Abstract
Kappaphycus alvarezii (Doty) L. M. Liao, a red seaweed widely cultivated for carrageenan polysaccharide, is also a potential source of the valuable pigment phycoerythrin (PE). Therefore, this study aims to extract phycoerythrin from K. alvarezii, evaluate its antimicrobial, antioxidant, and anticancer activities, and identify its biomedical potential for future therapeutic applications. The protein content of phycoerythrin pigment extracted from K. alvarezii was found to be 69.84% and showed excellent antimicrobial activity against Klebsiella oxytoca and Proteus mirabilis, with a minimum inhibition zone of 11 mm. It showed significant in vitro antioxidant activity, as analyzed using total antioxidant, hydrogen peroxide scavenging, reducing power, DPPH, and ABTS assays. Further, the pigment exhibited potent cytotoxicity against a human lung cancer cell line, with an IC50 value of 131.7 µg mL−1. Furthermore, increasing concentration of phycoerythrin pigment decreased the cell proliferation and induced apoptosis, as confirmed by Annexin V/PI staining. Comprehensive characterization using FT-IR, HPLC, and GC-MS analysis revealed the nature of pigment and functional groups, highlighting its potential for biomedical applications. The molecular docking of K. alvarezii-derived compounds revealed significant binding affinities with 13 antibacterial target proteins. These results highlight the potential of K. alvarezii bioactive compounds as promising antibacterial agents. The phycoerythrin extract from K. alvarezii demonstrated potent antimicrobial, antioxidant, and anticancer properties, with significant cytotoxicity against lung cancer cells and confirmed apoptosis induction. Structural analysis revealed its bioactive composition, emphasizing its potential as a natural therapeutic agent. These findings support its potential application in the biomedical and pharmaceutical industries.
Similar content being viewed by others
Introduction
Marine organisms produce a diversified array of bioactive compounds including carbohydrates, lipids, proteins, polyphenols, minerals, amines, amides, antioxidants, and pigments with unique molecular structures and varied biological functions1. Currently, these bioactive compounds are recognized as valuable sources for the food and health care sectors2. Therefore, there has been a rising attention in the research and development focusing on commercial exploitation of marine organisms derived bioactive primary and secondary metabolites as an ecofriendly and effective alternate for synthetic drugs3. In recent years, the discovery of unique bioactive substances with significant dietary, nutraceutical and medicinal advantages has attracted significant attention to marine algae.
Marine algae are essential components of the food chain and acts as a food supplier for the 70% of global living biomass2. Marine algae are photosynthetic organisms both macroscopic and microscopic, that have garnered attention due to their immense potential as a sustainable and environmentally friendly source for several bioactive compounds4. Marine algae type seaweeds are growing habitually through autotrophic, mixotrophic, or heterotrophic ways5. The potential of seaweeds to be cultivated in fresh, saline and waste waters, along with their fast multiplication, presents them as an attractive substitute for diverse products, thereby conserving time and resources6. According to the recent reports of FAO (2024)7the global seaweed productivity ranges about 37.8 million tonnes, they are subdivided into three categories based on their photosynthetic pigments: green macroalgae, brown macroalgae, and red macroalgae. Among these, red macroalgae account for over 60% of the 30,000 tons of algae produced globally. K. alvarezii is an inevitable and dynamic source yields 11.6 million tonnes feedstock worldwide, its productivity was extended upto the mark of 8,088 (dry) tonnes in India during 2005 to 20208. K. alvarezii is an effective producer of Phycoerythrin (PE), a natural protein pigment acts as the edible substances, rich source of antioxidants and extensive economic values9. A variety of red macroalgae are rich in bioactive compounds, making them a preferred choice for the production of commercially valuable bioproducts such as biofertilizers, biofuels, colorants, cosmetics, food, hydrocolloids, nutraceuticals, pharmaceuticals, plant stimulants and thickeners10.
Kappaphycus alvarezii (Doty) L. M. Liao (elkhorn sea moss) of family Solieriaceae, is a red seaweed (Rhodophyta) and is the primary carrageenophyte, commercially known as “cottoni”. It is mostly cultivated to produce commercial kappa-carrageenan hydrocolloids in both habitual and non-indigenous areas and has gained potential economic value in the seaweed industry. This macroalgae is the fifth most cultivated in the world, especially in various tropical countries of Asia and Africa, including India11due to its fast growing rates, simple cultivation process, high biomass yield in a short time with high polysaccharide content, and low investment requirements12. It also serves as a food source for local populations in Southeast Asia and is believed to possess various health benefits. The yellowish, reddish, green, and brown colours of K. alvarezii are based on the levels of phycoerythrin protein pigment13. The highly variable chemical components and quantities in K. alvarezii are influenced by the cultivation conditions, including water temperature, salinity, radiation, environmental variables, light level, profundity, and wave strength. On average, K. alvarezii consists of 50.8% carbohydrates, 15.6% ash, 12.4% sulphated groups, 3.3% proteins, 3.3% lipids, and 3.0% insoluble aromatics10. The literature indicates extensive use of K. alvarezii for carrageenan extraction. Furthermore, reports suggest that extracts of K. alvarezii possesses a multitude of essential compounds and high-value molecules with antioxidant and anti-inflammatory, antitumor, antiviral, antidiabetic, antihyperlipidemic activities, and for neural dysfunction properties11,14,15.
Cancer is a fatal disease that poses a significant risk to individuals worldwide, ranking as the second most common cause of mortality in numerous countries. In 2022, the global community experienced 20 million new cases of cancer and 9.7 million fatalities associated with this disease16. Lung cancer ranks among the most common cancers and is a primary contributor to cancer-related fatalities worldwide17. Patients diagnosed with lung cancer encounter a higher risk of meeting bacterial infections, which can vary from mild cases to those that pose significant threats to life. Both bacteria and viruses have the capability to trigger inflammatory cells and activate inflammatory signaling pathways. In particular, Escherichia coli (Migula 1895) Castellani and Chalmers 1919, Pseudomonas aeruginosa (Schröter 1872) Migula 1900, Staphylococcus aureus Rosenbach 1884, and Klebsiella sp. are gram-negative bacteria that are often found in the lungs of cancer patients18,19. Consequently, it is essential to explore novel anticancer medications sourced from nature that are safe, affordable, and less detrimental. The anti-cancer capability of K. alvarezii could be due to polysaccharides, nutrients, and other metabolites with antioxidant potential20. The phycobiliprotein (PBPs) family includes phycoerythrin (PE), which are macromolecular compounds in most macroalgal species. These proteins assemble to form a super-molecular protein complex called phycobilisome, with linkers joining the sub-units21. PBPs from red macroalgae species are essential as anticancer and anti-bacterial drugs. They have the potential to enhance the efficacy of conventional anticancer medications while simultaneously mitigating their adverse effects. Additionally, they can function as photosensitizers for the treatment of infected cells22.
