Abstract
The enterosalivary pathway generates systemic nitric oxide from dietary nitrate for vasodilation and blood pressure (BP) regulation, but standard antibacterial mouth rinses may disrupt this process. This study evaluated a bioactive mouth rinse infused with inorganic nitrate and antioxidants on mechanistic and clinical measures of the enterosalivary pathway, vascular health, and oral microbiome compared to an antibacterial mouth rinse containing chlorhexidine (CHX). Nine-week-old male Wistar rats were randomized to the bioactive or CHX rinse administered twice daily for one week. Systolic (SBP) and diastolic BP (DBP) were measured using tail cuff plethysmography. Blood and salivary nitrate and nitrite concentrations were determined by ozone-based chemiluminescence. Oral microbiome was assessed by 16S rRNA V4 gene amplicon sequencing. From baseline to week one, favorable changes in SBP (p = 0.008) and DBP (p = 0.016) were observed in the bioactive group compared to the CHX group. Blood and salivary nitrate and salivary nitrite concentrations (p < 0.05) were higher in the bioactive group. Compared to the CHX group, the bioactive group had significantly greater mean relative abundance of genera with established nitrate-/nitrite-reducing properties. Results suggest mouth rinse infused with inorganic nitrate and antioxidants favorably modulates the oral microbiome to support the enterosalivary pathway while reducing BP over one week.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) remains the leading cause of death globally, and currently affects over 500 million adults worldwide1. Oxidative stress and inflammation are fundamental to the onset and progression of CVD, as these processes underpin the development of subclinical disease, like elevated blood pressure and endothelial dysfunction2. Systemic oxidative and inflammatory stress influence the cells of the vasculature leading to structural and functional alterations observed in hypertension, as well as a reduction in nitric oxide (NO) bioavailability3.
Similar mechanisms underpin common oral conditions, including periodontal disease, such that detrimental bacteria promote the production of pro-inflammatory cytokines and reactive oxygen species (ROS) in the oral cavity4. The homeostatic imbalance that ensues is not limited to the oral cavity, rather inflammatory cytokines and ROS may be transmitted into circulation as a result of saliva’s constant flux with plasma5. Epidemiological and longitudinal studies have further expounded upon the relationship between oral and systemic health such that the poor condition of the oral cavity has been implicated in the onset of CVD6. Specifically, periodontal disease has been linked to a 19% increased risk of CVD development in young, healthy adults and a 44% increase in the older adult population7,8,9.
Supporting oral health comes in many forms, including daily brushing, flossing, and mouth rinse use, among others. Of interest, approximately 60% of adults in the United States adhere to a daily antibacterial mouth rinse regimen10. Common mouth rinses cannot differentiate among bacterial species and, thus, contribute to a reduced microbial diversity in the oral cavity11. Chlorhexidine is a common ingredient in mouth rinses prescribed by dental professionals, and it possesses broad spectrum anti-microbial properties12 .Unfortunately, an oral cavity devoid of commensal bacteria may have local and systemic consequences. Previous studies reported increases in blood pressure following the use of antibacterial mouth rinses in both normotensive and hypertensive individuals, as these rinses also reduce concentrations of protective bacteria critical to the enterosalivary nitrate-nitrite-NO pathway. The enterosalivary pathway uses dietary inorganic nitrate as a substrate to generate systemic nitric oxide for vasodilation and blood pressure regulation13,14,15.
Dietary inorganic nitrate is found most abundantly in green, leafy vegetables16. Common dietary patterns like the Dietary Approaches to Stop Hypertension diet safely provide nitrate levels up to 500% greater than the acceptable daily intake determined by the World Health Organization17. As inorganic nitrate is the primary substrate for the enterosalivary pathway, it is vital to blood pressure regulation and overall cardiovascular health as a result of its conversion to the vasodilatory molecule NO18,19. Briefly, ingested inorganic nitrate is digested, absorbed, and approximately 25% of circulating nitrate is concentrated in the salivary glands and secreted into the oral cavity. Facultative anaerobic bacteria present on the dorsal tongue facilitate the reduction of nitrate to nitrite via nitrate reductase enzymes. Genera such as Neisseria, Haemophilus, and Veillonella are recognized as key nitrate-reducers, yet the complete identification of bacteria with these properties is ongoing20,21,22. Nitrite is then converted to NO to influence vascular health under the acidic conditions of the stomach, in plasma or tissues by various nitrite reductase enzymes (such as deoxygenated hemoglobin, myoglobin, xanthine oxidoreductase, and aldehyde oxidase), or, in some instances, at the gingival margins23,24. While vasoprotective, these metabolites also support oral health through anti-microbial properties25.
Acknowledging the complex relationship between oral hygiene practices and cardiovascular health, this study aimed to target the prevention of both oral and vascular diseases by establishing the efficacy of a bioactive mouth rinse intervention: an alcohol-free, green tea-infused mouth rinse with added inorganic nitrates and antioxidants, specifically vitamin C and green tea-derived catechins. It was hypothesized that the bioactive mouth rinse would offset the rise in blood pressure that has been observed following the use of antibacterial mouth rinses, as it provided inorganic nitrate to support the enterosalivary pathway while concurrently preserving the oral microbiome. Furthermore, antioxidants with established oral health benefits were included to positively influence redox balance while also supporting the enterosalivary pathway through the enhancement of nitrate and nitrite reduction for increased generation of NO26,27.
