Abstract
This study aimed to investigate the therapeutic effects and radiographic results of percutaneous kyphoplasty (PKP) in patients with osteoporotic vertebral compression fractures (OVCFs) with or without intravertebral clefts (IVCs). Clinical data from 288 patients with OVCFs who underwent PKP surgery were retrospectively analyzed and stratified into the liquid-IVC group, gas-IVC group, and non-IVC group through magnetic resonance imaging. The baseline characteristics revealed that the liquid-IVC group included older patients and a greater proportion of male participants (P < 0.05). All patients reported pain relief, vertebral height (VH) improvement, and kyphotic angle correction immediately after PKP surgery (P < 0.05). The bone cement distribution score was significantly lower (P < 0.05) in the liquid-IVC group than in the other two groups, but the VH improvement rate (VHIR), VH change rate (VHCR) and recollapse rate were significantly greater (P < 0.05). Moreover, the non-IVC group demonstrated a significantly lower incidence of postoperative bone cement leakage than the other two groups did (P < 0.05). However, the VH and Cobb angle significantly decreased in all groups during the follow-up period (P < 0.05). Further analysis revealed a significant correlation between bone cement distribution and IVC (by evaluating the injury zone score) in the liquid-IVC group (R2 = 0.371, P < 0.05), whereas no significant difference was found in the other two groups (R2 = −0.123, P = 0.218). In conclusion, PKP surgery is effective in treating OVCFs with or without IVCs, but liquid-filled IVCs exhibit a worse therapeutic effect on radiographic parameters than gas-filled IVCs and non-IVCs during follow-up. Patients with liquid-filled IVCs also have limited and IVC-related bone cement distribution.
Similar content being viewed by others
Introduction
Intravertebral clefts (IVCs) frequently occur in patients with osteoporotic vertebral compression fractures (OVCFs) and have been extensively studied for over a century1. These clefts typically present as low-density cavities within the vertebral body on X-ray images and computed tomography (CT) scans2,3 and are considered as classic symptoms of vertebral avascular necrosis and Kummell’s disease4,5. Advancements in radiologic technology, particularly the increasing utilization of magnetic resonance imaging (MRI), have increased the detection rates of IVCs, with the incidence of IVCs reported to be 10–48%6,7. IVCs are characterized primarily by a well-defined high or low signal on T2-weighted MR images8,9. Liquid-filled IVCs exhibit low signal intensity on T1-weighted images and high signal intensity on T2-weighted images, whereas gas-filled IVCs display low signal intensity on both imaging modalities3,4,5.
Percutaneous kyphoplasty (PKP) is widely acknowledged as a safe and effective minimally invasive intervention for OVCFs10,11. Robust clinical evidence has demonstrated that PKP can alleviate the symptoms of OVCF patients with IVCs12,13,14. Nevertheless, the available data reveal divergent long-term outcomes between IVC-positive and IVC-negative cohorts13,14. Studies have revealed therapeutic differences across IVC subtypes, specifically between liquid-filled clefts and gas-filled clefts15. Surprisingly, few analyses have been performed to compare PKP efficacy in IVC-negative patients with that in liquid-filled and gas-filled patients.
In addition, studies have reported that variations in IVC morphology are associated with bone cement distribution patterns16,17. Radiographic data indicate positional concordance between the IVC and cement distribution, which limits cement distribution and compromises structural stability and long-term clinical outcomes18,19. However, the aforementioned studies did not specifically determine the manner in which IVC affects the distribution of bone cement.
To address these uncertainties, we compared clinical and radiological outcomes across three cohorts: OVCF patients with (1) fluid-filled IVCs, (2) gas-filled IVCs, and (3) non-IVCs, to investigate the differences in bone cement distribution and long-term efficacy among OVCF patients with various types of IVCs following PKP surgery. Through simulation of a computational image retrieval framework20,21, we aimed to quantify the positional congruence between the cement distribution and IVC location. The results indicate that different IVC types affect the distribution of bone cement, providing reference data that will guide future clinical practice.
