Abstract
The cigar wrapper leaves (CWLs), as a symbol of the intrinsic quality and appearance of cigars, reflects the overall quality of the cigar. The Shaoxing-flavored T3 Jiuqu used in Shaoxing wine production contains a large number of high-quality microorganisms, such as molds and yeasts, which play a significant role in enhancing flavor and quality. Among these microorganisms, several positively promote the fermentation of CWLs. A dominant strain, S1, was isolated and identified from the T3 Jiuqu and inoculated into the fermentation of CWLs. Gas Chromatography-Mass Spectrometry (GC–MS) was employed to analyze the volatile aroma components in the CWLs. The results showed that the contents of substances such as Phenethyl alcohol, Dihydroactinidiolide, Sclareol, and Farnesyl acetone were significantly increased compared to pre-fermentation (NF) and the natural fermentation with only water (WF) group. Specifically, Phenethyl alcohol content increased by 261.63% compared to WF group during the same turning-over period, while Farnesyl acetone content increased by 144.99%. The proportions of sugars and nicotine also increased significantly. Metagenomic analysis of the microbial samples on the surface of CWLs revealed that inoculating S1 significantly improved and altered microbial community structure. At the phylum level, the proportion of Pseudomonadota increased dramatically to 17%, while the proportion of Uroviricotasharply decreased sharply from 9% to 0. At the genus level, the previously dominant Staphylococcus genus was replaced by a balanced coexistence of Pantoea, Enterobacter, Cronobacter, and Aspergillus. This balanced microbial distribution significantly improved the quality of the CWLs.
Similar content being viewed by others
Introduction
Cigars are tobacco products made from leaves that have been harvested, cured, fermented, and aged, consisting of filler, binder, and wrapper. Fermentation is one of the most effective ways to enhance the quality of CWLs. This process is primarily dominated by complex microbial communities, which degrade certain macromolecules into smaller molecules and produce precursors of specific metabolites to improve the quality of the fermented product. Traditional fermentation is a spontaneous process that does not require artificial intervention and is highly influenced by environmental factors. However, due to advancements in technology and industry, various methods have been employed in the fermentation process, including modifications of fermentation parameters, introduction of fermentation media, and inoculation of characteristic microorganisms.
The fermentation effect of original microbial community in cigar tobacco leaves is not ideal, we can optimize or design the microbial community based on the fermentation function that the microbial community needs to achieve. Earlier research about inoculation of exogenous microorganism H1 and Acinetobacter indicus 3B2 were published to promote quality of cigar tobacco leaves and enhance content of conventional chemical components fermentation1,2. Staphylococcus capitis, a biosafety-level-1 (BSL-1) microorganism that is ubiquitous in natural environments and therefore considered low-risk, has demonstrated substantial potential in food fermentation and environmental adaptation (ATCC 146). In order to improve the fermentation quality of cigar tobacco leaves, the inoculation of exogenous Staphylococcus had a significant effect on improving the types of volatile aroma substances in cigar tobacco leaves, improved the aroma quality of cigar tobacco leaves and reduced the stimulation3.
In previous studies, extracts and products rich in microorganisms, such as rice paste, were added to the fermentation of CWLs. The co-fermentation of humi with CWLs significantly improved the fermentation quality, increasing the levels of nitrogen heterocyclic compounds and sugars associated with bacterial metabolism. Staphylococcus and other genera were identified as the dominant microbial groups during the fermentation4. Cai et al.5. prepared fermentation media from Fritillaria cirrhosa extract and loquat extract, which were added to the fermentation of CWLs for co-fermentation. After the fermentation treatment, the levels of saturated fatty acids and the number of operational taxonomic units (OTUs) in the CWLs exhibited an increasing trend, accompanied by changes in the microbial community structure. Overall, the addition of fermentation media played a promoting role in enhancing the quality of the CWLs. After co-fermenting with three types of Huangjiu starter cultures with flue-cured tobacco, significant differences in the number of OTUs were observed. Meanwhile, Staphylococcus emerged as the dominant microbial genus. The addition of the starter cultures enhanced the microbial richness and diversity of the cigar leaves6. The Huangjiu Jiuqu contains a rich microbial community, including various enzyme-producing and aroma-producing bacteria and fungi7, which play a crucial role in the fermentation process of Huangjiu. The main fermentation microbial community of Huangjiu is Lactobacillus and yeast, respectively. Previous studies have shown that yeast and Lactobacillus can promote the quality of cigar tobacco leaves8. In our previous studies, it was found that the pH of Huangjiu is acidic. Adding a certain concentration of Huangjiu during the fermentation process of cigar wrappers can alter the fermentation environment, which promotes the improvement of the fermentation quality of cigar wrappers9. Due to the complex composition and functions of microorganisms, their effects on the fermentation quality of CWLs are intricate. Therefore, it is not feasible to study the impact of any single characteristic microorganism on the fermentation quality of CWLs. In this study, screening and identification of the microorganisms present in the Jiuqu was applied to improve the quality of fermented CWLs.
In our preliminary work, a strain S1 was isolated from the Shaoxing-flavored T3 Jiuqu, it is hypothesized that it can improve the quality of ordinary CWLs during fermentation. In this study, whether strain S1 can enhance the quality of CWLs was investigated by GC–MS and chemical composition analysis. Further, metagenomic analysis of microbial samples on surface of CWLs was conducted to analyze dynamics of microbial community during fermentation.
Materials and methods
Materials
The Shaoxing-flavored T3 Jiuqu was purchased from Angel Yeast Co., Ltd., and the strain S1 was preserved by the Functional Yeast and Brewing Microbiology Team at Hubei University of Technology. The tobacco leaf samples CX-26 used for testing were produced in 2023 from the Tobacco Station in Enshi Prefecture, Hubei Province.
Selection and identification of strains
LB medium was applied to isolate strain, with NaCl at 10 g/L, peptone at 10 g/L, and yeast extract at 5 g/L, with pH adjusted to 7.0–7.2 and sterilized under high pressure at 121 °C for 20 min.
5 g of Shaoxing-flavored T3 Jiuqu powder were dissolved in sterile water at 30 °C for 30 min. The reconstituted starter culture powder was then spread on agar plates and incubated at 30 °C for 24 h. Single colonies with inconsistent morphology were picked onto new agar plates for further purification and cultivation until stable single colonies appeared.
