Abstract
We sought to investigate the factors influencing liver regeneration after hepatectomy for hepatocellular carcinoma and the relationship between liver regeneration and prognosis. This retrospective cohort study enrolled 92 hepatocellular carcinoma (HCC) patients undergoing hemihepatectomy at Qingdao University Affiliated Hospital (2014-2020) with complete CT imaging (postoperative day 3 and month 1) and clinical records. Using Hisense CAS software, we performed three-dimensional liver reconstruction to quantify standardized residual liver volume (SRLV) and calculate hepatic regeneration rate (HRR) at 1-month postoperation. Patients were stratified into high and low-regeneration groups based on median HRR. Univariate analysis and multivariate logistic regression were applied to identify factors influencing regeneration. Kaplan-Meier survival curves with log-rank tests analyzed tumor-free survival (TFS) and overall survival (OS) outcomes in relation to regeneration capacity. The cohort comprised 61 right and 31 left hemihepatectomies. Median 1-month HRR was 17.6% overall, with significant disparity between right (20.29%) and left hepatectomy subgroups (12.2%). Univariate analysis identified age, sex, alcohol history, hepatitis B status, cirrhosis severity, and SRLV as significant regeneration predictors (all P < 0.05). Multivariate modeling established cirrhosis severity (OR = 0.217, 95% CI:0.064–0.732, P = 0.014) and SRLV (OR = 0.989, 95% CI:0.982–0.995, P < 0.001) as independent determinants.Prognostically, high-regeneration patients exhibited extended median TFS (16 vs. 5 months, P<0.05) compared to low-regeneration counterparts, though no significant OS difference was observed (P>0.05). Cirrhosis severity and standardized residual liver volume (SRLV) independently predict post-hemihepatectomy liver regeneration in HCC patients. Preoperative 3D reconstruction-guided SRLV assessment combined with cirrhosis evaluation optimizes surgical planning. Enhanced hepatic regeneration correlates with shorter tumor-free survival (median 16 vs 5 months, P<0.05), necessitating intensified surveillance in high-regeneration cohorts to mitigate recurrence risks.
Similar content being viewed by others
Introduction
Primary hepatocellular carcinoma (PHC) is a common malignant tumor that seriously affects human survival. There are various treatment modalities for primary hepatocellular carcinoma, but radical hepatectomy, which can substantially prolong survival and improve quality of life, is still the preferred treatment for this disease1. After a patient has undergone hepatectomy, the strong regenerative capacity of the normal liver greatly reduces the impact of surgical injury on liver function. However, patients with chronic liver disease, such as cirrhosis, have a considerably prolonged liver regeneration process and insufficient regenerative capacity after surgery; when the RLV is insufficient after hepatectomy, patients are prone to liver failure or even liver failure2. These various factors have forced us to think about how to guarantee a safe level of liver regeneration in patients after surgery. In this study, instead of using the traditional animal liver resection model, we focused our research on clinical patients, utilized the advantages of clinical surgery, and accurately quantified the level of liver regeneration after hepatectomy based on three-dimensional medical image reconstruction technology. Additionally, we conducted follow-up surveys on postoperative patients, integrated the data, and explored and explored the influencing factors of liver regeneration after hepatectomy. Moreover, the tumor-free survival (TFS) and overall survival (OS) times of the patients were collected to investigate the relationship between the level of liver regeneration and prognosis.
Data and methods
Source of information
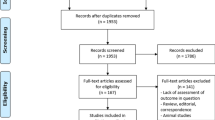
A total of 1,246 HCC patients underwent hepatectomy between January 2014 and December 2020. After applying strict inclusion/exclusion criteria (Fig. 1), 92 patients with complete preoperative assessment, serial CT imaging (postoperative day 3 and month 1), and follow-up records were included in the final analysis.This study was reviewed and approved by the Ethics Committee of the Affiliated Hospital of Qingdao University, and the entire study was strictly conducted in accordance with the Ethical Guidelines for Medical Research on Human Subjects. In the process of follow-up and obtaining the patients’ clinical data, we obtained the consent of the patients or their family members, and informed consent forms were signed.
The inclusion criteria were as follows:
(1) Postoperative pathologic diagnosis of hepatocellular carcinoma.
(2) Patients who underwent partial hepatectomy for hepatocellular carcinoma for the first time in our hospital.
(3) Preoperative assessment of Child‒Pugh class A or B.
(4) Patients with a 15-minute retention rate of < 10% on the preoperative ICG test.
The exclusion criteria were as follows:
(1) Patients who underwent surgical resection after tumor recurrence.
(2) The tumor was not completely resected during the operation.
(3) Patients who did not undergo single liver resection or resection of other organs.
(4) Patients who underwent TACE before or after surgery or who received other related treatments.
(5) Patients with a history of other malignant tumors.
(6) Patients with non-hemihepatectomy.
(7) Inadequate postoperative CT examination and incomplete information.
(8) Patients whose postoperative follow-up was lost or who refused to receive follow-up.
(9) Patients with incomplete clinical data from laboratory tests.
