Abstract
This study explores the enhancement of adsorption properties of penicillamine (PCA) in both neutral and zwitterionic forms through the use of magnesium oxide (Mg12O12) fullerene-like cages, supported by density functional theory (DFT) and molecular dynamics (MD) simulations. Results indicate that PCA adsorption is spontaneous and exothermic, with the cage preferentially abstracting a hydrogen atom from surface hydroxyl groups, leading to strong interactions evidenced by adsorption energies of -2.250 eV in water and − 2.204 eV in chloroform. Charge transfer from the Mg12O12 cage to PCA was approximately 0.299 |e| in water and 0.278 |e| in chloroform, resulting in decreased global hardness and increased chemical potential of the complex, suggesting enhanced reactivity. During the adsorption process, PCA in zwitterionic form exhibited the highest increase in dipole moment, with a value of 12.540 Debye (complex F), compared to the neutral form with the value of 11.382 (complex C) Debye, suggesting enhanced solubility of the system. Dynamic simulations showed that binding energies fluctuate and reach equilibrium after 120 ps. Time-dependent DFT analyses revealed solvent-dependent effects on exciton stability and absorption spectra: water stabilizes charges and enhances absorption, while chloroform induces peak broadening and redshift due to Coulomb interactions. Infrared spectroscopy demonstrated significant spectral shifts upon PCA adsorption, particularly via its carboxyl group, which exhibited the lowest energy gap (Eg) and favorable electronic interactions. Our findings suggest that Mg12O12 cages hold significant potential as biosensors and delivery vehicles for penicillamine, with solvent environment playing a crucial role in adsorption characteristics.
Similar content being viewed by others
Introduction
The pharmaceutical industry faces the ongoing challenge of developing drug delivery systems that simultaneously achieve high therapeutic efficacy and precise targeting of cells. While various strategies have been explored, the application of biocompatible nanomaterials as drug delivery vehicles shows considerable promise as a novel and potentially transformative technology1,2. Magnesium oxide (MgO) nanoparticles, characterized by their high surface area-to-volume ratio and unique physicochemical properties, have garnered significant attention across diverse scientific and technological domains. Their biocompatibility, non-toxic nature, and robust stability in abrupt conditions, renders them particularly promising for biomedical applications such as targeted drug delivery, biosensing, and bioimaging3,4,5. Beyond the biomedical realm, MgO nanoparticles exhibit remarkable catalytic activity, enabling their utilization in various chemical transformations and environmental remediation strategies6,7. Furthermore, the United States Food and Drug Administration regards magnesium oxide as a safe material for human consumption8. MgO nanoparticles possess several advantageous physicochemical characteristics, such as enhanced ionic character, substantial specific surface area, distinctive crystal structures, as well as oxygen vacancies, enabling seamless interaction with various biological systems9,10. Several nanomaterials, including nanocrystals, nanoclusters, and nanosheets, are attracting significant research interest due to their promising applications in drug delivery and biosensing11,12,13,14. Previous work has explored the use of non-biodegradable nanostructures such as MgO nanoparticles, nanoclusters, and nanotubes for anticancer drug delivery15,16. We focus on the drug loading and the influence of Mg-O bonds on catalytic activity of MgO carrier for PCA delivery and its biosensing application.
Penicillamine, a naturally occurring sulfur-containing amino acid, owes its diverse pharmacological activities to its unique functional groups. Its structure features a thiol (-SH) group, a primary amine (-NH2), and a carboxyl (-COOH) group, each contributing significantly to its chelating, antioxidant, and immunomodulatory properties17. Penicillamine (PCA) is a crucial medication for treating various conditions, including Wilson’s disease and rheumatoid arthritis. However, its clinical use is often hampered by its inherent limitations, such as poor bioavailability, short half-life, and significant side effects18,19. These drawbacks necessitate the development of advanced drug delivery systems to enhance its therapeutic efficacy and reduce its side effects. This study will explore the current landscape of penicillamine delivery systems by Mg₁₂O₁₂ fullerene-like cages, focusing on the challenges associated with its administration and the various strategies employed to overcome these limitations. We will examine different approaches, including but not limited to, liposomes, nanoparticles, polymeric micelles, and targeted delivery systems, analyzing their advantages, disadvantages, and potential for improving penicillamine therapy20,21,22. The ultimate goal is to critically assess the progress made in enhancing penicillamine delivery and identify future directions for research in this vital area. For example, Khaleghian et al. studied potential applications for three phosphorus-based nanocages (B12P12, Ga12P12, and B6Ga6P12) in controlled penicillamine drug delivery. Using natural bond orbital analysis and QTAIM, they found that PCA interacts significantly with all three cages via charge transfer, predominantly with gallium atoms. The interactions involved ionic bonds, ionic-hydrogen bonds, and van der Waals forces, with the strongest interaction observed in the Ga12P12 nanocage23. Huang et al. used DFT calculations to show that Penicillamine (PCA) effectively adsorbs onto Al- and Ga-doped B12N12 nanoclusters in both aqueous and chloroform solutions. This adsorption occurs preferentially through PCA’s nucleophilic sites, suggesting potential applications in drug delivery24. This work investigated the adsorption of various penicillamine (PCA) conformations in aqueous and chloroform solutions by DFT and MD simulations. Theoretical spectroscopic parameter (infrared) was computed for the most stable zwitterionic and neutral PCA conformations adsorbed onto Mg12O12 fullerene-like cages.