Methods
Patient selection and grouping
The study was approved by the Ethics Committee of the Affiliated Hospital of Southwest Medical University (KY2023328) and was conducted in accordance with the relevant guidelines and regulations. Since this was a retrospective radiographic study, the Ethics Committee of the Affiliated Hospital of Southwest Medical University waived the requirement of written informed consent. A total of 288 patients who underwent PKP from June 2019 to December 2023 were retrospectively enrolled. Patients were divided into three groups on the basis of whether they had IVCs and the type of IVCs on MRI: the liquid-IVC group (n = 74), the gas-IVC group (n = 112), and the non-IVC group (n = 102). The inclusion criteria were as follows: (1) History of severe low back pain. (2) Radiographic examination suggesting acute or subacute OVCF. (3) Presence of a single vertebral collapse. (4) Bone mineral density (BMD) ≤ -2.5. (5) A minimum follow-up duration if 6 months. (6) Stratification criteria were as follows3,4: (A) Liquid-IVC group: The IVCs exhibit low signal intensity on T1-weighted images and high signal intensity on T2-weighted images (Fig. 1). (B) Gas-IVC group: The IVCs exhibit low signal intensity on both T1- and T2-weighted imaging (Fig. 2). (C) Non-IVC group: no IVCs are shown on MRI. The exclusion criteria were as follows: patients with (1) symptoms of dural sac or nerve tissue compression; (2) severe comorbid diseases; (3) clinical or radiographic diagnosis of infection or vertebral malignancy; and (4) multiple vertebral fractures. All patients’ stratification and radiographic measurements were performed in a double-blinded fashion by two experienced spine surgeon (both 10 years of experience in spine surgery) independently.
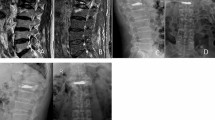
Radiological analyses of a 64-year-old female. A sagittal radiograph demonstrated a recollapse of an augmented vertebra during follow-up. (A–C) Radiography and computed tomography images showing an L1 compression fracture. (D–F) Fat saturation, T1W, and T2W MR images showing liquid-filled IVCs. (G,H) Immediate postoperative sagittal radiograph showing dispersive cement and re-expansion of the compressed vertebrae. (I,J) Severe recollapse developed at the 6-month follow-up.
Radiological analyses of a 72-year-old female. A sagittal radiograph demonstrated a recollapse of an augmented vertebra during follow-up. (A–C) Radiography and computed tomography images showing an L1 compression fracture. (D–F) Fat saturation, T1W, and T2W MR images showing gas-filled IVCs. (G,H) Immediate postoperative sagittal radiograph showing dispersive cement and re-expansion of the compressed vertebrae. (I,J) Recollapse developed at the 6-month follow-up.
Surgical procedure
All patients underwent the procedure in a prone position under local anesthesia with 1% lidocaine. The surgery was conducted by using a C-arm to locate the injured vertebra. The puncture point was positioned 4–5 mm lateral to the transverse process of the pedicle. A puncture needle was directed toward the medial edge of the pedicle, after which the needle body was gradually advanced. Lateral radiographs were used to confirm that the puncture needle had reached the central region of the anterior third of the vertebral body. The puncture needle was subsequently replaced with a working cannula, through which a balloon was introduced into the collapsed vertebra. A radiopaque medium was then injected to restore the vertebral height and correct kyphosis until satisfactory outcomes were achieved. Thereafter, the balloon was deflated and removed, and the cavity was filled with polymethylmethacrylate cement. All patients were discharged 3 days postsurgery and instructed to use a brace to avoid physical strain for 1 month. No contralateral cement injections were administered to the patients.
Outcome measures
Clinical parameters
The baseline information and clinical parameters of the patients included age, sex, duration, BMD, body mass index (BMI), trauma history, operative time, and number of fluoroscopies. Visual analog scale (VAS) scores were assessed preoperatively and postoperatively. The VAS score was used to evaluate pain intensity and efficacy.
Radiographic parameters
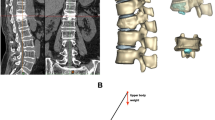
All patients underwent X-ray (ED150L X-ray machine, Shimadzu Corporation), CT (Philips iCT, 0.625 mm per layer, Koninklijke Philips Electronics N.V.), and MRI (Magneom Skyra 3.0 T, 3 mm per layer, SIEMENS AG.) scans before the surgical procedure. Postoperative radiographs were evaluated at two time points: one day after the operation and 6 months after the operation. The vertebral height (VH) was measured as the mean of the anterior VH (AVH), middle VH (MVH), and posterior VH (PVH) (Fig. 3). The VH and Cobb angle were measured at the X-ray workstation (iMedPacs Dicom Viewer) preoperatively, postoperatively, and 6 months after the operation. The VH improvement rate (VHIR) and the VH change rate (VHCR) were subsequently calculated using the following formulae: VHIR = (preoperative VH−postoperative VH)/(0-preoperative VH) × 100%; VHCR = (follow-up VH−postoperative VH)/(0-postoperative VH) × 100%. Recollapse of the augmented vertebrae was considered when the VHCR was > 20%. All the parameters were compared among the three groups.