Research design and methods
This study employed a self-developed 3D liver modeling system (based on CT/MRI data) to digitally quantify the dynamic process of liver regeneration with high precision. Specifically, the system automatically calculates standardized remnant liver volume (SRLV) and derives hepatic regeneration rate (HRR) using postoperative imaging data acquired at 3 days and 1 month. With HRR as the core evaluation metric, we systematically analyzed the impact of clinical factors—including age, gender, cirrhosis severity, and surgical approach (left/right hemihepatectomy)—on hepatic regenerative capacity. Concurrently, a comprehensive follow-up protocol integrating radiological imaging, serum biomarkers, and histopathological validation was implemented to track recurrence-free survival (time to first tumor recurrence) and overall survival (time to all-cause mortality), thereby investigating the correlation between hepatic regeneration levels and long-term prognosis.
The target hemiliver is mobilized by dividing its associated ligaments (e.g., falciform, coronary, and triangular ligaments), followed by exposure of the hepatic hilum. Selective ligation of the ipsilateral hepatic artery and portal vein branches is performed via Glissonean pedicle dissection. Total hepatic inflow occlusion (Pringle maneuver) is applied when necessary. The transection plane is determined along the Cantlie line, guided by ischemic demarcation or intraoperative ultrasound. Hepatic parenchymal dissection is conducted stepwise using ultrasonic dissectors, with meticulous ligation of hepatic venous tributaries.
Left hemihepatectomy: The branches of the middle hepatic vein (MHV) within the left lobe are typically transected without mandatory preservation of the MHV trunk, unless required for right anterior segment drainage.
Right hemihepatectomy: Preservation of the MHV trunk is prioritized to avoid residual liver congestion, though its sacrifice may be warranted in cases of tumor invasion or extended resections, contingent on collateral venous compensation.
After achieving meticulous hemostasis and biliary leakage testing (via intraoperative cholangiography or white gauze compression), a closed suction drain is placed adjacent to the resection margin, and the abdomen is closed in layers.
Liver Volume Calculation: Through the PACS management system, we captured the abdominal CT image data of the patient 3 days and 1 month after the operation, exported and saved them in Dicom file format; HisenseCAS was utilized to read the Dicom data for reconstructing the three-dimensional model of the liver(This software was originally developed by the Affiliated Hospital of Qingdao University, internal link is http:/192.168.99.233:6082/, version 1.25.2). For model reconstruction, Hisense CAS could automatically calculate the residual liver volume (RLV) and the future remnant liver volume (FRLV) 1 month after hepatectomy.(Examples of 3D reconstructions can be seen in Figs. 2, 3, 4 and 5.)
a example of 3D reconstruction (right liver tumor). (http:/192.168.99.233:6082/, version 1.25.2)
a example of 3D reconstruction (right liver tumor). (http:/192.168.99.233:6082/, version 1.25.2)
a example of 3D reconstruction (left liver tumor). (http:/192.168.99.233:6082/, version 1.25.2)
a example of 3D reconstruction (left liver tumor). (http:/192.168.99.233:6082/, version 1.25.2)
Observational indicators and follow-up
Patients’ general information (sex, age, height, weight, alcohol consumption history, hepatitis B status) and preoperative laboratory parameters (ALT, albumin, total bilirubin, AFP, AST) were systematically recorded. Intraoperative variables included surgical approach, operative time, blood loss, hepatic portal clamping frequency/duration, and transfusion requirements. Postoperative monitoring encompassed serial laboratory tests (ALT, AST, albumin, total bilirubin), liver failure incidence, and pathological findings (vascular tumor thrombus grade, satellite foci, lymph node metastasis, hepatocyte status).
This study established a systematic follow-up protocol to precisely evaluate postoperative prognosis in patients with hepatocellular carcinoma (HCC). Standardized follow-ups were conducted every 3 months for the first 2 years postoperatively, then every 6 months thereafter, continuing until 5 years post-surgery or patient death. The follow-up protocol included:
Clinical Evaluation: Outpatient records from hospital electronic medical systems were integrated to dynamically monitor symptoms, physical signs, and Child-Pugh scores.
Imaging Surveillance: Contrast-enhanced CT (arterial/portal venous phases) and hepatobiliary-specific contrast-enhanced MRI (EOB-Primovist) were alternated at baseline, 3/6/12 months postoperatively, and annually thereafter. New intrahepatic lesions required consistent findings across two imaging modalities (arterial phase enhancement with portal phase washout) and independent confirmation by two radiologists.
Biomarker Tracking: Serum alpha-fetoprotein (AFP) and protein induced by vitamin K absence/antagonist-II (PIVKA-II) levels were synchronously measured.
Recurrence Confirmation: After radical resection and during postoperative follow-up, new lesions detected in the liver or other organs were confirmed as HCC recurrence through imaging (≥ 2 diagnostic modalities) or histopathological verification via percutaneous biopsy.
Survival Status Verification: Mortality events were triple-verified through hospital death records, civil registration systems, and family interviews, with non-HCC-related deaths excluded.
Study Endpoints were defined as:
Tumor-Free Survival (TFS): Time from surgery to first confirmed recurrence.