The purified single colonies were then inoculated into fresh liquid medium and incubated at 30 °C for 12 h. Subsequently, the culture was sent for microbial identification and sequencing. The remaining culture was stored in glycerol tubes at − 80 °C for preservation. The sequencing work was conducted at Wuhan Aikangjian Biotechnology Co., Ltd. The sequencing results were compared with the NCBI database, and a phylogenetic tree of the strains was constructed using Mega 11 software10.
CWLs fermentation test
The strain was inoculated into a flask containing 50 mL of LB medium and incubated on a shaking incubator at 37 °C and 230 rpm for 36 h. Strain S1 was inoculated into the CWLs at a ratio of 20% (v/Kg), designated as the S1 group. Then the packaged leaves (100 kg) were placed in a fermentation tank and kept at 32 ± 2 °C and 80% humidity for 30 days. When the center temperature of the fermentation tank reached 50 °C, cigar tobacco leaves were turned over and sampled. The fermentation process included three turning points, during which samples were collected. The turning of the CWLs followed the principle of moving the upper layer to the lower layer and the outer layer to the inner layer principal. The initial fermentation temperature of the CWLs was set at 30 °C. Fermented CWLs were prepared for both the NF and WF groups, with all experiments conducted in triplicate.
Determination of conventional chemical components in CWLs
Following the method of Zhang et al.11, 0.25 g of dried CWLs powder was added to 25 mL of extraction solution (a water solution with acetic acid and ethanol at volume fractions of 1% and 2%, respectively). The mixture was shaken for 1 h, after which the filtered supernatant was subjected to routine chemical composition analysis using a FUTURA continuous flow analyzer.
Determination of volatile aroma substances in CWLs
10 g of CWLs powder were treated using simultaneous distillation extraction (SDE) techniques, utilizing saturated NaCl solution and dichloromethane as the extraction solvents. After extraction, the extract was collected and concentrated to 2 mL. Subsequently, 50 μL of phenethyl acetate (1.2028 mg/mL) was added as an internal standard, and the volatile aroma compounds were analyzed using Gas Chromatography-Mass Spectrometry (GC–MS).
Volatile aroma compounds in the CWLs samples were detected using an Agilent Technologies 7890A Gas Chromatograph (Agilent Technologies, Santa Clara, CA) coupled with a 5975C Mass Selective Detector (7890A-5975C; Agilent Technologies, Santa Clara, CA). An HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm) was utilized; the injection volume was set at 1 μL, with helium as the carrier gas at a flow rate of 1 mL/min and a split ratio of 10:1. The initial oven temperature was set at 40 °C and held for 2 min, followed by a ramp rate of 2 °C/min to 200 °C, held for 5 min, and then ramped to 280 °C at a rate of 10 °C/min. The electron impact ionization energy was set at 70 eV. The transfer line temperature was maintained at 250 °C, and the ion source temperature was set at 230 °C. The solvent delay time was 3 min.
The chromatograms were analyzed using the GC–MS solution software (Agilent Technologies, USA). The mass spectral data were compared with the spectra in the NIST reference library (NIST 14) within the GC–MS data system to identify the volatile compounds. Further statistical analyses were conducted using Excel 2017 software.
Determination of non-volatile organic acid and amino acid content in CWLs
The amino acid content was determined with reference to the industry standard YC/T 448–2012. The determination of non-volatile organic acid content was referred to YC/T 288–2009.
Sensory evaluation of CWLs
The fermented CWLs were hand-rolled into cigars for sensory evaluation. The main quality indicators were evaluated by a panel of experts from Tobacco Research Institute of Hubei Province. And the School of Life Sciences and Health Engineering, Hubei University of Technology, using a nine-point system, including seven indicators: aroma quantity, aroma quality, permeability, miscellaneous odor, strength, stimulation and aftertaste.
Nisin potency determination
The method was the same as previously published12. Micrococcus luteus suspension with a concentration of 108 CFU/mL was prepared. After sterilizing culture medium and cool to 50–55 °C, inoculate the medium with 1% of Micrococcus luteus suspension. Oxford cups were placed on the detection plate. 1 ml of Staphylococcus Capitalis S1 fermentation broth that has been incubated for 12 h with pH 2 was boiled for 3 min, then centrifuge under the conditions of 12,000 r/min, 4 °C for 2 min to get precipitates. 200 µl of the supernatant was added to the Oxford cup. Once the solution has completely permeated, incubate the plate in a culture chamber for 16–24 h, and measure the diameter of the transparent circle.
Collection and library construction of microorganisms on the surface of CWLs
30 g of CWLs samples were cut into small pieces to ensure adequate contact with the buffer solution and placed in 300 mL of buffer. The mixture was subjected to ultrasonic treatment for 30 min, followed by filtration through four layers of gauze to remove leaf fragments. The collected liquid was centrifuged at 4 °C and 6000 rpm for 10 min, and the supernatant was discarded. Buffer solution was then added to the centrifuge tube, and the precipitate was resuspended before continuing with centrifugation to discard the supernatant. This washing process was repeated until the supernatant was nearly colorless, resulting in the collection of microbial cells (with a precipitate weight exceeding 200 mg). The microbial pellet was rapidly frozen in liquid nitrogen for more than 30 min and then stored at -80 °C for future use.
Buffer Preparation: 100 mmol/L Tris–HCl, 50 mmol/L disodium ethylenediaminetetraacetate (EDTA-Na2), 20 g/L polyvinylpyrrolidone (PVP), 1 mL/L Tween-20, 1.4 mol/L NaCl, pH = 8.
Using the extracted DNA as a template, bacterial genes were amplified with the forward primer 515F (5’-GTGCCAGCMGCCGCGGTAA-3’) and the reverse primer 907R (5’-CCGTCAATTCMTTTRAGTTT-3’). PCR amplification method using the library building kit for Rapid Plus DNA Lib Prep Kit for Illumina (RK20208). Library construction and sequencing were performed at Beijing Novogene Technology Co., Ltd., with three replicates for each sample.
Data analysis
The differences in microbial communities before and after fermentation were analyzed using the bioinformatics analysis cloud platform of Beijing Novogene Technology Co., Ltd. (https://magic.novogene.com/). Significance difference analysis was conducted using Statistica 23.0 software (SPSS Inc., Chicago, IL, USA).