As such, the purpose of this study was to determine whether the use of the bioactive mouth rinse twice daily influenced mechanistic and clinical measures of the enterosalivary pathway, redox status, and vascular health over a one-week period compared to a standard mouth rinse containing chlorhexidine. This study also investigated changes in the oral microbiome that ensue following the use of the bioactive and chlorhexidine mouth rinses during the intervention period.
Methods
Experimental animals and study design
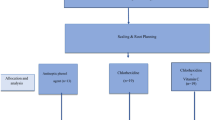
Male Wistar rats (n = 20, five weeks old) were purchased from Charles River Laboratories (Wilmington, MA, USA). Animals were individually housed in a climate-controlled environment (23 °C ± 1 °C, 12 h light-12 h dark cycle). Autoclaved water was replaced twice weekly to diminish the potential for bacterial overgrowth28. All procedures were carried out with accordance to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. All methods are reported in accordance with the ARRIVE guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Alabama (IACUC# 20-10-3981) (Tuscaloosa, AL, USA).
Following a seven-day acclimation period with free access to food and autoclaved water, rats were transitioned to a 10% fat-purified diet (Research Diets, New Brunswick, NJ, USA) (Supplemental Table 1) and were trained until nine weeks old through daily handling and restraint to reduce stress during intervention administration and experimental procedures. Following this three-week training period, rats were randomized to either the bioactive-infused mouth rinse group or the chlorhexidine-based (CHX) mouth rinse group for a one-week period (n = 10/group). A baseline assessment was conducted for both groups that consisted of oral microbiome sampling and blood pressure evaluation. After the intervention period began, the assigned rinse was administered twice daily (once in the morning and once in the evening) for a one-week period based on group assignment. Mouth rinse administration followed methods successfully employed in previous animal mouth rinse research29. In short, the rat was restrained in an upright position and 0.3 mL of the assigned mouth rinse was applied to the dorsal tongue using a micropipette in a controlled manner to ensure complete delivery without swallowing. The mouth rinse was delivered by the same study team members throughout the entire study period.
Due to the terminal nature of the salivary and blood collection procedures, described herein, and required sample volume, a separate group of male Wistar rats, raised under the same living and training conditions, underwent biospecimen collection followed by euthanasia without intervention assignment. These biospecimens were used to determine pre-intervention levels of nitrate, nitrite, and redox status in saliva and serum that could be compared to post-intervention levels.
Intervention details
The bioactive mouth rinse was composed of 5% green tea (Celestial Seasonings, Boulder, CO, USA), 14 mg sodium nitrate (Universal Preserv-A-Chem Inc., Mebane, NC, USA), and 40 mg ascorbic acid (Essential Depot, Sebring, FL, USA). All ingredients were food grade and available for safe use according to the Food and Drug Administration and the United States Department of Agriculture. To determine the dose of inorganic nitrate, principles of allometric scaling were employed and nitrate provision aligned with previous animal models30,31. Green tea concentration and vitamin C dose were based upon literature supporting the translocation of bioactive components from the oral cavity into circulation32,33.
The chlorhexidine-based mouth rinse was provided as a commercial antiseptic mouth rinse containing 0.2% chlorhexidine (Vedco Inc., Saint Joseph, MO, USA). This dose has been successfully implemented in previous animal studies29,34.
Outcome measure assessment
Oral microbiome
Rats were anesthetized using a combination of ketamine (80 mg/kg/body weight) and xylazine (5 mg/kg/body weight) injected into the intraperitoneal cavity (Patterson Veterinary, Loveland, CO, USA). Oral microbiome sampling followed a previously described protocol35. Briefly, the dorsal tongue of each rat was sampled using a sterile swab for 30 s. Swabs were stored at − 20 °C until time of analysis. Oral microbiome was assessed by 16S rRNA V4 gene amplicon sequencing using an Illumina MiSeq Platform (Illumina, San Diego, CA, USA)36. Amplicon based sequence variants (ASV) were analyzed by QIIME bioinformatics to determine relative abundance of taxa as well as alpha- and beta-diversity37.
Blood Pressure
Blood pressure was measured non-invasively using the BP-2000-R-2 Blood Pressure Analysis System (Visitech Systems, Inc., Apex, NC, USA)38. The platform was pre-heated to 38 °C, and a resting period of 20 min was implemented for each rat to ensure body temperature and sedation stabilization prior to beginning the measurement cycle.
Saliva collection
A stimulated saliva sample was collected following a previously described protocol39. Briefly, an intraperitoneal injection of pilocarpine hydrochloride (10 mg/kg/body weight) was administered to stimulate saliva secretion (Enzo Life Sciences, Inc., Farmingdale, NY, USA). Each rat was positioned laterally and approximately 1 mL of saliva was collected and stored at − 20 °C until time of analysis.
Blood collection
Following oral microbiome sampling, blood pressure, and saliva collection, rats were euthanized via carbon dioxide inhalation. Blood was collected from the vena cava, and 400 μL was immediately combined with 100 μl of a stop solution (0.8 M potassium cyanide, 0.1 M N-ethyl-maleimide, and 10% NP-40) and stored at − 80 °C for analysis of nitrite concentrations40. The remaining blood was centrifuged and the obtained serum was stored at − 80 °C until analysis of all other outcome measures.
