Abstract
Chronic kidney disease (CKD) is a significant global health issue associated with cardiovascular complications. While coffee, tea, and caffeine consumption have been linked to various health benefits, their relationship with CKD remains unclear. We conducted a cross-sectional analysis of NHANES 1999–2018 data, including 49,827 participants, assessing dietary intake through two 24-hour recalls and determining CKD status via the CKD-EPI equation. Our results showed that after adjusting for covariates, higher coffee consumption (> 352.5 g/day) was associated with lower odds of CKD (OR 0.760, 95% CI 0.701–0.823), as was tea consumption (OR 0.913, 95% CI 0.843–0.89). The highest tertile of caffeine consumption also showed a significantly reduced odds of CKD (OR 0.734, 95% CI 0.674–0.799). These findings suggest that coffee, tea, and caffeine consumption may be associated with better renal function, highlighting potential dietary strategies for CKD prevention.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a growing public health problem that significantly impacts patients’ quality of life and leads to higher rates of illness and premature death1,2,3. From 1990 to 2017, the global prevalence and mortality of CKD increased by 29.3% and 41.5%, respectively3. CKD can lead to renal dysfunction and progress to end-stage kidney disease and cardiovascular disease (CVD)4. In addition, CKD related complications can lead to the acceleration of disease progression and the increased risk of cardiovascular-related morbidity4. Multiple factors (i.e., alcohol use, smoking, and obesity) were found to be associated with the risk of CKD5,6. Recent research has increasingly focused on the relationship between dietary patterns and CKD risk7. As a crucial dietary component, the consumption of beverages is intricately intertwined with the preservation of overall health and renal function, as well as the modulation of high-risk factors for CKD progression (e.g., hypertension, obesity, and diabetes)7,8,9.
The association between coffee consumption and multiple health outcomes (i.e., stroke, hypertension, and CVD) has been extensively studied10,11. Multitude epidemiologic studies indicate that coffee may protect against various health outcomes, including hypertension, diabetes mellitus, and CVD10,11. The association between coffee consumption and CKD risk has been widely studied, while results are inconsistent. Previous studies reported that daily coffee intake was associated with a decreased risk of the development of CKD12,13. A cohort study found that coffee consumption was positively associated with CKD risk, but the association was not significant14. A meta-analysis suggested that coffee consumption might present potential harmful effects on kidney health15. Caffeine, a prominent bioactive compound found in coffee, has been reported to be associated with an increase in the estimated glomerular filtration rate (eGFR) and a reduced risk of developing CKD16. However, contrasting findings have also been reported where increased caffeine consumption did not significantly alter eGFR17.
Epidemiologic studies have also examined the association of tea consumption with CKD risk and the eGFR18,19,20. The majority of the studies indicated that increased tea consumption did not yield any significant impact on renal function improvement or delay in nephropathy progression14. However, a cohort study reported that oolong tea could promote the efficiency of eGFR compared to green tea and black tea19. In addition, it is reported that bioactive substances that existed in tea may improve risk factors for renal insufficiency, including blood pressure, oxidative stress, dyslipidemia, and insulin resistance or hyperglycemia21. In summary, whether tea consumption was inversely associated with CKD risk remains unclear.
The existing body of research has reported inconsistent results, making it difficult to draw definitive conclusions. Few studies have examined the relationship between different sources of caffeine intake and CKD risk. Most research has focused on coffee consumption’s impact on renal function, with only a handful examining the link between caffeine metabolites and renal function22. Moreover, earlier investigations into the long-term effects of caffeine have been hindered by the considerable error inherent in self-reported caffeine intake23. Urine caffeine metabolites are reliable markers for evaluating caffeine intake from food sources and can effectively avoid the above - mentioned limitations24.
To partially address these gaps, the present study adopts a cross-sectional design, utilizing data from the National Health and Nutrition Examination Survey (NHANES) 1999–2018. This allows us to assess the cross-sectional associations between coffee, tea, caffeine consumption and urine caffeine metabolites and CKD among adults in the United States.
Materials and methods
Data collection and study population
The data utilized in this study are obtained from the National Health and Nutrition Examination Survey (NHANES). NHANES was conducted by the Centers for Disease Control and Prevention of America, which is a two-year-cycle cross-sectional survey and aims at evaluating the health and nutritional status of American population25. Initially, participants were interviewed at their home to gather essential background information, such as socio-demographic details, medical history, and family history. Later on, they attended a mobile examination center (MEC) to provide additional data such as anthropometric measurements, blood pressure, laboratory tests, and other relevant information.
