Abstract
Inflammation plays a key role in the pathogenesis of various diseases, including inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease. Similar to animals, plant cells produce exosome-like nanovesicles (ELNs) that play a role in transmitting biological signals from specific types of cells or tissues to other cells or tissues. Here we show that ELNs derived from Peucedanum japonicum (PjELNs) exhibit potent anti-inflammatory effects on macrophages and are effective in a mouse colitis model induced by dextran sodium sulfate (DSS). RNA sequence analysis reveals that PjELNs suppress multiple inflammatory cytokine-mediated signaling pathways in LPS-stimulated macrophage cell lines. We further show that PjENLs promote the differentiation of goblet cells, thereby enhancing mucin production and providing a protective effect on mucosal damage in a DSS-induced murine model of colitis. We also demonstrate that PjELNs inhibit the DSS-induced reduction in the number of transit amplifying cells. PjELNs exhibit anti-inflammatory properties, partly due to the presence of miRNA-like small RNAs that directly regulate the expression of the inflammatory cytokine IL6. Importantly, these small RNAs exhibit cross-species effects in both humans and mice. These findings reveal the mechanism by which plant-derived ELNs modulate inflammatory responses, suggesting their potential as a preventive and therapeutic strategy for inflammatory diseases of the colon.
Similar content being viewed by others
Introduction
Inflammation is associated with various diseases, including inflammatory bowel diseases (IBD) such as ulcerative colitis (UC) and Crohn’s disease (CD)1,2,3. IBD is characterized by chronic inflammation of the intestinal tract, influenced by an overactive immune response and genetic factors. UC is limited to the colon, typically starting in the rectum and spreading continuously. In contrast, CD can occur anywhere in the digestive tract, often causing transmural inflammation. Molecular mechanisms involve the overproduction of cytokines, including TNF-α, IL-6, IL-1β, INF-γ, IL-17, IL12 and IL-23, and hyperactivation of immune cells such as T cells, macrophages, NK cells and granulocytes4,5. For mild to moderate UC, 5-aminosalicylic acid is an effective treatment6. On the other hand, moderate to severe UC can be treated with drugs targeting specific pathways of inflammation, including monoclonal antibodies to TNF-α, α4β7 integrins, IL12 and IL237. Small molecule drugs such as JAK inhibitors are also available8. In addition, novel therapies explore microbiota manipulation, emphasizing individualized approaches tailored to patients’ disease profiles9,10. However, there remains a need for the development of new treatments that are more effective and have fewer side effects.
Exosomes are small particles encapsulated in a lipid bilayer membrane and are generated by all cells. Once secreted, exosomes traverse the intercellular space and enter the circulatory system, where they can exert localized paracrine effects as well as systemic influences on distant sites. This makes them valuable as information carriers and key participants in various biological processes11,12. Exosome-like nanovesicles (ELNs) in plants are structurally and functionally similar to exosomes, and have the capability to regulate inflammatory responses13,14. For example, ginger-derived ELNs (GDELNs) are absorbed by intestinal macrophages and Lgr5-positive stem cells in mice, enhancing stem cell population. GDELNs upregulate HO-1 and anti-inflammatory cytokines Il10, while increasing Il6 expression in macrophages15. Malberry bark ELNs derived from white birch activate the AhR signaling pathway via HSPA8, a member of Hsp70, and protect against colitis in mice16. Lemon-derived ELNs induce pro-apoptotic gene expression and inhibit the proliferation of three types of cancer cells17.
In this study, we investigated the anti-inflammatory effects of ELNs purified from plants consumed as food in Okinawa Prefecture, Japan, a region renowned for its high population of long-lived individuals. As a result, we found that ELNs derived from Peucedanum japonicum, commonly known as coastal hog fennel, a perennial plant in the Apiaceae family, exhibit strong anti-inflammatory effects. We show that that ELNs derived from Peucedanum japonicum (PjELNs) suppress the expression of the inflammatory cytokines Il6 and Il1β during acute inflammatory responses in mouse macrophages, and also have the ability to suppress inflammatory responses in vivo in dextran sodium sulfate (DSS)-induced mouse colitis18. Furthermore, we demonstrate that PjELNs contain multiple miRNAs-like small RNAs that function across species and respond to inflammatory cytokines in both mice and humans. These results suggest that ELNs derived from edible plants could serve as valuable natural agents against acute and chronic inflammation.
Results
Isolation and characterization of exosome-like nanovesicles (ELNs) from Peucedanum Japonicum
We isolated ELNs from 14 types of vegetables, including Peucedanum japonicum (Fig. 1A), which are commonly consumed in Okinawa Prefecture, Japan, using the ultracentrifugation method. These ELNs were subsequently assessed for their effects on inflammatory responses in mammalian cells. ELNs were collected using sucrose density gradient ultracentrifugation, leading to the separation of distinct fractions designated as Band 1 and Band 2, which varied depending on the plant species (as an example, the results for Peucedanum japonicum are shown in Fig. 1B). Subsequently, ELNs were exposed to mouse macrophage Raw-Blue cells, and RT-qPCR was utilized to assess alterations in the expression of inflammatory cytokines Il6 and Il1β following LPS stimulation. ELNs derived from turmeric, crimson glory vine, grapefruit, maderira vine, lemongrass, garlic vine, bitter melon, and Peucedanum japonicum suppressed the expression of both Il6 and Il1β (Supplementary Fig. 1A). For this study, we chose to focus on Peucedanum japonicum-derived ELNs (PjELNs) for further detailed analyses.
