WTA extraction, 20 mL of bacterial culture with an initial OD600 ≈ 0.1 was incubated overnight at 37 °C in the presence of BBR. The turbidity of the bacterial culture was monitored by measuring the OD value, and the WTAs were extracted following the method described by Meredith et al.39. The gel electrophoresis was performed utilizing a Bio-Rad Protean II xi apparatus at 4 °C with a constant current of 80 mA in Tris-Tricine running buffer (0.1 M Tris base, 0.1 M Tricine, pH 8.2) for several hours. After electrophoresis, WTA was visualized with Alcian blue silver staining.
RNA extraction and qRT-PCR
Bacterial cultures were diluted to an initial OD600 ≈ 0.1, treated with different concentrations of BBR, and incubated at 37 °C for 3 h. The control group was treated with DMSO. RNA was extracted using the Qiagen RNeasy kit. qRT-PCR was conducted utilizing a two-step method. First, RNA was reverse transcribed into cDNA using the M-MuLV First Strand cDNA Synthesis Kit (Sangon Biotech).The reverse transcription reaction program was as follows: cDNA synthesis at 42 °C for 30–60 min; reaction termination at 70 °C for 10 min. Real-time PCR was then carried out using 2× SG Fast qPCR Master Mix (Low Rox) (Sangon Biotech) on a QuantStudio 5 Applied Biosystems (ABI) fluorescence quantitative PCR instrument (Thermo Fisher Scientific). The reaction program was: 95 °C for 3 min for pre-denaturation, 95 °C for 3 s for denaturation, 60 °C for 30 s for annealing and extension, for 40 cycles. Positive control: the target gene was positive, that is, the control group without berberine treatment; internal reference gene positive: 16 S rRNA was used as the internal reference gene to ensure stable expression of the internal reference gene. Negative control: H2O was used instead of the cDNA template to exclude reagent contamination.Gene expression changes were calculated using the 2-ΔΔCt method. All experiments were conducted with three independent biological replicates, and each gene was tested in triplicate for technical replicates.
Construction of gene deletion mutants
The tarO deletion mutant (▲tarO) was constructed by amplifying the upstream (~ 1.1 kb) (Table S2) and downstream (~ 1.0 kb) fragments of the target gene from S. aureus USA300 LAC genomic DNA using specific primers (Table S1). These fragments were fused by overlapping PCR and ligated into plasmid pKOR1, generating pKOR1::tarO. The plasmid was cloned in E. coli DH5α, sequenced, and confirmed to be free of mutations. It was then transferred into S. aureus RN4220 and subsequently into S. aureus USA300 LAC for allelic substitution, confirmed by PCR. The deletion of tarS, tarM, and tarSM genes followed the same procedure.
Construction of gene complementation plasmids
To improve cloning efficiency, AscI and PspOMI restriction sites were introduced into the pYJ335 plasmid by site-directed mutagenesis, resulting in pYJ335-1, which contains EcoRV, AscI, and PspOMI sites. The tarO gene was amplified from S. aureus USA300 LAC genomic DNA using primers tarO-F and tarO-R (Table S3), then digested with PspOMI, and ligated downstream of the xyl/tetO promoter in pYJ335-1, generating the p-tarO plasmid (Table S4).
Statistical analysis
Statistical analysis was performed utilizing GraphPad Prism 8.0 software. Data were analyzed by Student’s t-test and one-way analysis of variance (ANOVA). A p-value less than 0.05 was considered statistically significant (*p < 0.05, **p < 0.01).
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Zhou, F., Gu, X., Wang, W., Lin, M. & Wang, L. Advancements in MRSA treatment: the role of Berberine in enhancing antibiotic therapy. BMC Microbiol. 24 (1), 540. https://doi.org/10.1186/s12866-024-03692-9 (2024). PMID: 39731013; PMCID: PMC11674092.
Bilyk, B. L., Panchal, V. V., Tinajero-Trejo, M., Hobbs, J. K. & Foster, S. J. An interplay of multiple positive and negative factors governs methicillin resistance in Staphylococcus aureus. Microbiol. Mol. Biol. Rev. 86 (2), e0015921. https://doi.org/10.1128/mmbr.00159-21 (2022). Epub 2022 Apr 14. PMID: 35420454; PMCID: PMC9199415.