Measurements of radiology parameters. (A,B) Postoperative sagittal and coronal X-ray images revealed a bone cement distribution score of 8 (3 (sagittal quadrants) + 3 (coronal quadrants) + 2 (endplate)). (C,D) Measurement of the VH and Cobb angle. The AVH, MVH, PVH, and Cobb angle were measured via sagittal X-ray to determine the improvement ratio and kyphotic deformity. VH = (AVH + MVH + PVH)/3. VH vertebral height, AVH anterior vertebral height, MVH median vertebral height, PVH posterior vertebral height.
Injury zone score and bone cement distribution evaluation
The distribution of bone cement was determined according to the augmented vertebra in the coronal and sagittal X-ray images. Vertebra are divided into quadrants based on the anteroposterior and lateral positions. If one-third or more of a quadrant was filled with cement, it was considered an “effective quadrant.” If the bone cement touched the upper or lower endplates on the sagittal radiographs, each endplate touched by the bone cement was considered an independent effective quadrant. The number of effective distribution quadrants was described as the distribution of bone cement (4 + 4 + 2 = 10)22 (Fig. 3).
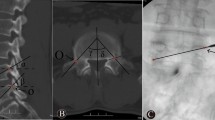
To calculate the injury zone score, the injured vertebra was divided into four quadrants according to the T2-weighted sagittal MR images and calculated as corresponding scores for each quadrant. The score was calculated if the signal-changed area passed through the quadrant. Each quadrant was assigned a numerical value: the upper left quadrant was assigned 1 point, the upper right quadrant 2 points, the lower left quadrant 3 points, and the lower right quadrant 4 points. If the signal change area extended through a given quadrant, the corresponding score for that quadrant was calculated. The total score for the entire damaged area ranged from 0 to 10 points (1 + 2 + 3 + 4 = 10). The same calculation was applied for the sagittal radiographs after the operation as for the sagittal bone cement distribution; the corresponding score was calculated if bone cement had filled one-third of a quadrant: 1 point for the upper left, 2 points for the upper right, 3 points for the lower left, and 4 points for the lower right (Fig. 4). The relationship between the injury zone score and the sagittal bone cement distribution was assessed.
Injury zone scoring and sagittal bone cement distribution. On sagittal X-ray and sagittal MRI T2-weighted images, the vertebral body is divided into left and right halves at the midpoint of its upper and lower endplates. Additionally, at the midpoints of the anterior and posterior edges, the vertebral body is further divided into upper and lower halves, resulting in the segmentation of the vertebral body into four quadrants. Each quadrant is individually assigned a value, which are subsequently summed to calculate the overall score. (A) MRI-T2 weighted images show that a gas-filled intravertebral cleft led to an injury zone score of 6 (1 (upper left) + 2 (upper left) + 3 (lower right)). (B) Post-operative sagittal X-ray images led to a bone cement distribution score of 6 (1 (upper left) + 2 (upper left) + 3 (lower left)).
Statistical analysis
SPSS version 27.0 (Chicago, IL, USA) was used for all the data analyses. The normality of the quantitative data was assessed by using the Kolmogorov‒Smirnov method. Levene’s test is used to assess the homogeneity of variances. Normally distributed data were analyzed in three groups by using single factor analysis of variance (ANOVA), and the least significant difference (LSD) test was used for multiple comparisons. Nonnormally distributed data were analyzed in three groups with the Kruskal‒Wallis test. The Mann‒Whitney test (nonnormally distributed) and t test (normally distributed) were used for comparisons between two groups. Pearson correlation analysis was used to analyze the correlation between the injury zone score and the sagittal bone cement distribution. The chi-square test was used for qualitative data. Nonnormally distributed data were presented as medians, whereas normally distributed data were expressed as the means ± standard deviations (x̄ ± s). P < 0.05 was considered statistically significant.