Characterization of PjELNs (Peucedanum japonicum exosome-like nanovesicles). (A) Peucedanum japonicum (coastal hog fennel). (B) Sucrose density gradient ultracentrifugation. White arrow, Band 1; Black arrow, Band 2. (C) (left panel) Particle distribution in the Peucedanum japonicum Band 2 fraction analyzed using NTA (Nanoparticle Tracking Analysis). (right panel) Average size of Band 2 particles (N = 48). (D) Transmission electron microscopy image of PjELNs (Scale bar: 100 nm). (E) Size distribution of RNAs in PjELNs (N = 6). (F) PjELNs inhibit mIl6 and mIl1β expression in LPS-treated Raw-Blue cells in a dose-dependent manner. (**: P < 0.01; N = 3).
For Peucedanum japonicum, we successfully recovered Band1 (1.02 ~ 1.12 mg/ml) and Band2 (1.12 ~ 1.18 mg/ml) fractions, each with distinct densities, and found that both effectively inhibited the expression of Il6 and Il1β (Fig. 1B and Supplementary Fig. 1A). However, Band2 exhibited a stronger suppressive effect, and as a result, we selected the Band2 fraction for subsequent analyses. Nanoparticle Tracking Analysis (NTA) revealed that PjELNs had an average size of 140 nm (Fig. 1C). Transmission electron microscopy confirmed that PjELNs has a double-membrane structure (Fig. 1D). We also found that PjELNs contained small RNAs of 100 nucleotides or less (Fig. 1E). Furthermore, PjELNs exhibited a concentration-dependent inhibitory effect on Il6 and Il1β expression in LPS-treated Raw-Blue cells (Fig. 1F) and no significant differences in Il6 expression were observed between several preparations (Supplementary Fig. 1B).
PjELNs significantly suppress LPS-induced inflammatory cytokine induction
To investigate the potential anti-inflammatory effects of PjELNs, we analyzed PjELNs-regulated genes in Raw-Blue cells. We extracted total RNA from three groups: non-stimulated Raw-Blue cells (None), LPS-stimulated Raw-Blue cells (LPS), and Raw-Blue cells pre-treated with PjELNs obtained from three independent preparations before LPS stimulation (LPS-PjELNs). We then subjected the samples to RNA-seq analysis. (Fig. 2A). We found that LPS stimulation induced the upregulation of 2,460 genes, while 2,746 genes were downregulated (Fig. 2B). Furthermore, comparing the gene expression profiles between LPS- and LPS-PjELNs-treated cells revealed that PjELNs treatment resulted in the upregulation of 187 genes and the downregulation of 351 genes (Fig. 2B). It is well-known that LPS stimulation induces the expression of inflammatory cytokines and activates macrophages. We therefore focused on genes upregulated by LPS stimulation and those downregulated by PjELNs treatment. Among the 2,460 genes induced by LPS stimulation, we identified 68 cytokine-related genes, 24 of which were downregulated by PjELNs treatment (Fig. 2C). Moreover, PjELNs significantly downregulated key inflammatory cytokines, including Il6, Il1β, Cxcl2, and Cxcl3 (Fig. 2D and E).
PjELNs significantly suppress inflammation-related pathways upregulated by LPS stimulation. (A) Samples used for RNA-seq analysis. (B) Volcano plots showing LPS versus None (left panel), and LPS-PjELNs versus LPS (right panel). Green dots represent significantly upregulated genes (FDR ≤ 0.05 and logFC > 0.6), while red dots represent significantly downregulated genes (FDR ≤ 0.05 and logFC < -0.6). (C) Venn diagram comparing upregulated genes in LPS versus None with downregulated genes in LPS-PjELNs versus LPS (upper panel). The bottom panel shows the results specifically for cytokines. (D) Heatmap of genes significantly differentially expressed in LPS-PjELNs versus LPS (Figure. 2B right panel). The upper panel displays gene expression levels standardized with z-scores across samples. The bottom panel shows the log fold change (logFC) values between the indicated conditions. (E) Boxplots of gene expression levels across conditions. (F,G) Dot plots showing Gene Set Enrichment Analysis (GSEA) results for pathways upregulated in LPS versus None (F) and downregulated in LPS-PjELNs versus LPS (G). Results are represented by size (gene count; legend in the upper right) and color (FDR; legend in the lower right). (H,I) Enrichment plots of the TNFA SIGNALING VIA NFKB (H) and IL6 JAK-STAT3 SIGNALING pathways (I) from Fig. 2F and G, respectively.
We next performed Gene Set Enrichment Analysis (GSEA) to elucidate the signaling pathways regulated by PjELNs. Consistent with a previous report19GSEA revealed that TNFα-NFκB signaling and IL6-JAK-STAT3 signaling were upregulated by LPS stimulation (Fig. 2F,G). LPS stimulation also activated epithelial-mesenchymal transition (EMT) and signaling pathways related to the inflammatory response, including INF-α signaling, INF-γ signaling, and IL2-STAT5 signaling (Fig. 2F). In contrast, PjELNs significantly suppressed LPS-induced activation of the TNFα-NFκB and IL6-JAK-STAT3 signaling pathways (Fig. 2H,I). Furthermore, treatment with PjELNs inhibited LPS-induced activation of the EMT, IFN-α, and IFN-γ signaling pathways (Fig. 2H).
Taken together, these results suggest that PjELNs significantly attenuate the inflammatory response induced by LPS stimulation by inhibiting the production of pro-inflammatory cytokines.
