Abstract
Knee osteoarthritis (OA) is a prevalent degenerative joint disease among middle-aged and older adults, leading to chronic pain, functional limitations, and decreased quality of life. While probiotics have shown anti-inflammatory potential in preclinical studies, their therapeutic role in OA has not been well established in human research. This exploratory randomized controlled trial (RCT) aimed to evaluate the efficacy and safety of Latilactobacillus sakei LB-P12 in improving knee OA symptoms. In this randomized, double-blind, placebo-controlled trial, 100 participants aged 40–75 years with chronic knee pain were assigned to receive either LB-P12 (350 mg/day, 10 billion CFU) or placebo for 12 weeks. The primary outcome was the change in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores. Secondary outcomes included visual analog scale (VAS) scores for pain, inflammatory markers (CRP, ESR, IL-1β, IL-6, TNF-α), cartilage degradation biomarkers (COMP), joint space width, and health-related quality of life (EQ-5D-5L). Safety was evaluated using laboratory tests and vital signs. After 12 weeks, the LB-P12 group showed significantly greater improvements in WOMAC total scores and VAS pain scores compared to the placebo group. All three WOMAC subdomains—pain, stiffness, and physical function—showed consistent reductions. Inflammatory cytokines, particularly IL-1β, were significantly reduced in the LB-P12 group. Quality of life also improved significantly. No adverse events or clinically meaningful abnormalities in laboratory values were observed. This exploratory RCT suggests that daily supplementation with LB-P12 is safe and may offer potential benefits in reducing knee pain and improving physical function and quality of life in individuals with knee OA. Further large-scale and long-term RCTs are needed to confirm these findings and elucidate the underlying mechanisms.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is the most common joint disease worldwide, affecting over 500 million people or approximately 7% of the global population. It disproportionately affects women, with prevalence rates of approximately 10% in men and 18% in women aged > 60 years 1,2. As a leading cause of disability, OA imposes a significant socioeconomic burden, accounting for approximately 1.0–2.5% of the gross domestic product, particularly in developed countries2. The socioeconomic impact of OA is expected to increase further owing to an aging population and rising obesity rates, emphasizing the urgent need for effective and sustainable treatment options1.
Nonsteroidal anti-inflammatory drugs (NSAIDs), commonly used as first-line OA treatment, provide symptomatic relie3,4,5,6 but are associated with well-documented adverse effects, including cardiovascular (CV)7,8,9, gastrointestinal (GI)8,9,10, and renal toxicity9,11, particularly with long-term use.
Pro-inflammatory cytokines such as IL-1β12 and TNF-α13 play a central role in OA by initiating and amplifying inflammatory cascades that lead to cartilage degradation and joint tissue damage14,15,16. The pivotal role of inflammation in OA pathogenesis led to recent developments in OA therapeutics that focused on novel disease-modifying OA drugs (DMOADs) to reduce inflammation and promote cartilage repair17. These drugs target the underlying pathophysiological mechanisms of OA and show promise in inhibiting disease progression and fostering cartilage restoration, thereby addressing the limitations of conventional treatments and demonstrating their potential for disease improvement.
Recent studies suggest that OA is not merely a localized joint disease but a complex condition closely associated with systemic inflammation18,19, metabolic syndrome20,21, and obesity19,22. These systemic factors not only exacerbate OA progression but also highlight the interconnected nature of bodily systems in the pathogenesis of the disease. Among these connections, the gut-joint axis has emerged as a critical area of interest, emphasizing the role of the gut microbiota in modulating inflammatory pathways and potentially influencing OA progression23,24. These systemic influences highlight the growing recognition of the gut–joint axis in OA pathogenesis. In this context, targeting the gut microbiota through probiotic interventions—such as Latilactobacillus sakei—may offer a novel and sustainable approach to modulating systemic inflammation and supporting joint health. As this concept has gained attention, research has increasingly focused on probiotic-based therapies as potential complementary approaches to regulate systemic inflammation and support joint health by targeting the gut microbiota.
Recent studies have highlighted the potential of Latilactobacillus sakei to alleviate inflammatory conditions, such as obesity25,26, inflammatory bowel disease (IBD)27,28, and atopic dermatitis29,30,31. Specifically, L. sakei produces various metabolites, including bacteriocins, exopolysaccharides, lactic acid, and γ-aminobutyric acid (GABA), which have been suggested to have multiple effects on gut immune regulation28,32,33,34,35. These metabolites are hypothesized to play a crucial role in modulating the gut microbiota composition, increasing the levels of short-chain fatty acids (SCFAs)25,36, and enhancing the expression of tight junction proteins 37,38, thereby maintaining intestinal barrier integrity. The mechanisms underlying these functions also include proposed effects on restoring the Th17/Treg cell balance and inhibiting NF-κB activation39. Through these pathways, L. sakei may contribute to reducing inflammatory responses, alleviates systemic inflammation, and mitigates the progression of inflammatory conditions. These findings highlight the potential of L. sakei as a therapeutic agent for the management of inflammation-related diseases via its immunomodulatory effects.
As a newly studied strain, L. sakei LB-P12 presents a unique opportunity for preliminary exploration of its specific functional properties, particularly its probiotic and possible therapeutic potentials. Among various L. sakei strains, LB-P12 has been shown in preclinical studies to suppress the NF-κB and HIF-2α signaling pathways, reduce levels of pro-inflammatory cytokines such as IL-6 and TNF-α, and protect cartilage integrity in osteoarthritic models, distinguishing it as a particularly promising candidate for joint health interventions40. Notably, this exploratory RCT is the first clinical investigation of L. sakei in the context of joint health, making a pioneering effort to evaluate its potential role in OA. Furthermore, this study seeks to provide initial insights into L. sakei LB-P12, focusing on its ability to regulate inflammation, protect cartilage, and promote joint function recovery, with the ultimate goal of generating hypotheses for future large-scale clinical trials on OA prevention and management.
