Abstract
Plant disease management based on pesticide use has numerous detrimental effects on health and the environment. As a result, the adoption of environment-friendly disease management options is the best alternative to pesticide use. Therefore, the identification of locally available bio-agents like Trichoderma species using morpho-cultural and molecular approaches specifically targeting the internal transcribed spacer (ITS) region, translation elongation factor 1-alpha (TEF 1-α) and RNA polymerase B subunit II (RPB2) is necessary. In this study, we characterized 24 Trichoderma strains isolated from the apple rhizosphere. Significant variations were observed in the morpho-cultural characteristics of Trichoderma isolates and categorized them into four groups (I-IV) that were identified as T. harzianum complex, T. koningiopsis, T. viride, and T. hamatum, comprising 4, 4, 6 and 10 isolates, respectively. The concatenated sequence data set derived from the ITS region, TEF 1-α and RPB2 grouped 24 Trichoderma isolates into 03 independent clades. Specifically, the sequencing based on ITS region grouped them into four sub-clades, which were identified as T. harzianum complex, T. viride, T. asperelloides, and T. koningiopsis, comprising 4, 6, 5 and 7 isolates, respectively, and two independent lineages, each represented by a single isolate. In contrast, sequencing of the TEF 1-α and RPB2 genes grouped 24 Trichoderma isolates into two distinct clades and six sub-clades that were identified as T. asperelloides, T. asperellum, T. hamatum, T. viride, T. koningiopsis and T. harzianum complex, comprising 5, 5, 3, 4, 3 and 4 isolates, respectively. Thus, the final identification of 24 Trichoderma strains was achieved through a combined morpho-cultural and molecular approach, resulting in the identification of six species viz., T. koningiopsis, T. viride, T. asperellum, T. asperelloides, T. hamatum and T. harzianum complex comprising 5, 5, 3, 4, 3 and 4 isolates, respectively in accordance with the reference sequences retrieved from NCBI. Notably, to our knowledge, this is the first report of T. koningiopsis, T. viride, T. asperellum, T. asperelloides, and T. hamatum from the apple rhizosphere.
Similar content being viewed by others
Introduction
Apple (Malus x domestica L.) is a premier table fruit cultivated in the temperate regions of the world as well as in the cool highlands of sub-tropical regions such as East Africa and the Northeastern parts of India. In India, apple cultivation accounts for 55% of the total area and 75% of the total production under temperate fruits1. In India, the commercial cultivation of apples is primarily confined to Jammu and Kashmir, Himachal Pradesh, and some selected parts of Uttarakhand, Arunachal Pradesh, Manipur, and Sikkim. In Jammu and Kashmir, apple is the most significant fruit crop, covering an area of 16,47,42,000 ha with an annual production of 18,82,319 metric tons2. However, apple is prone to various fungal, bacterial, and viral diseases affecting its quality and production. Among these, fungal diseases inflict substantial losses on the crop. Notably, soil-borne diseases such as white root rot and collar rot caused by Dematophora necatrix and Phytopthora cactorum, respectively, are significant threats after scab to the apple industry globally, including in Jammu and Kashmir3.
Strategies like pesticides and biological control agents are being used to manage plant diseases, especially soil-borne diseases4. However, the excessive use of pesticides leads to contamination of soil and water as many components of pesticides are recalcitrant and persist in the environment for a longer duration5. As a result, the application of biological control agents for plant disease management is gaining popularity as a way to reduce or eliminate the use of synthetic pesticides4. Among the various biological control agents of fungal origin, the Trichoderma species is the most intensively studied fungal biocontrol agent. Its biocontrol activity is of immense importance to agriculture and the environment, without any harmful effects on plants, animals, and humans6,7.
The genus Trichoderma is classified under the subdivision: Pezizomycotina (Ascomycota); class: Sordariomycetes; family: Hypocreaceae8,9. Over the past 35 years, the number of recognized Trichoderma aggregate and phylogenetic species has increased from nine to approximately eighty, respectively, indicating its discovery phase. It shows that several new Trichoderma species are likely to be identified through the exploration of new niches and new geographical regions. The identification and phylogenetic classification of strains have been supported by the development of new molecular tools, making DNA sequence data essential for the accurate identification of species within Trichoderma or any other economically important genera10,11. Some of the Trichoderma strains like T. viride, T. harzianum, T. longibrachiatum, T. hamatum, and T. koningii are recognized for their efficient biocontrol ability to inhibit the growth of soil-borne plant pathogens, hence improving overall plant health12,13. These strains are opportunistic, avirulent plant symbionts and parasites of other plant pathogenic fungi, commonly found in soil and root ecosystems14. For instance, Trichoderma virens isolate Tvr4 and Trichoderma harzianum isolate Thr15 have shown high effectiveness against the mycelial growth and microsclerotial suppression of Macrophomina phaseolina, causing charcoal rot of strawberry15. Rifai made an initial attempt to understand the diversity in Trichoderma spp. by introducing the concept of 9 “species groups”16. As is usually the case with other fungal genera, species of Trichoderma were also defined originally based on morphology by Rifai and Bissett16,17,18. The cultural sporulation pattern varied considerably within and between the two species of Trichoderma. Although conidial shape and arrangement, and hyphal branching pattern helped in distinguishing species from each other, they failed to designate Trichoderma species. Seaby19 also reported the difficulties in differentiation of Trichoderma spp. using classical microscopic features alone since cultural morphology and spore size significantly varied on different media and incubation temperature, respectively. Moreover, variation among the isolates based on phialide size, and their arrangements is very less. However, the sporulation pattern and spore size within the species are highly variable. Consequently, the morphological data can lead to misidentification, with 30–50% of identified characteristics potentially erroneous10. To overcome these limitations, the research over the past many years has increasingly focused on application of molecular approaches, resulting in the re-classification of several species and strains20. Molecular methods particularly based on multiple gene sequencing enhance the accuracy of the fungal species identification. Internal transcribed spacer (ITS) region of ribosomal DNA (rDNA) is one of the most consistently used targets to identify a Trichoderma strain at the species level21,22but some of the meticulously related species of Trichoderma share the sequences of their ITS regions, therefore cannot differentiate all the Trichoderma species [International Sub-commission on Trichoderma and Hypocrea Taxonomy (http://www.isth.info)]11,23. Conversely, translation elongation factor 1-alpha (TEF 1-α) and RNA polymerase B II (RPB2) genes are more variable and can effectively reflect the differences within and among the groups of closely related species in Trichoderma. Consequently, a combination of multigene sequencing based on TEF 1-α, and RPB2 genes, and the ITS region, proves invaluable for the proper identification of Trichoderma at the species level11,23. Besides, ITS, TEF 1-α, and RPB2 sequencing identified Trichoderma guizhouense in Türkiye24.