Nitrate and nitrite assessment in blood and saliva
Nitrite concentrations were determined using ozone-based chemiluminescence and triiode solution as previously described41. Briefly, blood samples were injected into a purge vessel containing triiode solution which rapidly converted not only nitrite, but also S-nitrosothiols, and iron nitrosyl compounds into free NO gas which was subsequently measured by the chemiluminescence analyzer (NOA 280i, Sievers, Boulder, CO, USA). Therefore, although nitrosothiols and iron nitrosyls make up a minor fraction of the compounds detected in plasma compared to nitrite40. It should be noted that this assay detects more than just nitrite. Absolute concentrations were calculated by comparing peak areas to a nitrite standard curve. Nitrate concentrations were determined by enzymatic reduction of nitrate to nitrite by incubation with nitrate reductase enzyme (Roche, Indianapolis, IN, USA) at 37 °C for 45 min followed by a previously described triiode assay42.
Redox status in serum and saliva
Malondialdehyde (MDA), a product of lipid peroxidation, is a biomarker of oxidative stress. Serum and salivary MDA levels were quantified using the thiobarbituric acid reactive substances assay as previously described43. Results are expressed as mM MDA.
Prior to assessing antioxidant capacity, serum was deproteinated using methanol/acetonitrile/acetone (1:1:1, v/v/v) as previously described44. The oxygen radical absorbance capacity (ORAC) assay on a FLUOstar Optima plate reader (BMG Labtech, Cary, NC, USA) was used to measure the antioxidant capacity of deproteinated serum and saliva45. The compound 2, 2-azobis(2—amidino-propane) dihydrochloride was used as the peroxyl radical generator and Trolox, a water-soluble analogue of vitamin E, served as the reference antioxidant standard. Results are expressed as μM Trolox equivalents.
Statistical analysis
As inorganic nitrate is the bioactive compound of highest priority in the intervention, sample size calculations were based on previously published research evaluating inorganic nitrate provision and blood pressure in an animal model. For example, Pinheiro et al. 2016 implemented a similar nitrate dose using drinking water and reported a significant increase in circulating nitrate/nitrite levels with reductions in blood pressure compared to controls (n = 10 Wistar rats/group). As such, the current study implemented a sample size of 10 Wistar rats per group for the provision of 14 mg nitrate twice daily in the bioactive mouth rinse. This number of animals and dose allowed for 80% power to detect significant differences at p < 0.05.
Data are presented as mean ± SEM or median (25th percentile, 75th percentile) based on normality. Differences between groups were evaluated using independent t-tests or Mann–Whitney U tests, and paired t-tests or Kruskal Wallis tests were used to determine within-group changes. Kruskal Wallis tests were used to compare genera-level relative abundance of bacterial operational taxonomic units between groups. Change variables were calculated and analyzed for SBP and DBP only due to significant differences in baseline SBP between groups (Completion–Baseline = Change). Associations between outcome measures were also assessed using Pearson’s or Spearman’s correlation coefficients based on normality of data. Significance was defined as p < 0.05. All analyses were performed using SPSS Statistics version 25 (SPSS, Inc., Chicago, IL, USA).
Results
Blood pressure
From baseline to study completion, SBP and DBP did not significantly change in the CHX rinse group (p = 0.501, p = 0.303). In contrast, SBP and DBP were significantly reduced in the bioactive rinse group such that SBP and DBP were reduced by 15.6% mmHg (p = 0.008) and 28.8% (p = 0.005), respectively. No significant changes in heart rate were observed in either group.
Overall, changes in SBP and DBP from baseline to study completion were significantly greater in the bioactive rinse group (SBP: − 19.2 ± 5.7 mmHg, DBP: − 18.4 ± 5.5 mmHg) compared to the CHX rinse group (SBP: + 3.5 ± 5.0 mmHg, DBP: + 6.3 ± 5.7 mmHg) (SBP: p = 0.008, DBP: p = 0.005). Changes in heart rate were not significantly different between the groups.
Circulating Nitrate and Nitrite Concentrations
There was not a significant increase in blood or salivary nitrate or nitrite concentrations in the CHX rinse group (Blood: p = 0.623, p = 0.098, respectively; Salivary: p = 0.572, p = 0.553, respectively) after one week of intervention. In contrast, significant alterations were observed in the bioactive rinse group such that blood and salivary nitrate increased by 32.4-fold (p < 0.001) and 5.7-fold (p = 0.004), respectively, after one week of intervention. Blood and salivary nitrite concentrations did not significantly change in the bioactive rinse group.
At study completion, blood nitrate concentrations were significantly greater in the bioactive rinse group compared to the CHX rinse group (p < 0.001, Fig. 1; Table 1). Blood nitrite was not significantly different between groups. Salivary nitrate and nitrite concentrations were also significantly greater in the bioactive rinse group compared to the CHX rinse group (p = 0.003, p = 0.017, respectively, Fig. 2).
Differences in (A) blood nitrate and (B) blood nitrite between the bioactive-infused mouth rinse group and the chlorhexidine mouth rinse group at week one. Bioactive = bioactive-infused mouth rinse group; CHX = chlorhexidine mouth rinse group. Data presented as mean ± standard deviation or median (25th percentile, 75th percentile) depending upon normality assessment. *denotes significance of p < 0.05.
Differences in (A) salivary nitrate and (B) salivary nitrite between the bioactive-infused mouth rinse group and the chlorhexidine mouth rinse group at week one. Bioactive = bioactive-infused mouth rinse group; CHX = chlorhexidine mouth rinse group. Data presented as mean ± standard deviation or median (25th percentile, 75th percentile) depending upon normality assessment. *denotes significance of p < 0.05.
