Abstract
Mastering the relationship between different numerical formats (i.e., digits, number words, and non-symbolic quantities) is an important foundational skill for later math competencies. However, the neurocognitive mechanisms of this relationship remain poorly understood in children. Consequently, the current study examines the integration between digits, number words, and dots in a sample of 34 primary school children (aged 7 to 14 years) with an electroencephalography paradigm tagging the frequency of stimulus presentation. In an oddball paradigm, we presented children with mixed notation sequences (i.e., dots – words, digits – dots, words – digits) at a rate of 166 ms per stimulus while manipulating the magnitude of the deviant numbers in an experimental (rule = standards < 5, deviants > 5) and control conditions (no rule). We observed deviant responses in the experimental but not in the control condition, with the strongest responses for dots – words, followed by words – digits and finally, digits – dots. These findings suggest that, in children, numerical magnitude information is automatically extracted irrespective of the format at least under implicit and rapid presentation conditions.
Similar content being viewed by others
Introduction
To acquire advanced mathematical competencies, children must learn to map culturally acquired symbolic numbers – such as Arabic numerals (e.g., “6”) and number words (e.g., “six”) – onto evolutionarily ancient, non-symbolic quantity representations, typically expressed as arrays of objects (e.g., “●●●●●●”;1). A central focus in numerical cognition research is understanding how these distinct numerical formats are integrated. Some accounts propose that the processing of symbolic numbers entails the automatic activation of their corresponding approximate, non-symbolic representations (i.e., “20” → “●●●●●●…”2,3,4). In contrast, other views suggest that symbolic and non-symbolic systems are largely distinct and interact only under specific conditions5.
A related, but distinct question is how this relationship between symbolic and non-symbolic formats evolves across development. Some researchers argue that the core number system supports the acquisition of small symbolic numbers (i.e., “one”/“1” →“●”, “two”/“2” → “●●”, etc.) up to around five, after which symbolic knowledge becomes increasingly independent6,7,8,9. According to this view, the two systems gradually estrange with age and experience10. However, the precise neurocognitive mechanisms underlying the relationship between symbolic and non-symbolic numbers in children remain poorly understood, particularly because most existing studies examine only two numerical formats, typically digits and dots (for review see11), and few include all three (e.g., dots, digits, and number words) in a single paradigm12,13.
In the current study, we recorded electroencephalography (EEG) responses tagged at the frequency of the stimulus presentation to shed further light on the neurocognitive development of numerical cross-format processing. Primary school children (aged 7 to 14) viewed cross-format number sequences (digits – words, words – dots, dots – digits) in an oddball design (standard stimuli < 5, deviant > 5) in an implicit task setting. This way, we probed whether children automatically integrated the numerical formats while avoiding potential confounds from task-related decisional strategies.
Mapping between digits, number words, and dots
It is widely accepted that humans possess two core systems for representing numerical information—often referred to collectively as the “number sense”14. The first, the Approximate Number System (ANS), encodes numerical magnitude imprecisely but has an unlimited capacity (e.g., “●●●●●●…” ≈ [iiiiii…]). The second, the Object Tracking System (OTS; also known as Parallel Individuation, PI), allows for the precise representation of small quantities (e.g., “●●●” = [i][j][k]), but is limited to sets of about 4–5 items.
While most developmental models in numerical cognition agree that these core systems support the acquisition of symbolic number knowledge15,16,17,18, they diverge in terms of the mechanisms involved and the specific role of each system. One line of research argues that symbolic numbers are learned via direct mappings onto the ANS, and that such mappings persist even for relatively large symbolic values in adults (e.g., > 1519). Supporting this claim, studies show that young children’s performance on numerical mapping tasks involving small numbers (1–6) varies systematically across format pairs: highest accuracy is typically found for words–dots, followed by dots–digits, and lowest for digits–words (Benoit et al., 2013). This has been interpreted as evidence that children initially map symbolic formats onto core representations and only later derive associations between symbolic formats themselves (e.g., “2” = “●●” → “two”). In contrast, other researchers propose that symbolic number knowledge builds initially on the OTS for learning small number words, but that acquisition of larger symbolic numbers (i.e., > 5) relies on semantic bootstrapping processes. In this view, the associations among symbolic representations become the primary mechanisms of further number learning5,6,20. For instance, Marinova et al.21 tested 195 kindergarten children aged between 2y6m and 5y2m and observed that children first learn to map between small (1 to 4) number words and dots, followed by number words and digits, and only after between dots and digits. Additionally, mediation analysis showed the best result when considering that the words – digits mapping performance predicted the relationship between dots – words and digits – dots. Together with previous findings22,23, these results suggest that when learning to map between dots and digits, children rely on their knowledge of how the two symbolic number formats (i.e. number words and digits) relate to each other and how symbolic number relates to non-symbolic quantities (i.e., “two” = “●●”, and “two” = “2”, then “2” → “●●”). These findings emphasize that core numerical representations are not the sole driving mechanisms behind the early mapping abilities and that their contribution, if any, might be limited to small quantities8,24,25.
Further evidence from primary school-aged children (6 to 11 years old) corroborates the findings in kindergarten children by demonstrating that the relationship between the symbolic and non-symbolic number representation is not as intuitive as previously thought, and the two representations may follow different developmental trajectories13,26,27. For instance, in a study by Marinova and Reynvoet27 primary school children performed an audio-visual number comparison in four conditions (number range 4–28): spoken number words – visually presented digits (i.e., purely symbolic), auditory presented tone sequence – visually presented dots (i.e., purely non-symbolic), number words – dots and tones – digits (i.e., mixed format). The results showed a ratio effect – that is a more efficient performance when the relative distance between two numbers is large (5 vs. 8) compared to small (7 vs. 8), in all conditions containing at least one non-symbolic stimulus (i.e., tones – digits, tones – dots, number words - dots), but not in the purely symbolic condition (i.e., number words - digits). Because the ratio effect is considered as an index of the underlying approximate representations, the authors concluded that its absence in the purely symbolic condition suggests that, already in children, a distinct cognitive system underlies the processing of symbolic numbers. Overall, although theories of numerical cognition emphasize that core numerical representations play a role during the acquisition of symbolic numbers, there is a growing body of research challenging the assumption that symbolic and non-symbolic numbers are automatically mapped onto each other in children.
Only a handful of studies have examined the cross-format mappings in children (e.g13,28). In a 2021 study, van Hoogmoed et al.13 examined the relationship between symbolic and non-symbolic numbers in a sample of older children (9 to 12 year-olds) using electroencephalography (EEG) and an event-related potentials methodology (ERP). In this study, 34 children performed a match-to-sample task (press a response key only when the prime and the target are the same in terms of their magnitude) in purely non-symbolic (dots – dots), purely symbolic (number words –digits) and mixed conditions (digits – dots, dots – digits). The number range varied between 20 and 40. The authors hypothesized that if the non-symbolic numerosities are automatically associated with the symbolic numbers one should observe a format-independent ratio effect and a similar ERP pattern for dots across conditions (i.e., regardless of whether they are primes or targets).
For the dots–dots condition, the results showed a late (after 650 ms) significant ratio effect within the parietal region in combination with early (200 ms) occipital responses to the non-numerical visual cues (i.e., surface and diameter). Interestingly, the symbolic task also had a ratio effect, but with a different pattern: early (50–300 ms) central and later left frontocentral responses (700–800 ms). Finally, comparison across conditions showed that the ERP responses for dots as primes did not differ across conditions, but the responses for the dots as targets differed, suggesting that the prime format (dots or digits) did influence the subsequent processing of the dots. Because the overall pattern of results varied across the numerical formats, the authors suggested that the symbolic and non-symbolic numbers are processed in separate cognitive systems and indicating their estrangement.
Yet, certain limitations of this study warrant further investigations. For instance, the authors used a match-to-sample task, requiring the participants to respond when the prime and target are of the same value. Although only the non-match trials were analysed in their study, the task is still explicit since the participants should actively process the magnitude or numbers. Indeed, earlier work by Cohen Kadosh and Walsh29 suggests that using implicit task instruction would allow probing the numerical representations, and presumably their relationship, per se and free of any decisional strategies. Additionally, behavioural and neurocognitive work in adults30,31 suggests that evidence for estrangement is more likely to occur under explicit task settings (e.g., magnitude comparison task), while integration is observed under implicit task settings (e.g., number – letter identification task), thus confirming earlier suggestions that the task instructions may influence whether the numerical format are processed in a format-(in)dependent manner in the brain.