Utilizing combinatorial strategies such as morpho-cultural and molecular studies based on DNA barcodes significantly enhances the capacity to accurately delineate various Trichoderma species22,25. Furthermore, as the identification of a plant disease/ pathogen is crucial for its effective management, the species identification of a biocontrol agent is equally essential for the development of commercial products. Therefore, keeping in view the lack of comprehensive studies regarding the variability of Trichoderma species in India particularly in Jammu and Kashmir, the present study aims to characterize the Trichoderma isolates collected from apple rhizosphere using conventional (morpho-cultural characteristics) and molecular methods based on ITS region, and TEF 1-α and RPB2 gene sequencing.
Results and discussion
Morpho-cultural characterization of the trichoderma isolates
Colony characteristics
Different Trichoderma isolates cultured on potato dextrose agar (PDA) medium (HiMedia Lab. Pvt. Ltd., Mumbai, India) showed notable variations in colony characteristics viz., colony texture, margins, colour, and shape (Table 1; Fig. 1). Based on texture, Trichoderma isolates were divided into Group I (cottony), Group II (Fluffy), and Group III (Velvety) comprising of 33.33% of isolates (Psh2, Psh3, PTi1, PTi2, PR3, NT2, Z1, and Z2), 41.67% (Psh1, PTi3, PNi1, PNi3, PR1, PR2, SR, SG, TB2, and TB3) and 25.0% of isolates (PNi2, SS, TB1, NT1, NT3, and Z3), respectively (Table 1). Further categorization of the isolates based on colony margins yielded two groups: Group I (regular margins) and Group II (irregular margins) (Table 1). Notably, the majority of isolates (87.50%) namely Psh1, Psh2, Psh3, PTi1, PTi2, PNi2, PNi3, PR1, PR2, PR3, SS, SR, SG, TB1, TB3, NT1, NT2, NT3, Z1, Z2 and Z3 were accommodated in Group I, whereas Group II comprised of only three isolates viz., PNi1, PTi3, and TB2 accounting for 12.50% of the remaining isolates. Furthermore, Trichoderma isolates were categorized based on colony colour, into four groups: Group I (light green with white centre), Group II (dark green with white centre), Group III (Blackish green with white centre), and Group IV (green with dull white centre) accommodating 20.83% of isolates (Psh1, PTi1, PNi2, PR3 and TB1), 45.83% (Psh2, Psh3, PTi2, PTi3, PNi1, Z1, SS, TB2, NT1, NT3 and Z3), 16.67% (PR1, PR2, SG and Z2) and 16.67% (PNi3, SR, TB3 and NT2) of isolates, respectively (Table 1). Based on colony shape, the isolates were divided into four groups accommodating 4 (16.67%), 4 (16.67%), 6 (25.00%), and 10 (41.66%) isolates in Group I (Psh2, Psh3, PTi2 and Z1), Group II (PTi1, PR3, NT2 and Z2), Group III (PNi2, SS, TB1, NT1, NT3 and Z3), and Group IV (Psh1, PTi3, PNi1, PNi3, PR1, PR2, SR, SG, TB2 and TB3), respectively (Table 1). Present results were in close agreement with the observations of Mohiddin et al., Kumar et al., Meena et al. and Shah et al. around the world26,27,28,29 who noted similar textures among the colonies of various Trichoderma isolates including flat cottony, fluffy or raised and flat granular forms with either regular or irregular margins on PDA medium. Additionally it has been documented that the majority of Trichoderma isolates showed diverse colony colours like dark green (12 isolates), whitish green (9 isolates), light green (6 isolates), and yellowish green (3 isolates)30. It has also been observed that the colony colour of 10 selected isolates of Trichoderma varied from dark green to light green or black green or whitish green. Similarly, colour variation from dark green or light green to yellowish green with flat to raised growth patterns has also been reported by Kamaruzzaman et al.31. These observations were in close agreement with our results. Furthermore, the colony shapes exhibited notable diversity among different Trichoderma isolates. The production of green spore mass was observed at the centre of the Petri plates containing PDA medium, often forming 1–2 concentric rings or spreading across the plate, accompanied by yellowish or whitish aggregated spore mass or white mycelium with green conidia towards the edges. Some isolates formed white mycelium with limited green conidial formation in concentric rings near the centre or dispersed along the margin. These observations regarding the colony shape are in close agreement with the findings reported by Ghutukade et al.32.
Conidial characteristics and shape of phialides
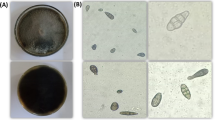
Different isolates of Trichoderma species cultured on PDA medium showed significant variations in their spore characteristics, such as colour, shape, arrangement, and shape of phialides (https://trichoderma.info/). Based on condial and phialide characteristics, Trichoderma isolates were categorized into four groups Group I-IV comprised of 10 (Psh1, PTi3, PNi1, PNi3, PR1, PR2, SR, SG, TB2 and TB3), 5 (PNi2, TB1, NT3, Z1 and Z3), 5 (PTi1, PR3, NT1, NT2 and Z2) and 4 (Psh2, Psh3, PTi1 and Z1) isolates. Based on conidial colour, four groups such as Group I with bright green coloured spores (Psh1, PTi3, PNi1, PNi3, PR1, PR2, SR, SG, TB2 and TB3), Group II (yellow-green in isolates PNi2, TB1, NT3, Z1 and Z3), Group III (pale green spores in isolates PTi1, PR3, NT1, NT2 and Z2), and Group IV (olive green spores in isolates Psh2, Psh3, PTi1 and Z1) ) were formed accommodating 10 (41.66%), 5 (20.84%), 5 (20.84%) and 4 (16.66%) isolates, respectively (Table 2). Likewise, the grouping of isolates based on spore shape also resulted in four groups, viz., Group I with sub-globose to obvoid conidia, Group II (globose to sub-globose), Group III (globose to sub-cylindrical), and Group IV (globose-shaped conidia), accommodating 10 (41.66%), 5 (20.84%), 5 (20.84%), and 4 (16.66%) isolates, respectively. Grouping based on spore arrangement, isolates were distributed into two groups namely Group I with smooth-walled conidia accommodated 79.16% of isolates (Psh1, PTi3, PNi1, PNi3, PR1, PR2, SR, SG, TB2, TB3, PTi1, PR3, NT1, NT2, Z2, Psh2, Psh3, PTi1 and Z1), and Group II (rough-walled) comprised 20.84% of isolates (PNi2, TB1, NT3, Z1 and Z3) (Table 2; Fig. 2). Additionally, the isolates were further grouped based on the shape of the phialides into four groups. Group I with flask-shaped phialides, Group II (slender), Group III (elongated), and Group IV (swollen) phialides were formed accommodating 4 (16.60%), 5 (20.80%), 5 (20.80%) and 10 (41.40%) isolates, respectively. Accordingly, grouping of the isolates such as Group I, II, III, and IV was carried out and detailed in Table 2; Fig. 3. Similar observations have been recorded by different researchers all over the world32,33,34,35,36,37,38,39 who noted the shape and arrangement of various Trichoderma isolates as globose, globose to sub-globose, globose to sub-cylindrical and sub-globose to obvoid having smooth and rough ornamentation. Similarly, it was found that conidial colour also varied widely, including typical green, olive green, grey, brown or even colourless40. It has also been reported that the conidia in Trichoderma species were olive green or dark green41. Furthermore, the grouping based on phialide shape also distributed different isolates into four groups viz., Group I with swollen-shaped phialides, Group II (slender), Group III (elongated), and Group IV (flask) accommodating 10 (41.66%), 5 (20.84%), 5 (20.84%) and 4 (16.66%) isolates, respectively. Other researchers across the globe16,27,36,39 have similarly documented that the shape of the phialide of various Trichoderma isolates varies from flask to slender, elongated, or swollen, confirming the results obtained from the present study.