The anti-inflammatory effects of PjELNs in vivo
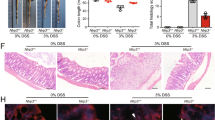
To examine the effects of PjELNs in vivo, we utilized a dextran sodium sulfate (DSS)-induced mouse colitis model20. Mice were orally administered with 1 × 1011 PjELNs daily for one week, followed by exposure to 3% DSS through their drinking water while continuing PjELNs administration. Compared to the PBS group, the PjELNs-treated mice exhibited a substantial increase in body weight starting on the third day of treatment. However, from the second day of DSS consumption onward, body weight decreased, resembling the PBS group (Fig. 3A). Additionally, no effect of PjELNs on cell proliferation in the large intestine was observed in the absence of DSS ingestion (Fig. 3B). Bleeding was observed in mice after 3 days of DSS treatment (Fig. 3C). In the PBS group, all mice displayed anal bleeding (category 5) starting on the 5th day after DSS ingestion. Conversely, in the PjELNs-treated group, bleeding was observed in only about 40% of the mice, even by the 7th day of DSS ingestion (Fig. 3C). Additionally, the PBS group displayed a progressive shortening of the large intestine from the cecum to the anus due to DSS intake, whereas in the PjELNs group, the reduction in intestinal length was significantly attenuated compared to the PBS group (Fig. 3D). Subsequently, the degree of inflammation was histological analysis evaluated using HE and Alcian blue-stained Sect.21. The total colitis score was determined based on inflammation severity, inflammation extent, crypt damage, and the ratio of the inflamed area to the total length (Supplementary Fig. 2). Based on the results, a significant reduction in inflammation was observed in the group administered PjELNs on days 5 and 7 of DSS intake (Fig. 3E).
PjELNs ameliorate DSS-induced colonic inflammation. (A) Body weight changes following administration of PBS (blue) or PjELNs at 1 × 1011 particles/day/mouse (green). (*: P < 0.05). (B) EdU uptake in the colon in the absence of DSS intake. (C) Bleeding severity categorized into five levels. (D) Impact of 3% DSS intake on colon length. (*: P < 0.05, **: P < 0.01; N = 3 for each time point). (E) total colitis score ((*: P < 0.05, **: P < 0.01; N = 3 for each time point).
In addition to HE (Fig. 4A-D) and Alcian blue staining (Fig. 4I–L), morphological analysis was performed using immunostaining (Fig. 4E–H) and in situ hybridization (Fig. 4M–P). In the large intestines of PBS-treated control mice, spanning from the cecum to the anus, tube-like structures formed by the tight junction marker ZO-1 and the epithelial cell marker β-catenin were consistently observed on the luminal surface layer (Fig. 4E. In mice that received DSS for 7 days, the epithelial cells displayed an irregular structure, with the disappearance of tight junctions (Fig. 4F). In this disrupted structural state, the number of mucin-producing cells significantly decreased, and Muc2-positive cells were nearly absent (compare Fig. 4I and J, or 4M and N, white arrows, respectively). In the large intestine, the mucus layer acts as a critical barrier that prevents direct contact between epithelial cells and dense populations of microorganisms22. Disruption of this system is a hallmark of colitis. However, even after 7 days of DSS ingestion, the ductal structure and expression of goblet cell markers Muc2, ClcaI, and Muc3 were preserved in the PjELNs-treated group (Fig. 4G and H, 4 K and L, 4O and P, black arrows and Supplementary Fig. 3A). Additionally, EdU-positive cells were retained (Supplementary Fig. 3B), indicating significantly reduced damage from DSS treatment.
PjELNs mitigate DSS-induced colonic structural damage. (A–D) Hematoxylin-Eosin staining (scale bar: 1.5 mm); (E–H) immunohistochemistry (scale bar: 100 μm); (I–L) Alcian blue staining (scale bar: 100 μm); (M–P) in situ hybridization (scale bar: 100 μm). White arrows indicate abnormal epithelial cell structures caused by DSS (uneven staining of β-catenin, absence of Alcian Blue staining, loss of Muc2 expression). Black arrows represent the amelioration of DSS-induced effects following PjELNs administration (uniform β-catenin staining, presence of Alcian Blue staining, maintenance of Muc2 expression).
PjELNs promote goblet cell differentiation and prevent the decline of transit amplifying cells
The Muc2 gene is typically expressed near the crypt base, with its expression decreasing as the distance from the cecum increases23. However, in the mice treated with PjELNs, we observed that the Muc2 gene expression in the distal colon, an area where the Muc3 gene is usually expressed (Fig. 5A and Supplementary Fig. 4). This distal expression was observed in intercrypt goblet cells (icGCs), the most differentiated goblet cells located on the colonic luminal surface24 (Fig. 5A, Black arrows), suggesting that PjELNs promote goblet cell differentiation toward icGCs.
PjELNs promote goblet cell differentiation and mitigate the reduction of transit amplifying cells. (A) Expression pattern of the Muc2 gene in the colon in the absence of DSS intake (a and e, whole colon image; P, proximal; M, middle; D, distal colon. Scale bar, 1 mm). (b-d and f-h, Close-up image. Scale bar, 100 μm). Black arrows indicate intercrypt goblet cells (icGCs). (B) Effect of PjELN administration on transit amplifying cells in the small intestine during DSS intake (white, OLFM4. Insets: green, β-catenin; blue, DAPI. Scale bar, 100 μm) (*, P < 0.05; **, P < 0.01; N = 3 for each time point).
We also observed a significant reduction in the number of transit amplifying cells positive for olfactmedin4 (OLFM4) at the crypt base after 7 days in the DSS-treated group (Fig. 5B). In contrast, this decrease was suppressed by approximately 20% in the PjELNs-administerd group (Fig. 5B). Consistent with our findings, it has been reported that certain plant-derived ELNs affect transit amplifying cells in the small intestine16. These findings suggest that the promotion of goblet cell differentiation and the prevention of transit amplifying cell reduction by PjELNs may play an important role in suppressing DSS-induced inflammation.
Small RNAs present in PjELNs have the potential to inhibit Il6 expression
To elucidate the mechanism of the anti-inflammatory effects of PjELNs, we performed GSEA using a collection of transcription factor targets (TFT) on the RNA-seq data in Fig. 2. The analysis revealed that genes induced by LPS stimulation were significantly regulated by transcription factors associated with key inflammatory signaling pathways such as NFκB (Supplementary Fig. 5A). Conversely, for genes suppressed by PjELN treatment, we did not observe significant transcription factor-mediated regulation (Supplementary Fig. 5B). PjELNs exerted a suppressive effect on NF-κB activation upon LPS stimulation, but this effect was comparable to that of ginger-derived ELNs, which lack the ability to suppress Il6 expression (Supplementary Fig. 1 and Fig. 6). Therefore, this action may contribute in part to the suppression of pro-inflammatory cytokine genes’ expression by PjELNs, suggesting the involvement of other mechanisms. We therefore speculated that small RNAs, particularly miRNAs present in PjELNs, might play an important role in regulating the expression of inflammatory cytokine genes.