Circulating redox measures
There was a significant reduction in serum hydrophilic and total AC by 14.8% (p = 0.012) and 16.0% (p = 0.038), respectively, in the CHX rinse group after one week of intervention. No significant changes in serum antioxidant capacity measures were observed in the bioactive rinse group. Both the CHX rinse group and the bioactive rinse group exhibited a significant decrease in salivary lipophilic antioxidant capacity after one week of intervention such that concentrations were reduced by 51.7% (p = 0.007) and 59.3% (p < 0.001), respectively. Serum and salivary oxidative stress levels were not significantly altered after one week of intervention in either group.
At study completion, all serum antioxidant capacity measures were significantly higher in the bioactive rinse group compared to the CHX rinse group (hydrophilic: p = 0.038, lipophilic: p = 0.002, total: p = 0.002). No significant differences in salivary antioxidant capacity measures were observed between groups. Similarly, serum and salivary oxidative stress levels were not significantly different between groups.
Analysis of oral microbe composition
Beta- and alpha-diversity
Beta diversity group analysis was significantly different between the bioactive-infused mouth rinse group and chlorhexidine mouth rinse group using Bray–Curtis (p = 0.012) or unweighted unifrac metrics (p = 0.001) (Fig. 3).
Overall oral microbiome composition by group visually represented using principle coordinate analysis (PCoA) plots at week one. Oral microbiome composition was significantly different between the bioactive-infused mouth rinse group and chlorhexidine mouth rinse group using (A) Bray–Curtis and (B) unweighted unifrac. Each point represents a single oral microbiome sample. Red points denote the bioactive-infused mouth rinse group; Grey points denote the chlorhexidine mouth rinse group. Significance defined as p < 0.05. No significant difference (p > 0.05) was observed between the groups using weight unifrac analysis.
From baseline to study completion, the alpha diversity metric Shannon Diversity Index did not significantly change in the CHX rinse group (p = 0.180); however, a significant reduction in the observed species level was observed (p = 0.003). In contrast, the bioactive rinse group exhibited a significant reduction in the Shannon Diversity Index from baseline to study completion (p = 0.049). No significant changes in the observed species level were observed.
At study completion, the Shannon Diversity index was not significantly different between groups, yet the observed species level was significantly lower in the CHX rinse group compared to the bioactive rinse group (p = 0.019).
Changes in mean relative abundance of nitrate- or nitrite-reducing bacteria
Kruskal–Wallis analysis revealed that the bioactive rinse group had significantly greater (p < 0.05) mean relative abundance of genera with established nitrate- or nitrite-reducing capabilities compared to the CHX rinse group, including Veilloneilla (CHX: 0.38%, Bioactive: 1.05%), Haemophilus (CHX: 0.05%, Bioactive: 0.11%), and Staphylococcus (CHX: 0.94%, Bioactive: 1.49%).
Correlations
Across both groups, significant inverse correlations between DBP and blood nitrate (r = − 0.619, p = 0.005), salivary nitrate (r = − 0.627, p = 0.007), and salivary nitrite (r = − 0.552, p = 0.022) were observed. DBP was also significantly inversely correlated with serum hydrophilic AC (r = − 0.478, p = 0.038), serum lipophilic AC (r = 0.456, p = 0.050), and serum total AC (r = − 0.542, p = 0.017).
In the bioactive rinse group only, DBP and salivary nitrate/nitrite were beneficially correlated with the relative abundance of Staphylococcus (Salivary nitrate: r = 0.795, p = 0.010), Veillonella (DBP: r = − 0.750, p = 0.020), Streptococcus (DBP: r = − 0.667, p = 0.050), and Neisseria (Salivary nitrite: r = 0.794, p = 0.011).
Discussion
The present study aimed to evaluate the influence of a novel bioactive mouth rinse containing inorganic nitrate and antioxidants on mechanistic and clinical outcomes of the enterosalivary pathway, redox status, and vascular health, as well as the oral microbiome, over a one-week period compared to a standard antibacterial mouth rinse containing CHX. In short, results suggest that a bioactive mouth rinse increases salivary and circulating levels of nitrate, favorably modulates the oral microbiome composition to support nitrate reduction, and reduces blood pressure compared to a CHX mouth rinse. To our knowledge, this is the first study to target the prevention of both oral and vascular diseases by evaluating the influence of a functional mouth rinse.
Previous research has established the blood pressure-lowering effects of dietary nitrate such that the provision of nitrate-rich foods or beverage resulted in improved vascular measures both short- and long-term46,47,48. The current study is unique in that it provided dietary nitrate in an oral hygiene intervention, thus relying on the translocation of compounds from the oral cavity into circulation at the salivary gland interface. Results demonstrated that the twice daily administration of a bioactive mouth rinse significantly lowered SBP and DBP over a one-week period.