Result
Identification of microbial strains screened in T3
The sequencing results of the selected strain were subjected to BLAST analysis against known bacterial 16S rDNA sequences in the NCBI database, revealing that strain S1 showed a high homology of 99% with Staphylococcus (Fig. 1). Phylogenetic analysis indicated that strain S1 clustered within the Staphylococcus genus, specifically with Staphylococcus capitis, thus identifying the strain as Staphylococcus capitis.
The effects of fermentation and inoculation with exogenous strain S1 on the volatile aroma compounds of CWLs
Qualitative and quantitative analyses of volatile aroma compounds in CWLs subjected to different treatment methods after fermentation revealed that the types and levels of volatile aroma compounds changed to varying degrees based on the treatment. The formation of aroma characteristics depends on several factors, such as fermentation conditions1,13, the addition of fermentation media9, and the inoculation of exogenous microorganisms8.
To investigate the impact of exogenous microorganisms on the metabolic functions of the indigenous microbial community in CWLs, the volatile aroma compounds in the leaves were analyzed, resulting in the selection of 34 representative volatile aroma compounds from hundreds identified is show in Fig. 2. The total content of shared aroma compounds in the S1 group peaked at 3108.95 µg/g after the first turning, representing an increase of 102.95% compared to the WF group at the same turning period. However, during subsequent turning processes, the content of volatile aroma compounds gradually decreased. Specifically, after natural fermentation, the levels of Geranyllinalool, Sclareol, Phytol, and Phenylacetaldehyde showed significant increases compared to pre-fermentation levels. In the S1 group, the content of Dihydroactinidiolide, Phenethyl alcohol, Geranyllinalool, Geranylgeraniol, Sclareol, Phytol, Farnesyl acetone, and Caryophyllene oxide was significantly higher than that of both the NF group and WF group. Conversely, the content of Neophytadiene exhibited a declining trend. Neophytadiene accounted for over 65% of the volatile aroma compounds in CWLs, contributing a grassy aroma to the smoking experience while reducing smoke irritation, leading to a smoother smoke. However, the content of Neophytadiene decreased in the later stages of fermentation, dropping from 1833.20 µg/g in the first turning of the S1 group to 815.72 µg/g in the third turning. Other groups also exhibited varying trends of reduction, likely due to the oxidation of Neophytadiene into unique low molecular weight compounds with distinct alcohol flavors14. The increase in the content of Geranyllinalool can contribute to a unique floral and woody aroma15. Sclareol can contribute to the aroma of ambergris16. Phytol is a degradation product of chlorophyll and is itself a fragrant compound that can contribute to a pleasant herbal aroma17. Phenethyl alcohol is derived from roses and can contribute to a rose-like aroma18. In the study, the maximum content reached 35.98 µg/g, representing a 262% increase compared to WF group, which had a content of 13.75 µg/g. GC–MS results indicated that the S1 group achieved optimal fermentation at 10 days. Prolonged fermentation time was found to be detrimental to the accumulation of volatile aroma compounds. Additionally, the inoculation of the exogenous strain resulted in a significant increase in the content of aroma compounds compared to the treatment with added starter cultures co-fermented with CWLs.
Effects of different treatments on sensory quality of CWLs
The sensory evaluation indicators of fermented cigar tobacco leaves showed various degrees of improvement after different processing methods (Table 1). Compared to WF group, smoking quality of CWLs significantly improved after inoculation with the exogenous S1 strain. The sensory evaluation results of the S1 group indicated that as fermentation time extended, the strength of CWLs decreased, while the scores for aroma quality and aroma quantity increased. The concentration, strength, and irritancy of the CWLs were positively correlated, and these three evaluation indicators in the S1 group were higher than those in the WF group. The aftertaste of the CWLs in the S1 group scored the highest during the first turn, then gradually decreased. In the combustibility evaluation, the WF group’s third turn and the S1 group’s second turn scored the highest, both above 8.5. In the ash color evaluation, the scores of all samples did not differ significantly, but they were consistent with the combustibility evaluation scores, with the WF group’s third turn and the S1 group’s second turn scoring higher. In the later stages of the CWLs fermentation process, the changes in various scoring indicators were not significant, but the S1 treatment group exhibited the best sensory quality during the first turn.
Effects of S1 fermentation on the content of major chemical components in CWLs
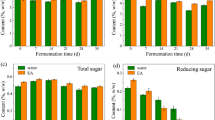
The main chemical components of tobacco leaves include nicotine, reducing sugars, total sugars, chlorine, total nitrogen, potassium, and others. The content of these components is closely related to the quality of cigar. An increase in sugar compounds can produce pleasant, mild, and non-irritating aroma during smoking, enhancing the overall smoking experience19.The changes in the content of major chemical components in different treatment groups of CWLs are shown in Fig. 3. During the fermentation process, the nicotine content in CWLs exhibited a significant upward trend. After the inoculation of the exogenous strain S1 group, the nicotine content peaked at 5.76%, representing an increase of 143.04% compared to the lowest nicotine level observed. The sugar content in CWLs is an important indicator of quality. The sugars can be transformed into acids, which help to mask the inherent irritation and unpleasant odors of the CWLs20. The content of reducing sugars and total sugars in the S1 group showed significant increases compared to NF group, with maximum values of 0.09% and 0.14%, respectively. In the WF group, the reducing sugars and total sugars measured during the third turning were similar to those in the first two turnings of the S1 group. However, the sugar content in the S1 group during the third turning exhibited a significant decrease compared to the first two turnings of the same treatment group, although it was significantly higher than that in the NF and WF groups. The presence of potassium facilitates the complete combustion of cigars, while the role of chlorine is to slow down the combustion process. The ratio of potassium to chlorine is an important indicator of the combustibility of cigars. Studies have shown that an ideal potassium to chlorine ratio falls within the range of 4–1021.
Effects of S1 fermentation on the content of non-volatile organic acids and amino acids in CWLs
Non-volatile organic acids, as crucial intermediates in the tricarboxylic acid (TCA) cycle, play a significant role in balancing the pH of the fermentation environment and improving the sensory quality of CWLs during fermentation. The content of non-volatile organic acids in different treatment groups of CWLs is shown in Fig. 4A. Throughout the fermentation process, malic acid dominated absolutely among all non-volatile organic acids. The common non-volatile organic acids in CWLs primarily included three types: malic acid (3.06 × 10⁶–5.77 × 10⁶ ng/g), citric acid (1.53 × 105–5.75 × 105 ng/g), and oxaloacetic acid (4.21 × 102–1.42 × 103 ng/g). In the WF group, the content of non-volatile organic acids initially decreased and then increased, with malic acid following the same trend as the overall non-volatile organic acids, while citric acid exhibited a gradual decline. During the third turning of the WF group, the non-volatile organic acid content reached its peak at 5.93 × 10⁶ ng/g, representing a 64.27% increase compared to the NF group (3.61 × 10⁶ ng/g). In the S1 group, the content of non-volatile organic acids first increased and then decreased, with malic acid mirroring this trend. However, citric acid content in the S1 group showed no significant changes.