Furthermore, van Hoogmoed et al.13 only included number words in their design as primes. Given the perceptual differences across conditions, analysing the associated ERP responses was impossible, thus leaving the question about the neurocognitive underpinnings of all three numerical formats (digits, words, and dots) open in children. Additionally, interpretation based on responses for larger number ranges (e.g., above 10) proves challenging across formats. On the one hand, double-digit numbers might require a decomposing strategy, as previous work suggests32. On the other, in languages such as Dutch or German, there is an inversion of the double-digit numbers (i.e., “three and twenty” instead of “twenty-three”), thus rendering a cross-format condition such as visually presented digits and number words (e.g., “23” vs. “five and twenty”) very different in cognitive and visual terms compared to dots – words or dots – digits condition, where it is unlikely that participants will have to do a decomposition and inversion at the same time to compare the numbers13. Consequently, single digits might be a better option if we want to test all three numerical formats.
To overcome these issues one could examine the relationship between the numerical formats using fast visual presentation (e.g., 100 ms per stimulus) and under implicit task settings, which would encourage automatic processing. An emerging EEG methodology in numerical cognition, referred to as fast periodic visual presentation or frequency–tagged EEG – seems promising. Frequency-tagging has already been successfully employed to study within and cross-format number processing in various adult populations33,34,35,36,37, and to study lateralisation patterns for digits and letters as well as cross-format number processing in canonical dot (e.g., dice patterns) and finger configurations in young children aged 6 to 10 years28,38. We discuss this methodology in more detail below.
Frequency-tagged EEG
The fundamental idea behind frequency-tagged EEG is similar to the well-known technique of steady-state visually evoked potentials (SSVEP)39,40,41. Concretely, if a stimulus (e.g., word) is presented at a particular frequency (e.g., F = 10 Hz or 100 ms per stimulus), it leads to a visual response at the frequency of presentation and its harmonics (2f, 3f, etc.). The oddball version of the SSVEP consists of the periodic presentation of two categorically different stimuli (for other paradigms, see40). For example, suppose standard category stimuli (e.g., words) appear at a fast rate of F = 10 Hz, with every nth item (e.g., every 8th stimulus) being a deviant (e.g., non-word). In that case, the presentation sequences look like this: W-W-W-W-W-W-W-NW-W-W…If the system automatically discriminates between the standard and the deviant categories (W vs. NW), we should observe a selective response at the deviant frequency F/n (i.e., 1.2 Hz) and its harmonics (i.e., 2.4 Hz, 3.6 Hz, etc.).
In a previous study, we used this paradigm to examine within and cross-format processing of digits, number words, and dots in adults34,35. Concretely, in the study by Marinova et al.,34, participants saw mixed format (digits – words, words – dots, and dots – digits) in an oddball design. Stimuli were presented at a fast rate of 10 Hz (i.e., 100 ms per item) and a deviant was inserted for every 8th item (i.e., at 1.25 Hz). In the experimental condition, the standard stimuli were numbers smaller than 5, and the deviants were numbers larger than 5. In the control condition, small and large numbers were randomly intermixed. The authors hypothesized that significant oddball responses in the experimental condition would indicate that adults automatically discriminate between small and large numbers, regardless of their format. Indeed, results showed evidence for automatic cross-format integration in the digits – words and dots – words condition, but not in the digits – dots condition. According to the authors, the results suggest that a direct cognitive link exists between the dots and number words and between the digits and number words, which can be observed early in the visual stream, while the link between dots and digits is probably indirect and occurs at a later processing stage. Later, Marlair et al.35 corroborated these findings by showing a cross-format distance effect in adults. Concretely, in this study, the authors stimulated participants at the 6/1.2hz rate and presented them mixed sequences of numerical representations (digits, words, canonical dot configurations and canonical finger configurations) while manipulating the numerical distance between the standard and the deviant (small numerical distance: standard = 2/ deviant = 3, and large numerical distance: standard = 2/deviant = 8). The results showed stronger right-lateralized oddball responses when the distance between the standard and the deviant was large, compared to when the distance was small. Overall, evidence from frequency-tagged EEG studies shows that adult participants automatically integrate magnitude information across formats, except in digits – dots conditions. However, the developmental perspective of the automaticity of this integration remains yet to be examined, as corresponding frequency-tagged EEG studies in younger populations are still missing.
The current study
Based on the findings and the experimental design of Marinova, et al.34, the current study examined cross-format integration of numerical information in primary school children using frequency-tagged EEG. More precisely, we used an oddball design with a stimulation rate of 6/1.2 Hz (i.e., 166 ms per stimulus, every 5th is a deviant) and presented children with mixed sequences of digits, words, and dots. In the experimental condition, the standard stimuli were numbers smaller than 5, while the deviants were numbers larger than 5. In the control condition the small and large category were randomly intermixed. In the current study, we used relatively small numbers (i.e., 1 to 9) because numbers within this range tend to be represented with a higher precision than larger numbers (i.e., > 1542), allowing us to compare the performance across conditions. We hypothesized that if children automatically integrate numerical magnitude, independently of the format, we should observe significant oddball responses in the experimental condition in response to the magnitude change but not in the control condition lacking such a magnitude-related difference between standard and deviant stimuli (H1). Alternatively, a lack of significant oddball responses would indicate that numerical magnitude across formats is not processed automatically in primary-school children (H0). Furthermore, based on previous developmental studies13,21,22,23,24, we expected that the oddball responses would be strongest in the digits – words and words – dots, and weaker, if present, in the dots – digits (H2a). Based on integration studies with adults (e.g34), we also expected an interaction between the sequence and the electrode site, suggesting different topographies (H2b): left lateralised for words – digits, and left occipital for dots – words, and right occipital for dots – digits (if present).
Methods
Participants
The sample consisted of 34 primary school children aged between 7 years 4 months and 14 years 4 months (Mage = 124.94 months (i.e., 10 years 5 months), SD = 19.80 months (i.e., 1 year 8 months), 12 females and 22 males). All children had normal or corrected-to-normal vision, no functional impairments, and followed a Luxembourgish school curriculum. All participants were multilingual (i.e., they spoke more than two languages). With respect to the language of instruction and the language of testing (i.e., German), 67.65% were L2-speakers (i.e., they spoke a language different than German or Luxembourgish with at least one of their parents/legal representatives), and 32.35% were L1 speakers (i.e., they spoke Luxembourgish or German with both their parents/legal representatives). A total of 79.41% of the children said that they preferred to count in German and/or Luxembourgish, 14.71% preferred Portuguese, and the final 5.88% preferred other languages. It is worth noting that although this sample seems unconventionally heterogeneous, it is representative of the Luxembourgish population, which is very diverse linguistically and culturally43,44. In addition, in the local educational context, it is not uncommon for some 13- or 14-year-olds (N = 2) to remain in primary school, particularly in cases of grade repetition or delayed school entry.
The Ethical Review Panel at the University of Luxembourg approved the experimental protocol for the current study (ERP 21–045). Approval for testing in public schools was obtained from Luxembourg’s Ministry of Education. All methods were performed in accordance with the relevant guidelines and regulations, including the Declaration of Helsinki and its latest amendments. Nineteen participants were tested at their primary school and 15 were tested at the cognitive neuroscience lab of the University of Luxembourg. Prior to participation, the parents/legal representatives received a detailed information sheet describing the procedure as well as the exclusion criteria (i.e., history of epilepsy, head trauma, learning and/or attentional difficulties). Only children whose parents/legal representatives signed the informed consent were tested.
Tasks and procedure
Each child completed three experimental tasks, performed in the order described below. The testing sessions lasted approximately 50 to 75 min per child with regular breaks. All tasks and instructions were executed in German by a native German/Luxembourgish-speaking experimenter.
Socio-demographic questionnaire
The questionnaire was completed by the child with the help of the experimenter and contained questions about general demographic information (i.e., biological sex and date of birth), the child’s language profile (i.e., which languages are spoken with the mother and with the father), language preferences (i.e., in which language(s) does the children prefer to speak and in which language(s) does the child prefer to count), as well screening questions for exclusion criteria (i.e., history of epilepsy; presence of any learning or attention difficulties diagnosed by qualifies mental health professional).
Speeded number words reading task
This task was used as a pre-screening measure to ensure that the child could read the number words presented on the screen at the speed of the periodic visual stimulation: 166 ms per stimulus. Concretely, children were presented with number words from “one” to “nine” in German and were asked to say out loud which number word they saw. Each trial started with a 500 ms fixation cross, followed by a number word presented for 166 ms. This was followed by a blank screen, which stayed until response. After 1500 ms intertrial interval, the next trial started. Children could respond verbally during the stimulus presentation and/or during the presentation of the blank screen. The responses (correct or incorrect) were noted in a separate sheet by the experimenter. After the response was recorded, the experimenter pressed “SPACE” for the next trial. Each number word appeared 3 times for a total of 27 randomly presented trials. There were no training trials. PsychoPy software45 was used for stimulus presentation. The task took approximately 2–3 min and children received positive feedback regardless of their performance. All children performed at 100% accuracy, suggesting that the sample comprised of proficient German speakers.