Significant alterations in blood pressure have also been observed in other animal studies providing a similar dose of dietary nitrate, albeit by drinking water or oral gavage29,31,34. Interestingly, the current study observed a greater magnitude of improvement in both SBP and DBP compared to the aforementioned publications. These discrepancies may stem from differences in the intervention vehicle and composition. Moreover, although nitrate was the bioactive compound of greatest interest in the bioactive mouth rinse intervention, other antioxidants were included, namely catechins from green tea and vitamin C. Independently, these antioxidants influence the mechanisms underlying blood pressure control in animal and human models, and they enhance the reduction of nitrate and nitrite in the enterosalivary pathway for increased generation of nitric oxide16,24,49,50. It is plausible that in combination, these factors resulted in an amplified blood pressure response over time. It is also important to consider the potential contributions of blood pressure variability among rodents owing to their outbred background. A significant difference in baseline SBP was observed between the bioactive and CHX mouth rinse groups despite standardized living and testing conditions. Accordingly, a change variable (Completion–Baseline = Change) was calculated for both SBP and DBP and used to investigate the hypothesis. It is possible that the difference in baseline SBP may have contributed to the magnitude of change observed. However, no significant differences in DBP were observed between groups at baseline, and a significant difference at study completion was observed in favor of the bioactive mouth rinse group (Supplemental Table 2). Taken collectively, the overall favorable results reported for blood pressure following a bioactive mouth rinse intervention are stronger for DBP and require further investigation in animal and human subjects.
To date, dietary nitrate interventions in humans have typically been provided in food or beverage form, including beet root juice, green leafy vegetables, or sodium nitrate, and in drinking water or oral gavage in preclinical models. Accordingly, the inorganic nitrate is swallowed, digested, and absorbed before undergoing concentration in the salivary glands for incorporation into the enterosalivary pathway19. This process results in an increase in circulating and/or salivary concentrations of nitrate and nitrite. In contrast, the current study primarily relied on the transfer of compounds from the oral cavity into circulation at the salivary gland interface since nitrate was delivered in an oral hygiene intervention. Results support the bioavailability of the bioactive mouth rinse such that blood and salivary nitrate concentrations were significantly increased after one-week, and these increases were significantly greater as compared to the CHX group. Significant inverse correlations were also observed between blood pressure and blood nitrate.
Of interest, the current study observed significant increases in blood nitrate, but blood nitrite was not significantly altered. It is unclear if other studies implementing a dietary nitrate intervention had a similar pattern of metabolite response, as most animal studies report a combined metric (NOx = nitrate + nitrite) or only report a single metabolite; however, Pinheiro et al. 2016 reported a significant increase in plasma nitrate by 60 – 90 μmol/L in non-hypertensive and hypertensive adult rats after a four-week inorganic nitrate intervention31. The direction of findings from the current study aligns with Pinheiro et al., yet the magnitude of increase was lower. It is plausible that differences in methodologies used to quantify nitrate and nitrite underpin this discrepancy. In the current study, blood nitrite was not significantly different between groups at week one. This is distinct from another one-week inorganic nitrate intervention study in which plasma nitrite levels were significantly increased34. These results may be indicative of nitrite in circulation rapidly converted to more vasoactive NO metabolites in the current study, as evidenced by the changes in SBP and DBP observed.
To our knowledge, this is the first study to quantify nitrate and nitrite concentrations in saliva of rats. Salivary nitrate and nitrite concentrations were significantly higher in the bioactive rinse group compared to the chlorhexidine rinse group at study completion. Similar increases have been reported in human studies following a dietary nitrate intervention14,51. Overall, these results support the bioavailability of inorganic nitrate in the bioactive mouth rinse, as well as successful incorporation into the enterosalivary pathway.
The bioactive mouth rinse was developed with additional bioactive compounds including green tea catechins and vitamin C, to support the efficiency of the enterosalivary pathway and for potential downstream redox benefits owing to their antioxidant properties. However, no significant alterations in serum or salivary oxidative stress were observed. Similarly, salivary AC did not significantly increase in the bioactive rinse group at week one as originally hypothesized. In fact, both the bioactive rinse and CHX rinse groups exhibited a significant reduction in salivary lipophilic AC. These findings underscore the need for continued research evaluating how conventional and alternative mouth rinse use influence redox status in the oral cavity and systemically. Nevertheless, a bioactive mouth rinse administered twice daily was successful in maintaining circulating antioxidant concentrations compared to the chlorhexidine group over a one-week period.
Differences in dietary patterns, oral hygiene habits, and medication use, among other factors, all contribute to the inter-individual variability observed in oral microbiome composition21,22. Such variability influences the efficiency of the enterosalivary pathway owing to varying concentrations in the relative abundance of bacteria with established nitrate-reducing properties. Nitrate-rich foods have been hypothesized to induce changes in the oral microbiome to support an increased efficiency of nitrate reduction to nitrite and, subsequently, NO21. Results from the current study align with this hypothesis such that the bioactive mouth rinse containing nitrate favorably modified the oral microbiome in support of the enterosalivary pathway compared to the chlorhexidine rinse over a one-week period. For example, at the genus level, the bioactive mouth rinse resulted in a significantly greater mean relative abundance of previously identified nitrate- and nitrite-reducers, Veillonella, Haemophilus, and Staphylococcus compared to the CHX rinse. These genera possess nitrate reductase enzymes and metabolic flexibility that support the conversion of nitrate to nitrite. Significant positive correlations were also observed between the aforementioned genera and serum nitrate concentrations and salivary nitrate/nitrite concentrations. Current findings build upon those of Hyde and colleagues in which a one-week dietary nitrate intervention increased the mean relative abundance of Haemophilus and Streptococcus in male Wistar rats29. Direct comparison of results to other animal studies is limited by inconsistencies in methodology of microbiome assessment (i.e., culture-based techniques vs. 16S rRNA gene sequencing). The genus-level changes observed in this study align with those previously reported in human subjects. Specifically, Veillonella have been identified as a predominant nitrate-reducing bacteria on the human tongue, and Haemophilus and Staphylococcus have been highlighted as minor contributors29,52. Although not all major nitrate-reducing species were altered in the current study, observed increases and correlations in key genera suggest that the bioactive rinse may positively influence the oral microbiome and warrants continued investigation.