Overall Survival (OS): Time from surgery to all-cause death or last follow-up.
Sensitivity analyses using the Fine-Gray competing risk model were applied to cases with single-modality imaging positivity.
Quality Control Measures: A multidisciplinary endpoint adjudication committee resolved disputed cases.The REDCap platform automated follow-up reminders with a tolerance window of ± 3 days.Standardized telephone questionnaires (including 6 recurrence-alert symptoms) and random audio audits ensured data reliability.
Results: This protocol reduced the median recurrence detection time by 2.1 months compared to conventional approaches, achieved a follow-up compliance rate > 95%, and provided high-level evidence for prognostic analysis.
Calculation formula
(1) Liver regeneration volume: liver regeneration volume = 1 month postliver surgery volume (FRLV) - residual liver volume (RLV).
(2) Liver regeneration rate: liver regeneration rate (%) = liver regeneration volume (∆LV)/residual liver volume (RLV) * 100%.
(3) Body surface area: body surface area (BSA): BSA (m2) = 0.0061*height (cm) + 0.0128*weight (kg) − 0.1529.
(4) Standard remnant liver volume (SRLV) = RLV/BSA.
Diagnostic criteria and definitions
(1) Given the patient’s first laboratory examination after admission, imaging manifestations, clinical symptoms, physical examination, etc., the patient’s liver function was scored and graded according to the Child‒Pugh classification.
(2) The degree of cirrhosis was graded according to the pathological METAVIR scoring system3. S2 and S3 were defined as hepatic fibrosis and S4 as cirrhosis.
(3) The median hepatic regeneration rate (HRR) observed at 1 month postoperatively was 17.6% (calculated from the cohort data). Based on this post hoc analysis, patients were stratified into low-regeneration group (HRR < 17.6%, n = 46) and high-regeneration group (HRR ≥ 17.6%, n = 46).
Statistical analysis
All statistical analyses were performed using SPSS 22.0 (IBM Corp., Armonk, NY). Continuous variables were first assessed for normality using the Shapiro-Wilk test. Normally distributed data were expressed as mean ± standard deviation (SD) and compared between groups using the independent samples t-test. Non-normally distributed variables were reported as median (interquartile range, IQR) and analyzed with the Mann-Whitney U test (for two groups) or Kruskal-Wallis H test (for multiple groups). Categorical variables were presented as frequencies (%) and evaluated using Pearson’s χ² test or Fisher’s exact test, as appropriate for expected cell frequencies.
Univariate analyses were conducted to identify potential predictors of liver regeneration. Variables with P < 0.05 in univariate screening were incorporated into a multivariable logistic regression model to determine independent risk factors, with results reported as adjusted odds ratios (ORs) and 95% confidence intervals (CIs). Spearman’s rank correlation coefficient (ρ) was utilized to assess bivariate associations between liver regeneration rates and continuous clinical parameters.
For survival outcomes, tumor-free survival (TFS) and overall survival (OS) were analyzed using Kaplan-Meier curves with log-rank tests to compare differences between high- and low-regeneration groups. Hazard ratios (HRs) were calculated via Cox proportional hazards regression models to quantify the prognostic impact of liver regeneration capacity. All tests were two-tailed, and a P < 0.05 was considered statistically significant. Sensitivity analyses, including competing risk models, were performed to validate the robustness of survival outcomes.
Results
Description of general information (Table 1: general information characteristics of the patients)
The association between the liver regeneration rate and the residual liver volume and change in the FLR within 1 month after hepatectomy.
Figure 6 revealed that the residual liver volume was negatively correlated with the liver regeneration rate at 1 month after surgery (r = −0.626, P < 0.001), and the standard residual liver volume was negatively correlated with the liver regeneration rate at 1 month after surgery (r = −0.542, P < 0.001).
The single analysis results shown suggested that the difference in the standard residual liver volume was statistically significant between the high regeneration and low regeneration groups (P < 0.001).(Table 2: Liver volume and liver regeneration).
Analysis of single factors affecting liver regeneration
The median regeneration rate of all patients at 1 month postoperatively was 17.6%, and the median liver regeneration rate at 1 month postoperatively was taken as the cutoff value. These 92 patients were categorized into two groups, namely, the high regeneration group (residual liver regeneration rate ≥ 17.6%, n = 46) and the low regeneration group (residual liver regeneration rate < 17.6%, n = 46). Single factor analysis was performed to assess the factors that might affect liver regeneration after hepatocellular carcinoma surgery and to screen the influencing factors of liver regeneration.
The results of univariate analysis of preoperative factors revealed that between the high-regeneration group and the low-regeneration group, there were statistically significant differences in terms of age (P < 0.05), sex (P < 0.05), alcohol consumption history (P < 0.05), and history of hepatitis B (P < 0.05), and these factors could be used as predictive factors in further analysis (Table 3: Single analysis of factors affecting liver regeneration (preoperative factors)).