Methodology and computational details
DFT calculations were performed using the CAM-B3LYP functional and the 6-311G** basis set, chosen for its suitability for inorganic nanomaterials25,26,27,28,29. Geometries of the Mg12O12, PCA and their adsorbed complexes were fully optimized. Solvent effects (water and chloroform) were included using the polarizable continuum model (PCM) [30]. The absence of imaginary frequencies confirmed that the optimized geometries corresponded to true minima on the potential energy surface. To analyze the interaction, frontier molecular orbital (FMO), total density of states (TDOS), and natural bond orbital (NBO) were conducted. Basis set superposition error (BSSE) was corrected using the counterpoise method. All DFT calculations were carried out using the Gaussian 09 package31. Optimized geometries and FMO plots were generated using the GaussView 5.0 program32, while vibrational frequencies and TDOS plots were obtained with the GaussSum 3.0 program33. Adsorption energy (Eads) was calculated as:
.
where EFullerene−PCA, EFullerene, and EPCA represent the total energies of the adsorbed complex, pristine Mg12O12 and NPX molecule, respectively. The computational details for calculating quantum molecular descriptors like hardness (η = (I - A) / 2), softness (S = 1 / 2η), chemical potential (µ = - (I + A) / 2), the maximum charge transfer (∆Nmax), nucleophilicity (υ = 1/ ω), and electrophilicity (ω = µ²/2η) using DFT are determined34,35. OpenMX software, with LCPAO parameters from Ref. [36, 37], was used to perform molecular dynamics simulations. A 2.5 ps production run with a 1 fs time step was conducted in an NPT ensemble, employing a Langevin thermostat for temperature control.
Measurement of bond lengths and interaction energies
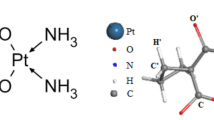
Mg12O12 nanocages, composed of interconnected six and four-membered rings via bridging bonds (b66 and b64), offer multiple adsorption sites for various molecules. Various adsorption sites on Mg12O12 include Mgtop (atop a Mg atom), Otop (atop an O atom), as shown in Fig. 1. Initial calculations for Mg and O atom adsorption converged to four distinct optimized geometries (A, B, C, and D, shown in Fig. 2). In complex C, the Mg-O and C-O bond lengths shift from 1.950 Å and 1.203 Å in the perfect Mg12O12 and PCA structures to 2.111 Å and 1.270 Å, respectively. In complex D, these bonds shift from 1.898 Å and 1.203 Å to 2.025 Å and 1.270 Å, respectively. Arshad and co-authors conducted a comprehensive investigation into the Mg-O bond lengths within a distinctive Mg12O12 fullerene-like cage. Their findings revealed that the Mg-O bond lengths measure approximately 1.77 Å and 1.80 Å at B3LYP/6-311G** level of theory38. Tazikeh et al. reported Mg-O bond lengths of 1.939 Å and 1.971 Å in the free Mg12O12 fullerene-like cage at the M06 and B97D levels of theory, respectively, values close to our calculations [39]. Cao et al. determined Mg-O bond lengths in Mg15O15 of 1.995 Å, 2.004 Å, and 2.018 Å in gas, toluene, and water environments, respectively, using the PBE-D functional40. In complexes C and D, the distance between the cage oxygen atom and the separated hydrogen atom was determined to be 0.980 Å and 0.981 Å, respectively. The C-OH bond length in the perfect PCA structure (1.338 Å) is shortened to 1.243 Å in complex C and 1.242 Å in complex D. In complex F, the C-O bond length of drug attached to the oxygen atom of cage decrease to 1.262 Å and the C-O bond length unattached (without hydrogen atom) increase to 1.234 Å, compared to complex G, the C-O bond length of drug unattached to the cage decrease to 1.200 Å and the C-S bond length closed to the Mg atom of cage slightly increased to 1.851 Å, compared to the C-O, C-OH, and C-S bond lengths of perfect drug are found to be 1.203, 1.338, and 1.849 Å in water phase, respectively. DFT-calculated adsorption energies, distances, and dipole moments are listed in Table 1. PCA adsorption, mediated by its –NH2 and –SH functional groups, showed weaker binding in complexes A and B.
Calculated adsorption energies in water were − 0.857 eV (complex A) and − 0.602 eV (complex B), while those in chloroform were − 1.157 eV (complex A) and − 0.683 eV (complex B). The corresponding distances between the drug’s functional groups and the nanocage were 2.184 Å (complex A) and 1.829 Å (complex B) in water, and 2.240 Å and 1.871 Å in chloroform, respectively (Figs. 2 and 3). In complex B, the drug-surface interaction at aqeuous media involves hydrogen bonding between the -SH group and surface oxygen atoms of the Mg12O12, contributing to the overall intermolecular forces. In complex A, PCA, via its positively charged -NH2 group, interacts electrostatically with negatively charged sites on the Mg12O12 surface. Whereas PCA drug via the –COOH functional group covalentlly interacts with the Mg12O12 fullerene-like cage and showed adsorption energies of approximately − 2.250 eV and − 2.232 eV in water, and − 2.204 eV and − 2.191 eV in chloroform for complexes C and D, respectively (Fig. 2). Our results demonstrate an exothermic interaction between the PCA drug and the Mg12O12 surface in complexes C and D. This interaction proceeds via the –COOH functional group in aqueous solution; a hydrogen atom is seprated and attaches to an oxygen atom of the cage. The drug-surface interaction in the complexes C and D are predominantly covalent in nature, but the strength varies considerably depending on the functional group on the drug molecule. Using DFT, Khaleghian investigated exothermic adsorption (negative Eads) of PCA onto B12P12, Ga12P12, and B6Ga6P12 nanocages. The lowest energy barrier resulted from O-H bond interaction. PCA@B6Ga6P12 exhibited the highest charge transfer (ΔN), indicating significant electron donation from PCA to the nanocage [23]. The –COOH group exhibits a significantly stronger interaction compared to –NH2 and –SH groups, possibly involving hydrogen bonding in addition to electrostatic forces. The solvent also influences the strength of the weaker electrostatic interactions. The different optimized geometries (A, B, C, and D) likely reflect variations in the precise binding sites and orientations of the drug molecule on the nanocage surface. The PCA adsorption via the –COOH functional group on the pristine Mg12O12 fullerene-like cage leads to the increment of dipole moment in complexes C (11.382 Debye) and complex D (9.922 Debye) in water phase compared to complexes C (9.097 Debye) and complex D (8.035 Debye) in chloroform phase. The higher dipole moments observed in the water phase compared to the chloroform phase strongly suggests increased polarity of the drug-nanocage complexes in aqueous phase.