Significant differences in alpha-diversity and beta-diversity were also observed between the bioactive rinse group and the CHX rinse group after one week which suggests that the rinses cultivated two distinct oral microbiomes. Specifically, a significantly lower observed species level in the CHX rinse group suggests that the antibacterial rinse was successful in disrupting the oral microbiome by lowering species richness. Differences in beta-diversity further support that the bioactive mouth rinse and the CHX mouth rinse cultivated two distinct oral microbiome compositions.
Collectively, significant alterations in the oral microbiome composition of the bioactive rinse group likely increased the efficiency of the enterosalivary pathway and, subsequently, increased nitrate/nitrite levels to support the observed changes in blood pressure.
This study advances the current understanding of dietary nitrate interventions on the enterosalivary pathway through the investigation of a dietary bioactive mouth rinse compared to a standard CHX mouth rinse. Results are strengthened by using purified diets and autoclaved water, as well as standardized animal training protocols to limit confounding variables. Additionally, gold standard methods, such as 16S rRNA gene sequencing with V4 amplification, non-invasive tail cuff blood pressure with plethysmography, and ozone-based chemiluminescence, were included and allometric scaling was employed to support translation of findings. Despite strengths, the study is not without inherent limitations. Although Wistar rats are a well-established model to evaluate outcomes related to the enterosalivary pathway, there are noteworthy differences between rodents and humans. For instance, nitrate is not concentrated in rodent salivary glands to the same degree as humans, although the mechanism underpinning this difference is unclear53. Additionally, it is common for rodents to have variability in blood pressure despite standardized conditions (i.e., like consistency in time of day of readings) owing to their outbred background54. Such variability impacts the precision of results.
Overall, results suggest a mouth rinse infused with inorganic nitrate and antioxidants favorably modulates oral microbiome composition in support of the enterosalivary pathway and reduces blood pressure compared to standard CHX rinse treatment over a one-week period. Acknowledging that 1.98 billion adults worldwide are living with hypertension, optimizing oral hygiene practices may have significant public health implications, as this represents a feasible intervention that can easily be integrated into an individual’s daily routine. To date, most nitrate interventions in humans have been delivered as beetroot juice, nitrate-rich vegetables, or nitrate salt capsules. As such, short- and long-term investigation of the bioactive rinse among humans is needed to provide insight regarding bioavailability and clinical vascular and oral health measures. Such studies will overcome the aforementioned limitations associated with animal models, and enable the consideration of baseline oral microbiome composition as a mediating variable. Ultimately, supporting the potential translation of this bioactive rinse into clinical practice.
Data availability
The oral microbiome datasets generated and analyzed during the current study are available in the National Institutes of Health Sequence Read Archive (SRA) repository, PRJNA1274611. The remaining data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AC:
-
Antioxidant capacity
- ASV:
-
Amplicon sequencing variants
- CHX:
-
Chlorhexidine
- CVD:
-
Cardiovascular disease
- DBP:
-
Diastolic blood pressure
- MDA:
-
Malondialdehyde
- NO:
-
Nitric oxide
- NOx :
-
Sum of nitrate and nitrite
- NP-40:
-
Nonidet P-40
- PCoA:
-
Principle coordinate analysis
- ROS:
-
Reactive oxygen species
- SBP:
-
Systolic blood pressure
References
Benjamin, E. J. et al. Heart disease and stroke statistics-2019 update: A report from the American heart association. Circulation 139, e56–e528. https://doi.org/10.1161/CIR.0000000000000659 (2019).
Siti, H. N., Kamisah, Y. & Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul. Pharmacol. 71, 40–56 (2015).
Cai, H. & Harrison, D. G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 87, 840–844. https://doi.org/10.1161/01.res.87.10.840 (2000).
Wang, Y., Andrukhov, O. & Rausch-Fan, X. Oxidative stress and antioxidant system in periodontitis. Front. Physiol. 8, 910 (2017).
Lima, D. P., Diniz, D. G., Moimaz, S. A., Sumida, D. H. & Okamoto, A. C. Saliva: Reflection of the body. Int. J. Infect Dis. 14, e184-188. https://doi.org/10.1016/j.ijid.2009.04.022 (2010).
Nazir, M. A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. (Qassim) 11, 72–80 (2017).
Janket, S. J., Baird, A. E., Chuang, S. K. & Jones, J. A. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod 95, 559–569. https://doi.org/10.1067/moe.2003.107 (2003).
Eke, P. I. et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 91, 914–920. https://doi.org/10.1177/0022034512457373 (2012).
Centers for Disease Control and Prevention. Periodontal disease. Centers for Disease Control and Prevention website. https://www.cdc.gov/oralhealth/periodontal_disease/index.htm. Published July 10, 2013. Updated March 10, 2015. Accessed January 30, 2018.
Statista. US population: do you use mouthwash/dental rinse? https://www.statista.com/statistics/276434/us-households-usage-of-mouthwash-and-dental-rinse/ Accessed June 7, 2019.