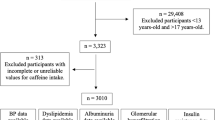
Ten cycles (1999–2000, 2001–2002, 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, 2017–2018) data included this study were downloaded from the NHANES website. A total of 101,316 participants from 1999 to 2018 enrolled in the survey. We excluded participants based on the following criteria: aged under 20 years old (n = 42,112), incomplete or unreliable CKD data (n = 4,134), pregnant (n = 1,575), and extreme total energy intakes (< 500 or > 8000 kcal/day for men, < 500 or > 5000 kcal/day for women) (n = 3,668)26. After exclusions,49,827 individuals were included in the final analysis (Fig. 1). NHANES was approved by the National Center for Health Statistics Research Ethics Review Board.
Chronic kidney disease assessment
CKD was defined as eGFR < 60 ml/min/1.73m2 or persistent albuminuria27. The eGFR was calculated using the 2021 chronic kidney disease-Epidemiology Collaboration Eq. (2021 CKD-EPI): eGFR = 142 × min(SCr/κ,1)^α × max(SCr/κ,1)^−1.209 × 0.9938^Age × 1.012 [if female], where SCr is serum creatinine in mg/dL, κ is 0.7 for females and 0.9 for males, α is −0.241 for females and − 0.303 for males, min denotes the minimum of SCr/κ or 1, max indicates the maximum of SCr/κ or 1, and Age is in years27,28. Albuminuria was defined as albumin-to-creatinine ratio ≥ 30 mg/g27,28. We did not define persistent proteinuria for most participants only measured once in the survey, and for the timing of second urine collection varied (first morning void vs. random) when albuminuria was repeated during selected cycles of NHANES29.
Exposure assessment
Frequency of coffee consumption, tea consumption, total caffeine consumption, caffeine from coffee, and caffeine from tea was obtained from two 24-hour dietary recall interviews, which were conducted by trained interviewers using an automated data collection system during the MEC examination30. Consumption of total caffeine and caffeine from tea and coffee was segmented into tertiles. Consumption of coffee and caffeine from coffee was divided into three categories. Participants with no consumption of coffee and caffeine from coffee were classified into group 1 (intake = 0), and individuals with consumption were divided into group 2 (< median) and group 3 (≥ median) based on its median intake (g/d) among the participants with consumption. Intake of tea and caffeine from tea was categorized into two groups. No consumption of tea and caffeine from tea was classified into group 1 (intake = 0), individuals with consumption were divided into group 2.
The measurement of urinary caffeine and its metabolite levels has been suggested as a reliable method for evaluating caffeine consumption31. Thus, we also evaluated the association of urinary caffeine metabolites and CKD using three cycles (2009–2010, 2011–2012, 2013–2014) data. All NHANES participants were required to provide urine samples in a mobile examination center (MEC). Ultra-high performance liquid chromatography-electrospray ionization-tandem quadrupole mass spectrometry was employed for the analysis of caffeine and its metabolites32including 1-methyluric acid (1U), 3-methyluric acid (3U), 7-methyluric acid (7U), 1,3-dimethyluric acid (13U), 1,7-dimethyluric acid (17U), 3,7-dimethyluric acid (37U), 1,3,7-trimethyluric acid (137U), 1-methylxanthine (1X), 3-methylxanthine (3X), 7-methylxanthine(7X), theophylline(13X), paraxanthine(17X), theobromine(37X), caffeine(137X) and5-acetylamino-6-amino-3-methy luracil (AAMU). Spearman correlation analysis revealed that all 15 metabolites exhibited a positive correlation with caffeine intake. Notably, 9 of these metabolites demonstrated a moderate positive correlation with caffeine intake (Spearman r, 0.501–0.578, P < 0.001) (Table S1). Therefore, we only assessed the relationship between 9 urinary caffeine metabolites and CKD. Bonferroni correction was applied to account for multiple comparisons, with a significance level of P < 0.05/9 for the nine metabolites assessed.