Material and Methods
Inclusion and exclusion criteria
Eligible participants were adults aged 40–75 years, who provided written informed consent after receiving a full explanation of the study’s objectives and procedures. Participants were included if they met the following criteria: (1) male or female aged 40 to 75 years; (2) reported a visual analog scale (VAS) pain score of ≥ 3/10; (3) suffered from Kellgren & Lawrence grade 1 or 2 knee osteoarthritis confirmed by bilateral knee joint anteroposterior (AP) X-rays; and (4) exhibited normal physical activity levels enabling participation in the trial.
Participants were excluded if they met any of the following criteria: (1) history of bone fractures within the past year; (2) had a meniscus tear; (3) were experiencing moderate osteoarthritis defined by radiographic findings such as periarticular osteophytes, irregular joint surfaces, or subchondral cysts; (4) were on COX-2 inhibitors, nonsteroidal anti-inflammatory drugs (NSAIDs), or joint-related dietary supplements (e.g., glucosamine, chondroitin, MSM) within 4 weeks of screening; (5) received probiotic or lactobacillus supplementation within 2 weeks of screening; (6) were diagnosed with thyroid disorders currently under treatment; (7) had kidney disease or serum creatinine levels ≥ 1.4 mg/dL; (8) had proteinuria ≥ 2 + ; (9) had liver disease or serum AST/ALT levels ≥ 100 IU/L; (10) were suffering from uncontrolled hypertension or history of angina or myocardial infarction; (11) had ongoing psychiatric conditions requiring medication (except intermittent use for sleep disorders); (12) were found to be using herbal medicines within 2 months of screening; (13) were participating in other clinical trials or use of investigational drugs within 4 weeks; (14) had conditions that may affect study outcomes as judged by the investigator; (15) had gastrointestinal surgery history (excluding appendectomy); (16) were pregnant or breastfeeding women; (17) were suffering from alcohol dependence or frequent alcohol consumption (≥ 4 times per week); (18) had hypersensitivity to the investigational product or its components; (19) any individuals deemed uncooperative or unsuitable for the study by the investigator; or (20) had arthritis attributed to factors other than degenerative conditions, as determined by the principal investigator.
Sample size calculation
To determine the appropriate sample size, a two-sided hypothesis test was conducted to evaluate the difference in the mean change in Korean Western Ontario and McMaster Universities Osteoarthritis Index (K-WOMAC) scores between the LB-P12 and placebo groups. The null hypothesis (H₀) assumed no significant difference in the mean K-WOMAC score change between the two groups, while the alternative hypothesis (H₁) proposed a significant difference. In this study, the independent variable was the type of intervention (LB-P12 vs. placebo), and the dependent variable was the change in K-WOMAC scores. Based on a previous study (Park et al., 2009)41, the standard deviation (σ) of K-WOMAC change in the intervention group was set at 14, and the expected mean difference (d) between the two groups was assumed to be 8.8, considering the 12-week intervention period. The statistical test was performed with a significance level (α) of 0.05 (two-sided, Zα/2 = 1.960) and a statistical power (1-β) of 80% (Zβ = 0.84).
Using G*Power 3.1.9.2, the minimum required sample size was determined to be 40 participants per group. Considering a 20% dropout rate, the final target sample size was set at 50 participants per group, totaling 100 participants.
Study design and intervention
One hundred participants were enrolled in the study, 50 of whom were assigned to the LB-P12 group and 50 to the placebo group. Both groups received identical 350 mg capsules to ensure blinding. The participants were instructed to take one capsule daily after dinner with water for 12 weeks. The probiotics used in this study were Latilactobacillus sakei LB-P12, originally isolated from Kimchi. The strain was developed, characterized, and standardized by LISCure Biosciences Inc. In the LB-P12 group, each capsule contained 233 mg of LB-P12, delivering 10 billion CFU per day. In contrast, the placebo group received capsules containing carboxymethylcellulose (CMC) without any active probiotic ingredients. The identical appearance and administration instructions for both groups ensured consistency and compliance throughout the study period.
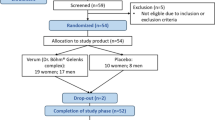
During the trial, eight participants withdrew from the study (five from the LB-P12 group and three from the placebo group) due to voluntary withdrawal of consent. The stated reasons included personal schedule conflicts, frequent business travel, and refusal to continue study product intake, with no adverse events or protocol-related concerns reported. Consequently, 92 participants completed the study and were included in the per-protocol (PP) analysis, whereas all 100 enrolled participants were included in the intention-to-treat (ITT) analysis.
The study adhered to the CONSORT guidelines. Figure 1 provides an overview of participant recruitment, randomization, dropouts, and final analysis.
Efficacy evaluations
Primary endpoints
The primary endpoint was evaluated using the WOMAC (Western Ontario and McMaster Universities Arthritis Index) score, a validated tool42,43,44 commonly used to assess pain, joint stiffness, and physical function in patients with osteoarthritis. The evaluation focused on four key aspects: overall symptom improvement through changes in the total WOMAC score, reduction in pain levels based on the WOMAC pain subscale, improvements in joint flexibility through the stiffness subscale, and enhanced daily activities and mobility assessed via the physical function subscale. The efficacy of LB-P12 was analyzed by comparing the changes in these scores before and after supplementation. Specifically, WOMAC scores were measured at visits 2 (week 0 before supplementation) and 4 (week 12 after supplementation). The primary method adopted for efficacy evaluation was per-protocol (PP) analysis, which ensured a comprehensive assessment of the effects of the treatment.