Results
Baseline characteristics and clinical outcomes of patients
No significant differences were found between the three groups in terms of disease course, BMD, BMI, or any other baseline feature (P > 0.05), except for age and sex. The liquid-IVC group (age: 75.34 ± 9.46, male: 42%) had a significantly greater mean age and a much greater proportion of male patients than did the gas-IVC (age: 71.22 ± 8.45, male: 20%) and non-IVC groups (age: 71.57 ± 9.9, male: 21%) (P < 0.05, Table 1). No significant difference in age or sex was found between the gas-IVC and non-IVC groups. The damaged segments and typical patients are presented in Figs. 1, 2 and 5 All the baseline characteristics of the patients are presented in Table 1.
No statistically significant differences were found among the three groups in terms of operative time or number of fluoroscopies (Table 2). The median postoperative VAS score was significantly lower (2, 2, 2; Table 3) than the preoperative score (7, 7, 7; Table 3) in all three groups, with no significant differences observed among the three groups pre- or postoperatively (Table 3).
Radiographic outcomes
No significant differences in bone cement volume or injury zone score were found among the three groups (P > 0.05). However, the liquid-IVC group (total: 7, sagittal: 6) presented a significantly lower total and sagittal bone cement distribution than the other two groups did (P < 0.05, Table 2), with no significant difference between the gas-IVC (total: 9, sagittal: 10) and non-IVC groups (total: 9, sagittal: 10) (P > 0.05). Additionally, the liquid-IVC group (VHIR: 11.39%, VHCR: 13.39%, recollapse rate: 32%) presented higher VHIR, VHCR, and recollapse rates than did the gas-IVC group (VHIR: 8.5%, VHCR: 6.98%, recollapse rate: 11%) and non-IVC group (VHIR: 6.29%, VHCR: 1.96%, recollapse rate: 9%) (P < 0.05, Table 2). No significant differences were found between the gas-IVC and non-IVC groups in terms of the VHIR and recollapse rates (P > 0.05). The VHCR was significantly greater in the gas-IVC group than in the non-IVC group (P < 0.05, Table 2). Moreover, the rate of bone cement leakage was significantly lower in the non-IVC group (18%) than in the liquid-IVC (34%) and gas-IVC groups (36%) (P < 0.05, Table 2). No procedure-related complications were recorded during the study, and none of the patients developed acute or delayed clinical or neurological symptoms during the follow-up.
Compared with the preoperative values, the postoperative VH and Cobb angle improved significantly in all groups (P < 0.05, Table 4). However, these improvements significantly decreased by the follow-up period (P < 0.05, Table 4). The non-IVC group (preoperative VH: 2.05 ± 0.40, follow-up VH: 2.10 ± 0.40; preoperative Cobb angle: 10.83 ± 5.01, follow-up Cobb angle: 9.01 ± 4.49) had the highest VH and the lowest Cobb angle compared with those of the liquid-IVC (preoperative VH: 1.77 ± 0.37, follow-up VH: 1.75 ± 0.33; preoperative Cobb angle: 13.46 ± 7.62, follow-up Cobb angle: 12.47 ± 6.40) and gas-IVC (preoperative VH: 1.82 ± 0.38, follow-up VH: 1.84 ± 0.37; preoperative Cobb angle: 14.33 ± 7.14, follow-up Cobb angle: 12.53 ± 5.76) groups preoperatively and at follow-up (P < 0.05, Table 4), and no significant differences were found between the liquid-IVC and gas-IVC groups (P > 0.05). A significant difference in the VH and Cobb angle was found between the gas-IVC (postoperative VH: 2.02 ± 0.35; postoperative Cobb angle: 9.83 ± 5.25) and non-IVC (postoperative VH: 2.21 ± 0.38; postoperative Cobb angle: 7.72 ± 3.75) groups postoperatively (P < 0.05, Table 4), whereas the liquid-IVC group (postoperative VH: 2.11 ± 0.72; postoperative Cobb angle: 8.64 ± 5.54) showed no significant differences in the VH and Cobb angle compared with the gas-IVC and non-IVC groups (P > 0.05).
Correlation outcome
A strong positive correlation was observed between injury zone scores and sagittal bone cement distribution in the liquid-IVC group (R2 = 0.371, P < 0.05). No significant correlations were found in the gas-IVC group (R2 = 0.151, P = 0.112) or the non-IVC group (R2 = −0.123, P = 0.218) (Fig. 6).