Kohler, T. et al. Chap. 5 - Teichoic acids, Lipoteichoic acids and related cell wall glycopolymers of Gram-positive bacteria. In Microbial Glycobiology 1st edn 75–91 (Academic Press, Ltd, 2009).
Myrbråten, I. S. et al. SmdA is a novel cell morphology determinant in Staphylococcus aureus. mBio 13 (2), e0340421 (2022).
Foster, T. J. Can β-lactam antibiotics be resurrected to combat MRSA? Trends Microbiol. 27, 26–38 (2019).
Brown, S. et al. Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proc. Natl. Acad. Sci. USA. 109, 18909–18914 (2012).
Farha, M. A. et al. Inhibition of WTA synthesis blocks the cooperative action of PBPs and sensitizes MRSA to β-lactams. ACS Chem. Biol. 8, 226–233 (2013).
Campbell, J. et al. Synthetic lethal compound combinations reveal a fundamental connection Betwee wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem. Biol. 6, 106–116 (2011).
Maki, H., Yamaguchi, T. & Murakami, K. Cloning and characterization of a gene affecting the methicillin resistance level and the autolysis rate in Staphylococcus aureus. J. Bacteriol. 176, 4993–5000 (1994).
Komatsuzawa, H. et al. Cloning and characterization of the Fmt gene which affects the methicillin resistance level and autolysis in the presence of Triton X-100 in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 41, 2355–2361 (1997).
Sewell, E. W. & Brown, E. D. Taking aim at wall teichoic acid synthesis: new biology and new leads for antibiotics. J. Antibiot. (Tokyo). 67, 43–51 (2014).
Xia, G. & Peschel, A. Toward the pathway of S. aureus WTA biosynthesis. Chem. Biol. 15, 95–96 (2008).
Swoboda, J. G., Campbell, J., Meredith, T. C. & Walker, S. Wall teichoic acid function, biosynthesis, and Inhibition. Chembiochem 11, 35–45 (2010).
Winstel, V., Xia, G. & Peschel, A. Pathways and roles of wall teichoic acid glycosylation in Staphylococcus aureus. Int. J. Med. Microbiol. 304, 215–221 (2014).
Lu, Y. et al. Modulation of MRSA virulence gene expression by the wall teichoic acid enzyme TarO. Nat. Commun. 14, 1594 (2023).
Gerlach, D. et al. Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity. Nature 563 (7733), 705–709 (2018).
Ciofu, O. et al. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 20, 621–635. https://doi.org/10.1038/s41579-022-00682-4 (2022).
Alolqa, R. N. et al. Pharmacokinetics of a multicomponent herbal Preparation in healthy Chinese and African volunteers. Sci. Rep. 5, 12961 (2015).
Yan, D. et al. Promotion of quality standard of herbal medicine by constituent removing and adding. Sci. Rep. 4, 3668 (2014).
Chu, M. et al. Role of Berberine in the treatment of Methicillin-Resistant Staphylococcus aureus infections. Sci. Rep. 6, 24748. https://doi.org/10.1038/srep24748 (2016). PMID: 27103062; PMCID: PMC4840435.
Qiu, M. & Xu, Z. Berberine hydrochloride reduces staphyloxanthin synthesis by inhibiting fni genes in methicillin-resistant Staphylococcus aureus. Mol Biol Rep. ;51(1):761. (2024). https://doi.org/10.1007/s11033-024-09698-w. PMID: 38874884.
Zhao, N. et al. Berberine disrupts Staphylococcal proton motive force to cause potent anti-staphylococcal effects in vitro. Biofilm 5, 100117. https://doi.org/10.1016/j.bioflm.2023.100117 (2023). PMID: 37090161; PMCID: PMC10113750.
Yu, H. H. et al. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food. 2005 Winter;8(4):454 – 61. https://doi.org/10.1089/jmf.2005.8.454. PMID: 16379555.
Luo, J. Y., Yan, D. & Yang, M. H. Study of the anti-MRSA activity of Rhizoma coptidis by chemical fingerprinting and broth microdilution methods. Chin J Nat Med. ;12(5):393–400. (2014). https://doi.org/10.1016/S1875-5364(14)60049-2. PMID: 24856764.
Wang, J. et al. The synthesis and antistaphylococcal activity of 9, 13-disubstituted Berberine derivatives. Eur. J. Med. Chem. 127, 424–433. https://doi.org/10.1016/j.ejmech.2017.01.012 (2017). Epub 2017 Jan 9. PMID: 28092858.