Amino acids are the building blocks of proteins, and the amino acids in CWLs can form flavors that harmonize with other volatile aromatic substances during combustion, enhancing the richness of the cigar. The amino acids in different treatment groups of CWLs are shown in Fig. 4B. Compared to the NF group, Leu, Glu, Iso, and Asp contents in the WF and S1 groups were increased, with Asp being an acidic amino acid. In the WF group, the contents of several amino acids gradually decreased as fermentation progressed, such as Lys, Try, and His, which are mostly alkaline amino acids. In the WF group, the contents of several amino acids initially decreased and then increased during fermentation, including Gly, Ser, Pro, Leu, Glu, Phe, Lys, Arg, His, Val, Ala, Iso, and Thr. In the S1 group, the change in Asp content was the most significant, with an increase of 60.80% from 127.09 µg/g in the first turn to 204.36 µg/g in the third turn. The total amino acid content peaked at 671.00 µg/g during the third turn, representing 20.08% increase compared to the NF group.
The influence of Staphylococcus on microbial community structure
α-Diversity analysis
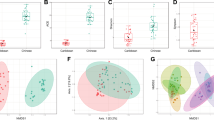
An α-diversity analysis of the microbial communities in CWLs revealed that all samples exhibited a coverage rate close to 1, indicating high coverage and reasonable sampling. This suggests that the sequencing results can accurately reflect the distribution of microbial communities on the surface of the CWLs, as shown in Fig. 5.
The Chao1 index reflects the richness of the microbial community, while the Shannon and Simpson indices indicate the diversity of the microbial communities. The results of the α-diversity analysis showed that the Shannon and Simpson indices in the S1 group were higher than those in the NF and WF groups, indicating a significant enhancement in microbial community richness and diversity. However, in terms of species diversity, the addition of strain S1 led to a decrease in species diversity on the surface of the CWLs, which further declined with extended fermentation time.
Researches have indicated that during the early stages of fermentation, the diversity of microbial species in CWLs is relatively high, but this gradually decreases over time. Additionally, during the co-fermentation process of flue-cured cigar with raw and cooked malt, as well as in the later stages of fermentation, both the richness and diversity of microbial communities exhibited a downward trend6. When exogenous strains are inoculated during the fermentation of CWLs, they can disrupt the original fermentation environment while simultaneously enhancing species richness10.
OTUs number
A Venn diagram at the OTUs level was established to describe the distribution of microorganisms among different treatment groups on the surface of CWLs. The number of OTUs for each CWLs group is illustrated in Fig. 6. The microbial OTU count on CWLs ranged from 5 to 1363. Compared to the NF group (1119) and WF group (1190), the S1 group (1363) exhibited a greater number of unique microorganisms, totaling 107, while NF group had the fewest unique microorganisms.
The results indicate that the inoculation of exogenous Staphylococcus facilitates the accumulation of microbial OTUs on the surface of CWLs, thereby enhancing the species diversity. Additionally, the fermentation process serves as an effective means of accumulating microbial OTUs on the surface of CWLs, promoting both species richness and diversity. Consistent with the findings of Yao et al.9, the inoculation of exogenous microorganisms promotes an increase in the number of microbial OTUs.
Changes in microbial community structure of CWLs
The growth and metabolism of microbial communities play a crucial role in the quality of CWLs. At the phylum level, microbial taxa with a relative abundance exceeding 0.5% are defined as dominant phyla, while at the genus level, those with a relative abundance exceeding 1% are classified as dominant genera. As shown in Fig. 7A, the microbial community in CWLs is primarily composed of five phyla: Bacillota, Proteobacteria, Myoviridae, Actinobacteria, and Ascomycota. In the NF group, Bacillota comprised the highest proportion, followed by Myoviridae. As fermentation progressed, the proportion of Myoviridae decreased, while Bacillota established absolute dominance, accounting for over 90% of the microbial community. In the S1 group, the proportion of Bacillota declined from above 90% in WF group to nearly 80%, while the proportion of Proteobacteria significantly increased.
At the genus level in Fig. 7B, the microbial community is primarily composed of five genera: Staphylococcus, Enterobacter, Cronobacter, Aspergillus, and Bacillus. In NF group and WF group, Staphylococcus accounted for the highest proportion, exceeding 95%. However, after the inoculation of exogenous Staphylococcus, the proportion of Staphylococcus decreased but remained above 75%. Additionally, genera such as Enterobacter, Cronobacter, and Aspergillus, which were not present in the other two groups, emerged. The appearance of these three genera contributed to a more balanced community structure. The results suggested that inoculation of exogenous strains possibly disrupted the growth environment for other microbial strains on the surface of the CWLs, leading to a reduction in the richness of previously dominant species and allowing other strains to proliferate rapidly. Alternatively, the exogenous strains might engage in antagonistic interactions with the indigenous Staphylococcus, reducing its inhibitory effects and increasing the proportions of other microbial species. In the study of co-fermentation of CWLs with roasted rice, Staphylococcus consistently emerged as the dominant genus throughout the entire fermentation process of the CWLs22.
Analysis of significantly differentiated microorganisms on the surface of CWLs
LEfSe analysis revealed significant differences in microbial composition among the different treatment groups of CWLs. The distribution of significantly differentiated microorganisms among the S1 group was observed at various taxonomic levels, including phylum, class, order, family, genus, and species, as illustrated in the Fig. 8. The NF group exhibited a greater number of significantly differentiated microorganisms. However, as fermentation progressed, the number of significantly differentiated microorganisms gradually decreased. Following the inoculation of the exogenous strain S1, there was a sharp decline in the number of significantly differentiated microorganisms. This reduction may be attributed to the antagonistic interactions between the exogenous strain S1 and the indigenous microorganisms, leading to a dramatic decrease in the abundance of the indigenous microbial community. Additionally, the changes in microbial community structure indicate that the introduction of the exogenous strain S1 caused significant alterations in the original community structure, resulting in a more balanced proportion of microorganisms other than Staphylococcus.