Discussion
OVCFs involving IVCs, initially identified as prominent radiolucency (gas-containing) in the vertebrae visible on CT or plain radiographs, are often considered typical symptoms of avascular necrosis (Kummell’s disease)1,23,24. Malghem et al.4 reported that clefts filled with gas or liquid were evident on T2 images, exhibiting low signal intensity on T1-weighted images and high signal intensity on T2-weighted images when filled with liquid and low signal intensity on both imaging modalities when filled with gas3,5. Recent studies have shown that IVCs may manifest as avascular necrosis (Kummell’s disease) and nonunion and pseudoarthrosis in OVCFs25.
The present study investigated the therapeutic effects of PKP on OVCFs with or without IVCs via MRI. The results demonstrated that PKP provided satisfactory short-term therapeutic effects, regardless of the presence or absence of various types of IVCs in the vertebrae. Patients across all three groups experienced significant improvements in pain relief, vertebral height restoration, and kyphotic angle correction immediately after PKP surgery, which is consistent with previous reports15,26. However, during long-term follow-up, patients in the three groups exhibited a gradient difference in vertebral height maintenance, with the liquid-IVC group showing the poorest outcomes and the non-IVC group exhibiting the best outcomes (Tables 2 and 4). This observation supports the findings of Yu et al.27 that the presence of IVCs contributes to the recollapse of augmented vertebrae following PKP. Notably, when a > 20% loss of vertebral height at follow-up was used as a criterion for vertebral recollapse28, the liquid-IVC group presented a significantly greater rate of recollapse than did the other two groups, with a statistically significant difference. This disparity may be influenced by several factors. One possible explanation could be that the formation of liquid-filled IVCs is frequently associated with nonunion of vertebral injury, indicating more severe microvascular damage within the vertebrae than in gas-filled IVCs, as well as more rapid osteoporosis29. Another possible explanation may be the limited bone cement distribution in patients with liquid-filled IVCs.
Analysis of the baseline data revealed that OVCF patients with liquid type IVCs had a greater mean age at onset than did those with gas type IVCs and without IVCs. Additionally, the proportion of males was greater in the liquid-IVC group than in the other two groups. Analysis of the radiographic data revealed that OVCF patients with IVCs had a lower mean VH before surgery than did those without IVCs. These findings may be attributed to the fact that older patients, particularly elderly men, generally exhibit greater pain tolerance30,31, often opting for conservative treatments postinjury. This may lead to repeated injury, contributing to the formation of IVCs or Kummell’s disease, ultimately causing more significant vertebral collapse. Notably, studies on Kummell’s disease have reported a greater incidence in men than in those with OVCFs32. However, the present study revealed no significant difference in the disease course among the three groups. This may be because patients neglect their initial vertebral injuries and delay medical consultation; thus, only the acute phase at the time of treatment is recorded15. Moreover, Oka et al.18 reported that the location of the affected vertebrae and the occurrence of IVCs were primarily concentrated at the thoracolumbar junction, which aligns with our findings.
Among the radiographic outcomes, patients in the liquid-IVC group presented poorer bone cement distribution, in both overall and the sagittal plane, which is consistent with the findings of Tang et al.15. A study by Oka et al.18 demonstrated that the distribution of bone cement may be limited by IVCs, with sagittal postoperative X-rays indicating spatial correspondence between cement dispersion patterns and preoperative IVC morphology/location observed on sagittal MR images. This finding is consistent with observations reported by Xiong et al.16, who noted that when bone cement was injected into vertebrae with IVCs, the cement was confined near the IVCs, promoting the formation of solid-pattern cement. Therefore, we modeled our approach on a computer image retrieval algorithm20,21 to simplify the conversion of images into data for quantitative analysis. Our findings revealed that the distribution of bone cement was significantly influenced by the location of IVCs in the liquid-IVC group. In contrast, the distribution of bone cement in the sagittal plane was more randomized in the gas-IVC and non-IVC groups following PKP. This finding also explains why the liquid-IVC group experienced greater vertebral height loss during follow-up. Bone cement was restricted to the area surrounding IVCs because of the existence of IVCs, resulting in limited distribution and the formation of solid-pattern cement with poor long-term efficacy33. This reduced the contact between the bone cement and trabecular bone, thereby weaking the structural support, leading to progressive vertebral height loss during follow-up34. The limited distribution of bone cement may be due to the formation of fibrous perichondrium in the liquid-filled IVCs and the surrounding sclerotic zone, and whether intraoperative disruption of the sclerotic zone may improve the distribution of bone cement and potentially enhance its long-term effectiveness warrants further investigation.