Yang, Y. S. et al. Synthesis and biological evaluation of 7-substituted Cycloberberine derivatives as potent antibacterial agents against MRSA. Eur. J. Med. Chem. 168, 283–292. https://doi.org/10.1016/j.ejmech.2019.02.058 (2019). Epub 2019 Feb 23. PMID: 30825723.
Lee, S. H. et al. TarO-specific inhibitors of wall teichoic acid biosynthesis restore β-lactam efficacy against methicillin-resistant Staphylococci. Sci. Transl Med. 8 (329), 329ra32 (2016).
Gerlach, D. et al. Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity. Nature 563 (7733), 705–709. https://doi.org/10.1038/s41586-018-0730-x (2018). Epub 2018 Nov 21. PMID: 30464342.
Zhou, F. et al. Advancements in MRSA treatment: the role of Berberine in enhancing antibiotic therapy. BMC Microbiol. 24, 540 (2024).
Zhou Fangfang, W. et al. May. Effect of Berberine on the whole genome transcription of methicillin⁃resistant Staphylococcus aureus.chin J lab med, 43, 5: 576–581. (2020). https://doi.org/10.3760/cma.j.cn114452-20190613-00350
Wolters, P. J., Hildebrandt, K. M., Dickie, J. P. & Anderson, J. S. Polymer length of teichuronic acid released from cell walls of Micrococcus luteus. J. Bacteriol. 172, 5154–5159 (1990).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 (2001).
Swoboda, J. G., Campbell, J., Meredith, T. C. & Walker, S. Wall teichoic acid function, biosynthesis, and Inhibition. Chembiochem 11 (1), 35–45. https://doi.org/10.1002/cbic.200900557 (2010). PMID: 19899094; PMCID: PMC2798926.
Campbell, J. et al. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem. Biol. 6 (1), 106–116. https://doi.org/10.1021/cb100269f (2011). Epub 2010 Nov 4. PMID: 20961110; PMCID: PMC3025082.
Biswas, R. et al. Proton-binding capacity of Staphylococcus aureus wall teichoic acid and its role in controlling autolysin activity. PLoS One. 7, e41415 (2012).
Qamar, A. & Golemi-Kotra, D. Dual roles of FmtA in Staphylococcus aureus cell wall biosynthesis and autolysis. Antimicrob. Agents Chemother. 56, e00187–e00112 (2012).
CLSI Performance standards for antimicrobial susceptibility testing. In Clinical and Laboratory Standards Institute M100–M128 (CLSI, 2022).
Kumarihamy, M. et al. Synthesis and inhibitory activity of Machaeridiol-Based novel Anti-MRSA and Anti-VRE compounds and their profiling for Cancer-Related signaling pathways. Molecules 27 (19), 6604. https://doi.org/10.3390/molecules27196604 (2022). PMID: 36235141; PMCID: PMC9570708.
Freitas, M. A., Pereira, A. H., Pinto, J. G., Casas, A. & Ferreira-Strixino, J. Bacterial viability after antimicrobial photodynamic therapy with Curcumin on multiresistant Staphylococcus aureus. Future Microbiol. 14, 739–748. https://doi.org/10.2217/fmb-2019-0042 (2019). Epub 2019 Jul 4. PMID: 31271058.
Acknowledgements
The authors declare no potential conflicts of interest.
Funding
This work was supported by grants from the Key Discipline of Xuhui District, Shanghai (SHXHZDXK202322);Medical Research Projects of Shanghai Eighth People’s Hospital(SHBY202516) Medical Research Projects of Shanghai Eighth People’s Hospital SHBY202517.
Author information
Authors and Affiliations
Contributions
GXM and ZFF designed the study and wrote the manuscript. LYN performed the formal analysis. LM and DY conducted the investigation. WW and XZB were responsible for the visualization. JMM contributed to the methodology. GXM and ZFF wrote the original draft. JMM and WL contributed to writing, review, and editing. WL also acquired funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, F., Gu, X., Lin, M. et al. Mechanistic studies on the effect of berberine on methicillin-resistant Staphylococcus aureus drug resistance through modulation of wall teichoteic acid. Sci Rep 15, 26003 (2025). https://doi.org/10.1038/s41598-025-11226-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-11226-0