Microorganisms, medicinal plants, and seaweeds are the primary sources of most cancer medications. Although red seaweed proteins and pigments hold significant prospects for the advancement of nutraceuticals and pharmaceuticals for cancer treatments, substantial gaps in our understanding remain14,23. Multiple factors, including their source, extraction methods, and human physiological reactions, effectiveness of algal compounds, requiring a thorough understanding to fully harness their advantages and commercial exploitation. Furthermore, the uses of these pigments and their derivatives in the context of cancer treatment remain largely unexamined. The present study aims to highlight the anticancer effects of phycoerythrin derived from K. alvarezii through its application in human lung cancer cell lines. This article examines the potential of phycoerythrin (PE), a red protein-pigment complex derived from K. alvarezii, as a promising candidate with potential for anticancer drug with possible treatment for lung cancer. The purified phycoerythrin was tested in vitro for its antimicrobial, antioxidant and anticancer activities, and K. alvarezii derived metabolites was evaluated for the first time through virtual screening and molecular docking analyses.
Materials and methods
Source of seaweed
The red seaweed K. alvarezii was collected alive and healthy from the Mandapam coast (9.2770° N, 79.1252° E) in Rameshwaram, Tamil Nadu, India (Fig. 1). We meticulously cleaned the seaweed samples in seawater to remove any superfluous material, including epiphytes and non-living matter, before transporting seaweed to the laboratory in sterilized portable sample storage ice-cooler box. Then, the seaweed was thoroughly cleaned and rinsed with sterile distilled water prior to the extraction process. Based on its morphological characters and partial rbcL gene sequencing (GenBank Accession No: MT478083), the seaweed was confirmed and designated as K. alvarezii isolate DBIS19.
Extraction of phycoerythrin
Kappaphycus alvarezii was sectioned into small fragments and measured to a weight of up to 10 g. The sodium phosphate buffer at pH 7.4 was prepared with the necessary chemicals. This buffer was then incorporated into the ground seaweed using a pre-cool sterile pestle and mortar placed on dry ice tray under aseptic lab environment under the room temperature of 28 ± 2 °C, followed by the transfer of mixture to a 250 mL beaker, and subject to a freeze-thaw cycle at temperatures of − 20 °C and 37 °C. The sample was subsequently centrifuged (REMI C-24 Plus) at 8000 rpm for a duration of 15 min. The supernatant was subjected to the analysis of phycoerythrin pigments utilizing a UV-Vis spectrophotometer at wavelengths of 562, 615, and 652 nm (Beckman, USA)24.
Purification of phycoerythrin
The isolated phycoerythrin pigment was saturated with ammonium sulfate 60% concentration by continuous stirring at 4 °C. Ammonium sulfate was added dropwise until a distinct precipitate formed at the bottom of the tube, indicating saturation. The phycoerythrin extract was stirred at 300 rpm overnight at 24 °C with 70% relative humidity and then next day centrifuged at 8000 rpm for 15 min at 4 °C. The obtained pellet was collected, dried, and prepared for further analysis. To partially purify the phycoerythrin, the extract was subjected to dialysis. The pellet obtained from ammonium sulfate precipitation was placed in a dialysis (12–14 kDa membrane) bag and dialyzed against sterile distilled water until the bag shrank, and the pigment was purified. Preliminary purification was further conducted using gel permeation chromatography with a Sephadex G-100 column. To achieve this process, a 30 × 2 cm column was prepared and equilibrated with 150 mL of sodium phosphate buffer (pH 7). A 10 mL dialyzed and filtered phycoerythrin sample was loaded onto the column. A linear gradient of sodium phosphate buffer, ranging from pH 5 to 8.2, was used to elute the sample. Fractions of 5 mL were collected at a constant flow rate of 20 mL h−1. The absorption spectrum of the purified phycoerythrin was measured using a UV-Vis spectrophotometer in the wavelength range of 300–750 nm. The total protein concentration was determined using Lowry’s method and the phycocyanin recovery was calculated25.
Characterization of phycoerythrin
Fourier transform infrared (FT-IR) spectroscopy analysis
The Perkin-Elmer FT-IR instrument was used to examine IR spectroscopy of phycoerythrin, facilitating the analysis of various sulfate, carboxyl, and hydroxyl groups contained in the purified phycoerythrin pigment.
High-performance liquid chromatography (HPLC) analysis
The phycoerythrin was separated using a C18 system column (LC-10VP Shimadzu) and eluted with sterile distilled water at a flow rate of 1.0 mL min−1 at 20 °C. A refractive index detector was used to keep track of the separated components. HPLC was used to examine the sugar composition of the seaweed extract after it had been hydrolyzed and treated with methanol. The column was calibrated using molecular mass standards and a standard curve was established.
Gas chromatography–mass spectrometry (GC-MS) analysis
The GC-2010 (Shimadzu GCM-QP 2010) gas chromatography apparatus, equipped with a flame-ionization detector (FID) and a split injector, was used to analyze phycoerythrin. High-purity helium served as the carrier gas at a flow rate of 1.40 mL min−1. The column temperature was maintained at 200 °C, while the splitter temperature was set to 240 °C. 1 µL sample of dichloromethane was injected through a glass-lined splitter with a split ratio of 1:90. Absorption was measured within the mass-to-charge (m/z) range of 40 to 800. To facilitate visualization by comparing the mass spectra of the components with those from the NIST 14 mass spectral database.