Fast periodic visual stimulation (FPVS) with EEG
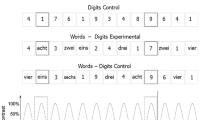
The task employed FPVS oddball design with a presentation rate of 6 Hz/1.2 Hz for the standard and deviant stimuli, respectively. Put differently, the stimuli were presented at a fast rate of 6 Hz (i.e., 6 stimuli per second or 166.66 ms per stimulus), with every 5th stimulus being a deviant (i.e., 1.2 Hz). The stimuli were depicted as digits, words, or dot configurations, thus giving raise to the following mixed-format conditions: Words–Digits, Dots–Words, and Digits–Dot (Fig. 1). The stimuli were presented as black symbols on grey background in a picture format. The final pictures were rescaled to 0.7 or their original size and presented centrally using 800 × 600 px resolution, while participants were seated conformably 1 m from the screen (LED monitor 540 × 330 mm). Consequently, the size for the digit stimuli, on average, varied between 21 × 45 px (i.e., 0.81 × 1.42 degrees of visual angle) and 30 × 48 px (i.e., 1.16 × 1.51 degrees of visual angle). The size of the words, on average, varied between 109 × 49 px (i.e., 4.21 × 1.54 degrees of visual angle) and 174 × 50 px (i.e., 6.72 × 1.58 degrees of visual angle). Finally, the random dots configurations were presented as pictures with size of 216.3 × 216.3 px (i.e., 8.35 × 6.80 degrees of visual angle). To control for perceptual habituation to the visual properties of the stimuli, the dot configurations as well as the fonts of the digits and words varied. For the dot stimuli, there were five different dot configurations per magnitude. The dot stimuli were generated with the MATLAB script by Gebuis and Reynvoet46, controlling for non-numerical cues (i.e., total surface, convex hull, density, dot size and circumference). For the words and digits stimuli, each stimulus was foreseen to appear in five different fonts (Arial, Agency FB, Comic sans MS, OCR A extended, Lucida sans). However, due to technical error, the stimuli were depicted only in four fonts (i.e., all but Lucida sans).
For each mixed-format condition, there was an experimental and control version. In the experimental condition, the standard category consisted of numbers smaller than 5 (i.e., 1,2,3,4), while the deviant category consisted of numbers larger than 5 (i.e., 6,7,8,9). The notation of the standard and deviant stimuli randomly varied depending on the format condition. For example, in the word-digits condition the standard stimuli contained both words and digits. Similarly, the deviant stimulus randomly appeared as a word or a digit. In the control condition, there was no rule, meaning that small and large numbers (in their various notations) were randomly intermixed. Each sequence in a condition started with 2 s fade-in phase, followed by 40 s of stimulation phase, after which 2 s fade-out followed, resulting in a 44s per sequence. Each condition contained 4 sequences, resulting in a total of 24 sequences per participant. The presentation was blocked per notation and per condition. For example, participants saw all four repetitions of the words – digits experimental condition, before moving to the control condition. The order of the notations as well as the conditions within the notations, were counterbalanced in a Latin square design. To maintain constant attention, participants were asked to fixate at the center of the screen, where a fixation cross was continuously presented. Their task was to press the space bar once the color of the cross changed from blue to red. The color changed occurred 8 times per sequence at random intervals.
Visual Depiction of the of the study’s design. Sequences were presented for 44 s in both experimental and control conditions. For the experimental conditions, participants saw mixed notation sequences (digits – dots, words – digits and dots – words). Irrespectively of the notation, numbers smaller than five were presented as standard stimuli (S) at the frequency of 6 Hz, and every 5th item, a number larger than 5 was inserted at the deviant (D) frequency of 1.2 Hz. In the corresponding control conditions, the categories (standard vs. deviant) were assigned randomly. The bottom panel illustrates the onset and the offset of each stimulus following the sinusoidal contrast modulation from a 0 to 100% contrast. The number words were presented in German, which translated into English as follows: “eins”—“one”, “zwei”—“two”, “drei”—“three”, “vier”—“four”, “sechs”—“six”, “sieben”—“seven”, “acht”—“eight”, “neun”—“nine”. This is conceptual, not an actual depiction of the stimuli’s appearance and their physical properties.
EEG data acquisition and preprocessing
Participants were seated comfortably 1 m from the screen. The EEG signal was acquired at 2048 Hz using a 64-channel Biosemi ActiveTwo system (Biosemi, Amsterdam, The Netherlands) with the electrode location according to the standard 10–20 system (for details see http://www.biosemi.com). The Common Mode Sense (CMS) and the Driven Right Leg (DRL) served as the reference electrodes. The electrode offset was held below 40 microvolts (µV). To decrease the processing load, recordings were resampled from 2048 to 512 Hz. Further data processing steps and analyses were done using Letswave 6 software (https://nocions.github.io/letswave6/). Data were filtered (band-pass cut off 0.1–100 Hz) and segmented into a 44 s sequences for visualisation and detecting artefact/noise. Noisy channels were interpolated using three of the closest neighbouring electrodes (no more than three electrodes, i.e., 5% of the electrodes per participant). Individual repetitions, where the EEG signal was too noisy to correct were removed. This resulted in the following total number of repetitions per condition (experimental vs. control): Words – Digits: 127 vs. 120 reps, Dots – Words: 128 vs. 121 reps, and Digits – Dots: 152 vs. 111 reps (It is worth noting that preliminary analyses of the data (including the noisy repetitions) yielded the same overall findings). Afterwards, the EEG signal was re-referenced to the common average and segmented in 40 s epochs (i.e., excluding 2 s fade-in and 2 s fade-out) containing 400 stimulus onsets and 20,480 data points. Then, we averaged the repetitions of each condition for each participant and applied Fast Fourier Transformation (FFT). Three regions of interests were defined a-priori based on previous studies34,37,38: Medial Occipital (MO: O1, Iz, Oz, O2), Left Occipito-Parietal (LOP: P5, P7, P9, PO7), and finally Right Occipito-Parietal (ROP: P6, P8, P10, PO8). As can be seen on Fig. 3, these a-priori ROI were compatible with the topographies obtained in the current study.
EEG data analysis
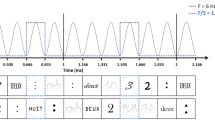
First, to estimate the Signal-to-Noise Ratio (SNR) across the spectrum, each frequency was divided by the mean amplitude of the twenty surrounding bins (10 on each side) to each bin, excluding the immediately adjacent bins and two most extreme bins38,39,47,48. We then averaged the data across participants and per notation condition. The amplitude spectra expressed as SNR for each notation condition is depicted in Fig. 2. Based on this visual inspection, deviant responses were present up to the 6th harmonic, excluding the standard frequency (i.e., 1.2 Hz, 2.4 Hz, 3.6 Hz, 4.8 Hz, 7.2 Hz, and 8.4 Hz), while the amplitude for the standard frequency was strongest at the first harmonic (i.e., 6 Hz). Figure 3 depicts the mean response for the deviant frequency and its 6 harmonics, expressed as SNR for each notation and condition.
Second, to assess the statistical significance of the oddball responses, we used z-scores38,39,47,48. These z-scores were obtained by first segmenting the individual FFT data around the response of interest and its harmonics (e.g., 1.2 Hz, 2.4 Hz, 3.6 Hz, 4.8 Hz, 7.2 Hz, and 8.4 Hz) into successive bins (12 on each side) and then summing across the frequency spectra. The same procedure was applied to the standard responses (i.e., 6 Hz). After that, we applied z-score transformations to each bin as a function of its twenty surrounding bins (10 on each side). Conventionally, z-scores above 1.64 (i.e., p < .05 one-tailed; hypothesis tested signal < noise) indicate significant brain responses to the deviant category. We applied the same procedure to examine the general visual response at the standard stimulation frequency (i.e., 6 Hz). However, based on visual inspection, we limited the analyses only to the first harmonic since that one yielded the highest amplitude.
Results
The study’s data set and statistical output are freely available on the Open Science Framework (https://osf.io/ktm94/).