Small RNAs in PjELNs suppress the expression of inflammatory cytokines. (A) Bar chart showing the alignment results of small RNA sequences extracted independently from PjELNs with known miRNAs in the miRBase database. The X-axis represents the proportion of small RNAs in PjELNs corresponding to known miRNAs. (B) Proportion of small RNAs in PjELNs corresponding to known miRNAs originating from different biological species. (C) Counts of human (left panel) and mouse (right panel) miRNAs similar to the small RNA sequences in PjELNs (top 15). (D) Counts of human (left panel) and mouse (right panel) miRNAs predicted to regulate IL6 expression (top 15). Similar small RNAs are found in PjELNs. (E) Schematic illustrating miRNAs predicted to regulate IL6 expression in both mouse and human.
To validate this hypothesis, we performed small RNA-seq analysis on PjELNs to investigate whether the small RNAs, including miRNAs, contained in PjELNs could suppress the expression of inflammatory cytokines. Small RNA-seq analysis showed that two independently prepared sets of PjELNs contained small RNAs equivalent to known miRNAs at rates of 12.8% (Prep-1) and 17.7% (Prep-2) (Fig. 6A). Additionally, we found that both PjELNs preparations contained an abundant small RNAs corresponding to miRNAs commonly found in either humans or mice (Fig. 6B and C). Furthermore, we found that both sets of PjELNs contained small RNAs that regulate the expression of inflammatory cytokines such as IL6 (Fig. 6D), IL1β (Supplementary Fig. 5C), CXCL2 (Supplementary Fig. 5D), and CXCL3 (Supplementary Fig. 5E). These results suggest that the small RNAs present in PjELNs may function as miRNA-like molecules, controlling the expression of inflammatory cytokines such as IL6 and IL1β. Among these miRNAs, mmu-miR-7006-3p and mmu-miR-12178-3p were predicted to regulate not only mouse IL6 expression but also human IL6 expression (Fig. 6E).
The small RNAs contained within PjELNs suppress the expression of inflammatory cytokine genes in both mice and humans
We investigated the expression levels of small RNAs with similarities to multiple human and mouse miRNAs in PjELNs using RT-qPCR. For mmu-Let7d-5p, which had a low read count in the RNA-sequence data, its content in PjELNs was less than 10% (Fig. 7A). In contrast, mmu-miR-8117, which had a high read count in the RNA-sequence data, was estimated to be present in PjELNs at levels 1 × 108 times greater than in Raw-Blue cells. Despite variations in concentration, other sequences were similarly enriched in PjELNs. We next investigated whether miRNAs homologous to the small RNAs in PjELNs contribute to the suppression of Il6 expression in Raw-Blue cells. We found that mmu-miR-6995-3p, mmu-miR-7006-3p, and mmu-miR-487b-5p effectively suppressed Il6 expression following LPS stimulation (Fig. 7B).
PjELNs contain functional small RNAs that regulate mouse and human IL6 gene expression. (A) Expression patterns of mouse-like miRNAs contained in ELNs (PjELNs); S10 (10,000 x g supernatant of Peucedanum japonicum); and Raw (Raw-Blue cell total RNA). (P < 0.05, **, P < 0.01; N = 3). (B) Effect of mmu miRNAs on mIl6 expression in Raw-Blue cells. (*, P < 0.05; **, P < 0.01; N = 3). (C) Expression patterns of human-like miRNAs contained in ELNs (PjELNs); S10 (10,000 x g supernatant of Peucedanum japonicum); HL60(S) (HL60(S) total RNA); and THP-1 (THP-1 total RNA). (*, P < 0.05; **, P < 0.01; N = 3). (D) Effect of hsa miRNAs and mmu miRNAs on IL6 expression in THP-1 cells. (*, P < 0.05; N = 3). (E) Effect of anti-mmu miR-7006-3p on mIl6 expression in Raw 264.7 cells. (*, P < 0.05; N = 3).
We also examined the expression of small RNAs homologous to human miRNAs in PjELNs. RT-qPCR analysis confirmed that multiple sequences were present in PjELNs at levels 1 × 103 times higher than in human macrophage cells (Fig. 7C). To explore whether these small RNAs also function in human macrophage THP-1 cells, we assessed their effects and found that hsa-miR-6737-3p reduced LPS-induced IL6 expression (Fig. 7D). Additionally, we observed that mmu-miR-7006-3p, which is not naturally present in human cells, also downregulated the human IL6 gene.
We further investigated whether the suppressive effect of PjELNs could be inhibited by expressing an antisense sequence to mmu miR-7006-3p. We infected Raw 264.7 cells with doxycycline (Dox)-inducible anti mmu-miR 7006-3p or Renilla luciferase short hairpin constructs, the latter used as negative control. After 24 h of construct induction, we assessed mIl6 expression via RT-qPCR. In cells expressing Renilla luciferase short hairpin, mIl6 expression was suppressed by PjELNs following 3 h of LPS stimulation. However, the suppressive effects of PjELNs were significantly reversed by anti-miR 7006-3p expression (Fig. 7E). These results suggest that PjELNs are enriched with multiple small RNAs capable of suppressing inflammatory cytokines across species.