KEGG is a database used for analyzing biological metabolic pathways, which integrates gene and genomic information with higher-level functional information to systematically analyze gene functions. The microbial community structure of CWLs exhibited significant changes among different treatment groups, leading to alterations in community metabolic pathways. The species contribution analysis to KEGG pathways is illustrated in Fig. 9A. The top five pathways with the highest relative abundance of microbial metabolic functions in CWLs include carbohydrate metabolism, amino acid metabolism, membrane transport, cofactor and vitamin metabolism, and energy metabolism. Among these, carbohydrate metabolism and amino acid metabolism pathways are closely related to the flavor formation of CWLs. Carbohydrate metabolism facilitates the entry of glucose into cells, promoting the glycolytic pathway within the cells, which effectively accumulates volatile aroma compounds. Significant differences were observed between the S1 group and the NF group in these two pathways. The robust activity of the membrane transport pathway may be attributed to the inoculation of the exogenous strain S1, which enhances the exchange and transport of substances across the cell membrane, thereby playing a facilitative role in the formation of flavor compounds.
The CAZy (Carbohydrate-Active enZYmes) database is specifically designed for the systematic classification and functional annotation of carbohydrate-active enzymes (Fig. 9B). These enzymes play a crucial role in the microbial degradation and utilization of carbohydrates and include several categories: CMB (Carbohydrate Binding Modules), AA (Auxiliary Activities, which are redox enzymes that work synergistically with CAZymes), PL (Polysaccharide Lyases, which non-hydrolytically cleave glycosidic bonds), CE (Carbohydrate Esterases, which hydrolyze carbohydrate esters), GH (Glycoside Hydrolases, which hydrolyze and/or rearrange glycosidic bonds), and GT (Glycosyltransferases, which form glycosidic bonds).Heatmap analysis of carbohydrate-active enzyme genes in CWLs revealed that among the various enzyme classes tested, the S1 group inoculated with the exogenous strain S1 exhibited strong activity across all enzyme functions. The exogenous strain S1 makes a significant contribution to the hydrolysis of carbohydrate ester bonds during fermentation, which is consistent with the KEGG metabolic pathway analysis results. Overall, the sustained high abundance of glycosyltransferases, carbohydrate-binding modules, and carbohydrate esterase genes in the S1 group likely facilitates the degradation and conversion of key carbohydrates during cigar fermentation. These carbohydrate compounds can be transformed into heterocycles such as hexose, Furfuryl alcohol, and Cyclotene, as noted in previous studies, thereby profoundly influencing the final chemical composition and flavor characteristics of the CWLs23.
Microbial interaction network with volatile aroma compounds
The interaction network among microorganisms spans the entire fermentation process and plays a decisive role in the formation of flavors and the changes in sensory characteristics of CWLs. To explore the relationship between the microbial community structure on the surface of CWLs, volatile aroma compounds, an interaction network diagram was constructed for the dominant microorganisms at the genus level and the volatile aroma compounds detected by GC–MS are shown in Fig. 10.
The network diagram illustrates that in NF and WF group, Staphylococcus showed a significant positive correlation with Neophytadiene, which is consistent with the heatmap data of volatile aroma compounds. In the NF group, the content of Neophytadiene was significantly higher compared to other groups. Staphylococcus exhibited a significant positive correlation with Pantoea while showing no correlation with Mammaliicoccus. In the S1 group, Staphylococcus displayed a significant positive correlation with Mammaliicoccus and a negative correlation with Pantoea. Regarding volatile aroma compounds, Staphylococcus had a negative correlation with β-ionol, geranyllinalool, phenethyl alcohol, and sclareol, which is clearly reflected in the heatmap of volatile aroma compounds.
Pantoea, which showed no significant correlation with these four alcohols in the NF and WF group, exhibited a significant positive correlation with them in the S1 group. Additionally, these four alcohols displayed significant positive correlations with each other. In the main diagram of the S1 group, the interactions between microorganisms, and between microorganisms and volatile aroma compounds are more comprehensive and complex, encompassing a greater number of elements.
Discussion
This study selected the CX-26 CWLs produced in Enshi Prefecture, Hubei Province, in 2023 as the research object. An exogenous strain S1, previously isolated from Shaoxing-flavored T3 Jiuqu in our laboratory, was inoculated. Following a fermentation period of 30 days, the quality of the CWLs was significantly improved. During the fermentation of CWLs, malic acid, citric acid, and oxalic acid collectively account for 70% of the organic acid content24. Non-volatile organic acids can adjust the pH of CWLs, thereby influencing the flavor and aroma of the smoke and balancing acidity-alkalinity during combustion. Additionally, malic acid generates subtle roasted aromas during metabolic processes. Higher malic acid content enhances the freshness and sweet–sour taste of CWLs, often perceived as a distinct “crispness” in the palate, reminiscent of fruity acidity25. In the S1 group, malic acid content significantly increased compared to WF group, leading to higher sweetness scores. However, excessive malic acid concentration during sensory evaluation may introduce sharpness to the taste, disrupt cigar balance, and reduce irritation of CWLs. Citric acid is closely tied to the fermentation process of CWLs. As fermentation progresses, citric acid is converted into other compounds such as alcohols, aldehydes, and esters, which impact the balance and refinement of cigar. Appropriate citric acid levels enhance the complexity of cigars, delivering a smoother and mellower inhalation experience. Among all treatment groups, differences of scores for cigars were minimal. However, the S1 group exhibited lower citric acid content yet higher scores for strength and irritation compared to other groups, suggesting that moderately lower citric acid content may help reduce cigar irritation. Amino acids are crucial chemical components in CWLs. The metabolism of amino acids generates compounds such as pyrazines, which impart baking and nutty aromas to cigars26. During the aging process of cigars, amino acids undergo the Maillard reaction with sugars to form a variety of volatile compounds that characterize cigar aroma, as well as furfuryl alcohol, furfural, and indole, which contribute to the roasted and sweet flavors of the smoke. Aromatic amino acids such as phenylalanine and tryptophan are key precursors for the formation of aromatic compounds27. Through Maillard reaction and thermal decomposition processes, these amino acids produce a variety of aromatic compounds, such as styrene, indole, and other substances with complex aromas like roasted and floral flavors. Aspartic acid reacts with glucose through Maillard reaction, similarly to glycine, alanine, valine, phenylalanine, and tyrosine, enhancing the flavor of CWLs effectively. The content of aspartic acid in group S1 is consistent with the trend of changes in related metabolic products in the heat map of volatile aroma substances. During the decomposition process of amino acids, such as leucine, isoleucine, and phenylalanine, they can generate ketones and aldehydes through complex reactions. The content of these metabolites peaks at the first turning of group S1. These substances typically possess rich aromas, such as baking, nutty, or caramel flavors, often making the aroma of cigars more intense. Such aromas frequently add unique floral, grassy, or smoky flavors to cigars.