Table 1 depicts the z-scores for both standard and deviant responses per Notation, Condition, and Region of Interest (ROI). The z-scores for the general visual responses (i.e., at the standard frequency of presentation 6 Hz) were all significant, confirming that participants attended to the stream of visual stimuli. The results concerning the oddball responses demonstrated first that all experimental conditions yielded stronger responses than the control conditions. Second, significant deviant responses were present for all mixed notations, but not across all ROIs. Concretely, the deviant responses were significant only in the Medial Occipital region (MO) for the Digits – Dots condition. Responses were present only in the Left Occipito-Parietal (LOP) region for Words – Digits. Finally, significant responses were present for Dots – Words in Right Occipito-Parietal (ROP) and MO.
To examine the standard and the deviant responses further, we conducted a repeated measures ANCOVA with Notation (3 levels: digits – dots, words – digits, dots – words), Condition (2 levels: experimental and control), and ROI (3 levels: ROP, LOP, and MO) as within-subject factors and age (in months) as a covariate. Data were analysed in classical and in Bayesian statistical frameworks. We report the Bayes factors (BF) - the ratio of the likelihood of the alternative hypothesis and the likelihood of the null hypothesis. Conventionally, the evidence provided by the BF values is categorised as “anecdotal” (for values between < 1 and 3), “moderate” (for values between 3 and 10), “strong” (or values between 10 and 30), “very strong” (for values between 30 and 100), and “extreme” (for values > 100). Given that the Bayesian framework allows us to quantify the evidence for both alternative and null hypotheses, we opted to report the Bayesian analyses. To obtain both classical and Bayesian results, we used JASP statistical package v 0.18.1.0 (https://jasp-stats.org/). Finally, the analyses reported here were run with the program’s default priors (i.e., r cale fixed effects = 0.5, random effects = 1, covariate = 0.354). The statistical output of using narrower (i.e., ½ lower than the default) and wider priors (i.e., ½ higher than the default) and using the classical statistical framework were similar to the ones reported here and are available on the OSF (https://osf.io/ktm94/).
Response to the standard frequency (6 Hz)
Assumption checks for the standard responses (i.e., general visual response) showed that the z-scores per Condition, as well as the Age, were all normally distributed (Shapiro-Wilk p > .05), apart from two conditions: digits – dots and dots – words, where the z-score for the experimental conditions in the Right and Left Occipito-Parietal ROIs showed slight deviations from normality (Skewness digits – dots exp ROP = 0.76, SE = 0.40, Kurtosis digits – dots exp ROP = -0.26, SE = 0.79, p = .03 Skewness dots – words exp LOP = 0.96, SE = 0.40, Kurtosis dots – words exp LOP = 0.78, SE = 0.79, p = .03). Visual inspection of the Q-Q plots (see Fig. 1S in Supplementary) showed that the residuals are mostly normally distributed. Mauchly’s test indicated that the sphericity assumption was violated for the interaction between Notation and ROI, p = .03, Greenhouse-Geisser correction was applied for the frequentist analyses. However, for the Bayesian analyses, sphericity corrections are not readily implemented49. Consequently, we cautiously approach any results where this assumption is violated.
Bayesian Repeated Measures ANCOVA (i.e., Bayesian Model Comparison) showed that the model that best explained the data was the model containing the main effect of Notation, the main effect of ROI and their interaction (i.e., Notation + ROI + Notation*ROI), BF10 = 7.85 × 1012 (see Table 2) This was further corroborated by the comparison of the model-averaged results outlined in Table 3. Here, the BF Inclusion (BFIncl) reflects the predictive strength of the effect with respect to the data by comparing all models that include the effect of interest to the models without this effect. The results showed that there is extreme support for the inclusion of the main effect of Notation, BFIncl = 2.96 × 106 and main effect of ROI, BFIncl = 2.76 × 106 and strong support the interaction Notation*ROI, BFIncl = 10.93 (see Fig. 4).
It is worth noting that in JASP, models that include interactions without the corresponding main effects are excluded because they are considered implausible due to violating the principle of marginality50,51. Therefore, to test the model-averaged results for the models including only the interactions, we computed the effects again, but instead of comparing all models, we compared them across matched models49. Put differently, models with the interaction effect are compared against all other models, including the same predictors but not the interaction effect and no higher-order interactions. The results of the matched models effect analysis remained similar: there was extreme support for the inclusion of the effect of Notation, BFIncl = 1.35 × 106, of the effect of ROI, BFIncl = 1.15 × 106, and moderate support for the inclusion of the Notation*ROI interaction, BFIncl = 5.12. In sum, a model including only the Notation*ROI, but not the corresponding main effects, demonstrated lower predictive power than the models with the main effects.
Post-hoc comparisons (posterior odds corrected for multiple comparisons, prior odds = 0.59, see Fig. 2S in Supplementary material) for Notation showed that the standard amplitude for the Words – Digits condition was strongest compared to both the Dots – Words (BF10,U = 1.24 × 106, posterior odds = 730369.41) and Digits – Dots conditions (BF10,U = 7.80 × 1019, posterior odds = 4.58 × 1019). The Dots – Words amplitude was also higher than the Digits – Dots (BF10,U = 32564.39, posterior odds = 419128.36). For the effect of ROI, the amplitudes were the strongest at the MO scalp region compared to both LOP (BF10,U = 7.38 × 1029, posterior odds = 4.34 × 1029) and ROP (BF10,U = 1.58 × 107, posterior odds = 9.27 × 106). The amplitudes recorded at LOP were the lowest also compared to the ROP, (BF10,U = 2.79 × 107, posterior odds = 1.64 × 107).
To disentangle the interaction between Notation and ROI, we conducted post-hoc Bayesian repeated measures ANOVA per Notation with ROI (3 levels: ROP, LOP, and MO) as a within-subject effect.
For the Digits – Dots notation, the Bayesian model comparison showed anecdotal support for the presence of main effect of ROI, BF10 = 8.37 × 106. The average z-scores were higher in the MO region, followed by the ROP and the LOP (see Fig. 4S in Supplementary material). This observation was supported by the difference between MO vs. LOP, (BF10,U = 1.01 × 106, posterior odds = 596064.29, prior odds = 0.59), and MO vs. ROP, (BF10,U = 66.21, posterior odds = 38.89, prior odds = 0.59), and the difference between ROP and LOP (BF10,U = 37.45, posterior odds = 22.00, prior odds = 0.59).
For the Words – Digits notation, there was again a main effect of ROI, BF10 = 21415.91. There was extreme evidence for a difference between MO vs. LOP, (BF10,U = 40398.63, posterior odds = 23730.20, prior odds = 0.59), and MO vs. ROP, (BF10,U = 62.85, posterior odds = 36.92, prior odds = 0.59), but no conclusive evidence for a difference between LOP vs. ROP (BF10,U = 0.60, posterior odds = 0.35, prior odds = 0.59). In sum, the responses to the standard frequency were again stronger in the MO, followed by ROP and LOP.
Finally, for the Dots – Words notation, there was also extreme evidence for the presence or a main effect of ROI, BF10 = 1269.19. Here, there were differences between MO and LOP, (BF10,U = 2731.30, posterior odds = 1604.37, prior odds = 0.59) and between MO and ROP, (BF10,U = 19.20, posterior odds = 11.28, prior odds = 0.59), but no conclusive evidence for a difference between LOP and ROP, (BF10,U = 0.43, posterior odds = 0.25, prior odds = 0.59)
In sum, the results showed that the general visual response at the standard frequency of stimulation (6 Hz) showed the strongest responses to the Words – Digits, followed by the Dots – Words and Digits – Dots notations. Most importantly for our current hypothesis – there was no evidence for an effect of Condition. Put otherwise, although there were differences in the amplitudes’ strength per Notation and ROI, the general visual response was unchanged across experimental and control conditions. Standard frequency amplitudes were the strongest in the MO region, followed by the ROP and LOR regions. There was also an interaction between the Notation and the ROI factors, suggesting somewhat different amplitude distribution across the ROI depending on the notation. However, for this interaction the assumption of sphericity was violated.
Response to the deviant frequency (1.2 Hz)
Assumption checks for the deviant responses showed that the z-scores per condition were all normally distributed (Shapiro-Wilk p > .05), apart from two conditions: Words – Digits and Dots – Words, where the z-score for the experimental conditions in the Medial Occipital ROI showed slight deviations from normality (Skewness words – digits exp MO = 0.26, SE = 0.40, Kurtosis words – digits exp MO = -1.32, SE = 0.79, p = .02 Skewness dots – digits exp MO = 1.00, SE = 0.40, Kurtosis dots – digits exp MO = 0.72, SE = 0.79, p = .02). The residuals are almost all normally distributed (see Fig. 1S in Supplementary Material). Mauchly’s test indicated that the sphericity assumption was violated for the factors of ROI, p = .03, and the interactions involving this factor (all ps < 0.05).