Discussion
In the present study, we have discovered that PjELNs derived from Peucedanum japonicum, a vegetable traditionally believed to confer health benefits, exhibit potent anti-inflammatory effects both in vitro and in vivo. Specifically, PjELNs inihibited LPS-induced upregulation of Il6 and Il1β in macrophage cell lines. RNA-seq analysis further revealed that PjELNs significantly attenuate LPS-induced enhancement of TNFα-NFκB and IL6-JAK-STAT3 signaling. Moreover, PjELNs exhibited anti-inflammatory effects in a DSS-induced mouse colitis model, suggesting a potential therapeutic role in mitigating inflammation associated with colitis. These cytokines and signaling pathways are also implicated in the pathogenesis of numerous other inflammatory diseases, including rheumatoid arthritis, cardiovascular diseases and cancer25,26,27. Our findings therefore raise the possibility that PjELNs could be valuable in the prevention and treatment of various inflammatory disorders.
In recent years, ELNs have been isolated from various plant species and shown to possess anti-inflammatory properties. Among ELNs purified from the 14 different plant species, we found that PjELNs exhibit a more potent in vitro anti-inflammatory effect compared to ginger28 and grape29. This is likely due to PjELNs’ ability to inhibit multiple signal transduction pathways, including the TNFα-NFκB signal, IL6-JAK-STAT3 signal, and IFNγ signaling pathways. Peucedanum japonicum has traditionally been used to treat ailments such as colds, coughs, physical weakness, and chronic bronchitis, particularly in the elderly, potentially due to its anti-inflammatory properties mediated by PjELNs.
Goblet cells produce the intestinal mucus layer and protect epithelial cells30. icGCs repersent the most differentiated stage of goblet cells and their dysfunction is closely linked to the onset of colitis22. In our in vivo inflammatory response study, we found that PjELNs promote goblet cell differentiation into icGCs and enhance mucin production. Thus, we speculate that PjELN-induced differentiation toward icGCs may contribute to the observed suppression of inflammation in PjELNs-treated mice. Although Spdef (SAM pointed domain containing ETS transcription factor) is known to play a role in goblet cell differentiation31the mechanism by which PjELNs exert their influence remains to be elucidated. Similar to ginger-derived ELNs, PjELNs are thought to be absorbed by macrophages in the intestinal mucosa upon oral ingestion, thereby suppressing the inflammatory response. However, the crosstalk between macrophage and goblet cells in this process has yet to be determined.
Transit amplifying cells are differentiating cells located at the crypt base that give rise to various cell types. These cells are maintained by niche factors such as R-spondin, Noggin and EGF, which are supplied by proximal lymph nodes or fibroblasts in the gastrointestinal tract32,33. In our study, we observed that PjELNs prevent the reduction of transit amplifying cells in the colitis model, suggesting that this effect may be critical for alleviating colitis. Further investigation into potential crosstalk between intestinal mucosal macrophages and fibroblasts could provide valuable insights into this mechanism.
We have discovered that small RNAs enclosed within PjELNs suppress the expression of inflammation cytokine genes. While previous reports have identified small RNAs in plant-derived ELNs34we successfully identified small RNAs that specifically target cytokines involved in the inflammatory response. Interestingly, these small RNAs exhibit cross-species effects in both humans and mice. It has been reported that miRNAs encapsulated in human and mouse exosomes are sorted by various RNA-binding proteins, and that miRNAs with characteristic sequences called EXOmotifs are selectively encapsulated35,36. We found that the functional small RNAs identified here are highly enriched within ELNs. Therefore, it would be interesting to investigate which plant RNA-binding proteins are responsible for this enrichment and whether EXOmotif-like sequences play a role in the selective packaging. Although identifying common small RNAs between PjELNs and ELNs from other anti-inflammatory plants remains a challenge due to limited genomic information, analyzing potential commonalities presents an exciting avenue for future research.
Methods
Preparation of plant-derived exosome-like nanovesicles (ELNs)
Approximately 500 g of each vegetable was purchased from a local grocery store. After washing the vegetables with water, they were crushed using a household juicer. The crushed material was then subjected to centrifugation at 3,000 x g for 20 min once, followed by two rounds of centrifugation at 10,000 x g for 40 min to remove solid debris. Subsequently, the fraction containing EVs was pelleted by ultracentrifugation at 150,000 x g for 90 min. The obtained pellet was resuspended in PBS and layered onto a sucrose density gradient consisting of 60%, 45%, 30%, and 8% sucrose solutions. The samples were then ultracentrifuged at 150,000 x g for 90 min, and fractions were collected. The fraction at the 30–45% sucrose density boundary was labeled as Band2, while the fraction at the 8–30% boundary was labeled as Band1. Each fraction was pelleted again by ultracentrifugation at 150,000 x g for 90 min, resuspended in PBS, and particle size and concentration were measured using Nanoparticle Tracking Analysis (NTA). The obtained EVs were stored at 4 °C or -80 °C.
Nanoparticle tracking analysis (NTA)
The particle size, size distribution, and concentration of extracellular vesicles obtained through sucrose density gradient ultracentrifugation were measured using the NanoSight NS300 (Malvern Instruments), equipped with a 405 nm blue laser.
Cell culture
Raw 264.7 (ATCC: TIB-71) mouse macrophage cells and HEK 293T (RIKEN BRC: RCB2202) cells were cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% FBS and Pen-Strep (100 U/ml). Raw-Blue (InvivoGen) mouse macrophage cells were cultured in DMEM supplemented with 10% FBS, 100 µg/ml Normocin (InvivoGen), 200 µg/ml Zeocin, and Pen-Strep (100 U/ml) at 37 °C in a 5% CO2 incubator. Human monocytic leukemia THP-1 (JCRB Cell Bank: JCRB0112) cells or human myeloid leukemia HL60(S) (JCRB Cell Bank: JCRB0163) cells were cultured in RPMI medium supplemented with 10% FBS and Pen-Strep (100 U/ml) at 37 °C in a 5% CO2 incubator. To assess inflammatory responses, THP-1 cells were first cultured in medium supplemented with 10 ng/ml PMA (Wako) for 24 h to differentiate them into macrophage-like cells. Following this, the cells were cultured in regular medium for an additional 24 h. To assess the effect of ELNs, they were added to the medium at a final concentration of 1 × 1010 particles/ml, and the cells were incubated for 16 h. The cells were then stimulated with LPS (Wako) at a final concentration of 1 µg/ml for 24 h to induce an inflammatory response. Doxycycline-inducible stable Raw 264.7 cell lines expressing anti-mmu miR7006-3p and a control (Renilla luciferase short hairpin RNA) were generated using a lentivirus system37,38. Anti-mmu miR7006-3p was amplified by PCR using the miRE-anti-mmu miR7006-3p oligo as a template, along with mire-XhoFw and Eco-oligo-rev primers. The XhoI-EcoRI fragment was inserted into pEN_TmiRc3 entry vector and subcloned into pSLIK-Neo vector using the Gateway LR Clonase II enzyme mix (Thermo Fisher).