Antibacterial activity of phycoerythrin
The antibacterial activity of phycoerythrin was evaluated using the well-diffusion method against seven clinical pathogens such as Bacillus subtilis, Escherichia coli, Klebsiella oxytoca, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pyogenes pure culture strains were obtained from Microbial Type Culture Collection and Gene Bank at the CSIR-Institute of Microbial Technology, Chandigarh and maintained in Department of Microbiology, Ayya Nadar Janaki Ammal College. Using a sterile cotton swab, 24-hour-old bacterial cultures were aseptically swabbed on Mueller-Hinton agar plates. Wells were loaded with phycoerythrin at a concentration of 30 µg mL−1, while Ampicillin at 50 µg mL−1 served as positive control. The plates were incubated at 35 °C for 24 h. After incubation, the diameter of the inhibitory zones around each well was measured (mm) and recorded.
In vitro antioxidant activity of phycoerythrin
Determination of total antioxidant capacity
The total antioxidant activity of phycoerythrin was assessed using the method described by26. A reaction mixture was prepared by combining 3.0 mL of reagent solution (containing 0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate) with 0.3 mL of the purified phycoerythrin absolute (100%) sample. The mixture was incubated in a water bath at 95 °C for 90 min. After 15 min of cooling, the absorbance of all samples was measured at 695 nm, along with ascorbic acid as a control.
Determination of reducing power
The reducing power of phycoerythrin was determined by using the method described by27. A 4 mL reaction mixture, consisting of absolute phycoerythrin sample at various concentrations (100, 250, 500, 750, and 1000 µg mL−1) in phosphate buffer (0.2 M, pH 6.6), was incubated with 1% (w/v) potassium ferricyanide at 50 °C for 20 min. The reaction was stopped by adding 10% (w/v) trichloroacetic acid (TCA) solution. The resulting solution was mixed with distilled water and 0.1% (w/v) ferric chloride solution. The absorbance was then measured at 700 nm using a UV-Vis spectrophotometer.
Hydrogen peroxide scavenging assay
The free radical scavenging activity of phycoerythrin was determined using the hydrogen peroxide assay28. A 10 mM hydrogen peroxide solution was prepared in phosphate-buffered saline (0.1 M, pH 7.4). To perform the assay, 1 mL of the absolute phycoerythrin sample at varying concentrations (100, 250, 500, 750, and 1000 µg mL−1) was mixed with 2 mL of the hydrogen peroxide solution. The mixture was incubated at 37 °C for 10 min. The absorbance was measured at 230 nm using a UV-Vis spectrophotometer, with a blank (without hydrogen peroxide) used as a reference.
DPPH radical scavenging assay
The free radical scavenging activity of phycoerythrin was assessed using the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) method using spectrophotometry29. DPPH reagent, was used to evaluate the oxidizable groups in natural or synthetic antioxidants. A 0.1 mM DPPH solution in methanol was prepared, and 1 mL of this solution was added with 3 mL of varying concentrations of phycoerythrin (100, 250, 500, 750, and 1000 µg). The absorbance was measured at 517 nm after 10 min.
ABTS inhibition assay
The ability of phycoerythrin to scavenge the 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) radical was determined using the method described in30. ABTS was made by combining 5 mL of 7 mM ABTS with 88 µL of 140 mM potassium persulfate, and the mixture was kept in the dark at room temperature for 16 h. The solution was then diluted with 50% ethanol until its absorbance at 734 nm reached 0.7 ± 0.05. For the assay, 5 mL of the prepared ABTS solution was mixed with 0.1 mL of phycoerythrin at various concentrations (100, 250, 500, 750, and 1000 µg mL−1). The final absorbance was measured at 743 nm using a UV–Vis spectrophotometer.
In vitro anticancer activity of phycoerythrin
Cell culture
The human lung cancer cell line (A549) was purchased from the National Centre for Cell Science (NCCS), Pune, and cultured in liquid Dulbecco’s Modified Eagle Medium (DMEM) (Sigma, St. Louis, MO, USA) supplemented with Fetal Bovine Serum (FBS) 10% (v/v) (Biochrom, Berlin, Germany), penicillin 100 µg/mL, and streptomycin sulphate 100 µg mL−1. The cells were maintained in an atmosphere of 5% CO2 and 100% relative humidity at 37 °C.
MTT assay for cell cytotoxicity
The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphentyltetrazoliumbromide] assay was performed to evaluate the in vitro cytotoxicity of phycoerythrin on A549 cells, as described in31. Cultured A549 cells were harvested by trypsinization and collected into a 15 mL tube. The cells were then plated into a 96-well tissue culture plate at a density of 1 × 105 cells mL−1 at the rate of 200 µL well−1 in DMEM media with 10% FBS and 1% antibiotic solution for 24–48 h at 37 °C. After incubation, the medium was replaced with serum-free DMEM, and wells were rinsed with sterile Phosphate Buffer Saline (PBS) and treated with different conentrations of phycoerythrin. Each sample was performed in triplicate, and the cells were cultured for 24 h at 37 °C in a humidified 5% CO2 incubator. Following, MTT (20 µL at 5 mg mL−1) was added to each well after the incubation period, and the cells were incubated for another 2–4 h until purple precipitates were visible under an inverted microscope observation. Finally, the medium was aspirated within the wells together with MTT (220 µL) and rinsed with 200 µL of 1x PBS. The formazan crystals were dissolved by adding 100 µL of Dimethylsulfoxide (DMSO) to each well, and the plate was gently agitated for 5 min. Absorbance at 570 nm was measured using a microplate reader (Thermo Fisher Scientific, USA), and the percentage cell viability and IC50 values were calculated using GraphPad Prism 6.0 software (USA).
Apoptosis assay
A549 cells were planted at a density of 5 × 105 cells mL−1 in a 96-well tissue culture plate in DMEM media with 10% FBS and 1% antibiotic solution for 24–48 h at 7 °C. After incubation, the medium was replaced with serum-free DMEM, and wells were rinsed with PBS before being treated with 44.33 µg mL−1 of phycoerythrin. Then, the plate was incubated at 37 °C in a 5% CO2 incubator for 24 h. Following the incubation, 10 µL Alexa Fluor and 10 µL propidium iodide were added to the wells, gently mixed, and incubated for 15 min. Subsequently, 400 µL of 1x Annexin binding buffer was added and gently mixed. The plate was centrifuged at 800 rpm for 2 min, and the cells were inspected using a fluorescence microscope with a fluorescent filter within 1 h32.