Covariates
Information of socio-demography, lifestyle, and health related factors had been collected by an interviewer using the Sample Person and Family Demographics questionnaires. Data included sex (male and female), age (20–39 years, 40–59 years, and ≥ 60 years), race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, and other races), educational level (below high school, High school, and above high school), marital status (married/living with partner, and widowed/divorced/separated/never married), body mass index (BMI) (normal: <25 kg/m2, overweight: 25 to < 30 kg/m2, obese: ≥30 kg/m2), physical activity (vigorous, moderate, and other), family income (Under $20,000, and $20,000 and over), smoking behavior (smoked at least 100 cigarettes in life or not), hypertension prevalence rate, diabetes mellitus prevalence rate, alcohol use (had at least 12 alcohol drinks/year or not), and total energy intake. We used the “dietaryindex” R package to calculate the AHEI based on dietary information33.
Statistical analysis
Statistical analysis was conducted using Stata 15.0 (Stata Corporation, College Station, TX). A new sample weight (the original 2-year sample weight divided by 2) had been constructed according to the analytical guidelines of NHANES when combing ten 2-year cycles of the continuous data34. In brief, we utilize the 4-year sample weights provided by NCHS instead of the 2-year sample weights. We conducted the Kolmogorov–Smirnov normality test to test the normality of continuous variables, and we described continuous variables with mean ± standard deviation (SD) (normally distributed) or median (interquartile range) (non-normally distributed). Student’s t-test (normally distributed) and the Mann–Whitney U test was used to examine the differences of continuous variable between the CKD group and the non-CKD group. Chi-square tests were chosen to examine the difference of categorical variables between the different groups.
In this study, we conducted binary logistic regression analyses to evaluate the association of coffee consumption, tea consumption, caffeine consumption and urinary caffeine and caffeine metabolite with CKD. Two models had been adopted to evaluate the association of coffee consumption, tea consumption, and caffeine consumption with CKD. Model 1, adjusted for age and sex; model 2 (fully adjusted model), adjusted for age, sex, race, marital status, educational level, family income, body mass index, physical activity, smoking status, alcohol consumption, hypertension, diabetes, alternative healthy eating index and energy intake. In addition, we performed subgroup analyses stratified by sex (male and female) and age (< 60 and 60~) to test sex and age as an interaction with coffee consumption, tea consumption, and caffeine consumption in the model that adjusted for the same covariates. As hypertension and diabetes may lie on the causal pathway between coffee/tea consumption and CKD, we conducted a sensitivity analysis excluding them. In addition, liner regression was conducted to evaluate the association of coffee consumption, tea consumption, and caffeine consumption with eGFR. A two-sided p < 0.05 was considered statistically significant.
Results
General characteristics
The baseline characteristics are presented in Table 1. A total of 49,827 individuals (mean age: 47.9 ± 19.2 years old) was included in this study, among whom 8,554 (17.2%) were diagnosed with CKD. Participants with CKD demonstrated a higher likelihood of being older, female, non-Hispanic Black individuals, having a lower educational attainment, being widowed/divorced/separated/never married, overweight, physically inactive, possessing lower family income and exhibiting a higher prevalence of hypertension and diabetes compared to those without CKD. Additionally, individuals with CKD reported higher coffee consumption but lower caffeine and energy intake.
The association of coffee consumption with CKD
There was a significant inverse association between coffee consumption and CKD (Table 2). In model 2, compared to individuals who reported no coffee consumption, those who reported consuming 0.01–352.5 g/day exhibited an odds ratio (OR) of 0.918 (95% confidence interval [CI]: 0.845–0.998; P = 0.044), while those who consumed more than 352.5 g/day had an OR of 0.760 (95% CI: 0.701–0.823; P < 0.001). Similarly, the OR for CKD was 0.902(95%CI :0.868–0.938; P < 0.001) in response to per SD increment in coffee consumption. The findings of the analyses, which were stratified by sex and age, exhibited consistent patterns (Fig. 2). A statistically significant association between coffee consumption and CKD was observed in females (P interaction = 0.021). Furthermore, a stronger association between coffee consumption and CKD was found among participants aged over 60 years (P interaction = 0.001). The results remained stable after sensitivity analysis.
Association of coffee, tea, and caffeine consumption with chronic kidney disease. The ORs were adjusted for potential confounders, including age (in sex group), sex (in age group), race, marital status, educational level, family income, body mass index, physical activity, smoking status, alcohol consumption, hypertension, diabetes, alternative healthy eating index and energy intake. The interaction terms were evaluated through likelihood tests. CI, confidence interval; OR, odds ratio. P interaction indicates the test for interaction terms by subgroup variables (sex and age).CI, confidence interval; OR, odd ratio. P interaction indicates the test for interaction terms by subgroup variables (sex and age).