Our study also revealed that patients in the liquid-IVC group experienced greater vertebral height restoration during PKP than those in the gas-IVC and non-IVC groups did, which is consistent with the results of Niu et al.35 and could be another factor contributing to the greater degree of vertebral height loss in the liquid-IVC group than in the other two groups during follow-up. Studies have demonstrated that greater initial vertebral height restoration during PKP is associated with a greater degree of vertebral height loss during follow-up36. In addition, the VH and Cobb angles were significantly different between the gas-IVC group and the non-IVC group postoperatively. This discrepancy may be attributed to the significantly lower VH and Cobb angle observed in the gas-IVC group than in the non-IVC group before surgery, although both groups achieved similar degrees of restoration.
Furthermore, the liquid-IVC and gas-IVC groups presented higher rates of bone cement leakage than did the non-IVC group. Tang et al.37 reported that IVCs were significantly correlated with cortical nonunion or defects at the vertebral walls or endplates. Similarly, Wang et al.38 proposed that a connection between IVCs and the basivertebral foramen may lead to direct cement leakage. A recent meta-analysis by Wu et al.39 revealed that IVCs were the primary determinants of bone cement leakage. These findings are consistent with our observations that the connection between IVCs and endplate, cortical bone, or basivertebral foramen defects may result in bone cement injection, leading to leakage into the intervertebral discs and extraspinal regions.
The available findings demonstrate that the short-term efficacy of PKP surgery is comparable in the treatment of OVCFs with or without IVCs15,40. Furthermore, given the current lack of clarity regarding the pathophysiological mechanisms responsible for the formation of liquid or gas within IVCs41, it is imperative to conduct further research into the pathological mechanisms of gas and liquid formation. This includes investigating the molecular pathways involved in IVCs formation to develop treatment strategies to prevent IVCs occurrence. Additionally, it is worth exploring whether disrupting the fibrocartilaginous layer covering IVCs during clinical surgery could increase the diffusion of bone cement in patients with IVC, thereby offering insights for optimizing clinical management.
In summary, this study which focused on bone cement distribution and long-term outcomes in OVCF patients with different different types of IVCs undergoing PKP surgery confirmed that PKP surgery is effective for treating OVCFs with or without IVCs. However, in OVCFs with liquid-filled IVCs, bone cement distribution was mostly confined to the area around the IVC—resulting in a limited cement distribution pattern—while simultaneously exhibiting excessive vertebral height restoration and increased recollapse risk compared to the other two groups, ultimately leading to the worst long-term outcome between three groups. In such cases, appropriate surgical timing for patients with liquid-filled IVCs is necessary.
Nevertheless, this study has several limitations. Firstly, this was a retrospective design with short-term follow-up and missing follow-up data on BMD, which may influence the findings. Secondly, since MRI was not performed in the coronal or axial plane, we only evaluated and compared the injury zone and the distribution of bone cement in the sagittal plane. Thirdly, CT imaging is superior to X-ray imaging in evaluating the cement distribution. Therefore, prospective, randomized controlled trials utilizing omnidirectional MRI scanning and postoperative CT scanning are necessary to further elucidate the impact of IVCs on bone cement distribution.
Conclusion
In summary, PKP is effective in treating OVCFs with or without IVCs. However, patients with liquid-filled IVCs had worse therapeutic effects on radiographic parameters than did those with gas-filled IVCs and non-IVCs patients during follow-up. The type of IVCs is an important factor that influences recollapse after vertebral augmentation. Patients with liquid-filled IVCs may have limited and IVC-related bone cement distribution. Therefore, careful surgical timing, rigorous observation and extended follow-up are strongly recommended for these patients, especially patients with liquid-filled IVCs.
Data availability
Correspondence and requests for materials should be addressed to S.W.
References
Kümmell, H. Ueber die traumatischen Erkrankungen der Wirbelsäule1). DMW Dtsch. Med. Wochenschr. 21, 180–181 (1895).
Libicher, M. et al. The intravertebral vacuum phenomen as specific sign of osteonecrosis in vertebral compression fractures: results from a radiological and histological study. Eur. Radiol. 17, 2248–2252 (2007).