Computational analysis
Target preprocessing
In the present study, the multitargeted proteins were chosen for the antimicrobial activity as Spore coat polysaccharide biosynthesis protein [PDB Id 1H7L], Staphylococcus aureus tyrosyl-tRNA synthetase [PDB id 1JIJ], Isoleucyl-tRNA synthetase [PDB ID QU3], Transcriptional regulator qacR [PDB ID 1RKW], HTH-Type Transcriptional Regulator MgrA [PDB ID 2BV6], YcgJ protein from Bacillus subtilis [PDB ID 2GLU], Dihydropteroate synthase [PDB ID 2VEG], Processed Glycerol Phosphate Lipoteichoic Acid Synthase 2 [PDB ID 2W8D], DNA Gyrase Subunit B, DNA Gyrase Subunit A [PDB ID 2XCT], DNA topoisomerase 4 subunit A [PDB ID 3RAE], Dihydrofolate reductase [PDB ID 3SRW], Penicillin-binding protein 3 [PDB ID 3VSL], Transcriptional regulator MvfR [PDB ID 4JVC] subjected for the molecular docking studies. These multitargeted proteins were retrieved from the Protein Data Bank (PDB). The preprocessing steps involved assigning bond orders, adding hydrogens, creating zero-order bonds to metals and disulfide bonds, converting seleno-methionines to methionine, and filling missing side chains using the Protein Preparation Wizard of the Maestro platform. The structures were then refined by optimizing hydrogen bonds and minimizing the structures using the OPLS4 force field (Maestro, Schrödinger 2021-2, NY, USA).
Molecular docking
The flexible ligand docking parameter was enabled via Glide’s XP (extra precision) function, and the target proteins and ligand molecules were docked using Maestro’s Glide docking module. We determined the optimistic pose of the ligand-protein complex molecule by examining the interaction between the ligand and protein during docking using the XP pose viewer. We used the ligand interaction module to acquire the 2D interaction diagram. We subsequently analyzed the acquired XP pose to investigate the binding interactions of ligand molecules with the target protein33,34.
Ligand preparation
The compound extracted from K. alvarezii, including (1,2-Benzisothiazol-3-amine, 2-Ethylacridine, 2,4-Dimethylbenzo[h]quinoline, 2-bromobutyloxychalcone), has been analyzed and profiled using GC-MS. We subsequently examined the 3D structures of the resultant compounds, retrieving them in the 3D structure data file format from the PubChem databases. We employed the LigPrep module to preprocess the structures, using the OPLS4 force field to minimize energy and generate 32 different stereoisomeric and tautomeric states (Schrödinger Release 2021-2: LigPrep, Schrödinger, LLC, NY, 2021).
Results and discussion
K. alvarezii holds significant importance as a red macroalga due to its bioactive compounds, which hold significant therapeutic potential. Different fields have utilized their diverse, unique, biologically active substances. The evaluation focused on the antioxidant, antimicrobial, and anticancer properties of the extract from K. alvarezii. We conducted a chromatographic examination of its phycoerythrin pigment to gain a deeper understanding of the metabolic constituents involved.
Results
Extraction and purification of phycoerythrin pigment
The centrifuged phycoerythrin pigment extracted from a K. alvarezii exhibited a red color. The purification and recovery involved four steps. The purity ratio of phycoerythrin, successively with ammonium sulfate (151.24 µg mL−1, purity 2.24, recovery 74%) dialysis (338.71 µg mL−1, purity 3.39, recovery 35%), and column chromatography yielded highest concentration and purity (554.12 µg mL−1, purity 5.63, recovery 11%) when compared to crude pigment were reported in Table 1. Also, the total protein concentration of phycoerythrin pigment was 69.84%.
Characterization of phycoerythrin
FT-IR analysis
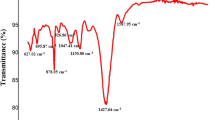
The phycoerythrin bond and functional group analysis of FT-IR is shown in Fig. 2. The peak 3446.56 cm−1 shows in O–H stretch and H–bonded stretching vibration presence of alcohols and phenols. The peak 3130.25 cm−1 was present in the O–H stretching vibration functional group of carboxylic acids. The peak was 2920.99 cm−1 C–H stretching vibration presence of alkanes. The peak 1719.42 cm−1 C = O stretching vibration presence of alpha, beta–unsaturated esters. Peaks 1610.45 cm−1, 1528.48 cm−1 and 1443.62 cm−1 C–C stretch (in–ring) presence of aromatics. The peak 1383.83 cm−1, 1264.25 cm−1, 1139.85 cm−1 and 1056.92 cm−1 C–O stretch presence of alcohols, carboxylic acids, esters, ethers. The peak 994.24 cm−1, 951.81 cm−1, 865.01 cm−1, 816.80 cm−1, 761.83 cm−1 and 619.11 cm−1 =C–H bend alkenes. The peak 535.21 cm−1 C–Br stretching vibration presence of alkyl halides. The distinct amide bands observed at 1610.45 and 3130.25 cm−1 in C-Phycoerythrin suggest that its secondary structure is predominantly composed of alpha helices.
HPLC analysis
HPLC analysis of phycoerythrin showed in Fig. 3. The obtained peaks were 2.110 (kaempferitrin), 2.823 (γ- tocopherol), 3.060 (β-sitosterol) and 4.353 (corilagin). The separation of its α-, β, and γ-subunits was successfully accomplished using a reversed-phase HPLC gradient semipreparative approach involving with C4 large-pore column, and a solvent system made up of 0.05% trifluoroacetic acid (TFA) in water and 0.05% TFA in acetonitrile.