The association of tea consumption with CKD
We observed a significant inverse association between tea consumption and CKD (Table 2). Compared to those who reported no tea consumption, individuals who consumed tea had a multivariate-adjusted OR of 0.913 (95% CI: 0.843–0.989; P = 0.025) for CKD. In addition, the OR for CKD was 0.955(95%CI:0.919–0.993; P = 0.020) in response to per SD increment in tea consumption. Stratification analyses based on sex and age consistently demonstrated similar patterns as the primary analysis (Fig. 2). Notably, no significant interaction effect of sex or age on the association between tea consumption and CKD was observed (P interaction > 0.05). The results remained stable after sensitivity analysis (Table S2).
The association of caffeine consumption with CKD
Caffeine consumption showed a significant inverse association with CKD (Table 2). Compared to individuals reporting a consumption of less than 44.5 g/day, those who reported consuming 44.5–153.0 g/day had an OR of 0.933 (95%CI: 0.862–1.010; P = 0.054), while those consuming more than 153.0 g/day had an OR of 0.734 (95% CI: 0.634–0.799; P < 0.001). Similarly, the OR for CKD was 0.876(95%CI: 0.840–0.914; P < 0.001) in response to per SD increment in caffeine consumption. The inverse association remained significant in the subgroup analyses stratified by sex and age (Fig. 2). Sex acted as a modifier to the effect of caffeine on CKD (P interaction = 0.031), while age did not modify the association (P interaction = 0.453). The results remained stable after sensitivity analysis (Table S2).
We found that coffee-sourced caffeine was significantly associated with CKD (OR for 1 SD increment:0.901, 95%CI: 0.865–0.938, P = 0.001) (Table 2). Sex serves as effect modifiers in the relationship between caffeine from coffee consumption and CKD (P interaction = 0.029) (Fig. 2). Tea-sourced caffeine was slightly associated with CKD (OR for 1 SD increment:0.948, 95%CI:0.911–0.987, P = 0.010) (Table 2). The association between tea-sourced caffeine and CKD was modified by age (P interaction = 0.001) (Fig. 2). In addition, an inverse association was observed between caffeine intake from tea and coffee and the odds of CKD (OR for 1 SD increment:0.886, 95%CI:0.851–0.923, P < 0.001) (Table 2). In the stratified analyses, a significantly stronger association was observed among females and older individuals (P interaction < 0.05) (Fig. 2). The results remained stable after sensitivity analysis (Table S2).
Associations of tea, coffee and caffeine intakes with eGFR
The associations between tea, coffee, and caffeine intakes with eGFR are presented in Table 3. A statistically significant increase of 0.229 in eGFR per 100 mg/day increment in total caffeine consumption was observed (β = 0.229, P < 0.001). Similarly, a statistically significant increase of 0.242 per 100 mg/day increment in eGFR was found for caffeine derived from coffee and tea consumption (β = 0.242, P < 0.001). Furthermore, an increase of 0.273 per 100 mg/day increment in eGFR was noted for caffeine sourced specifically from coffee (β = 0.273, P < 0.001). Additionally, each additional intake of coffee by the amount of 100 g/day was associated with a modest increase of eGFR by approximately 0.067 units. However, no significant association between tea or tea-sourced caffeine and eGFR was observed (all P > 0.05).
Association of urinary caffeine metabolites and CKD
We also evaluated the association between urinary caffeine metabolites and CKD (Table S3). We observed an inverse relationship between 17X and CKD risk (OR: 0.994, 95%CI: 0.990–0.999, P = 0.023), when accounting for multiple comparisons, the association no longer remains statistically significant. No significant associations were found between other urinary caffeine metabolites and CKD.
Discussion
In the present large cross-sectional study of US adults, there was a significant inverse association between consumption of coffee, tea and caffeine and CKD. These associations were robustly observed even after adjusted for potential mediators such as hypertension, and diabetes. Additionally, our findings suggest a positive correlation between the consumption of total caffeine, coffee-derived caffeine, and coffee with eGFR levels.
The protective effect of coffee on renal function has been a topic of extensive research. Our findings align with previous studies that have reported a beneficial role of coffee in renal health12,35. However, the literature is not entirely consistent, with some studies showing no significant association between coffee consumption and CKD risk14,18. This discrepancy may be attributed to differences in study design, population characteristics, and the control of confounding variables. A meta-analysis included four observational studies reported no impact of coffee consumption on CKD risk in males but found a decreased risk in females36. Another meta-analysis of cohort study reported that consumption of coffee was significantly associated with a lower risk of CKD37. In addition, a Mendelian Randomization (MR) study investigated the impact of coffee on the renal function and reported a beneficial effect of coffee consumption on renal function38. These findings collectively suggest that coffee may have a protective effect, particularly in certain subpopulations.