We evaluated the adsorption of two zwitterionic forms of penicillamine (PCA) onto an Mg12O12 fullerene-like cage in aqueous solution: an O-zwitterion (SH-NH3+-COO−) forming complex F, and an S-zwitterion (S−-NH3+-COOH) forming complex G. Complex F (O-zwitterion) exhibited a more favorable adsorption energy of approximately − 1.149 eV compared to complex G (S-zwitterion) at -0.856 eV. Both adsorption processes were exothermic. Next, we evaluated the covalent interaction between PCA and the Mg12O12 fullerene-like cage via the -COOH functional group using the M06-2X-D functional. The M06-2X-D functional is a meta-hybrid GGA functional that includes approximately 54% Hartree-Fock exchange and incorporates dispersion corrections41,42. In the aqueous phase, the adsorption energies are approximately − 2.250 eV for complex C and − 1.149 eV for complex F (O-zwitterion), indicating a more favorable interaction for complex C when using the CAM-B3LYP functional. In contrast, calculations with the M06-2X-D functional show that PCA forms covalent interactions with the Mg12O12 cage through the -COOH group, with adsorption energies of approximately − 2.653 eV for complex C and − 1.673 eV for complex F in water. The differences in adsorption energies between the two methods are approximately − 0.403 eV for complex C and − 0.524 eV for complex F (see Table 2).
Huwaimel and Alqarni investigated how functionalizing graphene oxide (GO) with hydroxyl (-OH), carboxyl (-COOH), and sulfonic (-SO₃H) groups significantly impacts the adsorption energy and charge transfer behavior of paclitaxel (PTX). Their findings revealed that the adsorption energies of PTX on reduced graphene oxide with hydroxyl and carboxyl groups (PTX@rGO-OH and PTX@rGO-COOH) were measured at -0.76 eV and − 0.91 eV, respectively. These values suggest primarily physical adsorption through hydrogen bonding. Conversely, when GO was functionalized with sulfonic groups (PTX@rGO-SO₃H), the adsorption energy increased substantially to -2.09 eV, indicating a transition towards chemisorption that involves stronger covalent interactions43.
Dipole moments of approximately 12.54 and 12.48 Debye were calculated for complexes F and G, respectively, suggesting that the zwitterionic forms of PCA enhance solubility compared to the neutral form upon adsorption onto the Mg12O12 surface (Fig. 4). A likely explanation for the observed difference in stability between the PCA forms involves the strength of intramolecular hydrogen bonding. The C-H···O hydrogen bond distance is 1.866 Å in Complex F and 1.942 Å in Complex G (in the aqueous phase). In complex E, we observe two intramolecular hydrogen bonding between PCA and Mg12O12, as one O-H—O hydrogen bond distance is 2.439 Å, and another C-H—O hydrogen bond distance is 2.489 Å. According to adsorption energy, dipole moment, and hydrogen bond distance analyzes in aqueous phase PCA in O-zwitterion form is more stable than S-zwitterion form, in agreement with theoretical and experimental evidences44,45. This contrasts with previous reports, which indicated bonding to the surface. In the adsorbed state on Fe(110), the S-zwitterionic form is 0.4 eV more stable than the O-zwitterionic form [46]. Analysis of complexes formed between PCA and a Mg12O12 fullerene-like cage reveals significant charge transfer from the nanocage to the PCA molecule, particularly in complex C. The charge on the O atom within the O–H bond and O atom within C = O bond in the PCA molecule (complex C) increased from − 0.365 to − 0.398 electrons to -0.527 and − 0.585 electrons, implying a charge transfer of 0.299 electron from the nanocage to PCA, reported by the NBO analysis. This charge transfer, evidenced by increased negative charges on oxygen atoms in the O-H and C = O bonds of PCA drugs, correlates with higher Eads and higher dipole moment, suggesting strong chemisorption. In complex D, the charge on the H and O atoms of the O–H bond and the O atom of the carbonyl group increased to 0.365 and − 0.529 electrons and − 0.583 electron, resulting in charge transfers of 0.246 electron from the nanocage to PCA, respectively. Complex D also shows considerable charge transfer and strong interaction. In contrast, complexes A (0.137 e) and B (0.128 e) exhibit minimal charge transfer and lower Eads, indicating weaker electrostatic interactions. The overall findings suggest the Mg12O12 fullerene-like cage is a promising candidate for PCA drug delivery due to its ability to form strong bonds with the drug. An assessment of the thermodynamic parameters like Gibbs free energy (ΔG), enthalpy (ΔH), total electronic energy (ΔEtotal), and zero-point correction energy (ΔEZPE) was conducted for the interaction of PCA with Mg₁₂O₁₂ fullerene-like cages in an aqueous environment (Table 2). Formation of the PCA/Mg12O12 complex is spontaneous (ΔG < 0) and exothermic (ΔH < 0). Complex C exhibits greater stability than complexes A and D, as indicated by its more negative values of ΔG (-1.697 eV), ΔH (-2.213 eV), ΔEtotal (-2.187 eV), and ΔEZPE (-2.230 eV). Complex D shows slightly less favorable thermodynamic parameters: ΔG = -1.659 eV and ΔH = -2.194 eV. The significant difference in stability between complex C and complex A is evident in the ΔEtotal (-2.187 eV vs. -0.790 eV) and ΔEZPE (-2.230 eV vs. -0.796 eV) values.
Figure 5 illustrates the molecular dynamic evolution of an Mg12O12 cage adsorbed with PCA over a time interval of 1200 picoseconds (ps). The time frames captured at 10 ps, 400 ps, 800 ps, and 1200 ps provide a detailed view of the structural changes and interactions between the Mg12O12 cage and the PCA molecule. Initially, at 10 ps, the system appears to be in a state of dynamic adjustment, with the PCA molecule beginning to interact with the Mg12O12 cage. As the simulation progresses to 400 ps and 800 ps, the interactions become more pronounced, indicating a stabilization of the molecular structure. By 1200 ps, the system reaches a relatively stable configuration, suggesting that the Mg12O12 cage effectively adsorbs the PCA molecule. The stability observed at this stage implies that the interactions between the Mg12O12 cage and PCA are strong enough to maintain the structure over time. Figure 6 depicts the interaction of a water molecule with the PCA-Mg12O12 complex at a specific snapshot taken at 500 picoseconds (ps). At this point in the simulation, the water molecule is observed to be positioned at a distance of approximately 2.7 angstroms from the PCA-Mg12O12 complex. This distance suggests a relatively close interaction, likely indicative of hydrogen bonding or other non-covalent interactions between the water molecule and the complex47. The proximity of the water molecule to the PCA-Mg12O12 complex at 500 ps highlights the dynamic nature of the system and the potential for water molecules to influence the stability and behavior of the complex.