The Bayesian Repeated Measures ANCOVA (i.e., Bayesian Model Comparison Across All Models) showed that the model that best explained the data was the model containing all the main effects and the interaction between Notation and Condition (i.e., Notation + Condition + ROI + Notation*ROI), BF10 = 6.89 × 1011 (Table 4) This was further corroborated by the comparison of the model-averaged results outlined in Table 5. Here, the results showed that there is extreme support for the inclusion of the main effect of Notation, BFIncl = 208.77 and main effect of Condition, BFIncl = 8.29 × 108 and strong support for the inclusion of the main effect of ROI, BFIncl= 13.82, and the interaction Notation*ROI, BFIncl= 13.60 (see Fig. 4).
The results remained largely similar across the matched models comparison: there was strong support for the inclusion of the effect of Notation, BFIncl = 47.38, extreme support for the inclusion of the effect of Condition, BFIncl = 9.75 × 108, anecdotal support for the inclusion of the effect of ROI, BFIncl = 2.42, and moderate support for the inclusion of the Notation*ROI interaction, BFIncl = 6.58. In sum, a model including only the Notation*ROI, but not the corresponding main effects, demonstrated lower predictive power than the models with the main effects.
Post-hoc comparisons (posterior odds corrected for multiple comparisons, prior odds = 0.59; see Fig. 4S in Supplementary material) for notation showed that the Dots – Words condition differed from both the Digits – Dots (BF10,U = 19.52, posterior odds = 11.46), and Words – Digits (BF10,U = 5183.06, posterior odds = 3044.53) conditions. We observed no evidence for a difference between the Digits – Dots and Words – Digits conditions (BF10,U = 0.21, posterior odds = 0.12). The overall amplitude was highest for the Dots – Words, followed by the Digits – Dots, and finally, Words – Digits. For the Condition, post-hoc comparisons showed extreme support for the difference between experimental and control conditions (BF10,U = 2.09 × 1032, posterior odds = 2.09 × 1032, prior odds = 1) with the experimental condition yielding higher amplitudes than the control one. Finally, concerning the effect of ROI, post-hoc comparisons (prior odds = 0.59) showed no support for the presence of an overall difference between the MO and LOP regions (BF10,U = 0.08, posterior odds = 0.05), moderate support for the presence of an overall difference between ROP and the LOP (BF10,U = 4.16, posterior odds = 2.44) and strong support for an overall difference between the ROP and MO (BF10,U = 24.66, posterior odds = 14.49). Overall amplitudes were higher for both MO and LOP, compared to the ROP.
Finally, there was an interaction between the Notation and ROI, suggesting a possible differential lateralisation pattern with the responses for Words – Digits and Dots – Words being mostly left lateralised, while for the Digits – Dots, the responses were strongest in the medial ROI. Nonetheless, this interaction should be approached with caution first because the assumption of sphericity was violated and second, because the experimental and control conditions are considered together. However, as depicted in Table 1, no significant z-scores were observed in any of the control conditions.
To disentangle the interaction between Notation and ROI, we conducted post-hoc Bayesian repeated measures ANOVA per Notation with ROI (3 levels: ROP, LOP, and MO) as a within-subject effect (see Fig. 5S in Supplementary material). For the Digits – Dots notation, the Bayesian model comparison showed anecdotal support for the presence of main effect of ROI, BFIncl = 1.66. The average z-scores were higher in the MO region, followed by the ROP and the LOP. This observation was moderately supported by the difference between MO vs. LOP, (BF10,U = 4.13, posterior odds = 2.43, prior odds = 0.59). There was anecdotal support for the difference between ROP and MO, (BF10,U = 1.00, posterior odds = 0.58, prior odds = 0.59), and the difference between ROP and LOP was inconclusive (BF10,U = 0.21, posterior odds = 0.12, prior odds = 0.59).
For the Words – Digits notation, the Bayesian model comparison showed moderate support for the presence of main effect of ROI, BFIncl = 4.80. The average z-scores were higher in the LOP, followed by the MO and ROP (see Fig. 5 in Supplementary material). This observation was moderately supported by the difference between LOP and MO, (BF10,U = 3.72, posterior odds = 2.19, prior odds = 0.59). There was anecdotal support for the difference between ROP and LOP, (BF10,U = 1.53, posterior odds = 0.90, prior odds = 0.59), and inconclusive for the difference between ROP and MO (BF10,U = 0.20, posterior odds = 0.12, prior odds = 0.59).
For the Dots - Words notation, the Bayesian model comparison showed moderate support for the presence of main effect of ROI, BFIncl = 9.67 (see Fig. 5 in Supplementary material). The average z-scores were somewhat similar in LOP and MO, followed by ROP. This observation was moderately supported by the difference between ROP and MO, (BF10,U = 5.17, posterior odds = 3.04, prior odds = 0.59), and ROP and LOP, (BF10,U =3.66, posterior odds = 2.15 prior odds = 0.59). There was inconclusive evidence for the difference between LOP and MO (BF10,U = 0.19, posterior odds = 0.11, prior odds = 0.59).
Taken together these results showed that the deviant frequency responses were strongest for the Dots – Words notation, followed by Words – Digits and finally Digits – Dots notation. There was some evidence that the response was also higher in the left and medial ROI, compared to the right ROI. Most importantly, unlike the results observed in response to the standard stimulation frequency (6 Hz), there was a main effect of condition in response to the deviant frequency (1.2 Hz) with the z-scores of the oddball stimuli being significantly present in the experimental, but not in the control condition.
Discussion
Mapping between different numerical formats (i.e., digits, number words, and non-symbolic quantities) constitutes and important building block of math competencies1,4,15,17,18,21,22,23. Yet, the neurocognitive mechanisms underlying the relationship between these three numerical formats remain poorly understood. In the current study, we examined whether digits, number words, and dots are automatically integrated in a sample of 7 to 14-year-old children using a frequency-tagged EEG technique.
We used an oddball design during which children passively viewed mixed notation sequences (i.e., digits – dots, words – digits, dots – words). In the experimental condition, these sequences were divided into standard (< 5) and deviant (> 5) magnitude categories and were presented at a fast rate of 6 Hz (i.e., 6 stimuli per second), with every 5th stimulus (i.e., at 1.2 Hz) being a deviant. In the control condition, stimuli of both magnitude categories were randomly intermixed. Based on previous developmental work21,22,23, we expected to observe responses at the deviant frequency 1.2 Hz in the experimental but not in the control conditions. In addition, we expected stronger deviant responses in the dots – words and words – digits, and weaker, if any, in digits – dots condition. Such results would indicate automatic integration of the magnitude information across these formats. We also anticipated notation-dependent lateralisation of the responses, characterised by the presence of an interaction between the notation and region of interest (ROI)29,34,35,38. Overall, the results provide evidence for partial representational overlap between digits, number words, and dots.
First, concerning the general visual response (i.e., the standard frequency of presentation 6 Hz), we observed evidence for the presence of main effects of notation, region of interest (ROI) and an interaction between these factors. The standard responses were strongest for the words – digits, followed by dots – words and digits – dots. The highest z-scores were recorded at the Medial Occipital (MO) region, followed by the Right-Occipito Parietal (ROP) and finally the Left Occipito-Parietal (LOP). There were also interactions between these two factors, suggesting a notation-depended lateralisation response. However, the lateralisation patterns were not pronounced, and the interaction probably reflects the fact that the amplitudes recorded at the LOP and ROP regions differed only in the dots – digits condition but not in the dots – words and words – digits (see also Figs. 2S and 3S in the Supplementary material). In addition, the assumption of sphericity for this interaction was violated. Therefore, we lack conclusive evidence to interpret this interaction definitively. Finally, and most importantly, there was no evidence for a main effect of the Condition, which indicates that the experimental and control conditions were well matched on their visual properties. Therefore, any significant harmonics in the oddball frequency are unlikely to be due to visual confounds.
In contrast, the main effect of the Condition was present when we analysed the magnitude-specific responses. Here, the results showed that the responses to the deviant frequency and its harmonics were present in the experimental but not in the control conditions, although, as it can be seen in Table 1, not all ROIs yielded significant z-scores (i.e., > 1.64). These findings are in line with previous studies using the same EEG-technique in adults and where the authors observed a magnitude discrimination response across digits, number words, and dots34,35, and across canonical dot and finger configurations in children28. Together, these findings suggesting that numerical formats are automatically integrated under implicit conditions [30, but see13].