GC-MS analysis
GC-MS analysis of the phycoerythrin revealed the following compounds such as 16.832 (-2-bromobutyloxychalcone), 17.024 (2-Ethylacridine), 17.072 (1,2-Benzisothiazol-3-amine) and 17.148 (Benzo[h]quinoline, 2,4-dimethyl-) within the peaks shown in Fig. 4. The analysis identified a variety of bioactive compounds, including undecane, 2-decyloxirane, methyl n-tridecanoate, n-hexadecanoic acid, eicosanoic acid, nonanoic acid, oleic acid, pentadecanoic acid, bicyclo[3.2.1]oct-3-en-2-one, 3,8-dihydroxy-1-1methoxy-7-(7-methoxy-1, 3 benzodioxol-5-yl)-6-methyl-5, N-(5-chloro-2-hydroxyphenyl) dodecanamide, and cholesta-8,24-dien-3-ol, 4-methyl.
Antibacterial activity of phycoerythrin
The antibacterial activity of phycoerythrin (30 µg mL−1) was evaluated against seven clinical pathogens. The maximum zone of suppression was found on Klebsiella oxytoca (20 mm), followed by Escherichia coli (17 mm), and Pseudomonas aeruginosa (18 mm), though generally less effective than Ampicillin (50 µg mL−1), which exhibited inhibition zones of 17–23 mm shown in Table 2.
Antioxidant activity of phycoerythrin pigment
The phycoerythrin pigment (1000 µg ml−1) showed a total antioxidant capacity of 73.27 ± 0.43%, reducing power of 71.09 ± 0.23%, hydrogen peroxide scavenging activity of 67.54 ± 0.41%. Also, it showed 58.13 ± 0.99% activity in the DPPH, and 61.92 ± 0.34% in the ABTS assay, portraying its antioxidant potential (Table 3; Supplementary Fig. 1–5).
Anticancer action of phycoerythrin pigment
Cytotoxicity of cells by MTT assay
This study utilized the MTT assay to investigate the ways phycoerythrin affects the growth of A549 cancer cells. The cells endured culture for a duration of 24 h across a range of phycoerythrin concentrations. The viability of the cells was assessed through the MTT assay following a 24-h incubation period. The alterations in the morphology of human lung cancer cells were observed following treatment with varying concentrations of phycoerythrin, as detailed in Table 4; Supplementary Fig. 6. The test showed a clear relationship between dosage and the level of toxicity in A549 human lung cancer cells. The cell viability showed a decline from 91.24 ± 0.09% at a concentration of 50 µg mL−1 to 46.36 ± 0.16% at 500 µg mL−1. Significant reductions were also observed at 200 µg mL−1 (74.29 ± 0.07%) and 400 µg mL−1 (54.85 ± 0.11%), emphasizing its promise as a potential anticancer agent. The concentrations of phycoerythrin at 50 µg mL−1 and 100 µg mL−1 indicated the presence of viable human lung cancer cells. The concentration of 100 µg mL−1 of phycoerythrin showed the existence of both viable and non-viable cells. The levels of phycoerythrin between 200 and 400 µg mL−1 indicated a minimal count of visible cells (Fig. 5).
Human lung cancer cells treated with phycoerythrin experience growth inhibition. The tested sample exhibited an IC50 value of 131.7 µg mL⁻¹. Ultimately, as the concentration of phycoerythrin increased, the presence of the cell steadily reduced. It is important to note that phycoerythrin demonstrates a more potent effect against human lung cancer cells shown in Table 5. Similarly, the various solvent extracts of Gracilaria edulis (Rhodophyta) were evaluated against cancer cell lines for their anti-proliferative capabilities35. The G. edulis ethyl acetate extract (GEEA) at a concentration of 100 µg mL−1 demonstrated a notable and highly significant reduction in growth within the A549 lung cancer cell line model. Additionally, when assessing a control group against a GEEA extract-treated group at different dosages (40, 60, 80, and 100 µg mL−1) after 48 h, a minimal level of LDH release was observed. The cell cytotoxicity assay36, revealed that phycoerythrin extracted from Microchaete acts as an anticancer agent in the Hep G2 cell line at specific doses (20–160 g mL−1), demonstrating an IC50 value of 105.77 g mL−1. In a similar way, anticancer efficacy of Gracilaria cortica (Rhodophyta) phycoerythrin was reported against the HepG2 cell line37.
Phycoerythrin induces apoptosis in cancer cells
The evaluation of apoptotic cells involved staining phycoerythrin with human lung cancer cells, as illustrated in Fig. 6. The control cells exhibited the highest number of viable cells, as indicated by green-coloured fluorescence. The percentage of apoptotic cells that tested positive for Annexin V/PI in human lung cancer cells treated with varying dosages of phycoerythrin (203.4 µg mL−1) increased in accordance with the dosage levels. Cells treated with fluorouracil demonstrated the presence of both viable and non-viable cells. In addition to identifying the morphological changes associated with apoptosis, we also assessed the apoptosis rate using the annexin V test. Annexin V has a strong and specific attraction for damaged and compromised plasma membranes, making apoptotic and necrotic cells stand out.
Phycoerythrin produces programmed cell death in human lung cancer cells. (a) Control cells exhibited a green color, signifying that the cells were alive; (b) Cells treated with phycoerythrin at a concentration of 203.4 µg mL−1 displayed live cells (green color), dead cells (red color), and apoptotic cells (yellow color).