The results of univariate analysis of intraoperative factors showed that there was no statistically significant difference between the high regeneration group and the low regeneration group in terms of the surgical approach, operating time, number of hepatic portal blocks, duration of hepatic portal blocks, intraoperative blood loss, presence or absence of intraoperative blood transfusion, or greatest tumor diameter (P > 0.05) (Table 4).
The results of univariate analysis for postoperative factors as well as pathological factors showed that the differences between the high-regeneration group and low-regeneration group in terms of postoperative alanine aminotransferase (ALT), albumin, total bilirubin, AFP, azelaic transaminase (AST), PT, INR, TNM stage and hepatic failure as well as in terms of the presence or absence of satellite foci in the pathological indications, the classification of vascular cancer thrombus, the presence or absence of lymph node metastasis, and the presence or absence of hepatocytes were not statistically significant (P > 0.05). As shown in Table 5, the differences in the degree of cirrhosis as indicated by pathology (P < 0.05) were statistically significant.
Multifactorial logistic regression analysis of factors affecting liver regeneration in patients
To further explore the independent influencing factors of liver regeneration after HCC resection, we included the factors with statistically significant differences between the two groups in the multivariate logistic regression analysis model. Ultimately, we found that the degree of cirrhosis (P < 0.05) and standard residual liver volume (P < 0.001) were independent factors of liver regeneration after hepatocellular carcinoma resection (Table 6).
The tumor-free survival, overall survival and survival status of patients
We also collected data on the tumor-free survival, overall survival, and survival status of the patients in this study. The median tumor-free survival time for all patients was 9 months. The median tumor-free survival in the high regeneration group was 5 months (95% CI: 3.523–6.477). The median tumor-free survival in the low regeneration group was 16 months (95% CI: 11.569–20.431). The difference in tumor-free survival between the two groups was statistically significant (p < 0.05) as shown in Fig. 7.
The median overall survival time for all patients was 36 months. The median OS in the high regeneration group was 34 months (95% CI: 17.987–50.013), and the median OS in the low regeneration group was 36 months (95% CI: 32.617–39.383). The difference in overall survival between the two groups was not statistically significant (P > 0.05) (Fig. 8).
Discussion
The liver has a very powerful regenerative function. When the human liver suffers from acute liver injury, such as during hepatectomy, the remaining liver rapidly activates the regenerative function of the liver to compensate for the insufficient liver function caused by acute liver injury, and this response tends to satisfy the normal needs of the human body. Gong2, in his study of regenerative follow-up of hepatocellular carcinoma patients after hepatectomy, reported that the liver regeneration rate reached 21.3% in the first week of postoperative recovery and 30.9% in the fifth week. However, as time progressed, the liver regeneration rate appeared to decrease gradually, and eventually, the phenomenon reached a steady state in the 9th-13th weeks. In other words, the process of liver regeneration was ongoing during the two to three months after liver resection, but the fastest rate of liver regeneration occurred in approximately the first month. This is why we chose the first postoperative month as the time point for our enrolled patients; we rationalized our study not only according to the availability of clinical data but also because of the pattern of liver regeneration. At the same time, to better observe the pattern of liver regeneration, we chose patients who underwent hemihepatectomy. It has been proven that without the interference of other factors, a larger liver resection volume is more likely to result in more extreme liver regeneration after surgery. According to the literature, comparing anatomical resections of various types of livers has revealed a negative correlation between the postoperative SRLV and the rate of liver regeneration at 1 month postoperatively. In other words, to better observe liver regeneration, we selected patients with larger hemihepatectomy resection volumes as the study subjects.
In this study, univariate analysis revealed that the rate of liver regeneration after hepatectomy was significantly greater in female patients than in male patients, and sex may affect liver regeneration after hepatectomy. In the study of Shan et al.4, liver regeneration ability was found to be greater in females than in males after hepatectomy, suggesting that sex was an influencing factor of liver regeneration, consistent with the findings of this study. In addition, hepatectomy induces the secretion of estradiol, which acts on estrogen receptor α in hepatocytes to promote liver regeneration, and estrogen receptor α deficiency leads to slower hepatocyte proliferation and delayed liver regeneration5. In an experimental study by Joonyong et al.6, pregnancy altered molecular signaling pathways controlling hepatic regeneration by enhancing EGFR and STAT5 signaling, which contributed to hepatocytes rapidly entering the cell cycle and cell mitosis 12 h earlier than usual, thus restoring the original volume and mass of the liver. This suggests that increased estrogen levels may be the main reason for the promotion of liver regeneration during pregnancy. Similarly, Chaturantabut et al.7 reported that estradiol activated G-protein-coupled estrogen receptor 1 (GPER1) to regulate phosphatidylinositol 3-kinase (PI3K) and mTOR signaling, thereby promoting zebrafish liver growth and human hepatocyte proliferation. However, Ibis et al.8 found that sex was not an influential factor in liver regeneration in their study of living donor livers after hepatectomy. Notably, the mean age of female patients in this study was 56 years, suggesting that a substantial proportion were postmenopausal with potentially significant reductions in endogenous estrogen levels compared to premenopausal women. However, the lack of longitudinal monitoring of postoperative estrogen concentrations or dynamic changes in hepatic ERα/GPER1 receptor expression limits mechanistic exploration of observed sex-based differences. Potential explanations—such as enhanced hepatocyte sensitivity to low estrogen levels or compensatory activation of receptor signaling pathways—require validation through molecular assays (e.g., receptor phosphorylation profiling or estrogen-responsive gene analysis).Additionally, the study by Ibis et al.8 focused on healthy living liver transplant donors, whose baseline hepatic status (absence of cirrhosis or tumor microenvironment) fundamentally differs from HCC patients. This disparity in study populations may partially account for inconsistencies in conclusions regarding estrogen-mediated hepatoprotective effects.Based on the above studies, sex-related differences may arise from differences in estrogen levels and estrogen receptor levels, so whether sex affects liver regeneration after resection and the role of estrogen signaling in liver regeneration after hepatectomy require further investigation.
