Abstract
Methamphetamine (MA) can cause functional abnormalities in the brain. However, the correlations between the abnormal brain activity and relapse in abstinent MA-dependent individuals (MADIs) were still unclear. This study aimed to explore the changes of regional brain activation associated with relapse in early abstinent MADIs, which could provide potential neuroimaging markers for predicting the relapse susceptibility. Twenty-seven individuals who relapsed to MA within 1 year after abstaining from MA, as well as 28 healthy controls (HCs), were enrolled in this study. Resting-state functional magnetic resonance imaging (rs-fMRI) collected from all HCs, and MADIs when they were abstinent from MA (Stage I) and relapsed to MA (Stage II). The measures for evaluating regional brain activity including regional homogeneity (ReHo) and fractional amplitude of low-frequency fluctuation (fALFF) were calculated for all participants. ReHo and fALFF values were compared between MADIs at Stage I, Stage II and HCs. Finally, relationships between altered brain regions and duration of MA use, duration of relapse were evaluated by Pearson correlation analysis. Compared with HCs, MADIs at Stage I demonstrated decreased brain activity in three cortical regions and increased brain activity in several subcortical regions, especially bilateral putamen. In addition, MADIs at Stage II exhibited a wider range of brain regions with abnormal activity, which presented as decreased activity mainly in the middle cingulate gyrus, parietal and occipital regions, and increased activity mostly in the subcortical regions (striatum, thalamus and hippocampal structure) and several prefrontal regions. Moreover, MADIs at Stage II showed decreased activity in the parietal regions and increased brain activity in the prefrontal and subcortical regions, especially bilateral caudate nucleus. The fALFF values of left and right caudate nucleus were negatively associated with duration of relapse. There were still abnormal activities in the brain of MADIs even when they were abstinent from MA. Increased activity in the bilateral putamen might be associated with early relapse in abstinent MADIs. Relapse to MA use could cause more wider range of abnormal brain activity, especially increased activity in the striatum, which might lead to more higher relapse rate of MA use.
Similar content being viewed by others
Introduction
Methamphetamine (MA) abuse has emerged as a pervasive global issue, affecting approximately 27 million individuals worldwide1. This highly addictive psychostimulant drug is particularly widespread in East and Southeast Asia, where its use has escalated into a major social and public health crisis2,3. MA abuse not only disrupts individuals’ physical and mental health but also places a significant burden on families, communities, and healthcare systems. The consequences of prolonged MA use include cognitive deficits, emotional instability, and severe psychiatric conditions, with relapse remaining one of the most persistent challenges in addiction recovery4,5. Alarmingly, up to 60% of individuals relapse within the first year of abstinence, underlining the urgent need for effective strategies to prevent recurrence6. Given these alarming relapse rates and their devastating consequences, understanding the neurobiological mechanisms underlying MA addiction and relapse has become imperative for developing more effective treatment strategies.
The neural mechanisms underlying MA addiction and relapse have garnered significant attention in neuroscience research5,7. Chronic MA use is known to induce widespread structural and functional changes in the brain, with key alterations observed in regions associated with reward processing, decision-making, and emotional regulation8,9,10. Studies have highlighted the role of the striatum, prefrontal cortex, and limbic system in mediating the reinforcing effects of MA and its impact on cognitive control11,12. Importantly, these neural changes often persist long after cessation of MA use, resulting in heightened vulnerability to relapse13,14. Such findings suggest that the brain’s reward and executive systems may serve as critical targets for predicting and mitigating relapse risk.
Relapse, a hallmark feature of MA addiction, poses significant challenges for both researchers and clinicians3. Current intervention strategies often focus on pharmacological approaches, yet their efficacy remains limited15,16,17. Understanding the neural underpinnings of relapse is crucial to improving these interventions and designing more precise, personalized treatments18,19. Advances in neuroimaging technologies, particularly resting-state functional magnetic resonance imaging (rs-fMRI), offer a non-invasive approach to probing the brain’s functional dynamics during different stages of addiction and recovery. Measures such as regional homogeneity (ReHo) and fractional amplitude of low-frequency fluctuation (fALFF) have proven effective in characterizing localized neural activity20,21, providing critical insights into brain function alterations associated with addiction22.
While prior research has extensively explored brain dysfunction in active MA users and abstinent individuals, studies focusing on relapse-specific neural mechanisms during early abstinence remain limited23. Early abstinence represents a critical window in addiction recovery, during which individuals often experience heightened cravings, impaired decision-making, and emotional instability, factors that collectively increase relapse risk24. Furthermore, no longitudinal studies have examined brain function across multiple stages of MA addiction—namely, abstinence and relapse—within the same individuals. Such research is essential for identifying neuroimaging markers that not only predict relapse susceptibility but also inform targeted therapeutic interventions.
In this context, the current study aimed to address these gaps by investigating the neural correlates of relapse in early abstinent MA-dependent individuals (MADIs) using rs-fMRI. By examining changes in regional brain activity during abstinence (Stage I), following relapse (Stage II), and comparing these patterns to healthy controls (HCs), we sought to identify distinct neural signatures of relapse vulnerability. Specifically, we hypothesized that early abstinent MADIs might exhibit unique patterns of regional brain activity that were predictive of subsequent relapse and that these patterns underwent further alterations following relapse. The findings of this study could provide valuable neuroimaging markers for relapse prediction and contribute to the development of more effective, evidence-based interventions for MA addiction.