The fermentation process can enhance the microbial richness and diversity in CWLs28. In the study by Hu et al.29 (Hu, et al., 2023) on the effects of fermentation media on the chemical composition and microbial community during the fermentation of CWLs, the distribution of the total number of microbial OTUs on the surface of the CWLs showed an increasing trend for both fungal and bacterial populations. Additionally, alterations in the composition of the fermentation medium had a significant impact on the changes in the number of microbial OTUs on the surface of the CWLs. The results of the microbial community structure on the surface of CWLs indicated that there were no significant changes in the community structure at the phylum and genus levels between NF group and WF group. However, after the inoculation of the exogenous strain S1, the microbial community on the surface of the CWLs exhibited notable changes at both the phylum and genus levels. At the genus level, following the inoculation of the exogenous strain S1, proportions of genera Pantoea, Enterobacter, Cronobacter, and Aspergillus increased, whereas the proportion of Staphylococcus decreased. This resulted in a more balanced genus structure. This shift may be attributed to competition or other negative interactions between the exogenous strain S1 and indigenous Staphylococcus, leading to a reduction in the diversity of indigenous microorganisms. Alternatively, the presence of indigenous Staphylococcus may have inhibited the growth of other microbial genera in the original microbial habitat, and the inoculation of the exogenous strain S1 may have altered the survival environment of indigenous Staphylococcus, weakening its competitive effect on other indigenous microorganisms and allowing the emergence of microbes not present in the other groups. Previous reports have identified that Staphylococcus capitis can encode a glycine endopeptidase that targets the cell wall of Staphylococcus aureus. This enzyme can render Staphylococcus aureus inactive18. Staphylococcus capitis was found to exhibit antimicrobial activity and has the potential to produce bacteriocins. Biotin is considered a probiotic, as it interacts with the immune system while eliminating competing microorganisms, thereby aiding the establishment of the producing strains within their ecological niches. It is possible that after the inoculation of strain S1, it generates enzymes or biotin that target indigenous Staphylococcus, leading to a disruption of the original community structure and allowing the exogenous strain S1 to occupy a dominant position within the microbial community structure30,31,32,33.
Enterobacter can rapidly convert sucrose to isomaltulose, and multiple studies have demonstrated that Enterobacter plays a significant role in flavor development during the fermentation process34. Although Aspergillus is primarily responsible for creating moldy environments and characteristic moldy flavors, research has shown that it also plays a significant role in determining the palatability of fermented foods and beverages35. During the fermentation process, the Aspergillus can contribute sweetness and reduce pungency, while compounds such as phenethyl alcohol can add sweetness to the smoking experience of cigars8. To investigate the effects of fermentation and the inoculation of the exogenous strain S1 on the degradation capacity of major chemical components and macromolecules, the research results indicated that the nicotine content showed a decreasing trend after the inoculation of the exogenous strain S1. However, compared to the NF and WF groups, the relative content of nicotine was significantly elevated. This may be attributed to the significant increase in reducing sugars and total sugars following the inoculation of the exogenous strain S1, which can enhance the smoking experience of CWLs by providing a smoother smoke. It is possible that the increase in the content of Aspergillus in the CWLs after the inoculation of the exogenous strain S1 contributes to this effect. Previous reports have indicated that Aspergillus can degrade macromolecules such as proteins and starches, converting them into smaller molecules like peptides, thereby improving the smoking quality of CWLs. In the study by Zhang et al.24 analyzing the structure and metabolic functions of microorganisms in CWLs during the agricultural processing stage, it was shown that the microbial community structure of the CWLs was suboptimal prior to treatment. In subsequent research, the microbial community structure of the CWLs was optimized through treatment, resulting in changes that could enhance the quality and flavor of the CWLs. In an environment where multiple microorganisms coexist, a phenomenon may occur where the abundance of one microorganism decreases while that of another increases. This is likely due to competitive and interactive relationships among the microorganisms. During the fermentation process, microorganisms compete for energy sources and nutrients. The introduction of an exogenous strain can overlap in nutrient utilization with indigenous microorganisms, leading to competition. If the inoculation of the exogenous strain is substantial, it may reduce the abundance of indigenous microorganisms. Alternatively, one microorganism may benefit from the interaction while the other remains unaffected. The addition of the exogenous strain could weaken the existing interactions among indigenous microorganisms, resulting in an increased proportion of the exogenous strain and consequently forcing a decrease in the abundance of indigenous microorganisms36. Research has shown that strain S1 possesses the ability to produce Nisin. As measured by vernier caliper, diameter of transparent circle is 8.0 mm, as shown in Fig. 11. According to the Nisin standard curve, the Nisin potency of Staphylococcus Capitalis S1 fermentation broth is calculated to be 138 IU/ml. Nisin is a short-chain hydrophobic small molecule lanthipeptide37, representing a rapidly expanding subset of the ribosomally synthesized and post-translationally modified peptide family. Nisin produced by exogenous strain S1 has been shown to effectively kill or inhibit Gram-positive bacteria, such as Lactobacillus, Clostridium botulinum, Staphylococcus, and Listeria38. Therefore, the inoculation of S1 strain mabe is the primary reason for the decrease in the abundance of the original Staphylococcus in cigar wrapper. This action helps suppress the inhibitors of aroma production during fermentation, thereby increasing the concentration of volatile aroma compounds33,39.