Molecular docking analysis
Computational biology combines computational techniques with biological systems to tackle disease-related challenges. Virtual screening, a key tool, reduces drug development costs and time by predicting receptor-ligand binding and affinities through docking and scoring. Molecular docking revealed significant binding affinities with 13 antibacterial target proteins for the first time with the K. alvarezii extracted bioactive compounds. These 13 antibacterial multitarget proteins were docked with 4 compounds detected in GC-MS analysis of the phycoerythrin, revealing that a lower docking score corresponds to higher binding affinity (Table 6; Supplementary Fig. 7–19). Among the results, the protein DNA gyrase subunits A (GyrA) and B (GyrB) showed a binding score of − 1.13 kcal/mol with 1,2-Benzisothiazol-3-amine. Staphylococcus aureus tyrosyl-tRNA synthetase protein scored − 2.86 kcal/mol with 1,2-Benzisothiazol-3-amine, while dihydropteroate synthase and transcriptional regulator qacR scored − 1.17 kcal/mol and − 1.21 kcal/mol, respectively, with 2-bromobutyloxychalcone. Penicillin-binding protein 3 (− 1.41 kcal/mol), DNA topoisomerase 4 subunit A (− 1.66 kcal/mol), and isoleucyl-tRNA synthetase (− 3.10 kcal/mol) also showed strong interactions with 1,2-Benzisothiazol-3-amine. Other substantial interactions included HTH-type transcriptional regulator MgrA (− 2.39 kcal/mol) and processed glycerol phosphate lipoteichoic acid synthase 2 (− 3.51 kcal/mol) with 2,4-dimethylbenzo[h]quinoline, and YcgJ protein from Bacillus subtilis (− 2.52 kcal/mol) with 2-bromobutyloxychalcone. Particularly, transcriptional regulator MvfR (− 4.88 kcal/mol) interacted strongly with 1,2-Benzisothiazol-3-amine, and dihydrofolate reductase exhibited the highest binding affinity (− 5.24 kcal/mol) with 2-ethylacridine. In general, 1,2-Benzisothiazol-3-amine and 2-bromobutyloxychalcone demonstrated strong interactions with multiple targets, while 2-ethylacridine showed the highest affinity with dihydrofolate reductase (− 5.24 kcal/mol), emerged as the most potent compound.
Discussion
This study investigated the production of phycoerythrin pigment from the red seaweed K. alvarezii, utilizing samples collected from the Mandapam shoreline in Rameshwaram, located on the southeast coast of India. Sharmila et al.33 have previously documented the collection of a live and healthy specimen of K. alvarezii from the Mandapam coast in Rameshwaram, India. Phycoerythrin was effectively extracted from microalgae through various cell disruption techniques, including sonication, mechanical maceration, and lysozyme treatments22. The protein content of 69.84% aligns with previous literatures38,39while our sequential purification process achieved superior purity compared to single-step chromatographic methods40. Notably, the anion-exchange chromatography with DEAE Sepharose fast flow column at 200 mM NaCl yielded a purity index (A565/A280) of 3.25, though process optimization remains necessary to mitigate recovery losses inherent in multi-stage purification. The selection of cell disruption methods proved critical, with mechanical and enzymatic techniques showing particular promise for phycoerythrin recuperation.
The FT-IR spectra are used to investigate the functional groups or chemical bonds within a molecule of an interaction system, assisting to analyze the functional groups or chemical bonds present in that molecule. Before extraction, the spectra reveal the absorption bands of Deep eutectic solvents (DES) at 1472 cm−1 and dialyzed R-PE at 1074 cm−1. The FT-IR analysis of C-phycoerythrin (CPE) revealed notable alterations in the amide III region, the protein backbone appeared to remain largely unchanged41, suggest specific side-chain modifications during extraction.Through, GC-MS analysis key bioactive compounds were identified including kaempferitrin (2.110), γ-tocopherol (2.823), β-sitosterol (3.060), and corilagin (4.353), consistent with phycoerythrin profiles from other red algae Porphyridium cruentum42. The methanol extract profile of our GC-MS analysis, highlighting their significant antimicrobial and free radical scavenging activities aligned with previous characterizations of Gracilaria corticata from the same geographic region43.
The antimicrobial screening revealed concentration-dependent inhibition of human pathogenic bacteria, with 0.1 mg mL−1 phycoerythrin demonstrating maximum efficacy consistent with Microchaete phycoerythrin activity36. Our findings support the proposed mechanism wherein phycoerythrin alters bacterial membrane permeability, causing macromolecule leakage and eventual cell death44,45. These findings are aligned with earlier reports of antibacterial properties in K. alvarezii, K. striatum, and Ulva lactuca against human pathogens46.
The antioxidant assessment yielded compelling results, with phycoerythrin showing remarkable free radical scavenging capacity. The DPPH radical assay revealed an IC50 of 0.043 mg mL−1, approaching ascorbic acid’s efficacy (0.031 mg mL−1). Similarly, ABTS radical scavenging (IC50 = 0.023 mg mL−1) nearly matched synthetic standard Butylated Hydroxyl Toluene (BHT) (0.031 mg mL−1), confirming phycoerythrin potential as natural antioxidant36. These findings align with documented protective effects against oxidative stress-related complications including cancer, diabetes, and neurodegenerative disorders. The concentration-dependent antioxidant activity mirrors observations in Nostoc linckia, where radical scavenging capacity associated with phycobiliprotein content47.
Our investigation of anticancer properties yielded significant findings. Phycoerythrin demonstrated dose-dependent cytotoxicity against A549 lung cancer cells (203.4 µg/mL), inducing apoptosis as evidenced by Annexin V/PI staining. This apoptotic effect surpassed fluorouracil treatments in specificity, supporting earlier reports of Microchaete phycoerythrin activity against HepG2 cells (IC50 = 105.77 µg mL−1)36 and Gracilaria cortica efficacy37. The observed caspase activation and cytochrome C release confirm the mitochondrial apoptotic pathway involvement48providing mechanistic insight into phycoerythrin anticancer properties. Molecular docking analyses further elucidated phycoerythrin therapeutic potential by predicting ligand-protein interaction strengths and binding orientations. This computational approach substantiates our experimental findings while offering avenues for structural optimization to enhance bioactivity34.
Conclusions
The scope of the present paper was to assess the potential of seaweed as a novel source for marine pharmaceuticals. This study successfully isolated and purified phycoerythrin from K. alvarezii using a process that included ammonium sulfate precipitation, dialysis, and column chromatography. In the FT-IR test, many different functional groups were found. In the HPLC test, bioactive compounds such as kaempferitrin, β-sitosterol, and 2-bromobutyloxychalcone were found. The antioxidant activity of phycoerythrin was very high, with a total antioxidant capacity of 73.27% and strong scavenging abilities in the DPPH and ABTS tests. Its antibacterial activity was most effective against K. oxytoca (20 mm) and moderately active against other pathogens. MTT assays verified that cytotoxicity against A549 human lung cancer cells was dose-dependent, and Annexin V/PI staining revealed increased apoptotic activity. Molecular docking further emphasized the potential of phycoerythrin as a natural source of bioactive compounds for therapeutic applications, highlighting its potential in antimicrobial target protein interactions. The outcome of this study demonstrates the potential of K. alvarezii phycoerythrin as a multifaceted bioactive compound with applications spanning antimicrobials, antioxidants, and anticancer therapeutics. However, scalability of purification processes and the optimization of recovery methods are required. Future research should explore suitable purification methods for seaweed biomolecules to study their structural and biological correlations using advanced modelling and confirm their effectiveness. This article offers a detailed plan that combines testing methods, biological assessments, and computer analysis to help improve the creation of phycoerythrin-based products for use in medicine and health supplements.