Linn, J., Birkenmaier, C., Hoffmann, R. T., Reiser, M. & Baur-Melnyk, A. The intravertebral cleft in acute osteoporotic fractures: Fluid in magnetic resonance imaging-vacuum in computed tomography?. Spine 34, E88–E93 (2009).
Maldague, B. E., Noel, H. M. & Malghem, J. J. The intravertebral vacuum cleft: A sign of ischemic vertebral collapse. Radiology 129, 23–29 (1978).
Theodorou, D. J. The intravertebral vacuum cleft sign. Radiology 221, 787–788 (2001).
Matzaroglou, C. et al. Kümmell’s disease: Is ischemic necrosis or vertebral “microcracking” the first step in the sequence?. Med. Hypotheses 80, 505 (2013).
Li, Z. et al. The therapeutic effects of percutaneous kyphoplasty on osteoporotic vertebral compression fractures with or without intravertebral cleft. Int. Orthop. 43, 359–365 (2019).
Kim, Y.-C., Kim, Y.-H. & Ha, K.-Y. Pathomechanism of intravertebral clefts in osteoporotic compression fractures of the spine. Spine J. 14, 659–666 (2014).
Yu, C. W., Hsu, C. Y., Shih, T. T. F., Chen, B. B. & Fu, C. J. Vertebral osteonecrosis: MR imaging findings and related changes on adjacent levels. AJNR Am. J. Neuroradiol. 28, 42–47 (2007).
Berenson, J. et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 12, 225–235 (2011).
Huang, Y. et al. The top 100 most-cited articles on kyphoplasty and vertebroplasty. World Neurosurg. 135, e435–e446 (2020).
Cho, C. H. et al. Guideline summary review: An evidence-based clinical guideline for the diagnosis and treatment of adults with osteoporotic vertebral compression fractures. Spine J https://doi.org/10.1016/j.spinee.2025.01.016 (2025).
Yu, W. et al. Efficacy and safety of the target puncture technique for treatment of osteoporotic vertebral compression fractures with intravertebral clefts. J. NeuroIntervent. Surg. 9, 1113–1117 (2017).
Liu, H. et al. Percutaneous kyphoplasty in patients with severe osteoporotic vertebral compression fracture with and without intravertebral cleft: A retrospective comparative study. Int. J. Gen. Med. 15, 6199–6209 (2022).
Tang, J., Liu, J., Gu, Z. & Song, B. Outcomes of augmentation in osteoporotic vertebral compression fractures showing a cleft sign on MRI. Cardiovasc. Intervent. Radiol. 44, 428–435 (2021).
Xiong, Y. et al. Refracture of the cemented vertebrae after percutaneous vertebroplasty: risk factors and imaging findings. BMC Musculoskelet. Disord. 22, 459 (2021).
Lv, N. et al. Study on the influence of balloon dilation mode on the intravertebral cleft of osteoporotic fracture. BMC Surg. 22, 351 (2022).
Oka, M., Matsusako, M., Kobayashi, N., Uemura, A. & Numaguchi, Y. Intravertebral cleft sign on fat-suppressed contrast-enhanced MR. Acad. Radiol. 12, 992–999 (2005).
Lv, N.-N. et al. Does the relationship between bone cement and the intravertebral cleft of kummell disease affect the efficacy of PKP?. World Neurosurg. 160, e430–e435 (2022).
Agrawal, S., Chowdhary, A., Agarwala, S., Mayya, V. & Kamath, S. S. Content-based medical image retrieval system for lung diseases using deep CNNs. Int. J. Inf. Technol. 14, 3619–3627 (2022).
Hussain, C. A., Rao, D. V. & Mastani, S. A. RetrieveNet: a novel deep network for medical image retrieval. Evol. Intell. 14, 1449–1458 (2021).
Liu, J., Tang, J., Zhang, Y., Gu, Z. & Yu, S. Percutaneous vertebral augmentation for osteoporotic vertebral compression fracture in the midthoracic vertebrae (T5–8): A retrospective study of 101 Patients with 111 fractured segments. World Neurosurg. 122, e1381–e1387 (2019).
Resnick, D., Niwayama, G., Guerra, J., Vint, V. & Usselman, J. Spinal vacuum phenomena: anatomical study and review. Radiology 139, 341–348 (1981).
Gou, P. et al. Magnetic resonance imaging negative spine trauma followed by a delayed intravertebral vacuum cleft–Kümmell’s disease: A case report and literature review. Orthop. Surg. 15, 366–370 (2023).