The GC–MS results indicate that the S1 group exhibited a significant increase in both the variety and quantity of volatile aroma compounds compared to the NF and WF groups. Among these, compounds such as Phenethyl alcohol, Geranylgeraniol, Geranyllinalool, and Sclareol reached their peak concentrations after the first turning of the exogenous microorganism S1 group. Subsequently, during the fermentation process, the concentrations of these volatile aroma compounds gradually decreased from the peak levels observed after the first turning. This suggests that the end of the first turning for the S1 group represents the optimal fermentation time. The trend in the change of volatile aroma compound concentrations in the S1 group aligns with that of WF group. It is possible that high ambient temperatures during the summer caused the fermentation chamber temperature to reach its peak too quickly, preventing the accumulation of volatile aroma compounds. Specifically, the aroma concentration of phenethyl alcohol increased slightly from 13.20 µg/g before fermentation to a maximum of 13.75 µg/g in WF group. Its concentration saw a significant rise to 35.98 µg/g after inoculation of the exogenous strain S1. Furthermore, Phenethyl alcohol contributes rose and balsamic aroma characteristics, providing sweetness to CWLs34. After natural fermentation treatment, the concentration of Phytol increased from 352.44 µg/g before fermentation to 504.76 µg/g at the first turning. Following the inoculation of the exogenous strain S1, the concentration of Phytol reached to 582.21 µg/g. The exogenous strain S1 is capable of encoding lipase, which can accelerate the synthesis of amino acids and free fatty acids during the fermentation of cigar leaves, thereby rapidly acidifying the fermentation environment and lipase can promote the generation of various aroma substances. The production of lipase can effectively improve the alkaline fermentation conditions, thus enhancing the quality of cigar leaves fermentation40. Fan et al.41 utilized HS–SPME–GC–MS to analyze the dynamic changes of volatile metabolites at different fermentation stages of CWLs. Various volatile metabolites exhibited an increasing trend at different stages of fermentation, and throughout the fermentation process, multiple volatile metabolites participated in various synthesis and degradation pathways. Shao et al.42 employed metagenomics and GC–MS techniques to investigate the flavor regulation mechanisms in fermented sausages inoculated with local strains. The study found that the sausages inoculated with exogenous strains contained a greater variety of flavor compounds, and S1 groups with exogenous strains exhibited higher levels of free amino acids and fatty acids.
The inoculation of the exogenous strain S1 significantly enhanced the functions of these four pathways (carbohydrate metabolism, amino acid metabolism, membrane transport, and energy metabolism) in CWLs, suggesting that the inoculation of strain S1 plays an important role in altering not only the indigenous microbial community structure but also the microbial metabolic pathways. Furthermore, the analysis of carbohydrate-active enzyme metabolic activities in the microbial community on the surface of CWLs revealed that S1 group exhibited significant activity across all related metabolic activities. Melissa Ivey et al.43 conducted a study on the interactions among microorganisms during food fermentation, discussing various aspects of these interactions, including nutritional interactions and cell signaling. The interactions among microorganisms play a significant role in the dominant microbial communities associated with various food fermentation processes. These interactions can occur in multiple ways: metabolite-mediated interactions involve substances such as ethanol, lactic acid, and acetic acid produced during microbial fermentation, which can interact with other microorganisms; cell signaling can trigger intercellular collective responses, thus influencing group behaviors during food fermentation, and quorum sensing behaviors are also present in various mixed cell cultures.
Thus, it can be inferred that the addition of the exogenous strain S1, following fermentation treatment, significantly enhanced both the quantity and variety of volatile aroma compounds in the CWLs, contributing to an overall improvement in the quality of the CWLs.
Conclusion
When traditional fermentation products fail to meet consumer demands, the addition of exogenous strains to alter the original microbial composition of CWLs can effectively enhance product quality. The results indicate that during the fermentation process of CWLs leaves, inoculation of the exogenous Staphylococcus capitis S1 could increase violate compound content and balance the microbial community structure in CWLs. During CWLs fermentation, richness and diversity of surface microorganisms was increased. There was a notable enhancement in both the variety and quantity of volatile aroma compounds. After the inoculation of the exogenous strain S1, the proportion of Staphylococcus decreased while the proportions of other microorganisms increased, leading to a more balanced microbial community structure. Additionally, the interactions among microorganisms and between microorganisms and volatile aroma compounds became more comprehensive. The follow-up work will include function of other microorganisms screened from T3 and application in fermentation quality improvement of cigar wrapper leaves.
Data availability
The raw data supporting the conclusions of this article will be made available by the corresponding author (Lan Yao), without undue reservation upon request. Sequence data associated with this project have been deposited in the NCBI Short Read Archive database (Accession Number: PRJNA1198196 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1198196)).
References
Yao, L. et al. Application of yeast in plant-derived aroma formation from cigar filler leaves. Front. Bioeng. Biotechnol. 10, 1093755 (2022).
Zheng, T. et al. Effects of inoculation with Acinetobacter on fermentation of cigar tobacco leaves. Front. Microbiol. 13, 911791 (2022).
Pei, Q. et al. Study on quality enhancement during cigar tobacco fermentation by Staphylococcus nepalensis: Insights into microbial community, volatile substances and sensory evaluation. Front. Microbiol. 16, 1526178 (2025).
Ren, M. et al. Effects of microbes and metabolites on tobacco quality in “humi” characteristic fermentation of cigar tobacco leaf. Process Biochem. 143, 186–197 (2024).
Wen, C. et al. Effects of fermentation medium on cigar filler. Front. Bioeng. Biotechnol. 10, 1069796 (2022).
Dong, G., Li, C. & Yang, W. et al. The dynamic analysis of the bacterial diversity of flue-cured tobacco K326 leaves surface during co-fermentation with Chinese rice wine Qu, (2022).
Liu, S. et al. A metagenomic analysis of the relationship between microorganisms and flavor development in Shaoxing mechanized Huangjiu fermentation mashes. Int. J. Food Microbiol. 303, 9–18 (2019).
Yao, L., Li, D. & Huang, C. et al. Screening of Cellulase-producing bacteria and their effect on the chemical composition and aroma quality improvement of cigar wrapper leaves. BioResources, 17(1), (2022).
Yao, L. et al. The impact of fangxian huangjiu on the fermentation quality and microbial community dynamics of cigar wrapper leaves. Front Bioeng Biotechnol 12, 1428750 (2024).
Zhang, L. et al. Study on tobacco quality improvement and bacterial community succession during microbial co-fermentation. Ind. Crops Prod. 208, 117889 (2024).
Zhang, Q. et al. Analysis of the structure and metabolic function of microbial community in cigar tobacco leaves in agricultural processing stage. Front. Microbiol. 14, 1230547 (2023).