Materials and methods
Participants
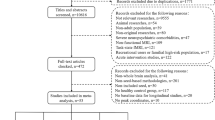
This study was approved by the ethics committee of Zhongda Hospital, School of Medicine, Southeast University (2024ZDSYLL159-P01). In addition, this study was performed in accordance with the Declaration of Helsinki. All participants were informed of the experimental procedure and signed informed consents before participating in this study. The sample size was estimated using fMRI power software package 7 allowing for an 80% calculation power and a 0.05 type I error rate. Initially, 45 individuals meeting DSM-IV criteria for methamphetamine dependence were recruited from the drug rehabilitation center. Of these, 18 participants were excluded for the following reasons: 3 for abuse of substances other than methamphetamine, 2 for neurological disorders, 1 for severe head trauma history, 2 for psychiatric disorder history, 1 for MRI contraindications, 2 for excessive head motion (> 2.0 mm translation or > 2.0° rotation), and 7 were lost to follow-up during the study period. Finally, 27 MADIs who relapsed within 1 year were included in the analysis. For healthy controls, 32 individuals were initially recruited, with 4 excluded due to excessive head motion, resulting in 28 HCs in the final analysis. This study included 27 MADIs who relapsed to MA (Stage II) within 1 year after abstaining from MA (Stage I), as well as 28 drug-free HCs matched for age, gender and education level. MADIs were recruited from the drug rehabilitation center in Jurong, Zhenjiang (Jiangsu, China) where the participants were treated without MA but with education, and physical exercise during abstinence. All MADIs fulfilled the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria for MA dependence assessed by the Structured Clinical Interview (SCID). MADIs were excluded if they met criteria for other substance dependence at any time. The demographic characteristics were shown in Table 1.
The inclusion criteria were as follows: (1) right-handed, Han Chinese, between 20 and 60 years of age, no less than 9 years of education; (2) positive urine test for MA and negative urine test for other drugs; (3) met the criteria for the diagnosis of MA addiction in DSM-IV, and were abstinent from using MA for 1 months, which was confirmed by guardians of the patients; (4) had normal visual acuity and hearing.
This study included only male participants for the following reasons: (1) In our rehabilitation center, male methamphetamine users constitute the vast majority (> 95%) of the population; (2) Gender differences in brain function and addiction mechanisms are well-documented, and including mixed gender could introduce significant heterogeneity in neuroimaging results; (3) Restricting the sample to males helps reduce confounding variables and increases sample homogeneity for this initial investigation.
The exclusion criteria were as follows: (1) abused harmful substances other than MA; (2) any general medical condition or neurological disorders that could confound brain function; (3) history of epileptic seizure, stroke, structural brain disease, severe head trauma or injury; (4) any current or previous psychiatric disorder, or were delirious; (5) family history of psychiatric disorder; (6) any contraindication for MRI including implanted metallic devices, ferromagnetic material or claustrophobia.
MRI data acquisition and preprocessing
All participants underwent MRI scans using a 3.0 Tesla Siemens MRI scanner. The T1-weighted images were acquired with the following parameters: repetition time (TR) = 1900ms; echo time (TE) = 2.48ms; flip angle (FA) = 9°; field of view (FOV) = 250 mm×250 mm; acquisition matrix = 250 × 250; slice thickness = 1 mm; number of slices = 176. The rs-fMRI data were acquired with the following parameters: TR/TE = 3000/40ms; FA = 90°; FOV = 240 mm×240 mm; acquisition matrix = 64 × 64; slice thickness = 4 mm; number of slices = 32; number of volumes = 133.
MRI data preprocessing was conducted using Data Processing and Analysis for Brain Imaging (DPABI) software in MATLAB25, which were according to our previous study26, including: (1) data format conversion; (2) removal of first 10 time points; (3) slice timing; (4) realignment; (5) T1 reorientation; (6) brain extraction; (7) segmentation; (8) normalization using T1 image unified segmentation. In this study, participants with head motion exceeding 2.0 mm or rotation greater than 2.0° were excluded.
Calculation of fALFF and ReHo values
The calculation of ReHo and fALFF values were performed by the software of DPARSF25, which were according to our previous study26. Finally, both ReHo and fALFF values were standardized using Fisher’s r-to-z transformation (zReHo and zfALFF), which could improve the normality. The zfALFF values were used for subsequent analysis. The smoothed zReHo (szReHo) values and zfALFF values were used for subsequent analysis.
Statistical analysis
AlphaSim is a Monte Carlo simulation-based multiple comparison correction program implemented in REST software27. This method estimates the probability of false positive clusters by generating random noise datasets and calculating the family-wise error rate under specified statistical thresholds and cluster sizes. The simulation process involves creating 1000 random datasets with the same dimensions and smoothness as the original data, applying the same statistical threshold, and determining the cluster size threshold that maintains the family-wise error rate below 5%.
The minimum cluster size of 13 voxels was determined through AlphaSim simulation results. Under the voxel-level threshold of P < 0.005, this cluster size effectively controls the family-wise error rate below 5%. This parameter setting is consistent with previous methamphetamine neuroimaging studies and represents a balance between sensitivity and specificity in detecting meaningful brain activation patterns.
Two simple t-tests and Chi-square tests were applied to explore the differences of demographic characteristics between MADIs and HCs using the Statistical Package for the Social Sciences (SPSS Inc, Chicago, IL, United States). P < 0.05 was considered statistically significant differences.