Similarly to the results of the general visual response analyses, we also observed a main effect of Notation. Here, the responses on the deviant frequency across both experimental and control conditions were strongest for the dots – words condition. The response for words – digits and digits – dots were similar in terms of amplitude strength. Finally, there was also some evidence for the presence of a main effect of ROI, yielding highest responses for the MO, followed by ROP and LOP, as well as evidence for an interaction between the ROI and notation. In our post hoc analysis, the evidence for a format-dependent lateralisation pattern was moderate at best and only for words – digits and dots – words. For the words – digits condition, the strongest amplitudes were in the LOP, while for the dots – words both the MO and the LOP showed similar amplitudes. Although the pattern of this interaction is in line with our expectations and previous studies (e.g34). These results should be taken with caution since the assumptions of sphericity for the factor ROI as well as the interactions involving this factor were violated. It could be argued that the inconclusive results concerning the interaction effects are due to insufficient data. However, as indicated in the results section, we analysed the data with narrow, wide, and flat priors. This did not lead to any substantial changes in the model comparison and the observed effects, thus showing that the current results are robust.
The current results concerning the main effect of Notation also corroborate to some extent previous developmental behavioral work by Marinova, et al.21. In this study, when the authors examined the cross-notation mapping performance of 3-, 4-, and 5- years-olds kindergarten children, it was found that for the 3- and 4-years-olds, the performance was significantly more accurate for the dots – words, followed by the words – digits and dots – digits (see also22,23). However, for the older children performance in the words – digits and dots – digits condition was similar. The significant main effect of notation in our current data showed that the overall deviant responses were strongest for dots – words, followed by words – digits and dots – digits, which seems to complement these previous behavioral findings.
Regarding the integration of magnitude-related information across various formats, however, our findings showed only a main effect of Condition, but not an interaction between the Notation and Condition as we initially anticipated. These findings suggest that the numerical integration in primary school children is notation-independent, at least during early processing stages targeted with the fast period stimulation of the present design (i.e., 166 ms). While evidence for an automatic integration across numerical formats, as measured in the current and previous studies28,34,35 does not directly imply that the cortical representations for these numerical formats are identical, it is nevertheless in line with the idea that numbers are represented in one common system in school-aged children or at least that there is a partial representational overlap2,3,4. However, it remains possible that at the later stages (> 166 ms), the processing becomes rather format-dependent (e.g13). Moreover, in contrast to the present results, our previous study in adults34 yielded an interaction between condition and notation. The significant differences between experimental and control conditions for dots – words, and words – digits, but not in dots – digits we observed in adults34, suggest that the automatic association between some numerical formats decreases substantially with further development and schooling. In support of this claim, previous neuroimaging studies in adults have demonstrated that a person’s experience and education (as indicated by their arithmetic proficiency) is negatively associated with the degree of representational overlap for digits and dots in the parietal cortex52.
Given the relatively wide age range (7–14 years), it is possible that subtle age-related differences in numerical format integration do exist throughout childhood but were not detected in the current study. For instance, a set of fMRI neuroimaging studies demonstrated that children around the age of 11 years, as well as adults recruit largely the same number processing neuronal networks for digits, dots, and number words11,12,53. Despite the activation of similar brain regions in children and adults, the functional connectivity of these regions does undergo a substantial change due to maturation mostly related to the domain-general aspects of cognitive control53. Finally, a growing body of evidence in both adults54,55,56,57 and children58 suggests that although symbolic and non-symbolic numbers draw on common neuronal substrates, their tunning patterns are format-dependent, thus suggesting distinct underlying neuronal populations. However, definitive conclusions about developmental trajectories of numerical mappings cannot be drawn from the present data. But the descriptively different neuronal response pattern reported in children and adult studies34 could be fruitful venues for future studies. In addition, since in the current study the standard stimuli were smaller than 5 and the deviants were larger than 5, it may be worthwhile to test the reverse direction to assess whether the strength of numerical format integration is symmetrical.
Another factor, possibly, influencing the automaticity of numerical format integration—particularly for number words—is the linguistic profile of the children; specifically, whether they are L1 or L2 speakers of the language of math instruction. As outlined in one of our reviews59, language background can significantly impact both basic (e.g., counting) and advanced (e.g., arithmetic) numerical skills. Prior evidence in children (e.g60), and adults (e.g61), further suggests that multilingual individuals may represent symbolic numbers and their lexico-semantic associations differently across languages. These differences may, in turn, influence whether symbolic and non-symbolic numbers are automatically mapped onto one another. Although we included a speeded number word reading task to confirm that children could read the German number words at the same presentation rate as in the frequency-tagged EEG task (i.e., 166 ms), it is important to note that the cognitive demands of these two tasks differ. Thus, potential differences in processing efficiency between L1 and L2 German speakers may also impact the strength of the observed oddball responses. Future studies could adopt similar paradigms to examine whether, and how, the automaticity of cross-format numerical mappings varies by language background, for instance by including number words presented in both L1 and L2, and assessing oddball responses both within and across individuals.
As the research on numerical format processing in children and adults continues, it is worth considering an additional line of research suggesting that both estrangement and integration are possible depending on the methodological choices and the underlying stimulus and task characteristics. Concretely, studies have demonstrated that the evidence for shared versus format-dependent representations also depends on methodological factors such as task instructions and even the use of single vs. mixed formats sequences, as well as the order of stimulus presentation31 but see62,63. For instance, behavioural and neurocognitive studies in adults suggest that cross-format integration or estrangement depends on task requirements with evidence for the estrangement being present in explicit (e.g., number comparison) condition, while integration is observed under implicit instructions (e.g31. On one side, behavioural studies using explicit task instructions that require processing the magnitude of numbers (e.g., number comparison, numerical matching task, match-to-sample task, etc.) have mostly found evidence for estrangement10,13,31,62,63,64. On the other, under implicit task instructions (e.g., letter-number discrimination task), previous research have reported evidence for an integration28,30,31,34. This means that, we may observe response pattern in line with integration or estrangement depending on the task due to top-down modulations29. For instance, goal-directed task instructions such as number comparison encourage participants to employ more cognitive resources. This leads to a finer tuning of the activated numerical representations, resulting in format-dependent performance. In addition, explicit task instructions require participants to retain the numerical stimuli in their working memory, thus potentially inhibiting the associative strength between symbolic and non-symbolic numbers31. Implicit task instructions on the other hand, may lead to broader neuronal activation, in which case there will be more overlap between the numerical format representations.
Another methodological factor influencing number representation may be the choice of presenting mixed or single format conditions. Similarly to previous studies employing within vs. cross-format conditions10,31,62, Fu and colleagues65 obtained different ERPs in response to numerical quantity depending on whether the block was within (dots - dots) or cross-format (dots - digits), with the latter condition eliciting overall larger response amplitudes. Drawing on working-memory literature, the authors propose that presenting numerical information in cross-format conditions could lead to a more precise coding of the underlying representation. Although it is often assumed that mixing across formats, will encourage participants to rely on an abstract numerical representation62,66,67, pinning formats against each other may also potentially increase the format-specific discrimination abilities. Future research should examine how instructions and presentation formats influences the numerical format integration across development.
In sum, in the current study we used an oddball frequency-tagged EEG methodology to examine whether primary school children (7 to 14 years old) automatically extract magnitude-related information across numerical formats (digits, words, and dots). In line with previous developmental studies, the results showed that overall deviant responses were strongest for dots – words, followed by words – digits and digits – dots. The presence of significant deviant responses in the experimental condition but not in the control condition, suggested that magnitude-related information is extracted independently of the format, at least under implicit and rapid representation conditions. Overall, these findings suggest that symbolic and non-symbolic numerical representations may partially overlap in school-aged children.
Data availability
The study’s data set and statistical output are freely available on the Open Science Framework (https://osf.io/ktm94/).
References
Dehaene, S. The number sense: How the mind creates mathematics. OUP USA (2011).
Dehaene, S. & Cohen, L. Towards an anatomical and functional model of number processing. Math. Cognition. 1 (1), 83120 (1995).
Nieder, A. & Dehaene, S. Representation of number in the brain. Annu. Rev. Neurosci. 32, 185–208. https://doi.org/10.1146/annurev.neuro.051508.135550 (2009).
Piazza, M., Pinel, P., Le Bihan, D. & Dehaene, S. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron 53 (2), 293–305. https://doi.org/10.1016/j.neuron.2006.11.022 (2007).
Carey, S. & Barner, D. Ontogenetic origins of human integer representations. Trends Cogn. Sci. 23 (10), 823–835. https://doi.org/10.1016/j.tics.2019.07.004 (2019).