Spore characteristics of Trichoderma isolates on PDA after 07 days of incubation at 28 ± 1oC; (a) Olive green, globose, smooth conidia; (b) Yellow green, globose to sub-globose, rough conidia; (c) Pale green, globose to sub-cylindrical, smooth conidia; (d) Bright green, sub-globose to obvoid, smooth conidia.
Trichoderma isolates’ conidial size (length x breadth) varied significantly, measuring between 2.50 and 5.00 μm x 2.00–3.80 μm, making it difficult to be grouped solely based on conidial dimensions (Table 3). The maximum mean conidial length of 4.75 μm and breadth of 3.65 μm was observed in isolate SR, followed by isolates PR2 (4.65 × 3.55 μm) and PR3 (4.65 × 3.40 μm) (Table 3). In contrast, the minimum mean conidial length of 2.50 μm and breadth of 2.20 μm was observed in isolate Z1. The highest length and breadth ratio of 1.43 μm was observed in isolate PNi2 and SS, whereas the lowest length and breadth ratio of 1.11 μm was observed in isolate Z1. Previous reports indicated that the conidial size varies from of 2–5 × 2–4 μm in different Trichoderma isolates40. Similar observations were also recorded by Soesanto et al.42who found variability in spore dimensions of four Trichoderma isolates. Furthermore, the conidial size was recorded in 30 different Trichoderma isolates, and reported that the conidial size and L/W ratio varied significantly in T. viride and T. asperellum41.
Cultural characterization
Colony diameter, growth rate and mycelial dry weight
Significant variations were recorded in growth characteristics such as colony diameter, growth rate, and mycelial dry weight of different Trichoderma isolates (Table 4). The colony diameter of different isolates ranged from 62.60 to 89.70 mm with the highest mean colony diameter (89.70 mm) recorded in isolate PR2, followed by SR (88.00 mm), PTi1 (84.40 mm), whereas, the least colony diameter (62.60 mm) was recorded in isolate Psh2 (Table 4). Grouping of Trichoderma isolates based on cultural characteristics was not possible due to their high level of variation. Similar results were also obtained by different researchers in terms of colony diameter of Trichoderma isolates on PDA medium 30,31,32,43,44,45. The average growth rates varied between 17.63 and 21.31 mm/day, with a maximum growth rate of 21.31 mm/ day recorded in isolates Psh2, TB3, NT2, and Z1 (Table 4). The minimum growth rate of 17.63 mm/ day was observed in Z2 isolate. Variations in growth rates shown by various Trichoderma isolates have been well documented by different researchers45,46,47. The average mycelial dry weight of different isolates ranged from 110.36 to 217.67 mg and the highest mycelial dry weight of 217.67 mg was recorded in isolate PR2 followed by SR (197.70 mg), whereas, the lowest mycelial dry weight (110.36 mg) was recorded in isolate PTi3 (Table 4). Significant variations in terms of mycelial dry weight of Trichoderma isolates were also reported from different countries28,47,48,49.
Thus, based on morpho-cultural characterization, the 24 isolates of Trichoderma were identified up to the species level and grouped into four species. The 4 isolates, namely Psh2, Psh3, PTi2 and Z1, belong to T. harzianum, whereas the 6 isolates PNi2, SS, TB1, NT1, NT3 and Z3 were identified as T. viride as described previously17,18,29,35,37,39,40. Four isolates namely PTi1, PR3, NT2 and Z2 were categorized as T. koningiopsis and 10 isolates Psh1, PTi3, PNi1, PNi3, PR1, PR2, SR, SG, TB2 and TB3 were grouped as T. hamatum based on the previous descriptions16,17,39 (Table 5).
Molecular characterization of Trichoderma species
The DNA of 24 Trichoderma isolates was quantified using NanoDrop and then diluted to 40-50ng/µl. High-quality DNA showing a single intact band on a 0.8% agarose gel, was stored at -80℃ in a deep freezer (Eppendorf India Pvt. Ltd.) (Fig. 4a).
Electrophoretic separation of PCR products of Trichoderma isolates (Images showing full length membranes are given in Supplementary Fig. 1) Lane L: DNA Ladder 500 bp; Lanes 1–24: Twenty four Trichoderma isolates;
a) DNA of 24 Trichoderma isolates.
b) PCR amplification of ITS region of 24 isolates using ITS1 & ITS4 primers.
c) Touchdown PCR amplification of TEF 1-αgene using TEF1 & TEF2 primers.
d) Internal PCR amplification of TEF 1-α gene using TEF3 & TEF4 primers.
e) Touchdown PCR amplification of RPB2 gene using RPB2-5 & RPB2-6 primers.
f) Internal PCR amplification of RPB2 gene using RPB2-5 & RPB2-7 primers.