Considering that there were two different groups of participants in the comparison, but their demographic characteristics were generally matched, two simple t-tests were used to compare the differences of ReHo and ALFF values between MADIs and HCs using demographic characteristics including age, educational level, height, weight, smoking and drinking as covariates by the REST Software (State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, Beijing, China)27. In addition, considering that the comparisons involved the same group of participants at different time periods, paired t-tests were used to compare the differences of ReHo and ALFF values between MADIs at Stage I and Stage II. The significant differences were set at P < 0.005 (a minimum cluster size of 13 voxels, corrected for multiple comparisons using the AlphaSim program in REST software). Finally, relationships between altered brain regions and duration of MA use, duration of relapse were evaluated by Pearson correlation analysis.
Results
Comparison of demographic data between MADIs and HCs
There were no significant differences in the age, gender, educational level, height, weight, marital status, employment and drinking between MADIs and HCs (P > 0.05). The duration of MA use was 6.74 ± 3.10 years in MADIs and the days that MADIs relapsed to MA from abstinence were 160.81 ± 72.037.
Comparison of ReHo and fALFF values between MADIs at stage I and HCs
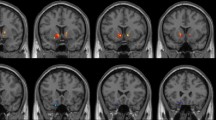
Compared with HCs, MADIs at Stage I demonstrated decreased ReHo values in the left superior parietal gyrus and left middle temporal gyrus, increased ReHo values in the left inferior temporal gyrus, right inferior temporal gyrus and right insula. MADIs at Stage I also exhibited decreased fALFF values in the right precentral gyrus, increased fALFF values in the left putamen and right putamen. (Table 2; Fig. 1)
Comparison of ReHo and fALFF values between MADIs at Stage I and HCs. MA: methamphetamine; MADIs: MA-dependent individuals; MADIs at Stage I: MADIs when they were abstinent from MA; HCs: healthy controls; ReHo: regional homogeneity; fALFF: fractional amplitude of low-frequency fluctuation. The significant differences were set at P < 0.005 (a minimum cluster size of 13 voxels, corrected by the AlphaSim program in REST software).
Comparison of ReHo and fALFF values between MADIs at stage II and HCs
In comparison with HCs, decreased ReHo values were identified in the left middle cingulate gyrus, left postcentral gyrus, left superior temporal gyrus, left middle occipital gyrus, left cuneus, right postcentral gyrus, right superior temporal gyrus, right calcarine fissure and increased ReHo values were found in the left opercular part of inferior frontal gyrus, left caudate, left hippocampus, right precuneus, right hippocampus, right parahippocampal gyrus of MADIs at Stage II. In addition, MADIs at Stage II had decreased fALFF values in the left middle cingulate gyrus, left precuneus, left postcentral gyrus, left paracentral lobule, right postcentral gyrus, right superior occipital gyrus, right angular gyrus, right lingual gyrus and increased fALFF values in the left parahippocampal gyrus, left putamen, left thalamus, right opercular part of inferior frontal gyrus, right anterior cingulate gyrus, right putamen, right hippocampus. (Table 2; Fig. 2)
Comparison of ReHo and fALFF values between MADIs Stage II and HCs. MA: methamphetamine; MADIs: MA-dependent individuals; MADIs at Stage II: MADIs when they relapsed to MA; HCs: healthy controls; ReHo: regional homogeneity; fALFF: fractional amplitude of low-frequency fluctuation. The significant differences were set at P < 0.005 (a minimum cluster size of 13 voxels, corrected by the AlphaSim program in REST software).
Comparison of ReHo and fALFF values between MADIs at stage II and stage I
MADIs at Stage II demonstrated decreased ReHo values in the left inferior temporal gyrus and increased ReHo values in the left fusiform gyrus, left olfactory when compared with those at Stage I. Moreover, decreased fALFF values in the left paracentral lobule, right postcentral gyrus and increased fALFF values in the left opercular part of inferior frontal gyrus, left caudate nucleus, right caudate nucleus were revealed in MADIs at Stage II. (Table 2; Fig. 3)
Comparison of ReHo and fALFF values between MADIs at Stage I and Stage II. MA: methamphetamine; MADIs: MA-dependent individuals; MADIs at Stage I: MADIs when they were abstinent from MA; MADIs at Stage II: MADIs when they relapsed to MA; ReHo: regional homogeneity; fALFF: fractional amplitude of low-frequency fluctuation. The significant differences were set at P < 0.005 (a minimum cluster size of 13 voxels, corrected by the AlphaSim program in REST software).
Relationships between altered brain regions and duration of MA use, duration of relapse
The fALFF values of left (r=−0.51; P < 0.01) and right (r=−0.39; P = 0.04) caudate nucleus were negatively associated with duration of relapse. (Fig. 4)
Discussion
In this comprehensive investigation of the neural mechanisms underlying MD addiction, we have uncovered distinctive patterns of brain activity changes that systematically progress from early abstinence through relapse28,29. The fALFF values of left and right caudate nucleus were negatively associated with duration of relapse. Through detailed analysis of both ReHo and fALFF measures, our findings reveal a complex evolution of neural circuit dysfunction that may explain both the persistent nature of addiction and the high rates of relapse observed among MADIs. These stage-specific neural adaptations provide unprecedented insights into the neurobiological basis of addiction vulnerability and suggest potential therapeutic targets for intervention at different stages of the addiction cycle.