Statement
We implemented all methods in accordance with pertinent regulations and guidelines. The authors have not conducted any experiments on humans or utilized human tissue samples. The ethics committee of the institute approved all experimental protocols and panelists involved in the study.
Data availability
The authors declare that data supporting the findings of this study will be available from the corresponding author upon reasonable request.
References
Menaa, F. et al. Marine algae-derived bioactive compounds: A new wave of nanodrugs? Mar. Drugs. 19, 1–36 (2021).
Ahmed, N. et al. Comprehensive exploration of marine algae diversity, bioactive compounds, health benefits, regulatory issues, and food and drug applications. Meas. Food. 14, 100163 (2024).
Banday, A. H. et al. Exploring the potential of marine natural products in drug development: A comprehensive review. Phytochem. Lett. 59, 124–135 (2024).
Eladl, S. N., Elnabawy, A. M. & Eltanahy, E. G. Recent biotechnological applications of value-added bioactive compounds from microalgae and seaweeds. Bot. Stud. 65, (2024).
Cupo, A. et al. Autotrophic vs. Heterotrophic cultivation of the marine diatom cyclotella cryptica for Epa production. Mar. Drugs. 19, 1–11 (2021).
Bora, A., Thondi Rajan, A. S., Ponnuchamy, K., Muthusamy, G. & Alagarsamy, A. Microalgae to bioenergy production: recent advances, influencing parameters, utilization of wastewater—A critical review. Sci. Total Environ. 946, 174230 (2024).
FAO. The State of World Fisheries and Aquaculture 2024 & FAO. (2024). https://doi.org/10.4060/cd0683en
Mantri, V. A. Twenty years of commercial farming of Kappaphycus Alvarezii in india: A look back at learnings and the way forward. Rev Aquac 17, (2025).
Ganesan, A. R. & Shanmugam, M. Isolation of phycoerythrin from Kappaphycus alvarezii: a potential natural colourant in ice cream. J. Appl. Phycol. 32, 4221–4233 (2020).
Solorzano-Chavez, E. G. et al. Evaluation of the Kappaphycus alvarezii growth under different environmental conditions and efficiency of the enzymatic hydrolysis of the residue generated in the Carrageenan processing. Biomass Bioenerg. 127, 105254 (2019).
Rudke, A. R., de Andrade, C. J. & Ferreira, S. R. S. Kappaphycus alvarezii macroalgae: an unexplored and valuable biomass for green biorefinery conversion. Trends Food Sci. Technol. 103, 214–224 (2020).
Das, D. et al. Phytochemical constituents, antimicrobial properties and bioactivity of marine red seaweed (Kappaphycus alvarezii) and Seagrass (Cymodocea serrulata). Foods 12, (2023).
Gereniu, C. R. N., Saravana, P. S., Getachew, A. T. & Chun, B. S. Characteristics of functional materials recovered from Solomon Islands red seaweed (Kappaphycus alvarezii) using pressurized hot water extraction. J. Appl. Phycol. 29, 1609–1621 (2017).
Sharma, R., Mondal, A. S. & Trivedi, N. Anticancer potential of algae-derived metabolites: recent updates and breakthroughs. Futur J. Pharm. Sci 9, (2023).
Nunes, A. et al. Uses and applications of the red seaweed Kappaphycus alvarezii: A systematic review. J. Appl. Phycol. https://doi.org/10.1007/s10811-024-03270-6 (2024).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Leiter, A., Veluswamy, R. R. & Wisnivesky, J. P. The global burden of lung cancer: current status and future trends. Nat. Rev. Clin. Oncol. 20, 624–639 (2023).
Lanoix, J. P. et al. Bacterial infection profiles in lung cancer patients with febrile neutropenia. BMC Infect. Dis 11, (2011).
Ye, M., Gu, X., Han, Y., Jin, M. & Ren, T. Gram-negative bacteria facilitate tumor outgrowth and metastasis by promoting lipid synthesis in lung cancer patients. J. Thorac. Dis. 8, 1943–1955 (2016).
Manohar, M., Sharan, V., Joshy, V. & L. & Recent advances in in vitro and in vivo studies on Kappaphycus alvarezii and its derivatives. Nat. Prod. Res. 1–14. https://doi.org/10.1080/14786419.2024.2354855 (2024).
Uju et al. Extraction of phycoerythrin from Kappaphycus alvarezii seaweed using ultrasonication. IOP Conf. Ser. Earth Environ. Sci 414, (2020).
Ulagesan, S., Nam, T. J. & Choi, Y. H. Extraction and purification of r-phycoerythrin alpha subunit from the marine red algae pyropia yezoensis and its biological activities. Molecules. 26, (2021).
Cotas, J., Leandro, A., Pacheco, D. & Gonçalves, A. M. M. & Pereira, L. A comprehensive review of the nutraceutical and therapeutic applications of red seaweeds (Rhodophyta). Life. 10, (2020).
Pugalendren, S., Sarangam, B. & Rengasamy, R. Extraction of R-Phycoerythrin from Kappaphycus alvarezii (Doty) Doty ex Silva and analyses of its physico-chemical properties. J. Acad. Ind. Res. 1, 407 (2012).
Shunmugiah et al. Isolation and purification of phycocyanin pigments from Spirulina sp. biomass and evaluation of its anticancer and antioxidant potential. Biomass Convers. Biorefinery. https://doi.org/10.1007/s13399-022-02765-x (2022).
Prieto, P., Pineda, M. & Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 269, 337–341 (1999).
Ramalingam, V. & Rajaram, R. Enhanced antimicrobial, antioxidant and anticancer activity of Rhizophora apiculata: An experimental report. 3 Biotech 8, (2018).