Phylogenetic analysis of multigene sequence data
The identification of various Trichoderma isolates initially based on morpho-cultural characterization was further validated by sequence analysis using molecular markers viz., internal transcribed spacer (ITS) region, translation elongation factor 1-alpha (TEF 1-α) and RNA polymerase B II (RPB2). The amplification of ITS region with primers ITS1 and ITS4 yielded a product of approximately 600 bp (Fig. 4b). The amplification of TEF 1-α gene carried out by touchdown PCR using primer pair 1 and 2 showed an amplicon size of approximately 920 bp. The internal PCR was subsequently performed using primers 3 and 4 amplifying a fragment of approximately 870 bp with the first PCR product serving as a template (Fig. 4c-d). Similarly, touchdown PCR of RPB2 gene using primer combinations 5 and 6 yielded approximately 1240 bp amplicon size, and the internal PCR showed an amplicon size of about 940 bp using primer pair 5 and 7, respectively (Fig. 4e-f). Accordingly, PCR amplified products of ITS region, and TEF 1-α and RPB2 genes from all the 24 isolates of Trichoderma were then custom sequenced (Fig. 4b-f) and results obtained in the form of chromatograms. Based on a concreated data set from ITS region, and TEF 1-α, and RPB2 genes, the 24 isolates of Trichoderma were broadly grouped into three major clades (A, B and C), then further sub-divided into sub-clades. Clade A based on ITS region sequences grouped the 24 isolates into five sub-clades viz., Clade I, II, III, IV and V accommodating 8.33, 16.67, 25.00, 20.84 and 29.16% of isolates, respectively (Table 6; Fig. 5). The isolates in clade I were identified as Trichoderma species (2 isolates), Clade II as T. harzianum (4 isolates), Clade III as T. viride (6 isolates), Clade IV as T. asperelloides (5 isolates) and Clade V as T. koningiopsis (7 isolates) (Table 6; Fig. 5). This study marks a novel attempt to study the phylogeny of the entire Trichoderma genus based on sequencing of ITS region, highlighting it as a powerful tool for authentic identification of Trichoderma species50. However, our findings indicated that the results obtained from the ITS region were not congruent with the morpho-cultural data. This could be possible because few morphological characters with limited variation may lead to overlap and misidentification of the isolates/strains also described by other researchers51,52,53,54. While the ITS region serves as a valuable identification tool, it is crucial to use identifications based on ITS region with caution, as closely related species such as T. viride, T. harzianum etc., can share the same ITS sequence, making accurate identification challenging. Thus, relying solely on ITS region is not sufficient for the precise identification and characterization of Trichoderma and Hypocrea at species level8,9,54,55 Therefore, to effectively resolve new species, in addition to ITS region, we employed multiple genes, including RPB2 and TEF 1-α, facilitating a more accurate delineation of Trichoderma species from the apple rhizosphere. Notably, over 80 species represented in GenBank are characterized by sequences from at least the ITS region of rDNA, and many are primarily identified using protein-coding genes like TEF 1-α and RPB2. This clearly underscores the importance of these genes for the precise identification of the Trichoderma genus at the species level10,56,57,58.
The phylogenetic analysis of sequences based on TEF 1-α (clade B) and RPB2 (clade C) revealed similar results (Fig. 5). Consequently, twenty-four isolates of Trichoderma were grouped into six sub-clades viz., Clade I-VI. The isolates in Clade I were identified as T. koningiopsis, Clade II as T. viride, Clade III as T. asperellum, Clade IV as T. asperelloides, Clade V as T. hamatum and Clade VI as T. harzianum accommodating 20.83, 20.83, 12.50, 16.67, 12.50 and 16.67% of isolates, respectively (Table 7; Fig. 5). Other researchers have similarly emphasized the significance of TEF 1-α and RPB2 sequencing in the identification and phylogenetic analysis of Trichoderma species59,60,61,62,63. Notably, despite the high conservation of the ITS region across the species, results obtained from TEF 1-α and RPB2 genes differed slightly from those obtained from the ITS region. This discrepancy may contribute to misidentifications, and could be attributed to the presence of non-orthologous copies of ITS in these isolates23,63,64,65,66. Consequently, the variability of TEF 1-α and RPB2 genes facilitates the differentiation among the closely related Trichoderma species11. Their presence as a single copy in the genomes of all eukaryotes combined with slow rate of divergence making these genes very useful for the higher level of phylogenetic reconstruction55,63,65,66,67.
This study demonstrated that the morpho-cultural or molecular characterization independently did not produce congruent results. Consequently, definitive identification of various Trichoderma species was achieved through an integrated approach that combined morpho-cultural as well as molecular characterization based on ITS region, and TEF 1-α and RPB2 genes. This analysis resulted in the categorization of 24 isolates into six distinct groups viz., Group I (T. koningiopsis), Group II (T. viride), Group III (T. asperellum), Group IV (T. asperelloides), Group V (T. hamatum) and Group VI (T. harzianum) accommodating 5 (PTi1, PR3, NT1, NT2, Z2), 5 (PNi2, SS, TB1, NT3, Z3), 3 (Psh1, PNi3, TB3), 4 (PTi3, PNi1, PR1, TB2), 3 (PR2, SR, SG) and 4 (Psh2, Psh3, PTi2, Z1) isolates, respectively. These findings indicated the presence of six species of Trichoderma in apple rhizosphere in two districts of Srinagar and Pulwama (Table 8). The consensus sequences based on ITS region, TEF 1-α and RPB2 genes of Trichoderma species were used in the nucleotide blast program of the NCBI database for the identification of each isolate and submitted to the GenBank NCBI, and Accession numbers obtained from MZ292909 to MZ292931 and MZ292986, MZ322871 to MZ322894 and MZ361055 to MZ361078, respectively (Table 8). To our knowledge, this study reports for the first time the occurrence of T. koningiopsis, T. viride, T. asperellum, T. asperelloides and T. hamatum in apple rhizosphere from India, although, their presence in the rhizosphere of other crops is well documented.
Materials and methods
Collection, isolation, purification, and maintenance of fungal isolates
Soil samples from apple rhizosphere were collected from various apple-grown areas viz., Nikas, Tiken, Rajpora, and Shadimarg in district Pulwama and Shalimar, New Theed, Harwan, and Zakura in district Srinagar of Kashmir valley (Table 9). Three soil samples from each location were collected in triplicates to a depth of 30 cm. The samples from each site were thoroughly mixed and foreign materials like roots, stones, pebbles, and gravel were removed. The bulk was reduced to 0.5–1.0 kg by quartering technique, where the thoroughly mixed samples were divided into four equal parts. The two diagonally opposite segments were discarded and the remaining two segments were recombined and remixed. This procedure was repeated until the desired sample size was achieved. The final samples were collected in paper bags, appropriately labeled, and used for the isolation of Trichoderma species. Trichoderma strains were isolated using serial dilution plate technique68 on Trichoderma specific medium (TSM)26,69,70,71,72 and incubated at 28 ± 1oC for one week until sporulation was observed. The different isolates were purified on a water agar medium using a single spore technique73. Twenty-four isolates belonging to different species of Trichoderma were obtained from two districts, namely Pulwama and Srinagar of Kashmir valley, and coded as per location, etc. (Table 9). The 24 Trichoderma cultures were maintained by sub-culturing on potato dextrose agar (PDA) medium, incubated at 28 ± 1oC, and stored at -80oC for further studies.