The beneficial effect of coffee may be attributed to the anti-oxidant and anti-inflammatory properties of coffee39,40. Oxidant stress (OS) is characterized by the pro-/antioxidant balance, could influence the function of cells and tissues due to the excessive generation of highly reactive oxygen (ROS)41. The kidney is an important metabolic organ, rich in oxidation reactions in mitochondria, which makes it vulnerable to damage caused by OS, and OS can accelerate the development of kidney disease41. Coffee contains various compounds, including hydroxyhydroquinone, and caffeine, which have known anti-oxidant effects42. In addition, increasing evidence showed that coffee exerted the protective effect by against OS39. A randomized controlled trail study showed that coffee consumption might increase the antioxidant capacity of plasma43. Given the role of OS in CKD development and the anti-inflammatory properties of coffee, it is plausible that coffee may contribute to CKD prevention.
The association between tea consumption and renal function has been extensively studied, while the results are inconsistent. A cohort study reported that there was no significant association between tea consumption and the change of eGFR18while another cohort study reported that high tea consumption was related to a decline of eGFR in 1 year44. In our study, we found that tea consumption might decrease the risk of CKD, whereas a study reported that there was no significant association between high tea consumption and CKD risk14. In addition, a MR study demonstrated that causal relationships existed between increased tea consumption and reduced risk of CKD and albuminuria and increased eGFR45. The protective effect of tea is likely due to its rich content of bioactive substances, such as phenolic compounds and minerals with antioxidant and anti-inflammatory properties46. Tea catechins, including epallocateigchin-3-gallate (EGCG), epigallocatechin, and epicatechin, may improve endothelial function and arterial vasodilation by reducing ROS levels47,48. These catechins act as metal ion chelators and ROS scavengers, and they induce antioxidant enzymes while inhibiting pro-oxidant enzymes47,48. Several animals and in vitro studies suggested that EGCG had potential effects on the renal function. Animal studies have shown that EGCG can ameliorate renal damage through antioxidant, anti-inflammatory, and apoptosis-inducing effects49,50.
Our study also observed an inverse association between caffeine consumption and CKD risk, as well as a positive association with eGFR levels. These results are supported by previous research showing that genetically predicted caffeine intake is associated with increased eGFR and reduced CKD risk16,51. The modulation of changes in eGFR by caffeine has been suggested to occur through diuresis and natriuresis via binding adenosine receptors, interference with the anti-inflammatory effects of adenosine, as well as stimulation of key proliferative mechanisms involved in glomerular remodeling and sclerosis52. This suggests that caffeine’s effects on renal function may be multifaceted, involving both hemodynamic and metabolic pathways.
The main advantage of this study is the data source. The data from NHANES with continuous quality assurance and quality control processes, therefore the data are timely and of high quality. They have employed population-based cluster random selection to identify a sample that is nationally representative, thus the data from NHANES can be extrapolated to the entire US population. Additionally, we controlled wide ranges of potential confounders to provide a better estimation of the association of coffee, tea, and caffeine intake with CKD risk.
Our study has several limitations. First, the cross-sectional design precludes establishing causality and exploring the temporal dynamics of the associations between tea, coffee, or caffeine consumption and CKD risk. To address this, we reviewed existing longitudinal studies and meta-analyses that support the robustness of our findings across different study designs. Second, dietary data were obtained from two 24-hour recall interviews, which may not accurately reflect long-term dietary intake. Future studies should consider using multiple 24-hour recalls or food diaries to better capture long-term dietary patterns. Additionally, dietary recording apps could enhance data accuracy and continuity. Finally, we did not account for variations in caffeine content due to differences in coffee bean type, origin, and processing methods. Future studies should consider these factors to provide a more comprehensive understanding of the relationship between coffee consumption and CKD risk.
Clinical implications
Our findings have several clinical implications. Given the inverse association between coffee, tea, and caffeine consumption and CKD risk, healthcare providers may consider discussing these dietary factors with patients at risk for kidney disease. Encouraging moderate consumption of coffee or tea could potentially serve as a simple, low-cost adjunct to traditional CKD prevention strategies. However, it is important to consider individual patient factors and not to replace established treatments. Further research is needed to determine optimal consumption levels and to confirm associations these in diverse populations.