The early abstinence period represents a critical window of vulnerability in addiction recovery, characterized by distinctive patterns of regional brain activity alterations that suggest disruption of multiple neural systems governing executive control, sensory processing, and reward-related behaviors30,31. Our analyses revealed decreased ReHo values in the left superior parietal gyrus and left middle temporal gyrus, reflecting compromised function in regions crucial for attention allocation and sensory integration. The superior parietal gyrus, serving as a key node in the dorsal attention network, normally facilitates voluntary control of attention and goal-directed behavior through its extensive connections with frontal executive regions and its role in spatial processing and action planning31,32. Its reduced activity during abstinence suggests impaired ability to maintain focus on recovery-related goals and resist drug-related distractions33. Similarly, the middle temporal gyrus plays an essential role in integrating complex sensory information and semantic processing, and its dysfunction may disrupt the normal processing of environmental stimuli, potentially biasing attention toward drug-related cues while diminishing the salience of natural rewards34,35.
Concurrent with these cortical alterations, we observed increased ReHo values in the left and right inferior temporal gyrus and right insula, alongside increased fALFF values in the bilateral putamen. This pattern of enhanced activity in specific brain regions provides crucial insights into the neural mechanisms that may promote relapse vulnerability. The heightened activity in inferior temporal regions suggests altered processing of visual and semantic information, potentially enhancing the cognitive processing of drug-related cues in the environment36,37. The right insula’s enhanced activity is particularly significant given its well-established role in interoceptive awareness and craving states38. This hyperactivity may reflect heightened sensitivity to internal physiological states that often trigger drug-seeking behavior, representing a potential mechanism through which bodily sensations may more readily precipitate craving and relapse during early abstinence.
Perhaps most critically, the observed bilateral putamen hyperactivity represents a fundamental neural signature of relapse risk. The putamen, as a key component of the dorsal striatum, plays a pivotal role in habit formation and motor learning through its extensive connections with motor and premotor cortices and its dense dopaminergic innervation39,40. Its enhanced activity during abstinence suggests persistent sensitization of habit-formation circuits that may maintain drug-seeking behavioral routines even in the absence of active drug use41. This finding provides a potential neurobiological explanation for the automatic, habitual nature of drug-seeking behaviors that often persist despite an individual’s conscious desire to maintain abstinence42.
The transition to relapse was marked by substantially more extensive neural disruptions, demonstrating the progressive and dynamic nature of addiction-related brain changes. Our ReHo analysis revealed decreased activity across an array of cortical regions, including the left middle cingulate gyrus, bilateral postcentral gyri, bilateral superior temporal gyri, left middle occipital gyrus, left cuneus, and right calcarine fissure. This widespread pattern of cortical hypoactivity suggests broad disruption of networks involved in multiple cognitive and sensory processes43,44. The middle cingulate gyrus, showing consistently reduced activity, serves as a crucial hub for error detection, behavioral adjustment, and emotional regulation through its extensive connections with the anterior cingulate cortex and prefrontal regions45,46,47. Its dysfunction may fundamentally impair an individual’s ability to monitor and regulate drug-seeking behaviors, potentially contributing to the persistence of addiction despite negative consequences48.
The observed reduction in activity across sensorimotor regions, including the bilateral postcentral gyri and visual processing areas, suggests compromised integration of sensory information that may affect how environmental stimuli are processed and interpreted49,50,51. This altered sensory processing could contribute to the enhanced salience of drug-related cues while diminishing the ability to engage with non-drug-related environmental stimuli28. The broad nature of these cortical alterations indicates that relapse involves not just discrete changes in reward-related circuits but rather a fundamental reorganization of how the brain processes and responds to both internal and external signals52,53.
Importantly, during the relapse phase, we observed a distinct pattern of increased activity in several key regions, including the left opercular part of inferior frontal gyrus, left caudate, bilateral hippocampus, right precuneus, and right parahippocampal gyrus. The fALFF analysis complemented these findings by revealing enhanced activity in additional regions, including the left putamen, left thalamus, right anterior cingulate gyrus, and right putamen. This complex pattern of subcortical hyperactivity suggests strengthened engagement of multiple parallel circuits involved in reward processing, memory formation, and behavioral control54. The enhanced activity in hippocampal and parahippocampal regions is particularly noteworthy, as these structures play crucial roles in contextual learning and memory consolidation55. Their hyperactivity during relapse may strengthen the associations between environmental contexts and drug-seeking behaviors, potentially contributing to the high rates of context-induced relapse observed in clinical settings56.
The direct comparison between relapse and abstinence stages revealed specific progression patterns in neural adaptation that provide crucial insights into the evolution of addiction-related brain changes. We observed decreased ReHo values in the left inferior temporal gyrus alongside increased values in the left fusiform gyrus and left olfactory region, suggesting altered processing of sensory and emotional information57,58,59. Most notably, the fALFF analysis demonstrated increased activity in the bilateral caudate nucleus, marking a significant shift in the locus of striatal involvement across addiction stages. This progression from predominantly putamen-centered alterations during abstinence to enhanced caudate nucleus activity during relapse represents a potential neural substrate for the transition from purely habit-based to more complex, goal-directed drug-seeking behaviors60,61. The caudate nucleus, as part of the associative striatum, integrates information from multiple cortical regions to guide goal-directed behavior, and its enhanced activation may reflect the increasing complexity of drug-seeking strategies as addiction progresses62.