Secondary endpoints
Secondary efficacy endpoints were used to evaluate the therapeutic effects of LB-P12 from multiple perspectives. Efficacy evaluation was primarily based on PP analysis, and secondary efficacy variables were assessed at visit 2 (week 0 prior to supplementation) and visit 4 (week 12 after supplementation).
First, the VAS, a well-established tool for measuring pain intensity, was used to assess changes in the patients’ subjective pain levels. The scale ranged from 0 to 10, with lower scores indicating less pain, thus allowing a clear assessment of pain relief. Secondly, systemic inflammatory markers, including serum C-reactive protein (CRP) levels and erythrocyte sedimentation rate (ESR), were measured to evaluate changes in inflammation. These biomarkers are widely recognized indicators of inflammatory responses and provide indirect evidence of LB-P12’s anti-inflammatory properties. Third, the levels of inflammatory cytokines such as IL-1β, IL-6, and TNF-α were analyzed using serum samples collected from participants. These cytokines play critical roles in the pathophysiology of osteoarthritis, and their measurement allows for the direct assessment of LB-P12’s potential to modulate inflammation. Fourth, serum levels of cartilage oligomeric matrix protein (COMP), a biomarker of cartilage damage, were measured to evaluate the extent of cartilage degradation and potential improvement following treatment. COMP serves as a reliable indicator of changes in cartilage integrity. Fifth, the joint space width (JSW) was measured using radiography to assess the changes in cartilage thickness and joint structure. This metric is a key indicator of cartilage regeneration and helps to evaluate the ability of LB-P12 to slow or reverse osteoarthritis progression. Finally, the EQ-5D-5L was used to assess health-related quality of life. This five-dimensional survey evaluated mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, providing a comprehensive overview of the impact of the treatment on the patients’ daily lives and overall well-being.
By analyzing these secondary endpoints, this study provided a holistic evaluation of LB-P12’s effects on pain relief, inflammation, cartilage health, joint structure, and quality of life. Measurements at weeks 0 and 12 allowed the assessment of changes over the treatment period, providing detailed insights into the therapeutic potential of LB-P12.
Biochemical assessment
To assess participants’ general health status and monitor potential adverse effects, clinical laboratory tests were conducted at each scheduled visit following a minimum 6-h fast. The 12 mL fasting blood samples were then centrifuged for 15 min at 1000g (3,000 rpm) within 30 min. After centrifugation, the plasma samples were separated into pyrogen-free tubes using pyrogen-free pipette tips and stored at − 80 °C until analysis. U2Bio CO., LTD. analyzed the samples. Each biochemical parameter was measured using a specific device to minimize errors. The levels of inflammatory cytokines such as IL-1β, IL-6, and TNF-α were analyzed using serum samples collected from participants. These cytokines were measured using a multiplex bead-based immunoassay (Milliplex® MAP Human Cytokine/Chemokine Magnetic Bead Panel, Millipore, USA) according to the manufacturer’s guidelines on a Luminex 200 system, enabling high-sensitivity detection of multiple cytokines simultaneously.
Safety evaluations
The occurrence, characteristics, duration, severity, relationship with the investigational treatment, and outcomes of all the adverse events were determined and documented at each visit (on days 0, 6, and 12). Safety assessments included physical examinations (vital signs, pulse rate, body temperature, systolic blood pressure, and diastolic blood pressure), laboratory test results (normal/abnormal changes in hematology, serum chemistry, and urinalysis), and allergic reactions (grade 4 or higher adverse events, according to the Common Terminology Criteria for Adverse Events [CTCAE v5.0]) during the screening period and at week 12. Blood samples for safety assessment were collected after a 6-h fast and analyzed at Dong-A University Hospital.
Statistical analysis
The general characteristics of the study population are summarized as follows: categorical data are expressed as frequencies and percentages, ordinal data as medians and interquartile ranges (IQRs), and continuous data as means and standard deviations (SDs). The missing values were imputed using the Last Observation Carried Forward (LOCF) method. The normality of continuous variables was assessed using the Shapiro–Wilk test. For between-group comparisons of categorical variables, the chi-square test or Fisher’s exact test was used depending on the expected cell counts. Differences in ordinal variables were evaluated using the Mann–Whitney U test, whereas continuous variables were analyzed using independent t-tests if normally distributed, or the Mann–Whitney U test if not. For within-group comparisons over time, paired t-tests were used for normally distributed data and the Wilcoxon signed-rank test was applied for non-normally distributed data. Comparisons between the two groups for key variables were verified using analysis of covariance (ANCOVA), adjusting for factors such as sex, alcohol consumption history, and baseline variable differences. Outliers identified during the analysis were removed and reanalyzed as reference data. The primary analysis of efficacy endpoints was conducted based on PP analysis, with additional reference analyses performed using ITT analysis. Study participants were classified into the PP and ITT groups based on randomization and availability of efficacy endpoint data. The PP analysis group included participants in the ITT group who completed the study by visit 4 without major protocol violations. The ITT group included participants who had consumed the investigational product at least once and had available data on the primary efficacy endpoints after consumption. All the statistical analyses were performed using SPSS Statistics for Windows (version 26.0; IBM Corp., Armonk, NY). The significance level was set at 0.05, and all the tests were two-sided. Interim analyses were not performed. These statistical methods ensured the reliability of the data and systematically validated the efficacy of the investigated products.