Conclusions
In conclusion, we found that tea, coffee or caffeine consumption was inversely associated with CKD, which may be attributed to the anti-oxidant and anti-inflammatory properties of the bioactive substances of coffee and tea. Our study may unravel the effect of coffee and tea consumption on CKD development. Large-scale, prospective studies are warranted to further validate the protective effects of tea, coffee or caffeine consumption on CKD risk in adults.
Data availability
The original data were retrieved from https://www.cdc.gov/nchs/nhanes/index.htm. All data generated or analysed during this study are included in this published article.
References
Xie, Y. et al. Analysis of the global burden of disease study highlights the global, regional, and National trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 94, 567–581. https://doi.org/10.1016/j.kint.2018.04.011 (2018).
Shi, Z. et al. Association between dietary patterns, cadmium intake and chronic kidney disease among adults. Clin. Nutr. 37, 276–284. https://doi.org/10.1016/j.clnu.2016.12.025 (2018).
Collaboration, G. B. D. C. K. D. Global, regional, and National burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 395, 709–733. https://doi.org/10.1016/S0140-6736(20)30045-3 (2020).
Evans, M. et al. A narrative review of chronic kidney disease in clinical practice: current challenges and future perspectives. Adv. Ther. 39, 33–43. https://doi.org/10.1007/s12325-021-01927-z (2022).
Zelle, D. M. et al. Physical inactivity: a risk factor and target for intervention in renal care. Nat. Rev. Nephrol. 13, 152–168. https://doi.org/10.1038/nrneph.2016.187 (2017).
Xia, J. et al. Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrol. Dial Transpl. 32, 475–487. https://doi.org/10.1093/ndt/gfw452 (2017).
Cosola, C., Sabatino, A., di Bari, I., Fiaccadori, E. & Gesualdo, L. Nutrients, nutraceuticals, and xenobiotics affecting renal health. Nutrients 10 https://doi.org/10.3390/nu10070808 (2018).
Pereira, M. A. Diet beverages and the risk of obesity, diabetes, and cardiovascular disease: a review of the evidence. Nutr. Rev. 71, 433–440. https://doi.org/10.1111/nure.12038 (2013).
Cirillo, M. et al. Relationship of the intake of water and other beverages with renal endpoints: Cross-Sectional and longitudinal Data-Observational, Population-Based study. J. Ren. Nutr. 32, 68–77. https://doi.org/10.1053/j.jrn.2021.06.013 (2022).
Chei, C. L., Loh, J. K., Soh, A., Yuan, J. M. & Koh, W. P. Coffee, tea, caffeine, and risk of hypertension: the Singapore Chinese health study. Eur. J. Nutr. 57, 1333–1342. https://doi.org/10.1007/s00394-017-1412-4 (2018).
Chieng, D. & Kistler, P. M. Coffee and tea on cardiovascular disease (CVD) prevention. Trends Cardiovasc. Med. 32, 399–405. https://doi.org/10.1016/j.tcm.2021.08.004 (2022).
Jhee, J. H. et al. Effects of Coffee Intake on Incident Chronic Kidney Disease: A Community-Based Prospective Cohort Study. Am J Med 131, 1482–1490 e1483 (2018). https://doi.org/10.1016/j.amjmed.2018.05.021
Tang, L. et al. Sex-specific association between coffee consumption and incident chronic kidney disease: a population-based analysis of 359,906 participants from the UK biobank. Chin. Med. J. (Engl). 135, 1414–1424. https://doi.org/10.1097/CM9.0000000000002234 (2022).
Gaeini, Z., Bahadoran, Z., Mirmiran, P. & Azizi, F. Tea, coffee, caffeine intake and the risk of cardio-metabolic outcomes: findings from a population with low coffee and high tea consumption. Nutr. Metab. (Lond). 16, 28. https://doi.org/10.1186/s12986-019-0355-6 (2019).
He, W. J. et al. Metabolites associated with coffee consumption and incident chronic kidney disease. Clin. J. Am. Soc. Nephrol. 16, 1620–1629. https://doi.org/10.2215/CJN.05520421 (2021).
Giontella, A. et al. Caffeine intake, plasma caffeine level, and kidney function: A Mendelian randomization study. Nutrients 15 https://doi.org/10.3390/nu15204422 (2023).
Mazidi, M. et al. The association between coffee and caffeine consumption and renal function: insight from individual-level data, Mendelian randomization, and meta-analysis. Arch. Med. Sci. 18, 900–911. https://doi.org/10.5114/aoms/144905 (2022).