Morpho-cultural characterization
Morphological and cultural characteristics of each isolate were observed on the PDA medium (HiMedia Lab. Pvt. Ltd., Mumbai, India). A 5-mm mycelial disc from each isolate from an actively growing region of a 7-day-old culture was aseptically punched and transferred to Petri plates containing PDA medium, followed by incubation at 28 ± 1 °C. After 5 days of incubation, colony characteristics such as colony texture, margins, colour, and shape were recorded. Conidial morphology was examined by preparing spore suspension from the seven-day-old culture of each isolate and observed under a microscope model CX31 (Olympus, Tokyo, Japan) to record shape, colour, septation, and size (length and width) for at least 25 conidia per isolate. Phialide shape was also assessed after 2 days, and the presence or absence of chlamydospores after 10 days of incubation at 28 ± 1ºC. Cultural characteristics such as colony diameter was recorded for 3 days and growth rate was calculated up to 4 days in all the isolates of Trichoderma. The mycelial dry weight of different isolates was calculated by drying the filter papers containing mycelium in a Thermotech hot air oven (MAC-230, Punjab Biotechnology, Chandigarh, India) at 60ºC for an hour for 3 consecutive days till a constant weight was attained. Three replications were maintained for each isolate for all the experiments. The data collected was analyzed by appropriate statistical method74.
Molecular characterization of various Trichoderma isolates
Genomic DNA extraction
Fungal cultures of all the isolates of Trichoderma spp. were separately cultured on potato dextrose broth (PDB) medium in 150 ml Erlenmayer flasks. After sterilization of PDB medium in 250 ml conical flasks at 15 lb psi in an autoclave (MAC-80 L, Punjab Biotechnology, Chandigarh, India) for 20–25 min., flasks were inoculated (at room temperature) with 5 mm mycelial discs of each Trichoderma isolate and incubated at 28 ± 1 °C for 7 days. Mycelium was filtered through double-layered sterilized filter paper, dried between two layers of filter paper in a laminar airflow cabinet (PCR 2.5, Macro Scientific Works, New Delhi, India), and stored separately at − 80 °C in a deep freezer (U 410, Eppendorf Hamburg · Germany) for further use. The total genomic DNA of each isolate was extracted using the CTAB (Cetyl trimethyl ammonium bromide) method75 with slight modifications and diluted to a final concentration of 25 ng/µl.
Quantification and quality check of DNA
The quantity of DNA was checked by agarose gel electrophoresis. In this, 0.8 g of agarose was dissolved in 100 ml of 0.5X Tris-acetate EDTA (TAE) (SRL Pvt. Ltd., Mumbai, India) electrophoresis buffer. The mixture was heated until the agarose was dissolved completely i.e. when the solution became transparent and clear. It was cooled down to 60 °C with constant stirring. Ethidium bromide was added to a final concentration of 0.5 µg/ml of buffer. Then the agarose solution was poured into an already prepared gel mould with combs and was left for 20–30 min for solidification. DNA samples for loading were prepared by adding 2 µl loading dye (6X) (0.25% w/v bromophenol blue and 50% glycerol in sterile water) (Thermo Fisher Scientific Pvt. Ltd., India) to 8 µl DNA so that the final concentration of loading dye was 1X. The DNA samples were loaded into wells with the help of a micropipette. Along with the DNA samples, a marker of known concentration was also loaded. The gel was run for about 1–2 h at 80 volts and visualized under a UV transilluminator using a photo gel documentation system (Alfa Imager EC, Protein Simple, USA), and a DNA sample was photographed. The intensity of fluorescence of each sample was compared with that of a standard marker and then the DNA concentration of each sample was ascertained. The quantity of DNA samples was also checked by Nano-drop (Bio-spectrometer, Eppendorf India Pvt. Ltd.). The quality was checked whether DNA formed a single high molecular weight band (good quality) or a smear (degraded/poor quality) in gel electrophoresis. The DNA of all the samples was diluted to 25 ng/µl by adding double-distilled sterile water, stored at − 80 °C in a deep freezer (U 410, Eppendorf Hamburg · Germany), and used for PCR amplification.
Polymerase chain reaction (PCR) using internal transcribed spacer (ITS) region, translation elongation factor 1-alpha (TEF 1-α), and RNA polymerase B subunit II (RPB2) gene-specific markers
Polymerase chain reaction (PCR) using ITS region, and TEF 1-α, and RPB2 gene-specific markers was performed in 0.2 ml PCR tubes in a T-Gradient thermal cycler (Whatman Biometra, T-Gradient, Goettingen, Germany) using 40–50 ng of genomic DNA of each isolate in a final volume of 50µl reaction mixture comprised of 31.6µl distilled sterilized PCR-water, 5.0µl (1.0X) PCR buffer, 3µl (1.5 mM) MgCl2), 4µl (0.2 mM) DNTPs, 2.0µl of each forward and reverse primers (0.4 pmol) and 0.4µl Taq polymerase (1.0 unit) provided by Thermo Fisher Scientific Pvt. Ltd., India. PCR amplification of ITS region was performed in a thermal cycler programmed for initial denaturation at 95℃ for 5 min. followed by 35 cycles of denaturation at 94℃ for 1 min., primer annealing for 2 min. at 56℃, primer extension for 3 min. at 72℃ with a final extension of 10 min52. The TEF 1-α was amplified using touch-down PCR (primer pair TEF 1 and 2) programmed with initial denaturation at 94℃ for 4 min. followed by 4 cycles at 94℃ for 1 min. and 90 sec. each at 70 and 68℃ followed by 26 cycles with annealing temperature decreasing by 0.5℃ per cycle from 68 − 55℃; 12 cycles with annealing at 55℃ and a final extension of 7 min. at 68℃. The internal PCR was carried out using primer pair TEF-3 and 4 programmed at 94℃ for 4 min. followed by 35 cycles at 94℃ for 30 sec., annealing at 50℃ for 15 sec., extension at 68℃ for 1 min. and 7 min., respectively62. Similarly, touch-down PCR was performed for RPB2 amplification using primer pair RPB2 5 and 6 programmed at 94℃ for 4 min. followed by 5 cycles at 94℃ for 45 sec., 60℃ for 45 sec. and 68℃ for 2 min. and 5 cycles with annealing temperature decreasing by 1.0℃ per cycle from 58 − 54℃ followed by 30 cycles of annealing at 54℃ and a final extension of 10 min at 68℃. The internal PCR was performed with primer pair RPB2 5 and 7 programmed at 94℃ for 4 min. followed by 35 cycles of denaturation at 94℃ for 30 sec., annealing at 50℃ for 15 sec., extension at 68℃ for 1 min. and a final extensioof 7 min. at 68℃ 622. A set of ITS region, and TEF1-α, and RPB2 gene-specific primers76,77,78,79 were used for molecular characterization of 24 isolates of Trichoderma (Table 10). The ITS region of rDNA was amplified using ITS1 (5’TCCGTAGGTGAACCTGCG3’) and ITS4 (5’TCCTCCGCTTATTTGATATGC3’) primers76. TEF 1-α78 and RPB2 79 genes were amplified according to already given procedure79. A fragment of the 5’ end of TEF 1-α gene containing three introns was amplified using a primer combination of 1 (5’CAAAATGGGTAGGAGGASAAGAC3’) and 2 (CAGTACCGGCRGCRATRATSAG3’) following touch-down PCR. This amplified product was re-amplification by an internal PCR using a primer combination of 3 (5’AGGACCAAGACTCACATCAACG3’) and 4 (5’AGTACCAGTGATCATGTTCTTG’3). A fragment of subunit 2 of RPB2 gene was amplified using primer pair of 5 (5’TGGGGWGAYCARAARAAGG’3) and 6 (5’CATRATGACSGAATCTTCCTGGT’3) following a touch-down PCR followed by an internal PCR using a primer pair of 5 (5’TGGGGWGAYCARAARAAGG’3) and 7 (5’GGTTGTGATCRGGRAARGGAATG’3).