Study approval
This study was conducted in accordance with the Declaration of Helsinki and the Korean Good Clinical Practice (KGCP) guidelines. The study protocol was approved by the Institutional Review Board (IRB) of Dong-A University Hospital (approval number: DAUHIRB-EXP-23-187, approved on 11 September 2024). The official trial registration page can be accessed at CRIS Trial Registration Page. The trial was registered at the Clinical Research Information Service (CRIS) on 21 February 2025 (registration number: KCT0010221), and the official registration page is available at https://cris.nih.go.kr/. All potential participants received detailed written information about the study and were thoroughly briefed on its purpose and procedures. Informed consent was obtained from each volunteer, and signed copies were provided for their records.
Results
Patient disposition, demographics, and baseline characteristics
Of the 100 randomized patients, 50 were assigned to the experimental group and 50 to the control group, with eight dropouts (five in the LB-P12 group and three in the placebo group) due to withdrawal of consent. All the withdrawals were confirmed via telephonic conversations without termination visits and dropouts were attributed to personal reasons. The participant flow, including enrollment and dropout details, is illustrated in Fig. 1. Patients were 9.8% male and 90.2% female, with a mean age of 62.79 ± 6.91 years and a mean body mass index (BMI) of 23.56 ± 0.57 kg/m2. Both groups had predominantly female populations, 91.1% in the experimental group and 89.4% in the control group. The Kellgren and Lawrence grading scores were consistent between the two groups, with both showing a mean grade of 1.0 (1.0). As shown in Table 1, the p-value (0.197) indicated no significant difference, confirming that the baseline severity of osteoarthritis was comparable between the experimental and control groups.
Per-protocol (PP) population results
Table 2 summarizes the comparison of both the primary and secondary efficacy endpoints based on the PP group analysis, highlighting the changes observed in the experimental and control groups after 12 weeks.
Primary efficacy endpoints
The primary efficacy endpoints, including changes in the total WOMAC score and its subcategories (pain, stiffness, and physical function), are detailed in Table 2. After 12 weeks, the total WOMAC score significantly decreased in the experimental group by -4.07 ± 12.76 points (p = 0.027) compared to the baseline, while the control group showed an increase of 2.15 ± 10.63 points (p = 0.340). The between-group comparison revealed a statistically significant reduction in the experimental group compared to the control group (p = 0.009). For the WOMAC pain score, the experimental group showed a decrease of -0.78 ± 2.77 points (p = 0.059), whereas the control group showed an increase of 0.49 ± 2.01 points (p = 0.087). While the reduction in the experimental group was not individually significant, the between-group comparison demonstrated a statistically significant reduction in the experimental group compared to the control group (p = 0.009). Regarding the WOMAC stiffness (joint rigidity) score, the experimental group experienced a statistically significant decrease of − 0.44 ± 1.31 points (p = 0.031), whereas the control group showed an increase of 0.26 ± 1.59 points (p = 0.256). The between-group comparison indicated a statistically significant reduction in the experimental group compared to the control group (p = 0.031). For the WOMAC physical function score, the experimental group showed a statistically significant decrease of − 2.84 ± 9.59 points (p = 0.034) after 12 weeks, whereas the control group demonstrated an increase of 1.40 ± 7.76 points (p = 0.407). The between-group comparison showed a statistically significant reduction in the experimental group compared to the control group (p = 0.023). These changes are visually represented in Fig. 2, which highlights the improvements in the primary efficacy endpoints observed in the experimental group compared to the control group.
Improvements in primary efficacy endpoints related to LB-P12 in the PP group. This figure summarizes the changes in primary efficacy endpoints in the PP group, including the WOMAC total score, pain, stiffness, and physical function subcategories, over 12 weeks between the control and treatment groups.
Secondary efficacy endpoints
Secondary efficacy endpoints, including changes in the VAS scores, IL-1β levels, and EQ-5D-5L quality of life scores, are also presented in Table 2. For the VAS score (mm), the experimental group showed a decrease of − 16.46 ± 15.40 points (p < 0.001) after 12 weeks, compared to a decrease of − 8.76 ± 15.68 points (p = 0.001) in the control group. Both groups exhibited statistically significant reductions, with the between-group comparison demonstrating a significantly greater reduction in the experimental group (p = 0.020). For the inflammatory marker IL-1β (pg/mL), the experimental group showed a decrease of -0.71 ± 1.08 pg/mL (p < 0.001), compared to a decrease of -0.06 ± 1.49 pg/mL (p < 0.001) in the control group. Between-group comparisons revealed a significantly greater reduction in the experimental group than in the control group (p = 0.009). For the EQ-5D-5L quality of life assessment, after 12 weeks, the experimental group showed an increase of 0.01 ± 0.15 points (p = 0.390) compared to baseline, while the control group demonstrated a decrease of − 0.04 ± 0.12 points (p = 0.009). The between-group comparison indicated a statistically significant increase in the experimental group compared to the control group (p = 0.021). These results are summarized in Fig. 3, which highlights the improvements in the secondary efficacy endpoints in the experimental group compared to the control group.
Improvements in secondary efficacy endpoints related to LB-P12 in the PP group. This figure illustrates the changes in key efficacy endpoints in the PP group, including after 12 weeks of intervention with LB-P12 compared to the control group. [(A) VAS (pain score), (B) IL-1β (inflammatory marker), (C) EQ-5D-5L (Quality of life), (D) C-reactive protein (CRP), (E) Erythrocyte Sedimentation Rate (ESR), (F) IL-6, (G) TNF-α, (H) Cartilage Oligomeric Matrix Protein (COMP), (I) Joint space width].