Figure 7a presents the binding energy (in KJ/mol) of a molecular system as a function of time (in picoseconds, ps). The plot likely illustrates the changes in binding energy over the course of a molecular dynamics simulation, providing insights into the stability and interactions within the system. At the beginning of the simulation (0 ps), the binding energy is relatively low, indicating weaker interactions. As time progresses, the binding energy fluctuates, suggesting dynamic changes in the molecular interactions. The presence of both positive and negative binding energy values indicates periods of stabilization (negative values) and destabilization (positive values) within the system. By the end of the simulation (120 ps), the binding energy appears to stabilize, implying that the system has reached a more equilibrium state. Figure 7b appears to represent a radial distribution function (RDF) plot, which is commonly used in molecular dynamics simulations to describe how the density of particles varies as a function of distance from a reference particle. The peaks in the plot indicate distances at which water molecules are more likely to be found around the PCA molecule. The first peak, typically around 0.3 nm, suggests the most probable distance for water molecules to be in close proximity to the PCA, likely due to hydrogen bonding or other interactions. The subsequent peaks at greater distances (e.g., 0.6 nm, 0.9 nm) represent secondary hydration shells, where water molecules are less densely packed but still influenced by the presence of the PCA. The decreasing amplitude of the peaks with increasing distance indicates a reduction in the correlation between the water molecules and the PCA as the distance grows. Figure 7c depicts the interaction energy (Eij) between two components of a molecular system as a function of time in picoseconds (ps). The plot illustrates how the interaction energy fluctuates over the course of the simulation. Initially, at 0 ps, the interaction energy is relatively low, indicating weaker interactions between the components. As time progresses, the energy values fluctuate, showing periods of both stabilization (negative values) and destabilization (positive values). The negative values of interaction energy suggest favorable interactions, such as attractive forces or bonding, while positive values indicate repulsive or less favorable interactions. By the end of the simulation at 1200 ps, the interaction energy appears to stabilize, suggesting that the system has reached a more equilibrium state with consistent interactions between the components. Figure 7d depicts the electrostatic energy (Ees) of molecular system as it changes over time in ps. Initially, at 0 ps, the electrostatic energy is relatively high (less negative), indicating weaker electrostatic interactions. As time progresses, the energy becomes more negative, reaching its lowest point around − 300 KJ/mol, which suggests stronger electrostatic interactions or stabilization of the system. The trend in the plot indicates that the system undergoes significant changes in electrostatic interactions over time, potentially due to the rearrangement of charged particles or the formation of stable electrostatic bonds. By the end of the simulation at 1000 ps, the electrostatic energy appears to stabilize at a more negative value, implying that the system has reached a state of electrostatic equilibrium. Figure 7e illustrates the number of hydrogen bonds formed over time in a water environment, measured in picoseconds. The plot shows how the number of hydrogen bonds fluctuates throughout the simulation. Initially, at 0 ps, the number of hydrogen bonds is relatively low, indicating fewer interactions. As time progresses, the number of hydrogen bonds increases, reaching peaks at certain intervals, this suggests the formation of stable hydrogen-bonded networks within the water environment. The fluctuations in the number of hydrogen bonds over time reflect the dynamic nature of hydrogen bonding in aqueous systems. By the end of the simulation at 1200 ps, the number of hydrogen bonds appears to stabilize, indicating that the system has reached a more equilibrium state with a consistent number of hydrogen bonds.
The binding energy (in KJ/mol) of a molecular system as a function of time (in picoseconds, ps), radial distribution function (RDF) (a) plot of water and PCA (b), interaction energy (Eij) between two components of a molecular system (c), the electrostatic energy (Ees) of molecular system (d) as it changes over time in picoseconds (ps), and the number of hydrogen bonds formed over time (e).
As shown in Fig. 8, the computed spectral profiles of the three adsorbed PCA forms on Mg12O12 fullerene-like cages exhibit significant differences, enabling the identification of PCA forms. The IR spectra of zwitterionic PCA, including O-zwitterion and S-zwitterion forms, exhibited intense peaks assigned to the asymmetric deformation of the –NH₃⁺ group δas(–NH₃⁺) at 1639 and 1662 cm− 1, respectively. The S-zwitterion also showed an intense peak assigned to the stretching vibration of the –NH₃⁺ group ν(–NH₃⁺) at 2786 cm− 1. In contrast, the natural form of PCA displayed moderate peaks at 1624 cm− 1 (assigned to the rocking vibration of the –NH₂ group, ρ(–NH₂) and 3594 cm− 1 (assigned to the stretching vibration of the –NH₂ group, ν(–NH2). Li et al.48 used FT-IR spectroscopy to characterize amphiphilic Janus Au nanoparticles functionalized with D-penicillamine and benzyl mercaptan. Their analysis identified various functional groups, including O-H (3393 cm− 1), aromatic and aliphatic C-H (3060 and 3022 cm− 1), primary amine N-H (1675 cm− 1), carboxylate C = O (1560 and 1349 cm− 1), and sulfhydryl S-H stretches (2598 cm− 1), based on characteristic peak assignments across a range of wavenumbers. A significant shift in the C = O stretching frequency was observed in the IR spectra upon zwitterion formation. In aqueous solution, the O-zwitterion (State F) showed a peak at 1738 cm− 1, and the S-zwitterion (State G) at 1857 cm− 1, compared to 1673 cm− 1 for the natural form (State C). The C = O stretching frequency varied among the three forms of PCA: 1849 cm− 1 (natural form), 1778 cm− 1 (O-zwitterion), and 1873 cm− 1 (S-zwitterion)49. The ν(S-H ) stretch, δ(-S-H) torsion, and δ(-NH2) bend were observed at 2765, 914, and 1609 cm− 1 respectively for PCA adsorbed on the Mg12O12 nanocage (State C). These values compare favorably with those of the free PCA molecule reported at 2762, 924, and 1633 cm− 1. FTIR analysis by Kandanapitiye et al. of Au@PCA nanoparticles (NPs) showed the disappearance of the thiol (S-H) stretching peak present in the free D-PEN ligand, indicating covalent bonding of D-PEN to the gold surface via the sulfur atom. Significant shifts and reductions in peaks associated with amine and carboxylate groups further suggested their interaction with the gold surface [50].