Herber-Gast, G. C. et al. Coffee and tea consumption in relation to estimated glomerular filtration rate: results from the population-based longitudinal Doetinchem cohort study. Am. J. Clin. Nutr. 103, 1370–1377. https://doi.org/10.3945/ajcn.115.112755 (2016).
van Hasselt, T. J. et al. Effects of tea consumption on renal function in a metropolitan Chinese population: the Guangzhou biobank cohort study. J. Ren. Nutr. 24, 26–31. https://doi.org/10.1053/j.jrn.2013.08.006 (2014).
Lew, Q. J., Jafar, T. H., Jin, A., Yuan, J. M. & Koh, W. P. Consumption of coffee but not of other Caffeine-Containing beverages reduces the risk of End-Stage renal disease in the Singapore Chinese health study. J. Nutr. 148, 1315–1322. https://doi.org/10.1093/jn/nxy075 (2018).
Dou, Q. P. Tea in health and disease. Nutrients 11 https://doi.org/10.3390/nu11040929 (2019).
Fujii, R., Nakatochi, M., Del Greco, M. F. & Coffee Intake Plasma caffeine levels, and kidney function: Two-Sample Mendelian randomization among East Asian and European ancestries. Kidney Int. Rep. 9, 1083–1092. https://doi.org/10.1016/j.ekir.2024.01.024 (2024).
Weng, Z. et al. Association of urinary caffeine and caffeine metabolites with cardiovascular disease risk in adults. Nutrition 84, 111121. https://doi.org/10.1016/j.nut.2020.111121 (2021).
Tan, N., Shen, W., Tang, M., Liu, M. & Zhang, Y. Association between urine caffeine metabolites and bone mineral density: A population-based study. Med. (Baltim). 102, e35674. https://doi.org/10.1097/md.0000000000035674 (2023).
National Health and Nutrition Examination Survey Questionnaires, Datasets, and Related Documentation., < https://wwwn.cdc.gov/nchs/ (.
Dong, X., Li, S., Sun, J., Li, Y. & Zhang, D. Association of coffee, decaffeinated coffee and caffeine intake from coffee with cognitive performance in older adults: National health and nutrition examination survey (NHANES) 2011–2014. Nutrients 12 https://doi.org/10.3390/nu12030840 (2020).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
Inker, L. A. et al. New Creatinine- and Cystatin C-Based equations to estimate GFR without race. N Engl. J. Med. 385, 1737–1749. https://doi.org/10.1056/NEJMoa2102953 (2021).
Saydah, S. H. et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National health and nutrition examination survey, 2009–2010. Clin. Chem. 59, 675–683. https://doi.org/10.1373/clinchem.2012.195644 (2013).
The Examination Protocol and Data Collection Methods., https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/manuals.aspx?BeginYear=2017/2017_MEC_In-Person_Dietary_Interviewers_Manual.pdf (.
Rybak, M. E., Sternberg, M. R., Pao, C. I., Ahluwalia, N. & Pfeiffer, C. M. Urine excretion of caffeine and select caffeine metabolites is common in the U.S. Population and associated with caffeine intake. J. Nutr. 145, 766–774. https://doi.org/10.3945/jn.114.205476 (2015).
Long, L. & Tang, Y. Urine caffeine metabolites and hearing threshold shifts in US adults: a cross-sectional study. Sci. Rep. 11, 21631. https://doi.org/10.1038/s41598-021-01094-9 (2021).
Zhan, J. J. et al. Dietaryindex: a user-friendly and versatile R package for standardizing dietary pattern analysis in epidemiological and clinical studies. Am. J. Clin. Nutr. 120, 1165–1174. https://doi.org/10.1016/j.ajcnut.2024.08.021 (2024).
National Health and Nutrition Examination Survey Tutorials. Module 3: Weighting., < https://wwwn.cdc.gov/nchs/nhanes/tutorials/module3.aspx (.
Kim, B. H., Park, Y. S., Noh, H. M., Sung, J. S. & Lee, J. K. Association between coffee consumption and renal impairment in Korean women with and without diabetes: analysis of the fourth Korea National health and nutrition examination survey in 2008. Korean J. Fam Med. 34, 265–271. https://doi.org/10.4082/kjfm.2013.34.4.265 (2013).
Wijarnpreecha, K., Thongprayoon, C., Thamcharoen, N., Panjawatanan, P. & Cheungpasitporn, W. Association of coffee consumption and chronic kidney disease: A meta-analysis. Int. J. Clin. Pract. 71 https://doi.org/10.1111/ijcp.12919 (2017).