pSLIK-miR-E-Neo vectors were cotransfected with pRSV-Rev, pMDLg/pRRE39and pMD2.G into HEK 293T cells for viral production. Virus particles were harvested using PEG-it (System Bioscineces), titrated by qPCR (Applied Biological Materials), and used to infect Raw 264.7 cells (MOI = 10.0). Stable cell lines were selected by culturing cells in the presence of 0.5 mg/ml G418. Anti-miRNA expression was induced by incubating the cells with 10 µg/ml doxycycline for 24 h. Subsequently, ELNs were added to the medium to reach a final concentration of 2 × 109 particles/ml for 16 h, and the cells were then incubated with 10 µg/ml LPS for 3 h.
Reverse transcription and quantitative PCR analysis
Following LPS stimulation, total RNA was extracted from the cells using Trisure (NipponGenetics), and cDNA was synthesized using the PrimeScript RT reagent kit (Takara Bio). Quantitative PCR (qPCR) was then conducted using TB Green Premix Ex Taq II (Takara Bio). Gene expression levels were normalized using the Gapdh gene for both mouse or human samples, and relative expression was calculated using the ΔΔCt method.
For the analysis of miRNA-like small RNAs in PjELNs, Peucedanum japonicum crushing solution (S10), or cultured cells, cel-miR-39 was introduced as a spike-in control (Norgen Biotek). RNA was then isolated using the Direct-zol RNA MicroPrep kit (Zymo Research). cDNA was synthesized from the extracted RNA using the Mir-X miRNA First-Strand Synthesis kit (Takara Bio), followed by qPCR analysis. All experiments were performed in triplicate (N = 3).
Primer sequences are listed in Supplementary Table 1.
Transfection
Synthesized mouse and human miRNAs (Ambion) were trasnfected using RNAiMax (Thermo Fisher) for 24 h according to the manufacturer’s instructions. After 24 h, they were treated with PjELNs at a concentration of 1 × 1010 particles/ml for 16 h. Following this ELNs exposure, the cells were stimulated with LPS at a final concentration of 1 µg/ml for 3 h and then collected. In all transfection assays, three independent assays were performed. The mouse and human miRNA sequences used in this study are listed in Supplementary Table 1.
Mouse
Male C57BL/6J mice, aged 8 to 12 weeks, were orally administered 3% dextran sodium sulfate (DSS; molecular weight: 36–50 kDa) (MPB) in their drinking water for 7 days to induce colitis. The DSS solution was replaced every other day. The ELNs administration group received 1 × 1010 or 1 × 1011 ELNs/100 µl/mouse using oral gavage (Fuchigami Instruments), while the control group received an equal volume (100 µl/mouse) of PBS orally, starting 7 days before DSS treatment. On days 0, 3, 5, and 7 of DSS treatment, mice were injected intraperitoneally with 50 mg/kg of EdU, and 30 min later, they were anesthetized with isoflurane and subjected to perfusion fixation using 4% PFA. Body weight measurements, stool examination, and monitoring for diarrhea and rectal bleeding were conducted daily throughout the treatment period. A minimum of N ≧ 3 mice were used at each time point. All animal experimental protocols were performed in accordance with the guidelines of the Animal Ethics Committee of the University of Tokyo. Mice were maintained in accordance with protocols approved by the Animal Care and Use Committee of the Institute of Quantitative Bioscience, The University of Tokyo (approval number: A2022IQB004-01).
Immunohistochemistry and in situ hybridization
After perfusion with PBS, the small intestine (from just below the stomach to the cecum) and the large intestine (from just below the cecum to the anus) were dissected, and their lengths were measured. Subsequently, the tissues were fixed overnight in 4% PFA. After fixation, the intestines were longitudinally incised to remove feces, and then they were rolled into a ‘Swiss roll.’ The samples were dehydrated in a series of ethanol solutions (20%, 40%, 60%, 80%, 100%) and subsequently substituted with xylene before being embedded in paraffin. Sections of 8 μm thickness were cut from the paraffin blocks using a microtome. The sections were deparaffinized in xylene and then rehydrated through a series of ethanol solutions (100%, 80%, 60%, 40%, 20%) before being subjected to morphological observations using Hematoxylin and Eosin (HE) staining or Alcian Blue staining (pH 2.5). EdU incorporation was detected using the Click-iT EdU Cell Proliferation Kit (Thermo Fisher) according to the manufacturer’s instructions.