The diminished Eg in all complexes strongly supports increased charge transfer. DFT calculations, using Kohn-Sham orbitals and Koopmans’ theorem, yielded electron affinity (A = -ELUMO) and ionization energy (I = -EHOMO) values. These calculations show an inverse relationship between electron affinity and ionization energy: as electron affinity increases, ionization energy decreases. The values of I and A of Mg12O12 were found to decrease upon solvation. In water, the values of I and A shifted from 7.94 eV and 0.30 eV to 7.36 eV and 0.47 eV, respectively. Similarly, in chloroform, the I and A changed from 8.04 eV and 0.43 eV to 7.19 eV and 0.58 eV, respectively. Concomitantly, the η value decreased from 3.82 eV (water) and 3.81 eV (chloroform) to 3.42 eV and 3.31 eV, respectively, for the complex C. The µ value also increased, from − 4.12 eV (water) and − 4.24 eV (chloroform) for the Mg12O12, to -3.89 eV for complex C in both solvents. Decreased global hardness and increased chemical potential indicate a decrease in the stability and an increase in the reactivity of the Mg₁₂O₁₂ fullerene [51, 52]. The results revealed that PCA adsorption reduced the global hardness and increased the chemical potential of Mg₁₂O₁₂, indicating a less stable and more reactive system53,54. The electronegativity (χ) and softness (S) of complex C were determined in both water and chloroform phases. In the water phase, χ decreased from 4.12 eV to 3.89 eV, while S increased from 0.130 eV to 0.146 eV (Table 2). In the chloroform phase, χ decreased from 4.24 eV to 3.88 eV, and S increased from 0.130 eV to 0.149 eV. The complexes exhibit increased chemical potential and softness, and a decreased nucleophilicity compared to perfect fullerene-like cage. While increased chemical potential and softness (indicating a greater tendency to donate and accept electrons, respectively) suggest enhanced reactivity. The nucleophilicity and electrophilicity values for complex C in comparison with other complexes is slightly increased and decreased respectively, as summarized in Table 3. Decreased nucleophilicity and electrophilicity may contribute to reduced toxicity and improved biocompatibility in some cases by lowering the molecule’s overall reactivity55,56
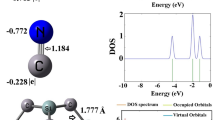
The adsorption characteristics of PCA molecules onto the Mg12O12 fullerene-like cages were additionally explored through FMO analysis. Phenomenon further amplifies the fullerene-like cages ability to adsorb PCA molecules. The values associated with EHOMO and ELUMO can be found in Table 1. In complex C, the electron density of the HOMO is primarily concentrated around the oxygen atoms within a cage-like structure of the molecule (Fig. 9). This means that in chemical reactions, these oxygen atoms are the most likely sites for donating electrons (acting as nucleophiles). The LUMO is primarily concentrated around the magnesium atoms of the cage. The LUMO represents the orbital that is most likely to accept electrons. Therefore, the magnesium atoms are the most likely sites for accepting electrons (acting as electrophiles). Significant changes occur upon complex formation, with the HOMO and LUMO becoming primarily localized on the PCA ligand, indicating strong interactions. This interaction is more pronounced in complexes C and D, where the HOMO is closer to the interaction site than in complexes A and B.
The proximity of both the HOMO and LUMO to the interacting atoms suggests substantial charge transfer, consistent with the NBO analysis. The HOMO energy of Mg12O12 with a value of -7.94 eV in water phase increased to -7.36 and − 7.31 eV in complexes C and D, while the HOMO energy in chloroform phase increased from − 8.04 eV to -7.23 and − 7.19 eV in complexes C and D by TDOS plots, respectively. In contrast, the LUMO energy of Mg12O12 with a value of -0.30 eV in water phase decreased to -0.43 and − 0.47 eV in complexes C and D, while the LUMO energy in chloroform phase reduced from − 0.43 eV to -0.53 and − 0.58 eV in complexes C and D, respectively, these results suggested that the HOMO and LUMO energies for complexes C and D in chloroform phase have more shifts compared to the water phase. This led to a moderate decrease in the energy gap (Eg). Evidently, the Eg values decreased for complexes C and D in both phases, underscoring a strong interaction between PCA and the Mg12O12 fullerene-like cage. In complex D, PCA adsorption onto the Mg12O12 fullerene-like cage shifted the Fermi level (EF) to -3.89 eV in both water and chloroform phases; the initial EF values were − 4.12 eV and − 4.24 eV, respectively. Upon PCA interaction with the Mg12O12 fullerene-like cage, the Eg of complexes C and D was reduced. In water, the Eg value decreased from 7.64 eV to 6.93 and 6.84 eV, respectively; in chloroform, the decreased from and 7.61 eV to 6.70 and 6.61 eV, respectively. In contrast, the Eg values calculated using M062X-D functional slightly decreased for complexes C (7.56 eV) and F (7.53 eV) in water phase, compared to pure Mg12O12 (7.65 eV). In complexes D and F, PCA adsorption onto the Mg12O12 fullerene-like cage shifted the EF to -4.17 and − 4.04 eV in the water phase; the initial EF value was − 4.16 eV, respectively (Table 2). The results suggest that PCA adsorption onto the Mg12O12 fullerene-like cage may lead to enhanced semiconductor behavior. A reduction in the density of states near the Fermi level is observed, implying a potential increase in electrical conductance relative to the pristine fullerene-like cage. Table 1 presents the changes in the Eg in complexes C and D, modified by PCA via its COOH functional group, decreased by 9.29% and 10.47% in water and by 11.96% and 13.14% in chloroform, respectively. These moderate changes suggest that the Mg12O12 fullerene-like cage may be a useful component in biosensors for drug detection in aqueous solutions. Hsu and co-authors found that amphetamine adsorption significantly altered the electronic properties of pure MgONC. The resulting more stable structure exhibited a release energy of approximately 3.75 eV and a 46.65% shift in the HOMO-LUMO gap57.