Intention-to-treat (ITT) population results
Table 3 presents the changes in both the primary and secondary efficacy endpoints based on the ITT group analysis, highlighting the outcomes observed in the experimental and control groups after 12 weeks.
Primary efficacy endpoints
As shown in Table 3, the total WOMAC score significantly decreased in the experimental group by − 3.66 ± 12.15 points (p = 0.027), while the control group increased by 2.02 ± 10.31 points (p = 0.340). The between-group comparison demonstrated a statistically significant reduction in the experimental group compared to the control group (p = 0.011). For the WOMAC pain score, the experimental group showed a decrease of − 0.70 ± 2.64 points (p = 0.059), and the control group showed an increase of 0.46 ± 1.95 points (p = 0.087). Although the reduction in the experimental group was not individually significant, the between-group comparison indicated a significant decrease (p = 0.009). For the WOMAC stiffness score, the experimental group showed a statistically significant decrease of − 0.40 ± 1.25 points (p = 0.031), while the control group showed an increase of 0.24 ± 1.55 points (p = 0.256). Between-group comparisons revealed a significant reduction in the experimental group (p = 0.036). The WOMAC physical function score decreased by − 2.56 ± 9.13 points (p = 0.034) in the experimental group, whereas the control group showed an increase of 1.32 ± 7.53 points (p = 0.407). Between-group comparisons confirmed a statistically significant reduction in the experimental group (p = 0.028).
Secondary efficacy endpoints
Table 3 shows the secondary efficacy endpoints. For the VAS score, the experimental group showed a decrease of − 14.81 ± 15.42 points (p < 0.001) compared to − 8.23 ± 15.34 points (p = 0.001) in the control group. Between-group comparisons indicated a significantly greater reduction in the experimental group (P = 0.035). For IL-1β, the experimental group demonstrated a reduction of − 0.63 ± 1.04 pg/mL (p < 0.001), while the control group exhibited a smaller decrease of − 0.06 ± 1.45 pg/mL (p < 0.001). Between-group comparisons revealed a significantly greater reduction in the experimental group (p = 0.031). In the EQ-5D-5L quality of life assessment, the experimental group showed an increase of 0.01 ± 0.14 points (p = 0.390), whereas the control group showed a decrease of − 0.04 ± 0.11 points (p = 0.009). Between-group comparison demonstrated a statistically significant improvement in the experimental group (p = 0.018).
Safety
The safety of the test product LB-P12 was assessed by evaluating 50 participants in the experimental group and 50 in the control group, all of whom consumed the test product at least once. Safety assessments included monitoring of adverse events, clinical laboratory tests, vital signs, and physical examinations conducted by the principal investigator and study staff. No adverse events or serious adverse events were reported among the 100 participants during the study period. Blood test results revealed no significant differences between the groups at visit four, with all observed variations falling within the normal clinical ranges. Detailed hematological results are presented in Supplementary Table S1.
Additionally, the vital signs and physical examination results showed no statistically significant differences between the experimental and control groups, confirming the safety of LB-P12.
Discussion
In this study, improvements in the WOMAC and VAS scores were observed in the LB-P12 group after 12 weeks. The total WOMAC score decreased by − 4.07 ± 12.76 points (p = 0.027) in the experimental group, while it increased by 2.15 ± 10.63 points (p = 0.340) in the control group, showing a significant difference between the groups (p = 0.009).
For the subcategories, the pain score decreased by − 0.78 ± 2.77 points (p = 0.059) in the experimental group and increased by 0.49 ± 2.01 points (p = 0.087) in the control group. The stiffness score decreased by − 0.44 ± 1.31 points (p = 0.031) in the experimental group, while it increased by 0.26 ± 1.59 points (p = 0.256) in the control group. The physical function score also decreased by − 2.84 ± 9.59 points (p = 0.034) in the experimental group and increased by 1.40 ± 7.76 points (p = 0.407) in the control group, with the experimental group showing better outcomes across all subcategories. For the VAS score, the experimental group showed a decrease of − 16.46 ± 15.40 mm (p < 0.001), while the control group showed a decrease of − 8.76 ± 15.68 mm (p = 0.001), with a statistically significant difference between the two groups (p = 0.020). Power analysis calculations confirmed that the study was adequately powered (80%) to detect a clinically relevant difference of 8.8 points in WOMAC scores between the two groups, based on a standard deviation of 14. These findings suggest that LB-P12 may be associated with improvements in pain and physical function in patients with knee osteoarthritis. However, as this was an exploratory study, the results should be interpreted with caution, and further validation in larger, long-term trials is warranted.
The observed reduction in IL-1β levels in the LB-P12 group suggests a potential immunomodulatory effect of the probiotic intervention. Pro-inflammatory cytokines such as IL-1β12 and TNF-α13 are known to play central roles in OA pathogenesis by promoting inflammatory cascades that lead to cartilage degradation and joint tissue damage. Therefore, modulation of these cytokines may represent a plausible mechanism through which LB-P12 contributes to symptom improvement.
To further contextualize these findings, previous in vitro and in vivo studies of Latilactobacillus sakei strains—including LB-P12—have demonstrated broad anti-inflammatory effects. Notably, L. sakei has been shown to produce bioactive compounds such as bacteriocins45,46,47, GABA48, and exopolysaccharides49, which are known to modulate the gut environment and support immune homeostasis. which are known to modulate immune responses by suppressing IL-1β, IL-6, and TNF-α50, and by enhancing IL-10 expression38. These effects are thought to occur via suppression of the NF-κB pathways26 and by promoting gut immune homeostasis. In preclinical models of rheumatoid arthritis and metabolic inflammation, L. sakei administration led to reduced inflammatory cytokine expression and improved systemic immune regulation, highlighting its translational potential51,52.