Carey, S. Where our number concepts come from. J. Philos. 106 (4), 220–254. https://doi.org/10.5840/jphil2009106418 (2009).
Reynvoet, B. & Sasanguie, D. The symbol grounding problem revisited: A thorough evaluation of the Ans mapping account and the proposal of an alternative account based on symbol-symbol associations. Front. Psychol. 7 (OCT), 1–11. https://doi.org/10.3389/fpsyg.2016.01581 (2016).
Krajcsi, A., Fedele, M. & Reynvoet, B. The approximate number system cannot be the leading factor in the acquisition of the first symbolic numbers. Cogn. Dev. 65 (October 2022), 101285. https://doi.org/10.1016/j.cogdev.2022.101285 (2023).
Sarnecka, B. W. Learning to represent exact numbers. Synthese 198 (Suppl 5), 1001–1018. https://doi.org/10.1007/s11229-015-0854-6 (2021).
Lyons, I. M., Ansari, D. & Beilock, S. L. Symbolic estrangement: evidence against a strong association between numerical symbols and the quantities they represent. J. Exp. Psychol. Gen. 141 (4), 635–641. https://doi.org/10.1037/a0027248 (2012).
Arsalidou, M., Pawliw-Levac, M., Sadeghi, M. & Pascual-Leone, J. Brain areas associated with numbers and calculations in children: Meta-analyses of fMRI studies. Dev. Cogn. Neurosci. 30 (July 2017), 239–250. https://doi.org/10.1016/j.dcn.2017.08.002 (2018).
Skagenholt, M., Skagerlund, K. & Träff, U. Neurodevelopmental differences in child and adult number processing: An fMRI-based validation of the triple code model. Developmental Cognitive Neuroscience, 48(December 2020), 1–9. (2021). https://doi.org/10.1016/j.dcn.2021.100933
van Hoogmoed, A. H., Huijsmans, M. D. E. & Kroesbergen, E. H. Non-Symbolic numerosity and symbolic numbers are not processed intuitively in children: evidence from an Event-Related potential study. Front. Educ. 6 (June), 1–16. https://doi.org/10.3389/feduc.2021.629053 (2021).
Feigenson, L., Dehaene, S. & Spelke, E. Core systems of number. Trends Cogn. Sci. 8 (7), 307–314. https://doi.org/10.1016/j.tics.2004.05.002 (2004).
Benoit, L., Lehalle, H., Molina, M., Tijus, C. & Jouen, F. Young children’s mapping between arrays, number words, and digits. Cognition 129 (1), 95–101. https://doi.org/10.1016/j.cognition.2013.06.005 (2013).
Le Corre, M. & Carey, S. One, two, three, four, nothing more: an investigation of the conceptual sources of the verbal counting principles. Cognition 105 (2), 395–438 (2007).
Odic, D., Le Corre, M. & Halberda, J. Children’s mappings between number words and the approximate number system. Cognition 138, 102–121. https://doi.org/10.1016/j.cognition.2015.01.008 (2015).
Von Aster, M. G. & Shalev, R. S. Number development and developmental dyscalculia. Dev. Med. Child Neurol. 49 (11), 868–873. https://doi.org/10.1111/j.1469-8749.2007.00868.x (2007).
Sullivan, J. & Barner, D. How are number words mapped to approximate magnitudes? Q. J. Experimental Psychol. 66 (2), 389–402. https://doi.org/10.1080/17470218.2012.715655 (2013).
Carey, S., Shusterman, A., Haward, P. & Distefano, R. Do analog number representations underlie the meanings of young children’s verbal numerals? Cognition 168, 243–255. https://doi.org/10.1016/j.cognition.2017.06.022 (2017).
Marinova, M., Reynvoet, B. & Sasanguie, D. Mapping between number notations in kindergarten and the role of home numeracy. Cogn. Dev. 57 (March 2020), 101002. https://doi.org/10.1016/j.cogdev.2020.101002 (2021).
Hurst, M., Anderson, U. & Cordes, S. Mapping among number words, numerals, and nonsymbolic quantities in preschoolers. J. Cognition Dev. 18 (1), 41–62. https://doi.org/10.1080/15248372.2016.1228653 (2017).
Jiménez Lira, C., Carver, M., Douglas, H. & LeFevre, J. A. The integration of symbolic and non-symbolic representations of exact quantity in preschool children. Cognition 166, 382–397. https://doi.org/10.1016/j.cognition.2017.05.033 (2017).
Hutchison, J. E., Ansari, D., Zheng, S., De Jesus, S. & Lyons, I. M. The relation between subitizable symbolic and non-symbolic number processing over the kindergarten school year. Dev. Sci. 23 (2), 1–13. https://doi.org/10.1111/desc.12884 (2020).
Sella, F., Slusser, E., Odic, D. & Krajcsi, A. The emergence of children ’ s natural number concepts: Current theoretical challenges. 1–9. (2021). https://doi.org/10.1111/cdep.12428
Goffin, C. & Ansari, D. How are symbols and nonsymbolic numerical magnitudes related?? Exploring bidirectional relationships in early numeracy. Mind Brain Educ. 13 (3), 143–156. https://doi.org/10.1111/mbe.12206 (2019).
Marinova, M. & Reynvoet, B. Can you trust your number sense: distinct processing of numbers and quantities in elementary school children. J. Numer. Cognition. 6 (3), 304–321. https://doi.org/10.5964/jnc.v6i3.292 (2020).
Marlair, C., Lochy, A., Buyle, M., Schiltz, C. & Crollen, V. Canonical representations of fingers and Dots trigger an automatic activation of number semantics: an EEG study on 10-year-old children. Neuropsychologia 157 (August 2020), 107874. https://doi.org/10.1016/j.neuropsychologia.2021.107874 (2021).
Cohen Kadosh, R. & Walsh, V. Numerical representation in the parietal lobes: abstract or not abstract? Behav. Brain Sci. 32 (3-4). https://doi.org/10.1017/S0140525X09990938 (2009).
Liu, R., Schunn, C. D., Fiez, J. A. & Libertus, M. E. The integration between nonsymbolic and symbolic numbers: evidence from an EEG study. Brain Behav. 8 (4), 1–12. https://doi.org/10.1002/brb3.938 (2018).
Ren, X., Liu, R., Coutanche, M. N., Fiez, J. A. & Libertus, M. E. Numerical estrangement and integration between symbolic and non-symbolic numerical information: Task-dependence and its link to math abilities in adults. Cognition 224 (February), 105067. https://doi.org/10.1016/j.cognition.2022.105067 (2022).
Verguts, T. & De Moor, W. Two-digit comparison: decomposed, holistic, or hybrid? Exp. Psychol. 52 (3), 195–200. https://doi.org/10.1027/1618-3169.52.3.195 (2005).
Buyle, M., Lochy, A., Vencato, V. & Crollen, V. Stronger neural response to canonical finger-number configurations in deaf compared to hearing adults revealed by FPVS-EEG. Hum. Brain. Mapp. 44 (9), 3555–3567. https://doi.org/10.1002/hbm.26297 (2023).
Marinova, M. et al. Automatic integration of numerical formats examined with frequency-tagged EEG. Sci. Rep. 11 (1), 1–12. https://doi.org/10.1038/s41598-021-00738-0 (2021).
Marlair, C., Crollen, V. & Lochy, A. A shared numerical magnitude representation evidenced by the distance effect in frequency-tagging EEG. Sci. Rep. 12 (1), 1–10. https://doi.org/10.1038/s41598-022-18811-7 (2022).
Van Rinsveld, A. et al. Automatic processing of numerosity in human neocortex evidenced by occipital and parietal neuromagnetic responses. Cereb. Cortex Commun. 1–12. https://doi.org/10.1093/texcom/tgab028 (2021).
Guillaume, M., Poncin, A., Schiltz, C. & Van Rinsveld, A. Measuring spontaneous and automatic processing of magnitude and parity information of Arabic digits by frequency-tagging EEG. Sci. Rep. 10 (1), 1–11. https://doi.org/10.1038/s41598-020-79404-w (2020).
Lochy, A. & Schiltz, C. Lateralized neural responses to letters and digits in first graders. Child Dev. 90 (6), 1866–1874. https://doi.org/10.1111/cdev.13337 (2019).
Liu-Shuang, J., Norcia, A. M. & Rossion, B. An objective index of individual face discrimination in the right occipito-temporal cortex by means of fast periodic oddball stimulation. Neuropsychologia 52 (1), 57–72. https://doi.org/10.1016/j.neuropsychologia.2013.10.022 (2014).