Ciancio, S. G. Mouthwashes: Rationale for use. Am J Dent 28, 4A-8A (2015).
Adams, D. & Addy, M. Mouthrinses. Adv. Dent. Res. 8, 291–301. https://doi.org/10.1177/08959374940080022401 (1994).
Kapil, V. et al. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med 55, 93–100 (2013).
Bondonno, C. P. et al. Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women. Am J Hypertens 28, 572–575. https://doi.org/10.1093/ajh/hpu192 (2015).
Senkus, K. E. & Crowe-White, K. M. Influence of mouth rinse use on the enterosalivary pathway and blood pressure regulation: A systematic review. Crit Rev Food Sci Nutr 60, 2874–2886. https://doi.org/10.1080/10408398.2019.1665495 (2020).
Ashworth, A. & Bescos, R. Dietary nitrate and blood pressure: evolution of a new nutrient?. Nutr. Res. Rev. 30, 208–219. https://doi.org/10.1017/S0954422417000063 (2017).
Hord, N. G., Tang, Y. & Bryan, N. S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 90, 1–10 (2009).
Duncan, C. et al. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med. 1, 546–551. https://doi.org/10.1038/nm0695-546 (1995).
Lundberg, J. O., Weitzberg, E. & Gladwin, M. T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 7, 156–167. https://doi.org/10.1038/nrd2466 (2008).
Smith, A. J., Benjamin, N., Weetman, D. A., Mackenzie, D. & Macfarlane, T. W. The microbial generation of nitric oxide in the human oral cavity. Microb. Ecol. Health Dis. 11, 23–27 (1999).
Tribble, G. D. et al. Frequency of tongue cleaning impacts the human tongue microbiome composition and enterosalivary circulation of nitrate. Front. Cell. Infect. Microbiol. 9, 39. https://doi.org/10.3389/fcimb.2019.00039 (2019).
Vanhatalo, A. et al. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free. Radic. Biol. Med. 124, 21–30. https://doi.org/10.1016/j.freeradbiomed.2018.05.078 (2018).
Castiglione, N., Rinaldo, S., Giardina, G., Stelitano, V. & Cutruzzola, F. Nitrite and nitrite reductases: From molecular mechanisms to significance in human health and disease. Antioxid Redox Signal 17, 684–716. https://doi.org/10.1089/ars.2011.4196 (2012).
Weitzberg, E. & Lundberg, J. O. Nonenzymatic nitric oxide production in humans. Nitric Oxide 2, 1–7. https://doi.org/10.1006/niox.1997.0162 (1998).
Alhulaefi, S. S. et al. Effects of dietary nitrate supplementation on oral health and associated markers of systemic health: A systematic review. Crit. Rev. Food Sci. Nutr. https://doi.org/10.1080/10408398.2024.2351168 (2024).
Dai, F., Chen, W. F. & Zhou, B. Antioxidant synergism of green tea polyphenols with alpha-tocopherol and L-ascorbic acid in SDS micelles. Biochimie 90, 1499–1505. https://doi.org/10.1016/j.biochi.2008.05.007 (2008).
Ferrazzano, G. F. et al. Antimicrobial properties of green tea extract against cariogenic microflora: An in vivo study. J. Med. Food 14, 907–911. https://doi.org/10.1089/jmf.2010.0196 (2011).
Peveler, J. L., Crisler, R. & Hickman, D. Quality testing of autoclaved rodent drinking water during short-term and long-term storage. La.b Anim. 44, 211–215. https://doi.org/10.1038/laban.734 (2015).
Hyde, E. R. et al. Characterization of the rat oral microbiome and the effects of dietary nitrate. Free. Radic. Biol. Med. 77, 249–257. https://doi.org/10.1016/j.freeradbiomed.2014.09.017 (2014).
Reagan-Shaw, S., Nihal, M. & Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 22, 659–661. https://doi.org/10.1096/fj.07-9574LSF (2008).
Pinheiro, L. C. et al. Oral nitrite circumvents antiseptic mouthwash-induced disruption of enterosalivary circuit of nitrate and promotes nitrosation and blood pressure lowering effect. Free Radic. Biol. Med. 101, 226–235. https://doi.org/10.1016/j.freeradbiomed.2016.10.013 (2016).
Yang, C. S., Lee, M. J. & Chen, L. Human salivary tea catechin levels and catechin esterase activities: Implication in human cancer prevention studies. Cancer Epidemiol. Biomarkers Prev. 8, 83–89 (1999).
Pharmacology/Toxicology NDA/BLA Review and Evaluation: Ascor® (Ascorbic Acid). Department of Health and Human Services, Public Health Service Food and Drug Administration, Center for Drug Evaluation and Research. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209112Orig1s000_PharmTox.pdf. Published August 31, 2016. Accessed October 24, 2020.
Petersson, J. et al. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic. Biol. Med. 46, 1068–1075. https://doi.org/10.1016/j.freeradbiomed.2009.01.011 (2009).
Abusleme, L. et al. Oral microbiome characterization in murine models. Bio Protoc 7, e2655. https://doi.org/10.21769/BioProtoc.2655 (2017).
Kumar, R. et al. Getting started with microbiome analysis: Sample acquisition to bioinformatics. Curr. Protoc. Hum. Genet. 82, 18.8.1-18.8.29. https://doi.org/10.1002/0471142905.hg1808s82 (2014).