Excessive or long-term alcohol consumption is one of the major causes of cirrhosis, and excessive alcohol consumption promotes oxidative stress in hepatocytes and the accumulation of acetaldehyde and lipopolysaccharides in the liver, exacerbating hepatic injury9,10. Shi et al.11 demonstrated that ethanol not only inhibits the proliferation of adult hepatocytes but also inhibits the proliferation of hepatic progenitor cells and inhibits cell differentiation through Snail signaling, which in turn affects liver regeneration. Dubuquoy et al.12 also demonstrated that the efficiency of hepatic progenitor cell differentiation into mature hepatocytes is reduced and liver regeneration is impaired in patients with alcoholic hepatitis. In this study, the incidence of poor liver regeneration was significantly greater in patients with long-term alcohol consumption than in patients without a history of alcohol consumption, suggesting that long-term alcohol consumption is one of the factors affecting postoperative liver regeneration. Therefore, for patients with a history of long-term alcohol consumption, postoperative individualized therapeutic measures are a key step in preventing hepatic regenerative dysplasia.
The RLV is an important factor affecting the level of postoperative hepatic regeneration. Inoue et al.13 reported that the rate of residual liver regeneration was closely related to the amount of resected hepatic parenchyma, and within a certain range of RLV, the more resected the hepatic parenchyma, the more rapidly the postoperative hepatic regeneration rate increased. Along with the restoration of RLV, hepatic function was gradually restored, likewise consistent with our study. Mohapatra et al.14 also showed that a lower RLV promotes liver regeneration after hepatectomy. However, in major hepatectomies with less than 20% RLV, there may be a rapid progression to liver failure or even death because the residual liver cannot meet metabolic physiological requirements15. Therefore, within a certain range, the RLV was negatively correlated with the level of postoperative liver regeneration, and the smaller the RLV, the greater the level of early postoperative liver regeneration. Due to the differences in liver reserve function between individuals of different heights and weights, we standardized the residual liver volume between individuals using body surface area to reduce the error of interindividual comparison. Multivariate logistic regression analysis revealed that the SRLV was an independent risk factor affecting hepatic regenerative capacity after hemihepatectomy (P < 0.001), and Spearman correlation analysis revealed that the nerve regeneration rate was negatively correlated with the SRLV. Fathi et al.16 demonstrated that the expression of interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α), the main inflammatory factors that promote liver regeneration, was significantly elevated after 70% liver resection and that the level of hepatocyte growth factor (HGF) increased rapidly and was positively correlated with the growth of residual livers after stimulation by IL-6 and TNF-α. The degree to which inflammatory factors or cytokines are released after hepatectomy affects liver regeneration17. Animal studies have shown that liver regeneration fails after major hepatectomy in mice with IL-6 gene knockout, and studies on mice with combined injection of IL-6 and sIL-6R have confirmed that such injection can accelerate liver regeneration after hepatectomy18.
Hepatic fibrosis and cirrhosis due to persistent liver injury are closely related to liver reserve function and regeneration of postoperative residual liver tissue19. Aierken et al.20 demonstrated that the severity of hepatic fibrosis is an important predictor of liver regeneration and that the regenerative capacity of the liver decreases significantly with increasing severity of fibrosis. Jang et al.21 also demonstrated a negative correlation between the degree of liver stiffness measured by MRI and liver regeneration ability in patients with hepatocellular carcinoma undergoing right hepatectomy. In this study, multivariate logistic regression analysis revealed that postoperative liver regeneration was greater in patients without cirrhosis than in patients with cirrhosis, and the liver regeneration rate was lower in patients with cirrhosis, confirming that cirrhosis is an independent risk factor affecting regeneration of the residual liver after hepatectomy. This may be due to the excessive accumulation of extracellular matrix proteins in cirrhotic livers, which leads to the distortion of liver structure and the formation of fibrous scarring and regenerative nodules, resulting in altered liver function and increased resistance to hepatic blood flow. Portal vein overperfusion and poor hepatic venous return after hepatectomy lead to inadequate hepatic arterial perfusion, decreased hepatic oxygen supply, and decreased hepatic regenerative capacity. In addition, hepatic sinusoidal endothelial cells are a critical source of cytokines and growth factors required for hepatocyte proliferation. The capillarization of endothelial cells in the hepatic sinusoids of cirrhotic livers and the disappearance of the original fenestrum structure prevent the exchange of substances between hepatic sinusoids and hepatocytes, increasing the susceptibility of hepatocytes to ischemia and nutrient damage19. In a study by Tiberio et al.22, the rate of increase and the peak levels of TNF-α mRNA and IL-6 mRNA in patients suffering from cirrhosis after hepatectomy were much lower than those in normal liver, and the low level of IL-6 resulted in decreased hepatic DNA synthesis and limited hepatocyte proliferation. IL-6 injection has a pro-regenerative effect on cirrhotic livers. Therefore, considering the relatively slow liver regeneration after hepatectomy in cirrhotic livers, when performing major hepatectomy in patients with cirrhosis, the degree of hepatic fibrosis and hepatic reserve function should be carefully evaluated before surgery. This evaluation may enable patients at different stages of fibrosis to obtain safer and sufficient RLVs to meet physiological metabolic requirements and avoid the occurrence of hepatic failure after hepatectomy.