The absorption spectra reveal key differences in the electronic transitions of Mg12O12-PCA complexes between aqueous and chloroform environments (Fig. 10). Theoretical analysis predicted a maximum absorption peak for the Mg12O12 fullerene-like cage in water at 224 nm (5.52 eV), with an oscillator strength of 0.354. Using UV-Vis spectroscopy, Somanathan et al. demonstrated the formation of nanosized MgO particles through the observation of a main absorption peak near 290 nm58. In water, the Water-C configuration shows the most intense absorption peak centered around 240–260 nm, corresponding to its exceptionally low energy gap of 6.93 eV and large dipole moment of 11.382 Debye. This strong absorption indicates efficient charge transfer between PCA and the Mg12O12 cage in polar media. By contrast, the same complex in chloroform (Chloroform-C) exhibits a redshifted peak near 260–270 nm with reduced intensity, despite having an even smaller energy gap of 6.70 eV. Lesiak et al. demonstrated that surface modification of cadmium nanoparticles with PCA resulted in a maximum absorbance at 402 nm59. This redshift and diminished absorption intensity in the less polar chloroform environment highlight the significant role of solvent polarity in modulating electronic transitions. The Water-F configuration, with its higher energy gap of 7.56 eV, demonstrates weaker absorption at 250 nm, illustrating how different adsorption configurations affect optical properties even within the same solvent. The solvent’s dielectric properties critically influence both the electronic structure and exciton behavior of these complexes. Water’s high dielectric constant (ε ≈ 80) effectively screens Coulombic interactions, leading to lower exciton binding energies (EBE ≈ 0.3–0.6 eV) that favor charge separation - a desirable feature for drug delivery applications where efficient charge dissociation could facilitate drug release. In contrast, chloroform’s weaker screening (ε ≈ 4.8) results in higher exciton binding energies (EBE ≈ 0.5-1.0 eV), making the excitons more stable but harder to dissociate. This fundamental difference in exciton stability between solvents manifests in their spectral characteristics: water enhances absorption intensity through better charge stabilization, while chloroform causes peak broadening and redshift due to stronger Coulombic interactions. The large dipole moments observed in these complexes, particularly in Water-C (11.382 Debye) and Chloroform-C (9.097 Debye), further modify exciton behavior by localizing electron-hole pairs, as evidenced by their distinct absorption profiles.
Conclusions
We investigated the adsorption of PCA onto perfect Mg12O12 fullerene-like cages in water chloroform phases. In the water phase, PCA adsorption on the Mg12O12 surface exhibited enhanced dipole moment and binding energy compared to the chloroform phase. Our analysis revealed that the carboxyll group of PCA, rather than its carbonyl group, interacts strongly with Mg atom in the Mg12O12 structure. This results in a significantly longer desorption time for Mg12O12 cage due to its large negative binding energy. DFT analysis revealed favorable intact adsorption of PCA (via the carboxyl group) from the aqueous phase, resulting in significant changes to the fullerene’s electronic structure. In contrast, adsorption via the –NH₂ and –SH groups was considerably weaker. Mg12O12 showed a strong tendency to abstract a hydrogen atom from a surface hydroxyl group (O–H) upon PCA adsorption; -NH₂ and –SH groups did not induce bond cleavage. Dynamic molecular interactions, evidenced by fluctuating binding energies, reach an apparent equilibrium after 120 ps. Water molecule distribution, as shown by the radial distribution function (RDF), reveals strong, short-range hydrogen bonding near the PCA. This gradually weakens with distance, forming progressively weaker secondary hydration shells. Infrared (IR) spectroscopy analysis of the νasym (COO⁻) and δsym (NH₂) modes demonstrated significant spectral shifts upon PCA encapsulation compared to PCA in aqueous solution. Analysis of the energetically most favorable complexes, including NBO analysis, indicated minor alterations to the electronic properties of Mg12O12, with minimal charge transfer from the fullerene cage to the adsorbed PCA. Furthermore, quantum molecular descriptor analysis revealed that PCA adsorption reduced the global hardness and increased the chemical potential of Mg12O12, indicating a less stable and more reactive system. The adsorption of PCA significantly alters the electronic properties of the Mg12O12 fullerene-like cage, resulting in a more semiconductor-like behavior and a predicted increase in electrical conductance. This is attributed to a decrease in the density of states near the Fermi level. Therefore, the lower binding energy and increased polarity of PCA-loaded Mg12O12 suggest its potential for enhancing solubility, a desirable characteristic for drug delivery systems in biological devices.
Data availability
All data generated or analysed during this study are included in this published article.
References
Tibbitt, M. W., Dahlman, J. E. & Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 138, 704–717 (2016).
Elumalai, K., Srinivasan, S. & Shanmugam, A. Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment. Biomed. Technol. 5, 109–122 (2024).
Verma, S. K. et al. Green synthesized MgO nanoparticles infer biocompatibility by reducing in vivo molecular nanotoxicity in embryonic zebrafish through arginine interaction elicited apoptosis. Sci. Total Environ. 713, 136521 (2020).
MubarakAli, D. et al. An investigation of antibiofilm and cytotoxic property of MgO nanoparticles. Biocatal. Agric. Biotechnol. 18, 101069 (2019).
Podder, S. et al. Effect of morphology and concentration on crossover between antioxidant and pro-oxidant activity of MgO nanostructures. Inorg. Chem. 57, 12727–12739 (2018).
Supin, K. K., Saji, A., Chanda, A. & Vasundhara, M. Effects of calcinations temperatures on structural, optical and magnetic properties of MgO nanoflakes and its photocatalytic applications. Opt. Mater. 132, 112777 (2022).
Karthik, K., Dhanuskodi, S., Gobinath, C., Prabukumar, S. & Sivaramakrishnan, S. Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. J. Photochem. Photobiol B. 190, 8–20 (2019).
Aničić, N., Vukomanović, M., Koklič, T. & Suvorov, D. Fewer defects in the surface slows the hydrolysis rate, decreases the ROS generation potential, and improves the non-ROS antimicrobial activity of MgO. Small 14, 1800205 (2018).