Collectively, these findings provide mechanistic support for the observed IL-1β reduction in this clinical trial and reinforce the hypothesis that LB-P12 may exert beneficial effects via gut-mediated systemic immunomodulation.
Latilactobacillus sakei LB-P12, as highlighted in this study, is a probiotic strain with significant potential for improving joint health, as demonstrated in a rigorous clinical trial. While previous studies on various L. sakei strains have primarily relied on preclinical models such as mouse experiments, this research represents an initial clinical investigation providing preliminary human-based data. By suggesting possible improvements in knee osteoarthritis symptoms, with a controlled randomized trial, this study provides valuable preliminary insights but requires further validation to establish clinical effectiveness.
This study provides valuable initial insights into the potential role of Latilactobacillus sakei LB-P12 in improving joint health; however, some limitations should be acknowledged. First, the study sample size (n = 100) was determined based on power analysis to detect differences in WOMAC scores. Although statistically powered, the limited sample size restricts the generalizability of the findings, and larger studies are needed to confirm these results. In particular, the study population was predominantly female (90.2%), which aligns with national epidemiologic data indicating a significantly higher prevalence of radiographic knee osteoarthritis in Korean women aged ≥ 50 years (37.3%) compared to men (19.2%)53,54. This distribution reflects the actual disease burden and enhances the demographic relevance of our findings. While this distribution reflects the real-world prevalence of knee osteoarthritis in older Korean women, the predominance of female participants (90.2%) may limit the generalizability of the findings to male populations. Future studies should aim to recruit a more gender-balanced cohort to validate the effects of LB-P12 in both sexes. Secondly, the study duration of 12 weeks may not have fully captured the long-term effects of LB-P12 on joint health or disease progression Longer follow-up periods are needed to assess whether these effects persist over time. Third, although reductions in inflammatory markers such as IL-1β were observed, this study did not explore the precise mechanisms by which LB-P12 influences joint health. These findings suggest that future studies should explore the gut–joint axis and cartilage degradation markers in greater depth to elucidate the mechanistic basis of LB-P12’s effects on osteoarthritis. In support of this, previous in vitro and in vivo preclinical studies have demonstrated that LB-P12 exerts broader anti-inflammatory effects. Specifically, LB-P12 administration was associated with downregulation of IL-6, TNF-α, and PGE2, and suppression of the NF-κB and HIF-2α signaling pathways in animal models of osteoarthritis. These findings suggest a plausible mechanistic basis for the observed effects on IL-1β in this clinical study. The present findings are consistent with preclinical evidence of anti-inflammatory effects of LB-P12, supporting the translational potential of probiotic interventions. The discrepancy between the clinical and preclinical biomarker profiles may reflect differences in study duration, immune response dynamics, or the sensitivity of systemic biomarkers to localized joint inflammation. Additionally, it is possible that the predominantly mild osteoarthritis among participants limited the baseline systemic inflammatory burden. In such populations, inflammatory markers like IL-6 and TNF-α may be less elevated and therefore less likely to demonstrate measurable reductions40. Studies involving patients with more severe or active disease may reveal clearer systemic anti-inflammatory effects of LB-P12. Future studies should investigate the mechanistic basis of LB-P12’s effects by incorporating (1) microbiome composition and metabolite profiling (e.g., short-chain fatty acids), (2) gut permeability markers, and (3) comprehensive inflammatory and cartilage degradation biomarker analysis. These investigations may provide deeper insights into the gut–joint axis and systemic immunomodulation associated with LB-P12. Despite these limitations, this study contributes to the growing body of evidence supporting the role of probiotics in musculoskeletal health. Future large-scale, multi-center randomized controlled trials are warranted to confirm these findings and further investigate the mechanistic basis of LB-P12’s effects.
Conclusion
In conclusion, L. sakei LB-P12 may offer potential benefits in improving knee osteoarthritis symptoms, as evidenced by reductions in pain (VAS) and functional impairment (WOMAC scores The findings, supported by power analysis, indicate a possible role for LB-P12 as a probiotic intervention for joint health; however, the exploratory nature of this study necessitates cautious interpretation. Given the study limitations, future large-scale RCTs with longer follow-up periods and a more comprehensive biomarker analysis are essential to fully establish the clinical applicability of LB-P12 in osteoarthritis management. This study provides preliminary evidence suggesting a potential role of L. sakei LB-P12 and underscores the need for continued research into probiotic-based interventions for musculoskeletal health.
Data availability
The datasets generated and/or analyzed in the current study are available from the corresponding author upon request. Requests to access these datasets should be directed to B.K.S.
References
Hunter, D. J., March, L. & Chew, M. Osteoarthritis in 2020 and beyond: A lancet commission. The Lancet 396, 1711–1712 (2020).
Allen, K., Thoma, L. & Golightly, Y. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 30, 184–195 (2022).
Magni, A. et al. Management of osteoarthritis: Expert opinion on NSAIDs. Pain Ther. 10, 783–808 (2021).
Bannuru, R. R. et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartil. 27, 1578–1589 (2019).
Bruyère, O. et al. Seminars in arthritis and rheumatism. 337–350 (Elsevier).
Kolasinski, S. L. et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 72, 220–233 (2020).