DNA sequencing and phylogenetic analysis
After PCR amplification using ITS, TEF 1-α, and RPB2 primers in a 50-µl reaction mixture, 5 µl of PCR amplified product of different isolates was electrophoresed to ensure successful amplification and the remaining 45 µl PCR amplified products of 24 isolates of Trichoderma spp. were sent for custom sequencing to Genei Labs, Bangalore, India. The sequences for each forward and reverse primer were retrieved from the chromatograms received. The sequence alignment was carried out for both forward and reverse sequences in BioEdit version 7.0 80 to obtain consensus sequences for each isolate. Similarly, all the 24 sequences for ITS region, and TEF1-α and RPB2 genes were obtained and analyzed through the BLASTn program of National Centre for Biotechnological Information (NCBI) (http://www.ncbi.nlm.nih.gov/BLAST) to compare the present sequences with the available database of NCBI (http://www.nlm.nih.gov/nuccore). The consensus sequences of ITS, TEF 1-α, and RPB2 for each isolate obtained were compared with three sequences of each of T. harzianum, T. viride, T. koningiopsis, T. hamatum, T. asperellum, T. asperelloides along with an outgroup sequence retrieved from NCBI database using molecular evolutionary genetic analysis (MEGA) software version 11 81. An optimal tree was generated using the Kimura-2-parameter (K2) substitution model and different taxa were clustered together in a bootstrap test with 1000 replicates82. The phylogenetic analysis confirmed the clustering of isolates into distinct clades using the best-fit model. The twenty-four sequences of each gene were submitted to GenBank, NCBI for the allotment of Accession numbers.
Conclusion
In conclusion, this study has unveiled a remarkable diversity of Trichoderma species within the apple rhizosphere of Jammu and Kashmir, India. It highlights the critical importance of integrating morpho-cultural and molecular methodologies for the precise characterization of these fungi. Our findings demonstrate that relying on a single approach is insufficient for identifying the various Trichoderma isolates at the species level. Thus, by employing a multifaceted strategy that combines morpho-cultural characterization with advanced molecular techniques—including the sequencing of the ITS region, TEF 1-α, and RPB2 genes—we can achieve a clearer delineation of these species. This approach led to the successful identification of T. koningiopsis, T. viride, T. asperellum, T. asperelloides, T. hamatum and T. harzianum, all of which were isolated from apple rhizosphere in Jammu and Kashmir, India. This research not only deepens our understanding of Trichoderma biodiversity but also opens avenues for future investigations into the ecological roles of these fungi and their potential as biocontrol agents in sustainable agricultural practices. To our knowledge, this is the first report of T. koningiopsis, T. viride, T. asperellum, T. asperelloides and T. hamatum from apple rhizosphere.
Data availability
The sequence data of this paper has been deposited in GenBank, NCBI and accession numbers (MZ292909 to MZ292931 and MZ292986, MZ322871 to MZ322894 and MZ361055 to MZ361078) are given in the paper.
References
Chadha, T. Textbook of temperate fruits. (2001).
Anonymous. Production and area statement for 2018–2019. Department Horticulture, Government of Jammu Kashmir (2019).
Grove, G. G., Eastwell, K. C., Jones, A. L. & Sutton, T. B. In Apples: Botany, Production and Uses459–488 (CABI Publishing Wallingford UK, 2003).
Vinale, F. et al. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 40, 1–10 (2008).
Hai, F. I. et al. Pesticide removal by a mixed culture of bacteria and white-rot fungi. J. Taiwan Inst. Chem. Eng. 43, 459–462 (2012).
Monte, E. & Llobell, A. in Proceeding V World Avocado Congress (Actas V Congreso Mundial del Aguacate). 725–733.
Reena, A. et al. Antagonistic activity of Trichoderma viride isolate on soil borne plant pathogenic fungi. Int. J. Bioassays. 2, 294–297 (2013).
Wijayawardene, N. N. et al. Outline of Fungi and fungus-like taxa. Mycosphere Online: J. Fungal Biology. 11, 1060–1456 (2020).
Maharachchikumbura, S. S. et al. Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers. 72, 199–301 (2015).
Kubicek, C. P., Bissett, J., Druzhinina, I., Kullnig-Gradinger, C. & Szakacs, G. Genetic and metabolic diversity of Trichoderma: a case study on South-East Asian isolates. Fungal Genet. Biol. 38, 310–319 (2003).
Samuels, G. J. Trichoderma: systematics, the sexual state, and ecology. Phytopathology 96, 195–206 (2006).
Chaverri, P., Castlebury, L. A., Overton, B. E. & Samuels, G. J. Hypocrea/Trichoderma: species with Conidiophore elongations and green conidia. Mycologia 95, 1100–1140 (2003).
Singh, A., Srivastava, S. & Singh, H. Effect of substrates on growth and shelf life of Trichoderma Harzianum and its use in biocontrol of diseases. Bioresour. Technol. 98, 470–473 (2007).
Harman, G. E., Howell, C. R., Viterbo, A., Chet, I. & Lorito, M. Trichoderma species—opportunistic, avirulent plant symbionts. Nature reviews microbiology 2, 43–56 (2004).
Korkom, Y. & Yildiz, A. Evaluation of biocontrol potential of native Trichoderma isolates against charcoal rot of strawberry. J. Plant. Pathol. 104, 671–682 (2022).
Rifai, M. A. A revision of the genus Trichoderma. (1969).
Bissett, J. A revision of the genus trichoderma. II. Infrageneric classification. Can. J. Bot. 69, 2357–2372 (1991).
Bissett, J. A revision of the genus Trichoderma. III. Section Pachybasium. Can. J. Bot. 69, 2373–2417 (1991).
Seaby, D. Differentiation of Trichoderma taxa associated with mushroom production. Plant. Pathol. 45, 905–912 (1996).
Hatvani, L. et al. Agricultural systems as potential sources of emerging human mycoses caused by Trichoderma: a successful, common phylotype of Trichoderma longibrachiatum in the frontline. FEMS Microbiol. Lett. 366, fnz246 (2019).
Kullnig-Gradinger, C. M., Szakacs, G. & Kubicek, C. P. Phylogeny and evolution of the genus Trichoderma: a multigene approach. Mycol. Res. 106, 757–767 (2002).