Anand, K. V., Anugraga, A. R., Kannan, M., Singaravelu, G. & Govindaraju, K. Bio-engineered magnesium oxide nanoparticles as nano-priming agent for enhancing seed germination and seedling vigour of green gram (Vigna radiata L). Mater. Lett. 271, 127792 (2020).
Manzetti, S. Quantum chemical study of regular and irregular geometries of MgO nanoclusters: effects on magnetizability, electronic properties and physical characteristics. Mater. Chem. Phys. 199, 7–17 (2017).
Yuan, G., Zheng, J., Lin, C., Chang, X. & Jiang, H. Electrosynthesis and catalytic properties of magnesium oxide nanocrystals with porous structures. Mater. Chem. Phys. 130, 387–391 (2011).
Zhu, K., Hu, J., Kübel, C. & Richards, R. Efficient Preparation and catalytic activity of MgO(111) nanosheets. Angewandte Chemie Int. 45, 7277–7281 (2006).
Chen, K. et al. Magnesium oxide nanotube as novel strategies to enhance the anticance activity of 5-Fluorouracil. J. Mol. Liq. 384, 122214 (2023).
Sun, N. et al. Chinh nguyen, improved anti-inflammatory and anticancer properties of celecoxib loaded zinc oxide and magnesium oxide nanoclusters: A molecular Docking and density functional theory simulation. Arab. J. Chem. 15, 103568 (2022).
Wu, S. et al. A DFT study of Sulforaphane adsorbed on M12O12 (M = Be, Mg and Ca) nanocages. Mater. Today Commun. 38, 107687 (2024).
Pishnamazi, M., Ahmed Algarni, M., Alshehri, A. A., Al Shmrany, H. & Alshehri, S. Adsorption of Thiotepa on B12N12, Mg12O12, and Si12C12 fullerene-like cages: A DFT study. Case Stud. Therm. Eng. 63, 105363 (2024).
Lange, E., Blizzard, L., Venn, A., Francis, H. & Jones, G. Disease-Modifying Anti-Rheumatic drugs and Non-Melanoma skin Cancer in inflammatory arthritis patients: A retrospective cohort study. Rheumatology 55 (9), 1594–1600 (2016).
Metushi, I. G., Zhu, X. & Uetrecht, J. D. -Penicillamine-Induced granulomatous hepatitis in brown Norway rats. Mol. Cell. Biochem. 393, 229–235 (2014).
Walshe, J. M. & Penicillamine The treatment of first choice for patients with wilson’s disease. Mov. Disord.. 14 (4), 545–550 (1999).
Erfani-Moghadam, V. et al. ST8 micellar/niosomal vesicular nanoformulation for delivery of Naproxen in cancer cells: physicochemical characterization and cytotoxicity evaluation. J. Mol. Struct. 1211, 127867 (2020).
Mokhtary, P., Javan, B., Sharbatkhari, M., Soltani, A. & Erfani-Moghadam, V. Cationic vesicles for efficient ShRNA transfection in the MCF-7 breast cancer cell line. Int. J. Nanomed. 13, 7107 (2018).
Ansari, M. J. et al. Sani sarjadi, in vitro release and cytotoxicity study of encapsulated sulfasalazine within LTSP micellar/liposomal and TSP micellar/niosomal nano-formulations. Alexandr Eng. J. 61, 9749–9756 (2022).
Khaleghian, M., Sheikhi, M., Shahab, S. & Kaviani, S. Investigation of adsorption behavior of penicillamine anticancer drug upon B12P12, Ga12P12, and B6Ga6P12 fullerene-like nano-cages: A DFT insight. Comput. Theor. Chem. 1237, 114616 (2024).
Jun Huang, S. M. et al. DFT study of D-Penicillamine adsorption on al and Ga doped Boron nitride (Al-B11N12 and Ga-B11N12) nanoclusters as drug delivery agents. J. Mol. Liq. 383, 122056 (2023).
Goerigk, L. & Grimme, S. A thorough benchmark of density functional methods for general main group thermochemistry, kinetics, and noncovalent interactions. Phys. Chem. Chem. Phys. 13, 6670–6688 (2011).
Padasha, R., Rabbani Esfahani, M., Shokuhi, A., Rad & DYNAMICS. The Computational Quantum Mechanical Study of Sulfamide Drug Adsorption Onto X12Y12 fullerene-like Nanocages: Detailed DFT and QTAIM Investigations1–11 J. Biomole. struct., (2020).
Wong, B. M. & Hsieh, T. H. Optoelectronic and excitonic properties of oligoacenes: substantial improvements from Range-Separated Time-Dependent density functional theory. J. Chem. Theory Comput. 6, 3704–3712 (2010).
Anderson, L. N., Belén Oviedo, M. & Wong, B. M. Accurate Electron affinities and orbital energies of anions from a nonempirically tuned Range-Separated density functional theory approach. J. Chem. Theory Comput. 13, 1656–1666 (2017).
Foster, M. E. & Wong, B. M. Nonempirically tuned Range-Separated DFT accurately predicts both fundamental and excitation gaps in DNA and RNA nucleobases. J. Chem. Theory Comput. 8, 2682–2687 (2012).
Khalili, A., Baei, M. T. & Hosseini Ghaboos, S. H. Improvement of Antioxidative Activity of Apigenin by B12N12 Nanocluster: Antioxidative Mechanism Analysis, ChemistrySelect 5 1829–1836. (2020).
Gaussian 09, Revision, D. 01 et al. J.B. (2009). J.V. Ortiz, J. Cioslowski, D.J. Fox, Gaussian, Inc., Wallingford CT.
O’Boyle, N. M., Tenderholt, A. L. & Langner, K. M. J. Comput. Chem. 29 839. (2008).
Dennington, R. D., Keith, T. A. & Millam, J. M. GaussView 5.0, Gaussian, (2008).
Parr, R. G. & Yang, W. Density-Fuctional Theory of Atoms and Molecules (Oxford University Press, 1989).
Parr, R. G., Szentpaly, L. V. & Liu, S. Electrophilicity index. J. Am. Chem. Soc. 121, 1922–1924 (1999).
Ozaki, T. & Kino, H. Efficient projector expansion for the Ab initio LCAO method. Phys. Rev. B. 72, 045121 (2005).