Gantar, M., Dhandayuthapani, S. & Rathinavelu, A. Phycocyanin induces apoptosis and enhances the effect of Topotecan on prostate cell line LNCaP. J. Med. Food. 15, 1091–1095 (2012).
Ramalingam, V. & Rajaram, R. Antioxidant activity of 1-hydroxy-1-norresistomycin derived from Streptomyces variabilis KP149559 and evaluation of its toxicity against zebra fish Danio rerio. RSC Adv. 6, 16615–16623 (2016).
Re, R. et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231–1237 (1999).
Mohan, H. et al. E-waste based graphene oxide/V2O5/Pt ternary composite: enhanced visible light driven photocatalyst for anti-microbial and anti-cancer activity. Colloids Surf. Physicochem. Eng. Asp. 607, 125469 (2020).
Ramalingam, V., Raja, S. & Harshavardhan, M. In situ one-step synthesis of polymer-functionalized palladium nanoparticles: an efficient anticancer agent against breast cancer. Dalt Trans. 49, 3510–3518 (2020).
Leslie, V. A., Alarjani, M., Malaisamy, K., Balasubramanian, B. & A. & Bacteriocin producing microbes with bactericidal activity against multidrug resistant pathogens. J. Infect. Public. Health. 14, 1802–1809 (2021).
Malaisamy, A. K. et al. Probing marine macroalgal phlorotannins as an antibacterial candidate against Salmonella typhi: molecular Docking and dynamics simulation approach. Curr. Plant. Biol. 40, 100418 (2024).
Sakthivel, R., Muniasamy, S., Archunan, G. & Devi, K. P. Gracilaria edulis exhibit antiproliferative activity against human lung adenocarcinoma cell line A549 without causing adverse toxic effect in vitro and in vivo. Food Funct. 7, 1155–1165 (2016).
Hemlata, Afreen, S. & Fatma, T. Extraction, purification and characterization of phycoerythrin from michrochaete and its biological activities. Biocatal. Agric. Biotechnol. 13, 84–89 (2018).
Zandi, K. et al. In vitro antitumor activity of gracilaria corticata (a red alga) against Jurkat and molt-4 human cancer cell lines. Afr. J. Biotechnol. 9, 6787–6790 (2010).
Sharmila, G. et al. Ultrasound assisted extraction of total phenolics from Cassia auriculata leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 84, 13–21 (2016).
Benavides, J. & Rito-Palomares, M. Bioprocess intensification: A potential aqueous two-phase process for the primary recovery of B-phycoerythrin from Porphyridium cruentum. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 807, 33–38 (2004).
Nguyen, H. P. T. et al. Purification of R-phycoerythrin from a marine macroalga Gracilaria gracilis by anion-exchange chromatography. J. Appl. Phycol. 32, 553–561 (2020).
Ghosh, T., Vyas, A., Bhayani, K. & Mishra, S. C-Phycoerythrin as a colorimetric and fluorometric probe for the sensitive, selective and quantitative detection of Cu2 + in aqueous samples. J. Fluoresc. 28, 671–680 (2018).
Bermejo, R., Talavera, E. M. & Alvarez-Pez, J. M. Chromatographic purification and characterization of B-phycoerythrin from Porphyridium cruentum-Semipreparative high-performance liquid chromatographic separation and characterization of its subunits. J. Chromatogr. A. 917, 135–145 (2001).
Ragunathan, V., Pandurangan, J. & Ramakrishnan, T. Gas Chromatography-mass spectrometry analysis of methanol extracts from marine red seaweed Gracilaria corticata. Pharmacogn J. 11, 547–554 (2019).
Abu-Khudir, R., Ismail, G. A. & Diab, T. Antimicrobial, antioxidant, and anti-tumor activities of Sargassum linearifolium and Cystoseira crinita from Egyptian mediterranean Coast. Nutr. Cancer. 73, 829–844 (2021).
El-Sheekh, M. & Sabbagh, E. Control of some microbial skin diseases by some marine algal extract. J. Agric. Chem. Biotechnol. 7, 67–74 (2016).
Sivakumar, K., Kannappan, S. & Vijayakumar, B. Molecular docking approaches of biomolecules extracted from red seaweed Kappaphycus alvarezii against hemolysin protein of bioluminescence disease-causing bacteria vibrio harveyi. Algal Res. 74, 103207 (2023).
Valuta, A. et al. Phycobiliprotein accumulation in Cyanobacterium Nostoc linckia and modification of antioxidant activity. Ann. Oradea Univ. Biol. Fascicle. 21, 13–19 (2015).
Logue, S. E. & Martin, S. J. Caspase activation cascades in apoptosis. Biochem. Soc. Trans. 36, 1–9 (2008).
Acknowledgements
The authors extend their deep appreciation to Ongoing Research Funding Program, (ORF-2025-741), King Saud University, Riyadh, Saudi Arabia. Authors would like to express their gratitude to Ayya Nadar Janaki Ammal College, Tamil Nadu, for providing the necessary facilities to conduct this research, as well as the International Centre for Genetic Engineering and Biotechnology, New Delhi for their assistance with molecular docking.
Funding
Ongoing Research Funding Program, (ORF-2025-741), King Saud University.
Author information
Authors and Affiliations
Contributions
H.B., G.R.P. and D.P. conceived and designed the experiments. P.S. and S.S. drafted the main manuscript text. H.B., S.M., M.M., J.P. and P.M. conducted the extraction, GC-MS analysis, and antibacterial, antioxidant, and anticancer experiments. M.A., S.V. and P.S. carried out the molecular docking analyses. D.P. and A.P. supervised the research project. F.U., H.O.E., M.N., A.A.F., M.A.R. and I.M.M. contributed to funding acquisition, manuscript review and editing, and project administration.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Balasundaram, H., Seethapathy, P., Sankaralingam, S. et al. Isolation, characterization, and biomedical potential of phycoerythrin phycobiliprotein from Kappaphycus alvarezii (Doty) L. M. Liao: antimicrobial, antioxidant, and anticancer activities. Sci Rep 15, 27904 (2025). https://doi.org/10.1038/s41598-025-11899-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11899-7