Druzhinina, I. S. et al. An oligonucleotide barcode for species identification in Trichoderma and hypocrea. Fungal Genet. Biol. 42, 813–828 (2005).
Hoyos-Carvajal, L., Orduz, S. & Bissett, J. Genetic and metabolic biodiversity of Trichoderma from Colombia and adjacent neotropic regions. Fungal Genet. Biol. 46, 615–631 (2009).
Korkom, Y. & Yıldız, A. First report of Trichoderma Guizhouense isolated from soil in Türkiye. J. Plant Dis. Prot. 131, 619–625 (2024).
Druzhinina, I. S., Kopchinskiy, A. G. & Kubicek, C. P. The first 100 Trichoderma species characterized by molecular data. Mycoscience 47, 55–64 (2006).
Mohiddin, F., Bashir, I., Padder, S. A. & Hamid, B. Evaluation of different substrates for mass multiplication of Trichoderma species. J. Pharmacognosy Phytochemistry. 6, 563–569 (2017).
Kumar, A., Sahu, T. K., Bhalla, A. & Jain, A. Morphological characterization of Trichoderma Harzianum from Madhya Pradesh. Annals Plant. Prot. Sci. 22, 228–229 (2014).
Meena, A. K. & Meena, A. K. Characterization and antagonistic effect of isolated Trichoderma sp. against pathogens under clusterbean (Cyamopsis Tetragonoloba L). Indian J. Agricultural Res. 50, 249–253 (2016).
Shah, S., Nasreen, S. & Sheikh, P. Cultural and morphological characterization of Trichoderma spp. Associated with green mold disease of pleurotus spp. In Kashmir. Res. J. Microbiol. 7, 139 (2012).
Sharma, K. & Singh, U. Cultural and morphological characterization of rhizospheric isolates of fungal antagonist Trichoderma. J. Appl. Nat. Sci. 6, 451–456 (2014).
Kamaruzzaman, M., Hossain, M. D. & Hossain, I. Antifungal and morphological assay of selective Trichoderma isolates against soil borne plant pathogenic fungi. Int. J. Innov. Appl. Stud. 16, 409 (2016).
Ghutukade, K., Deokar, C., Gore, N., Chimote, V. & Kamble, S. Molecular characterization of Trichoderma isolates by ISSR marker. J. Agric. Vet. Sci. 8, 1–5 (2015).
Hewedy, O. A. et al. Phylogenetic diversity of Trichoderma strains and their antagonistic potential against soil-borne pathogens under stress conditions. Biology 9, 189 (2020).
Choi, I. Y., Hong, S. B. & Yadav, M. C. Molecular and morphological characterization of green mold, Trichoderma spp. Isolated from oyster mushrooms. Mycobiology 31, 74–80 (2003).
Muthu, K. & Sharma, P. Molecular and morphological characters: an appurtenance for antagonism in Trichoderma spp. Afr. J. Biotechnol. 10, 4532–4543 (2011).
Mishra, S. Characterization of Trichoderma Strains Isolated from Bilaspur and Sarguja Divisions for their Plant Growth Promoting Activity and Disease Controlling Potential (Indira Gandhi Krishi Vishwavidyalaya, 2016).
Lieckfeldt, E., Samuels, G. J., Nirenberg, H. I. & Petrini, O. A morphological and molecular perspective of Trichoderma viride: is it one or two species? Appl. Environ. Microbiol. 65, 2418–2428 (1999).
Kabir, S., Debnath, S., Mazumder, A., Dey, T. & Bera, B. In vitro evaluation of four native Trichoderma spp. Isolates against tea pathogens. Indian J. Fund Appl. Life Sci. 6, 1–6 (2016).
Nagamani, P., Bhagat, S., Viswanath, K. & Biswas, M. Cultural and morphological variability among Trichoderma Harzianum and Trichoderma asperellum collected from Chickpea growing areas of Rayalaseema region of Andhra Pradesh. J. Food Legumes. 33, 93–100 (2020).
Gams, W. & Bissett, J. Morphology and identification of Trichoderma. Trichoderma Gliocladium. 1, 3–34 (2002).
Sriram, S., Savitha, M., Rohini, H. & Jalali, S. The most widely used fungal antagonist for plant disease management in India, Trichoderma viride is Trichoderma asperellum as confirmed by oligonucleotide barcode and morphological characters. Current Science 104, 1332–1340 (2013).
Soesanto, L., Utami, D. & Rahayuniati, R. Morphological characteristics of four Trichoderma isolates and two endophytic fusarium isolates. Can. J. Sci. Ind. Res. 2, 294–304 (2011).
Devi, Y. R. & Sinha, B. Cultural and anamorphic characterization of Trichoderma isolates isolated from rhizosphere of French bean (Phaseolus vulgaris L.) growing areas of Manipur. Bioscan 9, 1217–1220 (2014).
Saravanan, K. et al. Morphological and molecular characterization of Trichoderma isolates of Tamil Nadu. Eur. J. Mol. Biology Biochem. 1, 176–181 (2014).
Rashmi, K., Sinha, B. & Pramesh, K. Variability of native Trichoderma species isolated from rhizosphere of groundnut (Arachis hypogeae L.) in Manipur. Bioscan 10, 1707–1712 (2015).
Kumar, S. & Singh, O. Influence of media for growth of Trichoderma species. Annals Plant. Prot. Sci. 16, 513–514 (2008).
Srivastava, M., Singh, V., Shahid, M., Singh, A. & Kumar, V. Determination of biochemical and physiological aspects of a biocontrol agent Trichoderma Harzianum Th Azad. Int. J. Adv. Res. 2, 841–849 (2014).
Shahid, M., Singh, A., Srivastava, M., Mishra, R. & Biswas, S. Effect of temperature, pH and media for growth and sporulation of Trichoderma longibrachiatum and self life study in carrier based formulations. Annals Plant. Prot. Sci. 19, 147–149 (2011).
Jahan, N., Sultana, S., Adhikary, S., Rahman, S. & Yasmin, S. Evaluation of the growth performance of Trichoderma Harzianum (Rifai.) on different culture media. J. Agri Vet. Sci. 3, 44–50 (2013).
Kindermann, J., El-Ayouti, Y., Samuels, G. J. & Kubicek, C. P. Phylogeny of the genustrichodermabased on sequence analysis of the internal transcribed spacer region 1 of the rDNA cluster. Fungal Genet. Biol. 24, 298–309 (1998).
Kuhls, K. et al. Revision of Trichoderma sect. Longibrachiatum including related teleomorphs based on analysis of ribosomal DNA internal transcribed spacer sequences. Mycologia 89, 442–460 (1997).
Hermosa, M. et al. Molecular characterization and identification of biocontrol isolates of Trichoderma spp. Appl. Environ. Microbiol. 66, 1890–1898 (2000).