Van Der Pol, V. D. et al. In silico and experimental evaluation of primer sets for species level resolution of vaginal microbiota using 16S rRNA gene sequencing. J. Infect Dis. 219, 305–214 (2018).
Krege, J. H., Hodgin, J. B., Hagaman, J. R. & Smithies, O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25, 1111–1115. https://doi.org/10.1161/01.hyp.25.5.1111 (1995).
Lasisi, T. J., Shittu, S. T. & Alada, A. R. Switching to normal diet reverses kwashiorkor-induced salivary impairments via increased nitric oxide level and expression of aquaporin 5 in the submandibular glands of male Wistar rats. Appl. Physiol. Nutr. Metab. 44, 365–372. https://doi.org/10.1139/apnm-2018-0282 (2019).
Pelletier, M. M. et al. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic. Biol. Med. 41, 541–548. https://doi.org/10.1016/j.freeradbiomed.2006.05.001 (2006).
MacArthur, P. H., Shiva, S. & Gladwin, M. T. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 851, 93–105. https://doi.org/10.1016/j.jchromb.2006.12.012 (2007).
Kanady, J. A. et al. Nitrate reductase activity of bacteria in saliva of term and preterm infants. Nitric Oxide 27, 193–200. https://doi.org/10.1016/j.niox.2012.07.004 (2012).
Mohamadin, A. M., Hammad, L. N., El-Bab, M. F. & Abdel Gawad, H. S. Attenuation of oxidative stress in plasma and tissues of rats with experimentally induced hyperthyroidism by caffeic acid phenylethyl ester. Basic Clin. Pharmacol. Toxicol. 100, 84–90. https://doi.org/10.1111/j.1742-7843.2006.00003.x (2007).
Crowe, K. M. Optimizing protein precipitation efficiency for assessing the contribution of low molecular weight compounds to serum antioxidant capacity. Clin. Biochem. 47, 116–118. https://doi.org/10.1016/j.clinbiochem.2014.06.021 (2014).
Prior, R. L. et al. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J. Agric. Food Chem. 51, 3273–3279. https://doi.org/10.1021/jf0262256 (2003).
Kapil, V., Khambata, R. S., Robertson, A., Caulfield, M. J. & Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 65, 320–327. https://doi.org/10.1161/HYPERTENSIONAHA.114.04675 (2015).
Benjamim, C. J. R. et al. Nitrate derived from beetroot juice lowers blood pressure in patients with arterial hypertension: A systematic review and meta-analysis. Front Nutr 9, 823039. https://doi.org/10.3389/fnut.2022.823039 (2022).
Jackson, J. K., Patterson, A. J., MacDonald-Wicks, L. K., Oldmeadow, C. & McEvoy, M. A. The role of inorganic nitrate and nitrite in cardiovascular disease risk factors: A systematic review and meta-analysis of human evidence. Nutr. Rev. 76, 348–371. https://doi.org/10.1093/nutrit/nuy005 (2018).
Guan, Y., Dai, P. & Wang, H. Effects of vitamin C supplementation on essential hypertension: A systematic review and meta-analysis. Medicine 99, e19274. https://doi.org/10.1097/MD.0000000000019274 (2020).
Khalesi, S. et al. Green tea catechins and blood pressure: a systematic review and meta-analysis of randomised controlled trials. Eur. J. Nutr. 53, 1299–1311. https://doi.org/10.1007/s00394-014-0720-1 (2014).
Hohensinn, B. et al. Sustaining elevated levels of nitrite in the oral cavity through consumption of nitrate-rich beetroot juice in young healthy adults reduces salivary pH. Nitric Oxide 60, 10–15. https://doi.org/10.1016/j.niox.2016.08.006 (2016).
Doel, J. J., Benjamin, N., Hector, M. P., Rogers, M. & Allaker, R. P. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral. Sci. 113, 14–19. https://doi.org/10.1111/j.1600-0722.2004.00184.x (2005).
Montenegro, M. F. et al. Profound differences between humans and rodents in the ability to concentrate salivary nitrate: Implications for translational research. Redox. Biol. 10, 206–210. https://doi.org/10.1016/j.redox.2016.10.011 (2016).
Buttner, D., Hackbarth, H., Wollnik, F. & Borggreve, H. Blood pressure in rats: a comparison of a multifactorial experimental design to measurements in an outbred stock. Lab. Anim. 18, 110–114. https://doi.org/10.1258/002367784780891334 (1984).
Funding
This research was funded by the Academy of Nutrition and Dietetics Foundation 2019 Colgate Palmolive Fellowship in Nutrition, Oral Health/Dental Education. The funding source had no role in the design, analysis or writing of this article.
Author information
Authors and Affiliations
Contributions
The author contributions were as follows: Conceptualization (KES, KMCW); Funding acquisition (KES, KMCW); Investigation (KES, KMCW); Methodology (KES, KMCW, MZ, TL, ABB, WP, CM); Formal analysis (KES, AB); Project administration (KES, KMCW); Supervision (KMCW, MA, AB, HP, JRT); Roles/Writing - original draft (KES); and Writing - review & editing (KES, KMCW, MA, AB, HP, JRT, MZ, TL, ABB, WP, CM).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Senkus, K.E., Azrad, M., Bolland, A. et al. Functional mouth rinse containing inorganic nitrate and antioxidants bolsters the enterosalivary pathway and lowers blood pressure in Wistar rats. Sci Rep 15, 26827 (2025). https://doi.org/10.1038/s41598-025-12060-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-12060-0