Kim, D. Y., Lee, S. H., Jang, J. S., Chung, S. K. & Lee, H. Y. Intravertebral vacuum phenomenon in osteoporotic compression fracture: report of 67 cases with quantitative evaluation of intravertebral instability. J. Neurosurg. Spine 100, 24–31 (2004).
Yu, W. et al. Intravertebral vacuum cleft and its varied locations within osteoporotic vertebral compression fractures: Effect on therapeutic efficacy. Pain Phys. 20, E979–E986 (2017).
Yu, W. et al. The therapeutic effect of intravertebral vacuum cleft with osteoporotic vertebral compression fractures: A systematic review and meta-analysis. Int. J. Surg. 40, 17–23 (2017).
Liu, J. et al. A novel and convenient method to evaluate bone cement distribution following percutaneous vertebral augmentation. Sci. Rep. 10, 16320 (2020).
Qi, H. et al. Bone microarchitecture and metabolism in elderly male patients with signs of intravertebral cleft on MRI. Eur. Radiol. 32, 3931–3943 (2022).
Templeton, K. J. Sex and gender issues in pain management. J. Bone Jt. Surg. 102, 32–35 (2020).
Mullins, S., Hosseini, F., Gibson, W. & Thake, M. Physiological changes from ageing regarding pain perception and its impact on pain management for older adults. Clin. Med. 22, 307–310 (2022).
Steel, H. H. Kümmell’s disease. Am. J. Surg. 81, 161–167 (1951).
Lv, B. et al. Clinical efficacy of different bone cement distribution patterns in percutaneous kyphoplasty: A retrospective study. Pain Phys. 23, E409–E416 (2020).
Skripitz, R. & Aspenberg, P. Attachment of PMMA cement to bone: force measurements in rats. Biomaterials 20, 351–356 (1999).
Niu, J. et al. Percutaneous kyphoplasty for the treatment of osteoporotic vertebral fractures with intravertebral fluid or air: A comparative study. Clin. Spine Surg. Spine Publ. 30, 367–373 (2017).
Zhu, S. et al. Risk factors of cemented vertebral refracture after percutaneous vertebral augmentation: a systematic review and meta-analysis. Neuroradiology 62, 1353–1360 (2020).
Tang, B. et al. The impact of intravertebral cleft on cement leakage in percutaneous vertebroplasty for osteoporotic vertebral compression fractures: a case-control study. BMC Musculoskelet. Disord. 22, 805 (2021).
Wang, C. et al. Basivertebral foramen could be connected with intravertebral cleft: a potential risk factor of cement leakage in percutaneous kyphoplasty. Spine J. 14, 1551–1558 (2014).
Wu, Y. et al. Risk factors for cement leakage after percutaneous vertebral augmentation for osteoporotic vertebral compression fractures: a meta-analysis. Int. J. Surg. 111, 1231–1243 (2025).
He, W. et al. Effects of percutaneous kyphoplasty for the treatment of thoracic osteoporotic vertebral compression fractures with or without intravertebral cleft in elderly patients. Int. J. Gen. Med. 17, 193–203 (2024).
Lu, Y. et al. Unraveling the genetic basis of the causal association between inflammatory cytokines and osteonecrosis. Front. Endocrinol. 15, 1344917 (2024).
Acknowledgements
This work is jointly funded by the Natural Science Foundation of Sichuan (NO.2023NSFSC0333), the Sichuan Science and Technology Program (NO.2022-YFS0628) and the Strategic Cooperation Project of Luzhou Municipal People’s Government—Southwest Medical University (NO.2024LZXNYDJ031).
Author information
Authors and Affiliations
Contributions
Among the authors in the list, SW conceived and designed the study and gave final approval to the article. YZN co-designed the study, analyzed the data, and drafted the manuscript. SX made critical reviews and revisions to the manuscript for important intellectual content. YZN and SYX share the first authorship, due to equal contributions. YZN, SX and SW collected the data. YZN and YXS drew the images and analyzed the data. All the authors gave permission to publish this article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ning, Y., Sun, Y., Xu, S. et al. Impact of intravertebral cleft types on percutaneous kyphoplasty outcomes in osteoporotic vertebral compression fractures. Sci Rep 15, 26559 (2025). https://doi.org/10.1038/s41598-025-11749-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-11749-6