Srithongkul, T. & Ungprasert, P. Coffee consumption is associated with a decreased risk of incident chronic kidney disease: A systematic review and Meta-analysis of cohort studies. Eur. J. Intern. Med. 77, 111–116. https://doi.org/10.1016/j.ejim.2020.04.018 (2020).
Kennedy, O. J. et al. Coffee consumption and kidney function: A Mendelian randomization study. Am. J. Kidney Dis. 75, 753–761. https://doi.org/10.1053/j.ajkd.2019.08.025 (2020).
Martini, D. et al. Coffee consumption and oxidative stress: A review of human intervention studies. Molecules 21 https://doi.org/10.3390/molecules21080979 (2016).
Kolb, H., Kempf, K. & Martin, S. Health effects of coffee: mechanism unraveled?? Nutrients 12 https://doi.org/10.3390/nu12061842 (2020).
Daenen, K. et al. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 34, 975–991. https://doi.org/10.1007/s00467-018-4005-4 (2019).
Gomez-Ruiz, J. A., Leake, D. S. & Ames, J. M. In vitro antioxidant activity of coffee compounds and their metabolites. J. Agric. Food Chem. 55, 6962–6969. https://doi.org/10.1021/jf0710985 (2007).
Agudelo-Ochoa, G. M. et al. Coffee consumption increases the antioxidant capacity of plasma and has no effect on the lipid profile or vascular function in healthy adults in a randomized controlled trial. J. Nutr. 146, 524–531. https://doi.org/10.3945/jn.115.224774 (2016).
Diaz-Lopez, A. et al. Consumption of caffeinated beverages and kidney function decline in an elderly mediterranean population with metabolic syndrome. Sci. Rep. 11, 8719. https://doi.org/10.1038/s41598-021-88028-7 (2021).
Zhang, Y. et al. Causal association between tea consumption and kidney function: A Mendelian randomization study. Front. Nutr. 9, 801591. https://doi.org/10.3389/fnut.2022.801591 (2022).
Hodgson, J. M. & Croft, K. D. Tea flavonoids and cardiovascular health. Mol. Aspects Med. 31, 495–502. https://doi.org/10.1016/j.mam.2010.09.004 (2010).
Lee, M. J. et al. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol. Biomarkers Prev. 11, 1025–1032 (2002).
Bernatoniene, J. & Kopustinskiene, D. M. The role of catechins in cellular responses to oxidative stress. Molecules 23 https://doi.org/10.3390/molecules23040965 (2018).
Thangapandiyan, S. & Miltonprabu, S. Epigallocatechin gallate supplementation protects against renal injury induced by fluoride intoxication in rats: role of Nrf2/HO-1 signaling. Toxicol. Rep. 1, 12–30. https://doi.org/10.1016/j.toxrep.2014.01.002 (2014).
Luo, D. et al. -)-Epigallocatechin-3-gallate (EGCG) attenuates salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Sci. Rep. 10, 4783. https://doi.org/10.1038/s41598-020-61794-6 (2020).
Ma, Q., Song, S. & Xu, G. Inverse association between caffeine intake and albuminuria in US adults: an analysis of NHANES 2005–2016. Acta Clin. Belg. 78, 438–445. https://doi.org/10.1080/17843286.2023.2222243 (2023).
Marx, B., Scuvée, É., Scuvée-Moreau, J., Seutin, V. & Jouret, F. [Mechanisms of caffeine-induced diuresis]. Med. Sci. (Paris). 32, 485–490. https://doi.org/10.1051/medsci/20163205015 (2016).
Acknowledgements
The authors sincerely thank the NHANES of the CDC for providing the publicly available data.
Author information
Authors and Affiliations
Contributions
Panpan Gao and Zengli Yu were responsible for study design, data acquisition and interpretation, statistical analysis, and writing and editing of the manuscript. Xinrong Ji and Weiwei wang were responsible for drawing figure; Yao Chen and Zhan Gao aided in data acquisition and interpretation, statistical analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study used de-identified, nationally representative survey data that are publicly available upon request to the NHANES. The patients/participants provided their written informed consent to participate in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, P., Ji, X., Wang, W. et al. Association between coffee and caffeine consumption and chronic kidney disease. Sci Rep 15, 25228 (2025). https://doi.org/10.1038/s41598-025-11543-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11543-4