The prepared sections were deparaffinized using xylene and ethanol, followed by immersion in a 10 mM citrate buffer (pH 6.0) that was brought to a boil for 15 min to perform antigen retrieval for tissue staining. Afterward, the sections were blocked in a 2.5% BSA/PBS solution and incubated overnight at 4 °C with diluted primary antibody solutions. The primary antibody solutions used are anti-β-catenin (BD Biosciences: mouse monoclonal) antibody, anti-TJP1 (Human Protein atlas: rabbit polyclonal) antibody, and anti-OLMF4 (Cell signaling technology: rabbit monoclonal) antibody, all diluted to 1:500 in 1% BSA/PBS. Following incubation with the primary antibodies, the sections were washed with 0.1% saponin/PBS and then incubated for 60 min at room temperature with secondary antibodies, anti-mouse-Alexa 488 and anti-rabbit-Alexa 594 (Thermo Fisher), both diluted to 1:500. After counterstaining with 4’,6-diamidino-2-phenylindole (DAPI), the sections were mounted using VectaMount permanent mounting medium or Vector Shield mounting medium (Vector). Bright-field observations of the stained samples were performed using the BZ-X710 microscope (KEYENCE), while fluorescent observations were conducted using the FV-3000 confocal laser microscope (Olympus). Both observations were carried out using a 20x objective lens.
In situ hybridization was performed on deparaffinized and rehydrated sections under40 conditions, and observations were made using a 20x objective lens. For the probes, clones generated from cDNA isolated from the mouse digestive tract were used as templates for RT-PCR. These clones were labeled with DIG using T7 or SP6 RNA polymerase and the DIG RNA labeling reaction kit (Merk) for RNA probe labeling.
Histological scoring
The severity of colonic inflammation was assessed according to the method described in21. Inflammation severity was scored as follows: (none: 0, mild: 1, moderate: 2, severe: 3). Inflammation extent was scored as (none: 0, limited to the lamina propria: 1, extending into the submucosa: 2, reaching the muscularis propria: 3). Crypt damage was evaluated on the following scale: (none: 0, damage to the lower third of crypts: 1, damage to the lower two-thirds of crypts: 2, complete crypt loss with surface epithelium remaining: 3, complete loss of both crypts and surface epithelium: 4). In addition, the extent of lesions relative to the total colon length (Ratio) was evaluated using a four-point scale: (0%: 0, ≥ 1% and < 25%: 1, ≥ 25% and < 50%: 2, ≥ 50% and < 75%: 3, ≥ 75% and < 100%: 4). These scores were assessed using HE and Alcian blue-stained sections. The total colitis score was calculated by multiplying the sum of the above three parameters (inflammation severity, inflammation extent, and crypt damage) by the lesion extent ratio.
Immunoblotting
Western blots were carried out on total protein extract from PjELNs – LPS treated Raw-blue cells. Protein extracts were collected using PLC lysis buffer (50 mM Tris-HCl (ph7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and protease inhibitor). Protein lysates were separated by SDS-PAGE, transferred to PVDF membranes, blocked for 1% skim milk, and incubated overnight with Phospho-NFκB or NFκB antibody (CST, 1:500 in PBST). Protein bands were detected using Luminata Forte Western HRP Substrate (Merk Millipore) with HRP secondary antibodies and anti-GAPDH antibody conjugated HRP (MBL). The uncropped (full-size) gel images in Supplementary Fig. 6 are provided in Supplementary Fig. 7.
RNA-seq data analysis
Total RNA was extracted using RNA Clean & Concentrator. For RNA-seq analysis of RAW-Blue cells treated with PjELNs (Fig. 2A), sequencing library construction (mRNA-seq) and sequencing (paired-end 150 bp reads, 6G data) were performed using DNBSEQ-G50 by Beijing Genomics Institute (BGI). All sequenced libraries were aligned to the mouse reference genome build mm10 using STAR41. RSEM42 was used to calculate transcripts per kilobase million (TPM, Ensembl gene annotation GRCm38). Gene symbols and mRNA names were annotated according to mm10 using the UCSC database. For differential expression analyses, count data were analyzed using the R package ‘edgeR’43. The count data were fitted with a general linear model and differential expression was calculated using likelihood ratio tests. Genes with up-regulated (> 0.6-fold and FDR < 0.05) or down-regulated (< 0.6-fold and FDR < 0.05) were considered differentially expressed genes. Gene set enrichment analysis (GSEA) was performed using the R package ‘clusterProfiler’44.
Small RNA-seq data analysis
Small RNA-seq data analysis was performed according to the paper by Garg V and Varshney RV with slight modifications45. Small RNAs in PjELNs were extracted using RNA Clean & Concentrator and the quality and yield of small RNAs were measured with an Agilent 2200 TapeStation. Library construction and sequencing were performed using the NEBNext Multiplex Small RNA Library Prep Set for Illumina® (Set 1) and the Illumina Novaseq 6000, respectively. Adapter sequence and poly-A in the raw reads were trimmed with Trim Galore! (v. 0.6.7), retaining only reads of at least 20 bp. Pre-processed reads were aligned to existing miRNA sequences (Release 22, 271 organisms, 38,589 sequences) from miRbase (https://www.mirbase.org/) using bowtie46 with the -n 2 option. miRNA count data were generated from the alignment results using SAMtools47. A list of human and mouse miRNAs with a potential to regulate genes critical for inflammatory responses was obtained from the TargetScan database (https://www.targetscan.org/vert_80/). Subsequently, we identified small RNAs in PjELNs that are similar to human or mouse miRNAs predicted to regulate the expression of inflammatory cytokines.
Quantification and statistical analysis
Statistical analysis was performed using an unpaired two-tailed Student’s t-test with R version 4.2.2 (http://www.r-project.org/). A P-value of < 0.05 was considered statistically significant. Statistical details of experiments can be found in figure legends.
Data availability
The raw RNA-sequence and miRNA-sequence data acquired in this study have been deposited in GEO (GSE282037 and GSE282146).
References
Baumgart, D. C. & Carding, S. R. Inflammatory bowel disease: cause and immunobiology. Lancet 369, 1627–1640 (2007).
Kaser, A., Zeissig, S. & Blumberg, R. S. Inflammatory bowel disease. Annu. Rev. Immunol. 28, 573–621 (2010).
Neurath, M. F. Strategies for targeting cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 24, 559–576 (2024).
Neurath, M. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 14, 329–342 (2014).