Faris Alotaibi, H. et al. K. Kant joshi, comparative study of Mg12O12, Mg11AlO12, and Mg11GaO12 Fullerene-like cages for Naproxen sensing. Mater. Today Commun. 112438. (2025).
Arshad, M. et al. Transition Metal-Decorated Mg12O12 nanoclusters as biosensors and efficient drug carriers for the Metformin anticancer drug. ACS Omega. 8 (12), 11318–11325 (2023).
Tazikeh Lemeski, E., Bezi Javan, M., Soltani, A. & Azmoodeh, Z. Optical and electronic properties of Al-Doped Mg12O12 nanocluster: A theoretical study. Russ. J. Inorg. Chem. 64, 762–769 (2019).
Cao, Y. et al. Adsorption behavior of uracil on external surface of MgO nanotubes: A new class of hybrid nano-bio materials. J. Mol. Liq. 339, 116732 (2021).
Wu, S. et al. Density functional study of the adsorption behavior of 6-mercaptopurine on primary, si, al and Ti doped C60 fullerenes. Chem. Phys. Lett. 804, 139910 (2022).
Josa, D., Rodríguez-Otero, J., Cabaleiro-Lago, E. M. & Rellán-Piñeiro, M. Analysis of the performance of DFT-D, M05-2X and M06-2X functionals for studying π⋯π interactions. Chem. Phys. Lett. 557, 170–175 (2013).
Huwaimel, B. & Alqarni, S. A DFT investigation of the adsorption mechanism of Paclitaxel on functionalized graphene oxide for enhanced drug delivery. Sci. Rep. 15, 13889 (2025).
Mateo Marti, E., Methivier, C. & Pradier, C. M. S)-Cysteine chemisorption on Cu(110), from the gas or liquid phase: an FTRAIRS and XPS study. Langmuir 20 (23), 10223–10230 (2004).
Gaillard, T., Trivella, A., Stote, R. H. & Hellwig, P. Far infrared spectra of solid state L-serine, L-threonine, L-cysteine, and L-methionine in different protonation States. Spectrochimi Acta A. 150, 301–307 (2015).
Kokalj, A., Behzadi, H. & Farahati, R. DFT study of aqueous-phase adsorption of cysteine and penicillamine on Fe(110): role of bond-breaking upon adsorption. Appl. Surf. Sci. (2020).
Sun, L. et al. Chiral differentiation of l- and d-penicillamine by β-cyclodextrin: investigated by IRMPD spectroscopy and theoretical simulations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 241, 118653 (2020).
Lia, D. et al. One-pot synthesis of amphiphilic Janus gold nanoparticles with dpenicillamine and benzyl mercaptan ligands by toluene/water emulsion reaction. Appl. Surf. Sci. 475, 615–620 (2019).
Cao, Y. et al. A.B.Albadarin, penicillamine functionalized B12N12 and B12CaN12 nanocages act as potential inhibitors of Proinflammatory cytokines: A combined DFT analysis, ADMET and molecular Docking study. Arab. J. Chem. 14 (7), 103200 (2021).
Kandanapitiye, M. S., Gunathilake, C., Jaroniec, M. & Huang, S. D. Biocompatible D-Penicillamine conjugated Au nanoparticles: targeting intracellular free copper ions for detoxification. J. Mater. Chem. B.. 3 (27), 5553–5559 (2015).
Wang, Q. et al. Chinh nguyen, electrostatic interaction assisted ca-decorated C20 fullerene loaded to anti-inflammatory drugs to manage cardiovascular disease risk in rheumatoid arthritis patients. J. Mol. Liq.. 350, 118564 (2022).
Soltani, A., Baei, M. T., Tazikeh Lemeski, E. & Pahlevani, A. A. The study of SCN adsorption on B12N12 and B16N16 nano-cages. Superlattice Microst.. 75, 716–724 (2014).
Cao, Y. et al. Molecular Docking evaluation of celecoxib on the Boron nitride nanostructures for alleviation of cardiovascular risk and inflammatory. Arab. J. Chem. 15, 103521 (2022).
Baei, M. T., Kanani, Y., Joveini Rezaei, V. & Soltani, A. Adsorption phenomena of gas molecules upon Ga-doped BN nanotubes: A DFT study. Appl. Surf. Sci. 295, 18–25 (2014).
Shi, Y. & Carroll, K. S. Parallel evaluation of nucleophilic and electrophilic chemical probes for Sulfenic acid: reactivity, selectivity and biocompatibility. Redox Biol. 46, 102072 (2021).
Cao, Y. et al. Predicting adsorption behavior and anti-inflammatory activity of Naproxen interacting with pure Boron nitride and Boron phosphide fullerene-like cages. J. Mol. Liq. 339, 116678 (2021).
Hsu, C-Y. et al. Talib abed, utility of (MgO)12 nanocage as a chemical sensor for recognition of amphetamine drug: A computational inspection. Chem. Phys. Impact.. 7, 100382 (2023).
Somanathan, T., Mohana Krishna, V., Saravanan, V., Kumar, R. & Kumar, R. MgO nanoparticles for effective uptake and release of doxorubicin drug: pH sensitive controlled drug release. J. Nanosci. Nanotechnol.. 16, 9421–9431 (2016).
Lesiak, A. et al. Surface modification of cadmium-based nanoparticles with D-penicillamine—study of pH influence on ligand exchange reaction. J. Nanopart. Res. 22, 238 (2020).
Acknowledgements
The authors extend their appreciation to Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia for funding this work under researcher supporting project number (PNURSP2025R205).
Funding
This work was supported by Princess Nourah bint Abdulrahman University researchers supporting project number (PNURSP2025R205), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
H.F.A. and A.K. conceptualized the study and designed the methodology. Y.J. and S.B. performed data curation and formal analysis. S.A.-H. and A.S. contributed to software and validation. T.K. and S.R. prepared the original draft and visualization. K.K.J. supervised the project and reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alotaibi, H.F., Kumar, A., Jadeja, Y. et al. Theoretical investigation of penicillamine adsorption on mg12o12 fullerene-like cages considering solvent effects, electronic properties, and potential biomedical applications. Sci Rep 15, 25547 (2025). https://doi.org/10.1038/s41598-025-11275-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11275-5