Kullnig, C. M. et al. Confusion abounds over identities of Trichoderma biocontrol isolates. Mycol. Res. 105, 769–772 (2001).
Kubicek, C. P., Komon-Zelazowska, M. & Druzhinina, I. S. Fungal genus hypocrea/trichoderma: from barcodes to biodiversity. J. Zhejiang Univ. Sci. B. 9, 753–763 (2008).
Cai, F. & Druzhinina, I. S. In honor of John bissett: authoritative guidelines on molecular identification of Trichoderma. Fungal Divers. 107, 1–69 (2021).
Latha, J., Verma, A. & Mukherjee, P. K. PCR-fingerprinting of some Trichoderma isolates from two Indian type culture collections–a need for re-identification of these economically important fungi. Curr. Sci. 83, 372–374 (2002).
Chakraborty, B., Chakraborty, U., Saha, A., Dey, P. & Sunar, K. Molecular characterization of Trichoderma viride and Trichoderma Harzianum isolated from soils of North Bengal based on rDNA markers and analysis of their PCR-RAPD profiles. Global J. Biotechnol. Biochem. 5, 55–61 (2010).
Savitha, M. & Sriram, S. Morphological and molecular identification of Trichoderma isolates with biocontrol potential against Phytophthora blight in red pepper. Pest Manage. Hortic. Ecosyst. 21, 194–202 (2015).
Oskiera, M., Szczech, M. & Bartoszewski, G. Molecular identification of strains collected to develop plant Growth-Promoting and biocontrol agents. J. Hortic. Res. 23, 75–86 (2015).
Jaklitsch, W. M., Kubicek, C. P. & Druzhinina, I. S. Three European species of hypocrea with reddish brown stromata and green ascospores. Mycologia 100, 796–815 (2008).
Yu, Z. F., Qiao, M., Zhang, Y. & Zhang, K. Q. Two new species of Trichoderma from yunnan, China. Antonie Van Leeuwenhoek. 92, 101–108 (2007).
Smith, A. et al. Diversity of soil-dwelling Trichoderma in Colombia and their potential as biocontrol agents against the phytopathogenic fungus sclerotinia sclerotiorum (Lib.) de Bary. J. Gen. Plant Pathol. 79, 74–85 (2013).
Prabhakaran, N., Prameeladevi, T., Sathiyabama, M. & Kamil, D. Multiplex PCR for detection and differentiation of diverse Trichoderma species. Ann. Microbiol. 65, 1591–1595 (2015).
Cheney, S. A., Lafranchi-Tristem, N. J., Bourges, D. & Canning, E. U. Relationships of microsporidian genera, with emphasis on the polysporous genera, revealed by sequences of the largest subunit of RNA polymerase II (RPB1). J. Eukaryot. Microbiol. 48, 111–117 (2001).
Hirt, R. et al. Microsporidia are related to fungi: evidence from the largest subunit of RNA polymerase II and other proteins. Proc. Natl. Acad. Sci. 96, 580–585 (1999).
Hassan, M. M., Farid, M. A. & Gaber, A. Rapid identification of Trichoderma Koningiopsis and Trichoderma longibrachiatum using sequence-characterized amplified region markers. Egypt. J. Biol. Pest Control. 29, 1–8 (2019).
Brito-Vega, H. The morphological and molecular characterization of Trichoderma spp. In cocoa agroforestry systems. Open Sci. Journal 5, 1–14 (2020).
Johnson, L. Effect of antibiotics on the numbers of bacteria and fungi isolated from soil by the dilution-plate method. Phytopathology, 47, 630–631 (1957).
Elad, Y., Chet, I. & Henis, Y. A selective medium for improving quantitative isolation of Trichoderma spp. From soil. Phytoparasitica 9, 59–67 (1981).
Baba, Z. et al. Incidence of fusarium wilt of Chilli (Capsicum annum L.) in Kashmir Valley and its management by Trichoderma spp. Mycopath 12, 1–8 (2014).
Gupta, S., Mahajan, S., Gupta, M. & Sharma, D. Screening Of native isolates Of Trichoderma spp. Of Jammu for their biocontrol potential through hydrolytic enzyme activities. Indian Phytopathol. 69, 173–180 (2016).
Mohiddin, F., Bashir, I., Padder, S. A. & Bhat, M. A. Characterization of biocontrol agents isolated from temperate region of India. J. Pure Appl. Microbiol. 11, 379–385 (2017).
Tuite, J. Plant pathological methods. Fungi and bacteria. (1969).
Gomez, K. A. & Gomez, A. A. Statistical Procedures for Agricultural Research (Wiley, 1984).
Murray, M. & Thompson, W. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8, 4321–4326 (1980).
White, T. J., Bruns, T., Lee, S. & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: Guide Methods Appl. 18, 315–322 (1990).
White, T. PCR Protocols: A guide to methods and applications, 315 (1990).
Shoukouhi, P. & Bissett, J. Preferred primers for sequencing the 5’end of the translation elongation factor 1-alpha gene (eEF1a1). ISTH available from: (2008). http://www.isth.info/methods
Shoukouhi, E. Preferred primers for sequencing the 5’end of the translation elongation factor 1-alpha gene (EF1-α1) and subunit 2 of the RNA polymerase B gene (RPB2). (2008). http://www.isth.info/methods
Hall, T. A. in Nucleic acids symposium series. 95–98 (Oxford).
Tamura, K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (2011).
Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120 (1980).
Acknowledgements
The authors are thankful to all scientists, technical- and non-technical staff as well as students of Plant Pathology, SKUAST-K who directly or indirectly helped us in conducting the present study.
Funding
No funding was available and the corresponding author has paid all the cost incurred on current research personally.
Author information
Authors and Affiliations
Contributions
SS-carried the major work and wrote the paper SH-wrote the paper and data analysis MM-reviewed paper MSD-Sequence data analysis BAP-Data analysis IK-Data analysis and interpretations of results KM-Guidance and review the results during experimentation MM-isolation and characterization of some isolates BCASK-reviewed the paper AN-Data analysis and did morphology of isolates MAL-Data analysis and interpretations of results SSM-Review of the paper and writing OC-Review and language editing MDS-Concept of the work, writing, reviewing and data analysis (Major Advisor).
Corresponding author
Ethics declarations
Compliance with ethical standards
There was no involvement of animals in this study. All authors declare that there are no conflicts of interest.
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was received from all the individual participants included in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Surma, S., H., S., M., M. et al. Morpho-cultural and molecular characterization of trichoderma species from the northwestern himalayan apple rhizosphere of India. Sci Rep 15, 26320 (2025). https://doi.org/10.1038/s41598-025-12086-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12086-4
Keywords
This article is cited by
-
Decoding Plant Growth-Promoting Rhizobacteria Volatile Organic Compounds Mediated Growth Promotion in Malus domestica
Journal of Plant Growth Regulation (2025)