These systematic neural adaptations provide crucial insights into the mechanisms underlying addiction chronicity and the challenges of maintaining long-term recovery. The progression from focused subcortical alterations during abstinence to more widespread circuit dysfunction following relapse suggests a cascade of neural adaptations that may increasingly strengthen drug-seeking behaviors while progressively weakening cognitive control mechanisms63,64. The bilateral nature of many observed changes, particularly in striatal regions, indicates systematic adaptation of parallel neural circuits that may contribute to the persistent and treatment-resistant nature of addiction65.
Our findings show both convergent and divergent patterns when compared to neuroimaging studies of other substance addictions. Convergent findings include striatal dysfunction and prefrontal hypoactivity, which have been consistently reported across cocaine, alcohol, and opioid addiction studies31,66,67. The progression from subcortical to more widespread cortical involvement during relapse aligns with theories of addiction as a progressive brain disorder affecting multiple neural networks68. However, our study reveals methamphetamine-specific patterns that distinguish it from other substances. The extent and persistence of cortical hypoactivity we observed appears more pronounced than typically reported in cocaine addiction studies, possibly reflecting methamphetamine’s unique neurotoxic effects55. Additionally, the specific putamen-to-caudate progression pattern, while theoretically consistent with habit-to-goal-directed transition models, has not been systematically documented in other substance addiction longitudinal studies65. These comparisons suggest that while addiction involves common neural mechanisms across substances, methamphetamine’s distinctive pharmacological profile necessitates substance-specific considerations in both research and treatment approaches. The broader cortical involvement we observed may explain the particularly challenging nature of methamphetamine addiction recovery and the high relapse rates associated with this substance69.
Our research provides a comprehensive framework for understanding methamphetamine addiction as a dynamic disorder involving progressive adaptation of multiple neural circuits. By combining ReHo and fALFF analyses, we revealed for the first time the dynamic progression of neural adaptations from abstinence to relapse, particularly the shift in striatal involvement from putamen to caudate nucleus. This transition reflects the evolution from habit-based to goal-directed drug-seeking behaviors, extending previous models of addiction progression. The systematic characterization of stage-specific changes suggests potential therapeutic targets at different stages of the addiction cycle, underscoring the importance of targeted, stage-specific interventions. While our findings highlight the critical importance of early intervention in preventing the cascade of neural adaptations that promote chronic addiction, the cross-sectional design limits causal inferences about their temporal sequence. Future longitudinal studies with higher temporal resolution could better characterize these neural dynamics and their relationship to clinical outcomes. Understanding how these circuit-level changes interact with environmental triggers could inform more effective prevention strategies and potentially improve long-term outcomes for individuals struggling with methamphetamine dependence.
Based on these neurobiological insights, our findings suggest potential for developing personalized, stage-specific therapeutic interventions. During early abstinence (putamen-dominant phase), treatment strategies should focus on habit disruption and automatic behavior modification, such as cue exposure therapy and response prevention techniques that specifically target the dorsal striatal habit system70,71. Interventions like cognitive behavioral therapy with emphasis on breaking automatic drug-seeking routines may be most effective during this phase72. In contrast, during the relapse phase (caudate-dominant phase), therapeutic approaches should shift toward enhancing goal-directed cognitive control and executive function. This could include working memory training, cognitive control enhancement programs, and motivational interviewing techniques that engage the associative striatum’s role in goal-directed behavior72. The caudate nucleus’s integration of cortical information for complex decision-making suggests that interventions targeting cognitive flexibility and decision-making processes may be particularly beneficial during this stage31. This neurobiologically-informed, stage-specific treatment approach represents a significant advancement over traditional one-size-fits-all addiction therapies and could potentially improve treatment outcomes by targeting the specific neural circuits most relevant to each recovery stage67,73.
An important limitation of this study is the inclusion of only male participants, which limits the generalizability of our findings to female populations. Future studies should include female participants to explore gender-specific neural mechanisms of methamphetamine addiction and relapse, as gender differences in addiction neurobiology are increasingly recognized as clinically significant74,75. Another important limitation of this study is the potential confounding effect of rehabilitation interventions, including education and physical exercise programs, on brain activity patterns. Previous research has demonstrated that physical exercise can improve striatal function and cognitive control abilities, potentially influencing the neural activity patterns we observed76,77. Similarly, educational interventions may affect cognitive networks and decision-making processes78. Finally, in rs-fMRI studies, many multiple comparison correction methods can be used for statistical analysis. Due to the limitation of sample size, this study adopted the AlphaSim correction method, however, in future research, we will further expand the sample size and use more multiple comparison correction method for statistical analysis to validate the results of this study, such as permutation test with Threshold-Free Cluster Enhancement (TFCE), a strict multiple comparison correction strategy, which can reach the best balance between family‐wise error rate and reproducibility79.
While all participants in our study received standardized rehabilitation protocols, we cannot definitively separate the neural effects of abstinence/relapse from those of therapeutic interventions. Future studies should implement more rigorous control conditions, such as comparing different rehabilitation protocols or including treatment intensity as a covariate in analyses80. Additionally, control groups receiving different intervention types (e.g., exercise-only vs. education-only vs. combined interventions) would help isolate the specific neural effects of abstinence and relapse from treatment-related changes. Despite this limitation, our findings remain valuable as they reflect the real-world clinical context in which recovery occurs, and the observed neural patterns may represent the combined effects of both neurobiological recovery processes and therapeutic interventions.