Schjerning, A.-M., McGettigan, P. & Gislason, G. Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat. Rev. Cardiol. 17, 574–584 (2020).
Lanas, A., Tornero, J. & Zamorano, J. L. Assessment of gastrointestinal and cardiovascular risk in patients with osteoarthritis who require NSAIDs: the LOGICA study. Ann. Rheum. Dis. 69, 1453–1458 (2010).
Bindu, S., Mazumder, S. & Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 180, 114147 (2020).
Domper Arnal, M.-J., Hijos-Mallada, G. & Lanas, A. Gastrointestinal and cardiovascular adverse events associated with NSAIDs. Expert Opin. Drug Saf. 21, 373–384 (2022).
Knights, K. M., Tsoutsikos, P. & Miners, J. O. Novel mechanisms of nonsteroidal anti-inflammatory drug-induced renal toxicity. Expert Opin. Drug Metab. Toxicol. 1, 399–408 (2005).
Yu, C., Miao, W., Zhang, Y., Zou, M. & Yan, X. Inhibition of miR-126 protects chondrocytes from IL-1β induced inflammation via upregulation of Bcl-2. Bone Joint Res. 7, 414–421 (2018).
Min, S. et al. Serum levels of the bone turnover markers dickkopf-1, osteoprotegerin, and TNF-α in knee osteoarthritis patients. Clin. Rheumatol. 36, 2351–2358 (2017).
Chow, Y. Y. & Chin, K.-Y. The role of inflammation in the pathogenesis of osteoarthritis. Mediators Inflamm. 2020, 8293921 (2020).
Kapoor, M., Martel-Pelletier, J., Lajeunesse, D., Pelletier, J.-P. & Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 7, 33–42 (2011).
Sellam, J. & Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 6, 625–635 (2010).
Oo, W. M., Little, C., Duong, V. & Hunter, D. J. The development of disease-modifying therapies for osteoarthritis (DMOADs): The evidence to date. Drug Design, Development and Therapy, 2921–2945 (2021).
Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 21, 16–21 (2013).
Wang, T. & He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 44, 38–50 (2018).
Yoshimura, N. et al. Association of knee osteoarthritis with the accumulation of metabolic risk factors such as overweight, hypertension, dyslipidemia, and impaired glucose tolerance in Japanese men and women: the ROAD study. J. Rheumatol. 38, 921–930 (2011).
Shin, D. Association between metabolic syndrome, radiographic knee osteoarthritis, and intensity of knee pain: Results of a national survey. J. Clin. Endocrinol. Metab. 99, 3177–3183 (2014).
Lee, S. et al. Obesity, metabolic abnormality, and knee osteoarthritis: A cross-sectional study in Korean women. Mod. Rheumatol. 25, 292–297 (2015).
Gleason, B., Chisari, E. & Parvizi, J. Osteoarthritis can also start in the gut: the gut–joint Axis. Indian J. Orthop. 56, 1150–1155 (2022).
Hao, X. et al. The gut microbiota in osteoarthritis: Where do we stand and what can we do?. Arthritis Res. Ther. 23, 1–11 (2021).
Lim, S., Moon, J. H., Shin, C. M., Jeong, D. & Kim, B. Effect of Lactobacillus sakei, a probiotic derived from kimchi, on body fat in Koreans with obesity: a randomized controlled study. Endocrinol. Metab. 35, 425–434 (2020).
Jang, H. M. et al. Lactobacillus sakei alleviates high-fat-diet-induced obesity and anxiety in mice by inducing AMPK activation and SIRT1 expression and inhibiting gut microbiota-mediated NF-κB activation. Mol. Nutr. Food Res. 63, 1800978 (2019).
Seo, S. et al. Anti-colitis effect of Lactobacillus sakei K040706 via suppression of inflammatory responses in the dextran sulfate sodium-induced colitis mice model. J. Funct. Foods 29, 256–268 (2017).
Liu, Y. et al. Intraspecific difference of Latilactobacillus sakei in inflammatory bowel diseases: Insights into potential mechanisms through comparative genomics and metabolomics analyses. Imeta 2, e136 (2023).
Park, C. W. et al. New functional probiotic Lactobacillus sakei probio 65 alleviates atopic symptoms in the mouse. J. Med. Food 11, 405–412 (2008).
Rather, I. et al. Oral administration of live and dead cells of Lactobacillus sakei proBio65 alleviated atopic dermatitis in children and adolescents: a randomized, double-blind, and placebo-controlled study. Probiot. Antimicrob Proteins 13, 315–326 (2021).
Legras, J. L., Merdingolu, D., Cornuet, J. M. & Karst, F. Bread, beer and wine: saccharomyces cerevisiae diversity reflects human history. Mol. Ecol. 16, 2091–2102 (2007).
Gu, Q. et al. Bacteriocins: Curial guardians of gastrointestinal tract. Compr. Rev. Food Sci. Food Saf. 23, e13292 (2024).
Auteri, M., Zizzo, M. G. & Serio, R. GABA and GABA receptors in the gastrointestinal tract: From motility to inflammation. Pharmacol. Res. 93, 11–21 (2015).
Vinderola, G., Perdigón, G., Duarte, J., Farnworth, E. & Matar, C. Effects of the oral administration of the exopolysaccharide produced by Lactobacillus kefiranofaciens on the gut mucosal immunity. Cytokine 36, 254–260 (2006).
Perdigon, G., Maldonado Galdeano, C., Valdez, J. & Medici, M. Interaction of lactic acid bacteria with the gut immune system. Eur. J. Clin. Nutr. 56, S21–S26 (2002).