Norcia, A. M., Appelbaum, G., Ales, L., Cottereau, J. M., Rossion, B. & B. R., & The steady-state visual evoked potential in vision research: A review. J. Vis. 15 (6), 1–46. https://doi.org/10.1167/15.6.4 (2015).
Regan, D. Some characteristics of average steady-state and transient responses evoked by modulated light. Electroencephalogr. Clin. Neurophysiol. 20 (3), 238–248. https://doi.org/10.1016/0013-4694(66)90088-5 (1966).
Verguts, T., Fias, W. & Stevens, M. A model of exact small-number representation. Psychon. Bull. Rev. 12, 66–80. https://doi.org/10.3758/BF03196349 (2005).
Greisen, M., Georges, C., Hornung, C., Sonnleitner, P. & Schiltz, C. Learning mathematics with shackles: how lower reading comprehension in the Language of mathematics instruction accounts for lower mathematics achievement in speakers of different home Languages. Acta. Psychol. 221, 103456. https://doi.org/10.1016/j.actpsy.2021.103456 (2021).
Martini, S., Schiltz, C., Fischbach, A. & Ugen, S. Identifying math and reading difficulties of multilingual children: effects of different cut-offs and reference groups. Divers. Dimensions Math. Lang. Learning: Perspect. Cult. Educ. Multiling. 200-228 https://doi.org/10.1515/9783110661941-011 (2021).
Peirce, J. et al. PsychoPy2: experiments in behavior made easy. Behav. Res. Methods. 51, 195–203. https://doi.org/10.3758/s13428-018-01193-y (2019).
Gebuis, T. & Reynvoet, B. Generating nonsymbolic number stimuli. Behav. Res. Methods. 43 (4), 981–986. https://doi.org/10.3758/s13428-011-0097-5 (2011).
Retter, T. L. & Rossion, B. Neuropsychologia Uncovering the neural magnitude and spatio-temporal dynamics of natural image categorization in a fast visual stream. Neuropsychologia 91, 9–28. https://doi.org/10.1016/j.neuropsychologia.2016.07.028 (2016).
Retter, T. L., Rossion, B. & Schiltz, C. Harmonic amplitude summation for frequency-tagging analysis. J. Cogn. Neurosci. 33 (11), 2372–2393. https://doi.org/10.1162/jocn_a_01763 (2021).
Van Den Bergh, D. et al. A tutorial on conducting and interpreting a bayesian ANOVA in JASP. Annee Psychologique. 120 (1), 73–96. https://doi.org/10.3917/anpsy1.201.0073 (2020).
Rouder, J. N., Engelhardt, C. R., McCabe, S. & Morey, R. D. Model comparison in ANOVA. Psychon. Bull. Rev. 23, 1779–1786. https://doi.org/10.3758/s13423-016-1026-5 (2016).
Rouder, J. N., Morey, R. D., Speckman, P. L. & Province, J. M. Default Bayes factors for ANOVA designs. J. Math. Psychol. 56, 356–374. https://doi.org/10.1016/j.jmp.2012.08.001 (2012).
Bulthé, J., De Smedt, B. & de Op, H. P. Arithmetic skills correlate negatively with the overlap of symbolic and non-symbolic number representations in the brain. Cortex 101, 306–308. https://doi.org/10.1016/j.cortex.2018.01.008 (2018).
Skagenholt, M., Skagerlund, K. & Träff, U. Neurodevelopmental differences in task-evoked number network connectivity: comparing symbolic and nonsymbolic number discrimination in children and adults. Dev. Cogn. Neurosci. 58, 101159. https://doi.org/10.1016/j.dcn.2022.101159 (2022).
Cai, Y., Hofstetter, S. & Dumoulin, S. O. Nonsymbolic numerosity maps at the occipitotemporal cortex respond to symbolic numbers. J. Neurosci. 43 (16), 2950–2959. https://doi.org/10.1523/JNEUROSCI.0687-22.2023 (2023).
Castaldi, E. et al. Mapping subcomponents of numerical cognition in relation to functional and anatomical landmarks of human parietal cortex. NeuroImage J. 215 (November 2020), 104815. https://doi.org/10.1016/j.cognition.2021.104815 (2021).
Kutter, E. F., Bostroem, J., Elger, C. E., Mormann, F. & Nieder, A. Single neurons in the human brain encode numbers. Neuron 100 (3), 753–761e4. https://doi.org/10.1016/j.neuron.2018.08.036 (2018).
Wilkey, E. D., Conrad, B. N., Yeo, D. J. & Price, G. R. Shared numerosity representations across formats and tasks revealed with 7 Tesla fMRI: decoding, generalization, and individual differences in behavior. Cereb. Cortex Commun. 1 (1), 1–19. https://doi.org/10.1093/texcom/tgaa038 (2020).
Nakai, T., Girard, C., Longo, L., Chesnokova, H. & Prado, J. Cortical representations of numbers and nonsymbolic quantities expand and segregate in children from 5 to 8 years of age. PLoS Biol. 21 (1), 1–25. https://doi.org/10.1371/journal.pbio.3001935 (2023).
Schiltz, C., Lachelin, R., Hilger, V. & Marinova, M. Thinking about numbers in different tongues: an overview of the influences of multilingualism on numerical and mathematical competencies. Psychol. Res. 88, 2416–2431. https://doi.org/10.1007/s00426-024-01997-y (2024).
Marchand, E., Wade, S., Sullivan, J. & Barner, D. Language-specific numerical Estimation in bilingual children. J. Exp. Child Psychol. 197, 104860. https://doi.org/10.1016/j.jecp.2020.104860 (2020).
Lachelin, R., Marinova, M., Reynvoet, B. & Schiltz, C. Weaker semantic priming effects with number words in the second Language of math learning. J. Exp. Psychol. Gen. 153 (3), 706–719. https://doi.org/10.1037/xge0001526 (2024).
Marinova, M., Sasanguie, D. & Reynvoet, B. Symbolic estrangement or symbolic integration of numerals with quantities: methodological pitfalls and a possible solution. PLoS ONE. 13 (7). https://doi.org/10.1371/journal.pone.0200808 (2018).
Marinova, M., Sasanguie, D. & Reynvoet, B. Numerals do not need numerosities: robust evidence for distinct numerical representations for symbolic and non-symbolic numbers. Psychol. Res. 85 (2), 764–776. https://doi.org/10.1007/s00426-019-01286-z (2021).
van Hoogmoed, A. H. & Kroesbergen, E. H. On the difference between numerosity processing and number processing. Front. Psychol. 9 (SEP), 1–13. https://doi.org/10.3389/fpsyg.2018.01650 (2018).
Fu, W., Dolfi, S., Decarli, G., Spironelli, C. & Zorzi, M. Electrophysiological signatures of numerosity encoding in a delayed Match-to-Sample task. Front. Hum. Neurosci. 15 (January), 1–17. https://doi.org/10.3389/fnhum.2021.750582 (2022).
Barth, H., La Mont, K., Lipton, J. & Spelke, E. S. Abstract number and arithmetic in preschool children. Proc. Natl. Acad. Sci. U.S.A. 102 (39), 14116–14121. https://doi.org/10.1073/pnas.0505512102 (2005).
Sasanguie, D., De Smedt, B. & Reynvoet, B. Evidence for distinct magnitude systems for symbolic and non-symbolic number. Psychol. Res. 81 (1), 231–242. https://doi.org/10.1007/s00426-015-0734-1 (2017).
Acknowledgements
The authors would like to thank Maryse Georges, Aurélie Marochi, and Zuzana Brandt for their help with the data collection.
Funding
MM: Luxembourg National Research Fund (C20/SC/14629532/SymNumDev). CS: Luxembourg National Research Fund (C20/SC/14629532/SymNumDev).
Author information
Authors and Affiliations
Contributions
Study design conceptualization: MM & CS; Design, methods, and experimental protocol implementation: MM & CS; Data curation and Data analysis: MM with input from CS; Manuscript preparation and revisions: MM & CS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This research was conducted and written according to APA ethical standards. The Ethical Review Panel at the University of Luxembourg approved the experimental protocol for the current study (ERP 21–045). Approval for testing in public schools was obtained from Luxembourg’s Ministry of Education. All methods were performed in accordance with the relevant guidelines and regulations, including the Declaration of Helsinki and its latest amendments. Prior to participation, written informed consent was sought from each participant’s their legal representative.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marinova, M., Schiltz, C. Numerical format integration in primary school children examined with frequency-tagged electroencephalography. Sci Rep 15, 28641 (2025). https://doi.org/10.1038/s41598-025-11281-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11281-7