Conclusion
Our findings demonstrate persistent brain activity alterations in MADIs during abstinence, characterized by decreased ReHo values in attention-related regions alongside increased activity in the inferior temporal gyrus, right insula, and notably, the bilateral putamen. The enhanced putamen activity during abstinence represents a potential neural signature for early relapse risk. Relapse was marked by more extensive neural disruptions, progressing from focused putamen-centered changes to widespread network dysfunction, particularly enhanced activity across the striatum. This progression, especially the shift from putamen to caudate nucleus dominance, suggests a transition from habit-based to goal-directed drug-seeking behaviors. Our findings not only extend the current understanding of the neural basis of methamphetamine addiction but also provide a theoretical foundation for developing stage-specific therapeutic interventions. Early intervention targeting these distinct patterns of neural dysfunction might be crucial in preventing the cascade of maladaptive neural adaptations that characterize chronic addiction and contribute to high relapse rates. Future research should employ more intensive longitudinal designs with multiple assessment points (e.g., 1, 3, 6, and 12 months post-abstinence) to more precisely characterize the temporal dynamics of neural adaptation. Additionally, multimodal studies incorporating biomarkers, cognitive assessments, and environmental factors will be essential for developing comprehensive relapse prediction models. Long-term follow-up studies tracking participants beyond the first year could provide insights into the stability of these neural patterns and their relationship to sustained recovery outcomes.
Data availability
The datasets used and analysed during the current study were available from the corresponding author on reasonable request.
References
Drugscrime uoo. World Drug Report 2021[J]. CrimRxiv, (2020).
Kwon, N. J. & Han, E. A commentary on the effects of methamphetamine and the status of methamphetamine abuse among youths in South korea, japan, and China[J]. Forensic Sci. Int. 286, 81–85 (2018).
Xu, S., Zhang, K. & Luo, T. Development of the risk of relapse assessment scale for methamphetamine abusers in China[J]. Drug Alcohol Depend. 227, 108992 (2021).
Luo, Y. L. et al. Effects of methamphetamine abuse on Spatial cognitive function[J]. Sci. Rep. 8 (1), 5502 (2018).
Zerekidze, A. et al. Neural correlates of impaired cognitive control in individuals with methamphetamine dependence: An fMRI study. Brain Sci. 13(2), 197. https://doi.org/10.3390/brainsci13020197 (2023).
Brecht, M. L. & Herbeck, D. Time to relapse following treatment for methamphetamine use: a long-term perspective on patterns and predictors[J]. Drug Alcohol Depend. 139, 18–25 (2014).
Nestor, L. J., Ghahremani, D. G. & London, E. D. Reduced neural functional connectivity during working memory performance in methamphetamine use disorder[J]. Drug Alcohol Depend. 243, 109764 (2023).
Dakhili, A. et al. Cue-induced craving and negative emotion disrupt response Inhibition in methamphetamine use disorder: behavioral and fMRI results from a mixed Go/No-Go task[J]. Drug Alcohol Depend. 233, 109353 (2022).
Bischoff-Grethe, A. et al. Altered reward expectancy in individuals with recent methamphetamine dependence[J]. J. Psychopharmacol. 31 (1), 17–30 (2017).
Mizoguchi, H. & Yamada, K. Methamphetamine use causes cognitive impairment and altered decision-making[J]. Neurochem Int. 124, 106–113 (2019).
Crane Na, Molla, H. & De Wit, H. Methamphetamine alters nucleus accumbens neural activation to monetary loss in healthy young adults[J]. Psychopharmacol. (Berl). 240 (9), 1891–1900 (2023).
Hoffman, W. F. et al. Psychopathy and corticostriatal connectivity: the link to criminal behavior in methamphetamine Dependence[J]. Front. Psychiatry. 11, 90 (2020).
Tobias Mc, O’neill, J. et al. White-matter abnormalities in brain during early abstinence from methamphetamine abuse[J]. Psychopharmacol. (Berl). 209 (1), 13–24 (2010).
Simon, S. L. et al. Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence[J]. J. Stud. Alcohol Drugs. 71 (3), 335–344 (2010).
Karila, L. et al. Pharmacological approaches to methamphetamine dependence: a focused review[J]. Br. J. Clin. Pharmacol. 69 (6), 578–592 (2010).
Hamel, C. et al. Psychosocial and Pharmacologic interventions for methamphetamine addiction: protocol for a scoping review of the literature[J]. Syst. Rev. 9 (1), 245 (2020).
Chan, B. et al. Pharmacotherapy for methamphetamine/amphetamine use disorder-a systematic review and meta-analysis[J]. Addiction 114 (12), 2122–2136 (2019).
Yan, C. et al. Neurofunctional changes related to methamphetamine and sexual cues in methamphetamine dependence from short- to long-term abstinence[J]. Addict. Biol. 29 (6), e13405 (2024).
Stewart, J. L. et al. Striatum and Insula dysfunction during reinforcement learning differentiates abstinent and relapsed methamphetamine-dependent individuals[J]. Addiction 109 (3), 460–471 (2014).
Zang, Y. et al. Regional homogeneity approach to fMRI data analysis[J]. Neuroimage 22 (1), 394–400 (2004).
Zou, Q. H. et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF[J]. J. Neurosci. Methods. 172 (1), 137–141 (2008).