With respect to age, Pibiri et al.23 concluded that liver regenerative capacity decreases gradually with increasing age. Imamura et al.24 suggested that aging liver wounds lead to impaired liver function and poor liver regeneration after transplantation, so donor age should be taken into account during liver transplantation. However, the decline in hepatic regenerative capacity in elderly patients is caused by a variety of interacting intracellular and extracellular factors. In recent years, with the rapid development of digital medicine technology in the field of hepatic surgical techniques, the great improvement of perioperative management, the wide application of personalized precision medicine, and the relaxation of the indications for major hepatectomy in liver surgery, it is increasingly believed that advanced age is no longer an absolute contraindication for surgery. Yasuda et al.25, based on a study of liver regeneration after large hepatectomy in 41 patients with hepatocellular carcinoma, proposed that postoperative complications, survival time, and liver regeneration volume at 1 and 6 months after hepatectomy surgery did not differ between the older and younger groups; therefore, liver regeneration after hepatectomy is not affected by age. This is consistent with the results of this study.
Finally, in terms of the prognosis of patients after surgery, some studies have shown that large-volume liver resection may promote tumor growth26. In terms of the recurrence of hepatocellular carcinoma, some studies have shown that the vast majority of recurrent hepatocellular carcinoma lesions originate from microscopic metastasis. A study by Matsui S et al.27 also verified at the molecular level that increases in c-met, VEGFR-2 and other molecular components in the experimental body after surgery were significantly correlated with the volume of recurrent tumors as well as the aggressiveness of the tumors. Depending on the existing medical conditions, it is not only the presence of a tumor at the primary site but also the presence of tumor cells in the circulating blood or even the presence of micrometastases at other sites, that must be considered. Studies have shown that clinical operations such as biopsies and surgical resection not only have an impact on the primary site but can also accelerate the entry of tumor cells into the blood28,29. Similar conclusions have been reached in animal experiments. In the detection of with malignant lung tumors or malignant colon tumors in mice, we found that each gram of tumor tissue can release 105 to 106 malignant tumor cells into the blood, which is quite alarming30. After liver resection, the recurrence of tumors is mainly dependent on such micrometastases. Thus far, because of the existing medical conditions and technology, the detection of these micrometastases has been almost impossible to accomplish, and the occurrence and developmental mechanism of micrometastases have not been fully elucidated. The balance between proliferation and decline of tumor cells in completed hepatectomy patients may be disrupted, and a change in the dormancy of micrometastases could lead to tumor proliferation and recurrence. This dynamic balance is disrupted by the process of liver regeneration after hepatectomy. At the molecular level, a considerable number of molecules, such as HGF, angiogenic factors, growth factors, and matrix proteins, are involved in the regulation of tumor cell proliferation and decline31. These factors undergo considerable changes in the process of liver regeneration after hepatectomy. At the genetic level, tumor genes or metastasis suppressors can also be involved in regulating the status of micrometastases32.
Our center has established a nationally advanced laparoscopic minimally invasive surgery unit, achieving a 90% minimally invasive rate for Grade IV procedures and successfully transitioning from traditional open surgery to a minimally invasive paradigm. Through continuous refinement of surgical expertise under strict adherence to patient safety protocols, we have optimized both the precision and applicability of minimally invasive techniques. Retrospective data demonstrate that intraoperative blood loss in laparoscopic hepatectomies is consistently controlled below 200 mL, with a 20–30% reduction compared to open approaches (excluding converted cases). Open surgery, while associated with higher blood loss (typically 400–800 mL), remains indispensable for complex cases involving large tumors, vascular invasion, or extended resections (Table 7).
Key Lessons from 3D Reconstruction-Guided Hepatectomy:
Preoperative Imaging Optimization: Enhanced integration of contrast-enhanced CT and hepatobiliary-specific MRI (e.g., EOB-Primovist) improves tumor boundary delineation and vascular mapping. Discrepancies > 10% in liver volume measurements between modalities trigger recalibration or manual verification.
Intraoperative Validation: Real-time intraoperative ultrasound coupled with 3D model alignment corrects 10–15% of vascular trajectory deviations, minimizing critical structure injury.