Friedrich, M., Pohin, M. & Powrie, F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 50, 992–1006 (2019).
Kornbluth, A. & Sachar, D. B. & Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am. J. Gastroenterol. 105, 501–23; quiz 524 (2010).
Danese, S. et al. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann. Intern. Med. 160, 704–711 (2014).
Bechman, K., Yates, M. & Galloway, J. B. The new entries in the therapeutic armamentarium: the small molecule JAK inhibitors. Pharmacol. Res. 147, 104392 (2019).
Guo, X. Y., Liu, X. J. & Hao, J. Y. Gut microbiota in ulcerative colitis: insights on pathogenesis and treatment. J. Dig. Dis. 21, 147–159 (2020).
Shan, Y., Lee, M. & Chang, E. B. The gut Microbiome and inflammatory bowel diseases. Annu. Rev. Med. 73, 455–468 (2022).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367, eaau6977 (2020).
Isaac, R., Reis, F. C. G., Ying, W. & Olefsky, J. M. Exosomes as mediators of intercellular crosstalk in metabolism. Cell. Metab. 33, 1744–1762 (2021).
Dad, H. A., Gu, T. W., Zhu, A. Q., Huang, L. Q. & Peng, L. H. Plant Exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol. Ther. 29, 13–31 (2021).
Xu, Z. et al. Plant-derived extracellular vesicles (PDEVs) in nanomedicine for human disease and therapeutic modalities. J. Nanobiotechnol. 21, 114 (2023).
Mu, J. et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 58, 1561–1573 (2014).
Sriwastva, M. K. et al. Exosome-like nanoparticles from mulberry bark prevent DSS-induced colitis via the AhR/COPS8 pathway. EMBO Rep. 23, e53365 (2022).
Raimondo, S. et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 6, 19514–19527 (2015).
Eichele, D. D. & Kharbanda, K. K. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our Understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 23, 6016–6029 (2017).
Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2, 1–9 (2017).
Wirtz, S. et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 12, 1295–1309 (2017).
Williams, K. L. et al. Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in Transgenic mice that overexpress growth hormone. Gastroenterology 120, 925–937 (2001).
Bergstrom, K. et al. Proximal colon-derived O-glycosylated mucus encapsulates and modulates the microbiota. Science 370, 467–472 (2020).
Velcich, A. et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 295, 1726–1729 (2002).
Nyström, E. E. L. et al. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Science 372, (2021).
Ridker, P. M. & Rane, M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ. Res. 128, 1728–1746 (2021).
Singh, S., Singh, T. G., Mahajan, K. & Dhiman, S. Medicinal plants used against various inflammatory biomarkers for the management of rheumatoid arthritis. J. Pharm. Pharmacol. 72, 1306–1327 (2020).
Ren, L., Zhang, D., Pang, L. & Liu, S. Extracellular vesicles for cancer therapy: potential, progress, and clinical challenges. Front. Bioeng. Biotechnol. 12, 1476737 (2024).
Zhuang, X. et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles. 4, 28713 (2015).
Ju, S. et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 21, 1345–1357 (2013).
Johansson, M. E. V. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. U S A. 105, 15064–15069 (2008).
Gregorieff, A. et al. The ets-domain transcription factor Spdef promotes maturation of goblet and Paneth cells in the intestinal epithelium. Gastroenterology 137 (45.), 1333 (2009).
Goto, N. et al. Lymphatics and fibroblasts support intestinal stem cells in homeostasis and injury. Cell. Stem Cell. 29, 1246–1261e6 (2022).
Niec, R. E. et al. Lymphatics act as a signaling hub to regulate intestinal stem cell activity. Cell. Stem Cell. 29, 1067–1082e18 (2022).
Urzì, O., Raimondo, S. & Alessandro, R. Extracellular vesicles from plants: current knowledge and open questions. Int. J. Mol. Sci. 22, 5366 (2021).
Fabbiano, F. et al. RNA packaging into extracellular vesicles: an orchestra of RNA-binding proteins? J. Extracell. Vesicles. 10, e12043 (2020).
Garcia-Martin, R. et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature 601, 446–451 (2022).
Shin, K. J. et al. A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proc. Natl. Acad. Sci. U S A. 103, 13759–13764 (2006).
Fellmann, C. et al. An optimized MicroRNA backbone for effective single-copy RNAi. Cell. Rep. 5, 1704–1713 (2013).
Dull, T. et al. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 72, 8463–8471 (1998).
Saito, Y., Kojima, T. & Takahashi, N. Mab21l2 is essential for embryonic heart and liver development. PLoS ONE. 7, e32991 (2012).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 12, 323 (2011).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. EdgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. ClusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Garg, V. & Varshney, R. K. Analysis of small RNA sequencing data in plants. Methods Mol. Biol. 2443, 497–509 (2022).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Leinonen, R., Sugawara, H. & Shumway, M. International nucleotide sequence database collaboration. The sequence read archive. Nucleic Acids Res. 39, D19–21 (2011).
Acknowledgements
This work was supported by Ido Medical Corporation and Grant-in Aid for Scientific Research (No.21H02693, 21K07146, 22K06922) from MEXT, Project for Cancer Research and Therapeutic Evolution (P-CREATE, no. 17cm0106103h0002) from the Japan Agency for Medical Research and Development.
Author information
Authors and Affiliations
Contributions
T.K., T.H., Y.K., T.N. and T.A. designed research. T.K. performed experiments. T.H. performed bioinformatics analysis. T.K., T.H., Y.K., T.N. and T.A discussed and reviewed the manuscript. T.K., T.H. and T.A. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kojima, T., Hayashi, T., Kageyama, Y. et al. Exosome-like nanovesicles from Peucedanum Japonicum directly regulate inflammatory cytokines via small RNAs. Sci Rep 15, 27424 (2025). https://doi.org/10.1038/s41598-025-12175-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-12175-4