Ersche, K. D. et al. Brain networks underlying vulnerability and resilience to drug addiction[J]. Proc. Natl. Acad. Sci. U S A. 117 (26), 15253–15261 (2020).
Nestor, L. J. et al. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects[J]. Psychiatry Res. 194 (3), 287–295 (2011).
Huang, S. et al. Craving responses to methamphetamine and sexual visual cues in individuals with methamphetamine use disorder after Long-Term drug Rehabilitation[J]. Front. Psychiatry. 9, 145 (2018).
Chao-Gan, Y. & Yu-Feng, Z. DPARSF: A MATLAB toolbox for pipeline data analysis of Resting-State fMRI[J]. Front. Syst. Neurosci. 4, 13 (2010).
Ma, M. et al. Functional whole-brain mechanisms underlying effects of tDCS on athletic performance of male rowing athletes revealed by resting-state fMRI[J]. Front. Psychol. 13, 1002548 (2022).
Song, X. W. et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing[J]. PLoS One. 6 (9), e25031 (2011).
Wang, G. et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers[J]. PLoS One. 8 (7), e68791 (2013).
Li, X. et al. The central amygdala nucleus is critical for incubation of methamphetamine craving[J]. Neuropsychopharmacology 40 (5), 1297–1306 (2015).
Paulus, MP & Stewart, JL. Interoception and drug addiction. Neuropharmacology 76 (Pt B(0 0)), 342–350 (2014).
Goldstein, R. Z. & Volkow, N. D. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications[J]. Nat. Rev. Neurosci. 12 (11), 652–669 (2011).
Corbetta, M. & Shulman, G. L. Control of goal-directed and stimulus-driven attention in the brain[J]. Nat. Rev. Neurosci. 3 (3), 201–215 (2002).
Potvin, S. et al. Cognitive deficits in individuals with methamphetamine use disorder: A meta-analysis[J]. Addict. Behav. 80, 154–160 (2018).
Robinson, T. E., Berridge, K. C. & Review The incentive sensitization theory of addiction: some current issues[J]. Philos. Trans. R Soc. Lond. B Biol. Sci. 363 (1507), 3137–3146 (2008).
Newton, T. F. et al. Theories of addiction: methamphetamine users’ explanations for continuing drug use and relapse[J]. Am. J. Addict. 18 (4), 294–300 (2009).
Lafer-Sousa, R. & Conway, B. R. Parallel, multi-stage processing of colors, faces and shapes in macaque inferior Temporal cortex[J]. Nat. Neurosci. 16 (12), 1870–1878 (2013).
Chan, D. et al. Patterns of Temporal lobe atrophy in semantic dementia and alzheimer’s disease[J]. Ann. Neurol. 49 (4), 433–442 (2001).
Naqvi, N. H. et al. Damage to the Insula disrupts addiction to cigarette smoking[J]. Science 315 (5811), 531–534 (2007).
Lee, B. et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity[J]. J. Neurosci. 29 (47), 14734–14740 (2009).
Volkow, N. D. et al. Unbalanced neuronal circuits in addiction[J]. Curr. Opin. Neurobiol. 23 (4), 639–648 (2013).
Everitt, B. J. & Robbins, T. W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion[J]. Nat. Neurosci. 8 (11), 1481–1489 (2005).
London, E. D. et al. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging[J]. Brain Res. 1628 (Pt A), 174–185 (2015).
Li, W. et al. White matter impairment in chronic heroin dependence: a quantitative DTI study[J]. Brain Res. 1531, 58–64 (2013).
Goldstein, R. Z. & Volkow, N. D. Drug addiction and its underlying Neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex[J]. Am. J. Psychiatry. 159 (10), 1642–1652 (2002).
Rolls, E. T. et al. The human orbitofrontal cortex, vmpfc, and anterior cingulate cortex effective connectome: emotion, memory, and action[J]. Cereb. Cortex. 33 (2), 330–356 (2022).
Shen, C. et al. Anterior cingulate cortex cells identify Process-Specific errors of attentional control prior to transient Prefrontal-Cingulate Inhibition[J]. Cereb. Cortex. 25 (8), 2213–2228 (2015).
Westendorff, S. et al. Prefrontal and anterior cingulate cortex neurons encode attentional targets even when they do not apparently bias behavior[J]. J. Neurophysiol. 116 (2), 796–811 (2016).
Gowin, J. L. et al. Altered cingulate and insular cortex activation during risk-taking in methamphetamine dependence: losses lose impact[J]. Addiction 109 (2), 237–247 (2014).
Cappadocia, D. C. et al. Temporal evolution of target representation, movement direction planning, and reach execution in Occipital-Parietal-Frontal cortex: an fMRI Study[J]. Cereb. Cortex. 27 (11), 5242–5260 (2017).
Mastria, G. et al. Morphology, connectivity, and encoding features of tactile and motor representations of the fingers in the human precentral and postcentral Gyrus[J]. J. Neurosci. 43 (9), 1572–1589 (2023).
Gu, J., Cao, L. & Liu, B. Modality-general representations of Valences perceived from visual and auditory modalities[J]. Neuroimage 203, 116199 (2019).
Liang, X. et al. Interactions between the salience and default-mode networks are disrupted in cocaine addiction[J]. J. Neurosci. 35 (21), 8081–8090 (2015).
Kohno, M. et al. Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence[J]. JAMA Psychiatry. 71 (7), 812–820 (2014).