Risk Stratification: A dual-threshold model (Fibrosis Index ≥ 3.25 and ICG-R15 > 10%) effectively reduces postoperative hepatic insufficiency. For HBsAg-positive patients without radiographic cirrhosis, routine intraoperative frozen biopsy guides resection planning.
Regeneration Monitoring: High-regeneration cohorts undergo biweekly serum HGF, IL-6, and AFP monitoring starting at postoperative week 1. A “1-1-3” imaging protocol (MRI/CT alternated at 1 month, 3 months, and quarterly intervals) enhances early recurrence detection.
Technological Integration: A PACS-3D modeling platform streamlines preoperative planning, while 3D-printed models and dynamic simulations improve patient comprehension of surgical risks.
Limitations and Innovations: Severe hepatic steatosis necessitates MRI-PDFF-based liver volume correction. Future initiatives include machine learning-driven HRR prediction tools (input: SRLV, liver stiffness, etc.) to preemptively identify high-risk patients.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Chen, Y. S. et al. Surgical resection significantly promotes the overall survival of patients with hepatocellular carcinoma: a propensity score matching analysis[J]. BMC Gastroenterol. 21 (1), 220. https://doi.org/10.1186/s12876-021-01807-4 (2021).
Gong, W. F. et al. Evaluation of liver regeneration and post-hepatectomy liver failure after hemihepatectomy in patients with hepatocellular carcinoma[J]. Biosci. Rep. 39 (8). https://doi.org/10.1042/bsr20190088 (2019).
Bedossa, P. & Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR cooperative study Group[J]. Hepatology 24 (2), 289–293. https://doi.org/10.1002/hep.510240201 (1996).
Shan, Y. S., Hsieh, Y. H., Sy, E. D., Chiu, N. T. & Lin, P. W. The influence of spleen size on liver regeneration after major hepatectomy in normal and early cirrhotic liver[J]. Liver Int. 25 (1), 96–100. https://doi.org/10.1111/j.1478-3231.2005.01037.x (2005).
Tsugawa, Y., Natori, M., Handa, H. & Imai, T. Estradiol accelerates liver regeneration through Estrogen receptor α[J]. Clin. Exp. Gastroenterol. 12, 331–336. https://doi.org/10.2147/ceg.S214196 (2019).
Lee, J., Garcia, V., Nambiar, S. M., Jiang, H. & Dai, G. Pregnancy facilitates maternal liver regeneration after partial hepatectomy[J]. Am. J. Physiol. Gastrointest. Liver Physiol. 318 (4), G772–g780. https://doi.org/10.1152/ajpgi.00125.2019 (2020).
Chaturantabut, S. et al. Estrogen Activation of G-Protein-Coupled Estrogen Receptor 1 Regulates Phosphoinositide 3-Kinase and mTOR Signaling To Promote Liver Growth in Zebrafish and Proliferation of Human Hepatocytes[J]1561788–1804e13 (Gastroenterology, 2019). 610.1053/j.gastro.2019.01.010
Ibis, C. et al. Factors affecting liver regeneration in living donors after Hepatectomy[J]. Med. Sci. Monit. 23, 5986–5993. https://doi.org/10.12659/msm.908136 (2017).
Kong, L. Z. et al. Pathogenesis, early diagnosis, and therapeutic management of alcoholic liver Disease[J]. Int. J. Mol. Sci. 20 (11). https://doi.org/10.3390/ijms20112712 (2019).
Ganesan, M., Eikenberry, A., Poluektova, L. Y., Kharbanda, K. K. & Osna, N. A. Role of alcohol in pathogenesis of hepatitis B virus infection[J]. World J. Gastroenterol. 26 (9), 883–903. https://doi.org/10.3748/wjg.v26.i9.883 (2020).
Shi, X. et al. Alcohol disrupts human liver stem/progenitor cell proliferation and Differentiation[J]. J. Stem Cell. Res. Ther. 4 (5). https://doi.org/10.4172/2157-7633.1000205 (2014).
Dubuquoy, L. et al. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis[J]. Gut 64 (12), 1949–1960. https://doi.org/10.1136/gutjnl-2014-308410 (2015).
Inoue, Y. et al. Volumetric and functional regeneration of remnant liver after Hepatectomy[J]. J. Gastrointest. Surg. 23 (5), 914–921. https://doi.org/10.1007/s11605-018-3985-5 (2019).
Mohapatra, N., Sinha, P. K., Sasturkar, S. V., Patidar, Y. & Pamecha, V. Preoperative Alanine aminotransferase and remnant liver volume predict liver regeneration after live donor Hepatectomy[J]. J. Gastrointest. Surg. 24 (8), 1818–1826. https://doi.org/10.1007/s11605-019-04332-8 (2020).
Memeo, R. et al. Optimization of the future remnant liver: review of the current strategies in Europe[J]. Hepatobiliary Surg. Nutr. 10 (3), 350–363. https://doi.org/10.21037/hbsn-20-394 (2021).