Won, S.-M. et al. Lactobacillus sakei ADM14 induces anti-obesity effects and changes in gut microbiome in high-fat diet-induced obese mice. Nutrients 12, 3703 (2020).
Lim, S.-M., Jeong, J.-J., Woo, K. H., Han, M. J. & Kim, D.-H. Lactobacillus sakei OK67 ameliorates high-fat diet–induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr. Res. 36, 337–348 (2016).
Eun, S.-H., Lim, S.-M., Jang, S.-E., Han, M. J. & Kim, D.-H. Lactobacillus sakei K17, an inducer of IL-10 expression in antigen-presenting cells, attenuates TNBS-induced colitis in mice. Immunopharmacol. Immunotoxicol. 38, 447–454 (2016).
Lee, S.-Y., Jeong, J.-J., Kim, K.-A. & Kim, D.-H. Lactobacillus sakei OK67 ameliorates collagen-induced arthritis in mice by inhibiting NF-κB activation and restoring Th17/Treg cell balance. J. Funct. Foods 18, 501–511 (2015).
Song, M., Kim, W. J., Shim, J. & Song, K. Latilactobacillus sakei LB-P12 Ameliorates Osteoarthritis by Reducing Cartilage Degradation and Inflammation via Regulation of NF-κB/HIF-2&alpha, Pathway. J. Microbiol. Biotechnol. 35, 1–10. https://doi.org/10.4014/jmb.2504.04013 (2025).
Park, S.-H., Kim, S.-K., Shin, I.-H., Kim, H.-G. & Choe, J.-Y. Effects of AIF on knee osteoarthritis patients: Double-blind, randomized placebo-controlled study. Korean J. Physiol. Pharmacol. 13, 33–37 (2009).
Bae, S.-C. et al. Cross-cultural adaptation and validation of Korean Western Ontario and McMaster Universities (WOMAC) and Lequesne osteoarthritis indices for clinical research. Osteoarthritis Cartilage 9, 746–750 (2001).
McConnell, S., Kolopack, P. & Davis, A. M. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): A review of its utility and measurement properties. Arthritis Care Res. Off. J. Am. College Rheumatol. 45, 453–461 (2001).
Gandek, B. Measurement properties of the Western Ontario and McMaster Universities Osteoarthritis Index: A systematic review. Arthritis Care Res. 67, 216–229 (2015).
Gao, Y., Jia, S., Gao, Q. & Tan, Z. A novel bacteriocin with a broad inhibitory spectrum produced by Lactobacillus sake C2, isolated from traditional Chinese fermented cabbage. Food Control 21, 76–81 (2010).
Todorov, S. D. et al. Characterization of a bacteriocin produced by Lactobacillus sakei R1333 isolated from smoked salmon. Anaerobe 17, 23–31 (2011).
Todorov, S. D. et al. Partial characterization of bacteriocins produced by three strains of Lactobacillus sakei, isolated from salpicao, a fermented meat product from North-West of Portugal. Food Control 30, 111–121 (2013).
Kook, M.-C., Cho, S.-C., Kang, J., Song, Y. & Park, H. Effect of gamma-aminobutyric acid produced by Lactobacillus sakei B2–16 on diet and exercise in high fat diet-induced obese rats. Food Sci. Biotechnol. 23, 1965–1970 (2014).
Bajpai, V. K., Rather, I. A. & Park, Y. H. Partially purified exo-polysaccharide from lactobacillus Sakei Probio 65 with antioxidant, α-glucosidase and tyrosinase inhibitory potential. J. Food Biochem. 40, 264–274 (2016).
Rather, I. A. et al. Effect of a bioactive product SEL001 from Lactobacillus sakei probio65 on gut microbiota and its anti-colitis effects in a TNBS-induced colitis mouse model. Saudi J. Biol. Sci. 27, 261–270 (2020).
Jhun, J. et al. Lactobacillus sakei suppresses collagen-induced arthritis and modulates the differentiation of T helper 17 cells and regulatory B cells. J. Transl. Med. 18, 1–11 (2020).
Ji, Y. et al. Dose-dependent and strain-dependent anti-obesity effects of Lactobacillus sakei in a diet induced obese murine model. PeerJ 7, e6651 (2019).
동욱신, 수정남, 윤식방 & 종연이. Estimation of the prevalence of Korean adults aged 50 years or more with knee osteoarthritis based on the data from fifth Korea National Health and Nutrition Examination Survey. J. Korean Med. Assoc. 56, 431–436 (2013).
Han, S. Epidemiology and pathogenesis of osteoarthritis. J. Korean Med. Assoc. 67, 620–628 (2024).
Funding
This study received no external funding.
Author information
Authors and Affiliations
Contributions
B.K.S., S.H.H., and Q.L. conceived and designed the study. M.S., K.S., and J.S. assisted with data collection and analysis. The initial draft of the manuscript was written by B.K.S. and Q.L., while S.H.H. critically reviewed and revised the manuscript. All authors reviewed the final version, provided their approval, and agreed to be accountable for all aspects of the study to ensure its integrity and accuracy. Informed consent was obtained from all participants before their inclusion in the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was performed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of the Dong-A University Hospital Institutional Review Board (DAUHIRB-EXP-23–187, 11 Sept. 2024).
Informed consent
Informed consent was obtained from all the participants before their inclusion in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shine, BK., Li, Q., Song, M. et al. Efficacy and safety of Latilactobacillus sakei LB-P12 in patients with knee osteoarthritis: an exploratory randomized, double-blind, placebo-controlled clinical trial. Sci Rep 15, 25980 (2025). https://doi.org/10.1038/s41598-025-11250-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11250-0