Jasinska, A. J. et al. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies[J]. Neurosci. Biobehav Rev. 38, 1–16 (2014).
Thompson, P. M. et al. Structural abnormalities in the brains of human subjects who use methamphetamine[J]. J. Neurosci. 24 (26), 6028–6036 (2004).
Goodman, J. & Packard, M. G. Memory systems and the addicted Brain[J]. Front. Psychiatry. 7, 24 (2016).
Chatterjee, A. et al. The neural response to facial attractiveness[J]. Neuropsychology 23 (2), 135–143 (2009).
Conway, B. R. The organization and operation of inferior Temporal Cortex[J]. Annu. Rev. Vis. Sci. 4, 381–402 (2018).
Simonini, L. A comprehensive review of COVID-19-related olfactory deficiency: Unraveling associations with neurocognitive disorders and magnetic resonance imaging findings. Diagnostics (Basel) 14(4), 359. https://doi.org/10.3390/diagnostics14040359 (2024).
Willuhn, I. et al. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use[J]. Proc. Natl. Acad. Sci. U S A. 109 (50), 20703–20708 (2012).
Balleine, B. W., Delgado, M. R. & Hikosaka, O. The role of the dorsal striatum in reward and decision-making[J]. J. Neurosci. 27 (31), 8161–8165 (2007).
Yin, H. H., Ostlund, S. B. & Balleine, B. W. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks[J]. Eur. J. Neurosci. 28 (8), 1437–1448 (2008).
Hu, Y. et al. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction[J]. JAMA Psychiatry. 72 (6), 584–592 (2015).
Kalivas, P. W. & O’brien, C. Drug addiction as a pathology of staged Neuroplasticity[J]. Neuropsychopharmacology 33 (1), 166–180 (2008).
Everitt, B. J. & Robbins, T. W. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction[J]. Neurosci. Biobehav Rev. 37 (9 Pt A), 1946–1954 (2013).
Volkow, N. D., Koob, G. F. & Mclellan, A. T. Neurobiologic advances from the brain disease model of Addiction[J]. N Engl. J. Med. 374 (4), 363–371 (2016).
Koob, G. F. & Volkow, N. D. Neurobiology of addiction: a neurocircuitry analysis[J]. Lancet Psychiatry. 3 (8), 760–773 (2016).
Everitt, B. J. & Robbins, T. W. Drug addiction: updating actions to habits to compulsions ten years On[J]. Annu. Rev. Psychol. 67, 23–50 (2016).
Darke, S. et al. Major physical and psychological harms of methamphetamine use[J]. Drug Alcohol Rev. 27 (3), 253–262 (2008).
Everitt, B. J. & Robbins, T. W. Drug addiction: updating actions to habits to compulsions ten years on[J]. Ann. Rev. Psychol. 67 (1), 23–50 (2016).
Volkow, N. D. et al. Addiction: beyond dopamine reward circuitry[J]. Proc. Natl. Acad. Sci. U S A. 108 (37), 15037–15042 (2011).
Mchugh, R. K., Hearon, B. A. & Otto, M. W. Cognitive-behavioral therapy for substance use disorders[J]. Psychiatr. Clin. North Am. 33 (3), 511 (2010).
Luigjes, J. et al. Defining compulsive behavior[J]. Neuropsychol. Rev. 29, 4–13 (2019).
Becker, J. B. & Koob, G. F. Sex differences in animal models: focus on addiction[J]. Pharmacol. Rev. 68 (2), 242–263 (2016).
Quigley, J. A. et al. Sex differences in vulnerability to addiction[J]. Neuropharmacology 187, 108491 (2021).
Linke, S. E. & Ussher, M. Exercise-based treatments for substance use disorders: evidence, theory, and practicality[J]. Am. J. Drug Alcohol Abus. 41 (1), 7–15 (2015).
VOSS, M. W. et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention[J]. Hum. Brain. Mapp. 34 (11), 2972–2985 (2013).
Garland, E. L. et al. Mindfulness-oriented recovery enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: A randomized controlled trial[J]. J. Consult. Clin. Psychol. 87 (10), 927 (2019).
Chen, X., Lu, B. & Yan, C. G. Reproducibility of R-fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes[J]. Hum. Brain Mapp. 39 (1), 300–318 (2018).
Lomas, C. Breaking the cycle: a systematic review of Neurobiological mechanisms and psychotherapeutic innovations in ketamine addiction. J. Addict. Dis. 6, 1–22. https://doi.org/10.1080/10550887.2025.2464356 (2025).
Funding
The work was supported by the grants of: Sports Research Project of Nanjing Sports Bureau (No. NJTY2024-101; NJTY2023-102).
Author information
Authors and Affiliations
Contributions
Ming Ma and Guoxiang Wang designed the experiments. Ming Ma, Tiancheng Yu, Zhi Qin, Peng Zhao, Mudi Zhao, Jianye Guo, Ningqing Huang, Mengqian Liu, Kaixi Zhang and Qian Cai contributed to clinical data collection and assessment. Ming Ma, Jianhuai Chen and Guoxiang Wang analyzed the results. Ming Ma, Jianhuai Chen and Guoxiang Wang wrote the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, M., Yu, T., Qin, Z. et al. Abnormal regional brain activity associated with relapse in early abstinent methamphetamine-dependent individuals. Sci Rep 15, 26657 (2025). https://doi.org/10.1038/s41598-025-11438-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11438-4