Fathi, F., Sanei, B., Ganjalikhani Hakemi, M., Saidi, R. F. & Rezaei, A. Liver Resection Promotes (Regulates) Proinflammatory Cytokines in Patients with Hepatocellular Carcinoma[J]. Can J Gastroenterol Hepatol, 2021: 5593655. (2021). https://doi.org/10.1155/2021/5593655
Sparrelid, E. et al. Serial assessment of growth factors associated with liver regeneration in patients operated with associating liver partition and portal vein ligation for staged Hepatectomy[J]. Eur. Surg. Res. 59 (1–2), 72–82. https://doi.org/10.1159/000488078 (2018).
Li, D., Wang, Z., Zhang, C. & Xu, C. IL-1R1 deficiency impairs liver regeneration after 2/3 partial hepatectomy in aged mice[J]. Turk. J. Biol. 45 (2), 225–234. https://doi.org/10.3906/biy-2010-51 (2021).
Zuñiga-Aguilar, E. & Ramírez-Fernández, O. Fibrosis and hepatic regeneration mechanism[J]. Transl Gastroenterol. Hepatol. 7, 9. https://doi.org/10.21037/tgh.2020.02.21 (2022).
Aierken, Y. et al. Liver fibrosis is a major risk factor for liver regeneration: A comparison between healthy and fibrotic liver[J]. Med. (Baltim). 99 (22), e20003. https://doi.org/10.1097/md.0000000000020003 (2020).
Jang, S. et al. Value of MR elastography for the preoperative Estimation of liver regeneration capacity in patients with hepatocellular carcinoma[J]. J. Magn. Reson. Imaging. 45 (6), 1627–1636. https://doi.org/10.1002/jmri.25517 (2017).
Tiberio, G. A. et al. IL-6 promotes compensatory liver regeneration in cirrhotic rat after partial hepatectomy[J]. Cytokine 42 (3), 372–378. https://doi.org/10.1016/j.cyto.2008.03.012 (2008).
Pibiri, M. Liver regeneration in aged mice: new insights[J]. Aging (Albany NY). 10 (8), 1801–1824. https://doi.org/10.18632/aging.101524 (2018).
Imamura, H. et al. A Donor Age-Based and Graft Volume-Based Analysis for Living Donor Liver Transplantation in Elderly Recipients[J]3e168 (Transplant Direct, 2017). 710.1097/txd.0000000000000688
Yasuda, S. et al. Liver regeneration after major liver resection for hepatocellular carcinoma in the Elderly[J]. J. Invest. Surg. 33 (4), 332–338. https://doi.org/10.1080/08941939.2018.1517839 (2020).
Umeda, Y. et al. Refractory response to growth factors impairs liver regeneration after hepatectomy in patients with viral hepatitis[J]. Hepatogastroenterology 56 (93), 971–977 (2009).
Matsui, S. et al. Clinical significance of aggressive hepatectomy for colorectal liver metastasis, evaluated from the HGF/c-Met pathway[J]. Int. J. Oncol. 37 (2), 289–297. https://doi.org/10.3892/ijo_00000677 (2010).
Floriani, I. et al. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis[J]. J. Magn. Reson. Imaging. 31 (1), 19–31. https://doi.org/10.1002/jmri.22010 (2010).
Wang, J. Y. et al. Molecular detection of Circulating tumor cells in the peripheral blood of patients with colorectal cancer using RT-PCR: significance of the prediction of postoperative metastasis[J]. World J. Surg. 30 (6), 1007–1013. https://doi.org/10.1007/s00268-005-0485-z (2006).
Joyce, J. A. & Pollard, J. W. Microenvironmental regulation of metastasis[J]. Nat. Rev. Cancer. 9 (4), 239–252. https://doi.org/10.1038/nrc2618 (2009).
Kisseleva, T., Gigante, E. & Brenner, D. A. Recent advances in liver stem cell therapy[J]. Curr. Opin. Gastroenterol. 26 (4), 395–402. https://doi.org/10.1097/MOG.0b013e32833a6bec (2010).
Wikman, H., Vessella, R. & Pantel, K. Cancer micrometastasis and tumour dormancy[J]. Apmis, 116(7–8): 754 – 70. (2008). https://doi.org/10.1111/j.1600-0463.2008.01033.x
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research was supported by National Natural Science Foundation of China (82073078); Natural Science Foundation of Shandong Province (ZR2022MH122); China Postdoctoral Foundation Project (2019M662304).
Author information
Authors and Affiliations
Contributions
HL: Writing – original draft & review & editing, Data curation, Investigation, Methodology. XG: Writing –review. XW: Validation, Visualization. LL: Writing –review. LT: Funding acquisition. BZ: Funding acquisition. Writing – review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Human Ethics and Consent to Participate declarations
This study meets the requirements of the Declaration of Helsinki, and all patients sign an informed consent form before surgery.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, H., Gai, X., Wang, X. et al. Analysis of the factors influencing liver regeneration after hepatectomy in hepatocellular carcinoma patients and the relationship between liver regeneration and prognosis. Sci Rep 15, 26874 (2025). https://doi.org/10.1038/s41598-025-11520-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11520-x