Wolf, C. E. & Gibbons, W. R. Improved method for quantification of the bacteriocin nisin. J. Appl. Bacteriol. 80(4), 453–457 (1996).
Yao, L. et al. Heterogeneity changes of active bacterial community on cigar filler leaves after fermentation based on metagenome. Biosci. Biotechnol. Biochem. 87(9), 1056–1067 (2023).
Hu, H. et al. Research progress in factors influencing nephytadiene content in tobacco leaves. Acta Agric. Jiangxi 22, 17–20 (2010).
Chang, J. et al. Engineering Escherichia coli via introduction of the isopentenol utilization pathway to effectively produce geranyllinalool. Microb. Cell Fact. 23(1), 292 (2024).
Diao, M. et al. Probing the biotransformation process of sclareol by resting cells of Hyphozyma roseonigra. J. Agric. Food Chem. 70(34), 10563–10570 (2022).
Islam, M. T. et al. Phytol in a pharma-medico-stance. Chem. Biol. Interact. 240, 60–73 (2015).
Sakai, M. et al. Production of 2-phenylethanol in roses as the dominant floral scent compound from L-phenylalanine by two key enzymes, a PLP-dependent decarboxylase and a phenylacetaldehyde reductase. Biosci. Biotechnol. Biochem. 71(10), 2408–2419 (2007).
Zong, P. et al. Effects of adding cocoa fermentation medium on cigar leaves in agricultural fermentation stage. Front. Bioeng. Biotechnol. 11, 1251413 (2023).
Yan, S. et al. Soil carbon supplementation: Improvement of root-surrounding soil bacterial communities, sugar and starch content in tobacco (N. tabacum). Sci. Total Environ. 802, 149835 (2022).
Yin, F. et al. Contribution of tobacco composition compounds to characteristic aroma of Chinese faint-scent cigarettes through chromatography analysis and partial least squares regression. J. Chromatogr. B 1105, 217–227 (2019).
Fang, X. et al. Roles of cigar microbes in flavor formation during roasted-rice leachate fermentation. Appl. Microbiol. Biotechnol. 108(1), 457 (2024).
Cho, H., Park, M. K. & Kim, Y. Study on volatile compounds formed from the thermal interaction of hydrolyzed vegetable proteins with reducing sugars. Food Sci. Biotechnol. 32(3), 283–298 (2023).
Zhang, W., Yang, X. A. & Zhang, Q., et al. Effect of exogenous neutral protease fermentation on cigar leaf quality: BIO Web of Conferences, (EDP Sciences, 2023).
Fan, X., Zi, W. & Ao, J., et al. Analysis and application evaluation of the flavour-precursor and volatile-aroma-component differences between waste tobacco stems. Heliyon, 8(9), (2022).
Wanrong, H. U. et al. Influence of fermentative medium on the chemical compositions and microbial communities of cigar tobacco leaves. J. Light Ind. 38(1), 90–100 (2023).
Maoz, I., Lewinsohn, E. & Gonda, I. Amino acids metabolism as a source for aroma volatiles biosynthesis. Curr. Opin. Plant Biol. 67, 102221 (2022).
Zhang, Q. et al. Microbial and enzymatic changes in cigar tobacco leaves during air-curing and fermentation. Appl. Microbiol. Biotechnol. 107(18), 5789–5801 (2023).
Hu, W. et al. Sensory attributes, chemical and microbiological properties of cigars aged with different media. Front. Bioeng. Biotechnol. 11, 1294667 (2023).
Cotter, P. D., Hill, C. & Ross, R. P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 3(10), 777–788 (2005).
Dobson, A. et al. Bacteriocin production: A probiotic trait?. Appl. Environ. Microbiol. 78(1), 1–6 (2012).
Małaczewska, J. et al. In vitro immunomodulatory effect of nisin on porcine leucocytes. J. Anim. Physiol. Anim. Nutr. 103(3), 882–893 (2019).
O’Sullivan, J. N. et al. Human skin microbiota is a rich source of bacteriocin-producing staphylococci that kill human pathogens. FEMS Microbiol. Ecol. 95(2), fiy241 (2019).
Tian, Z. et al. Characterization of physicochemical properties, microbial diversity and volatile compounds of traditional fermented soybean paste in Henan province of China. Food Biosci. 50, 102045 (2022).
Pennerman, K. K., Al-Maliki, H. S. & Lee, S., et al. Fungal volatile organic compounds (VOCs) and the genus Aspergillus//New and future developments in microbial biotechnology and bioengineering. pp. 95–115 (Elsevier, 2016).
Sieuwerts, S. et al. Unraveling microbial interactions in food fermentations: From classical to genomics approaches. Appl. Environ. Microbiol. 74(16), 4997–5007 (2008).
Gharsallaoui, A. et al. Nisin as a food preservative: Part 1: physicochemical properties, antimicrobial activity, and main uses. Crit. Rev. Food Sci. Nutr. 56(8), 1262–1274 (2016).
Field, D. et al. In vitro activities of nisin and nisin derivatives alone and in combination with antibiotics against Staphylococcus biofilms. Front. Microbiol. 7, 508 (2016).
Davies, E. A., Bevis, H. E. & Delves, B. J. The use of the bacteriocin, nisin, as a preservative in ricotta-type cheeses to control the food-borne pathogen Listeria monocytogenes. Lett. Appl. Microbiol. 24(5), 343–346 (1997).
Pei, Q. et al. Exploring the synergistic effect of Lactiplantibacillus plantarum 1–24-LJ and lipase on improving Quality, Flavor, and safety of Suanzharou. Food Res. Int. 200, 115432 (2025).
Fan, J. et al. Widely targeted metabolomic analysis reveals that volatile metabolites in cigar tobacco leaves dynamically change during fermentation. Biochem. Biophys. Rep. 35, 101532 (2023).
Shao, X. et al. Decoding the flavor regulation mechanism of fermented sausages inoculated with indigenous strains via metagenomic and GC-MS analysis. LWT 206, 116604 (2024).
Ivey, M., Massel, M. & Phister, T. G. Microbial interactions in food fermentations. Annu. Rev. Food Sci. Technol. 4(1), 141–162 (2013).
Acknowledgements
This work was financially supported by the Major Special Projects of China Tobacco Corporation (110202101059 (XJ-08), 110202201040(XJ-11), 110202201035 (XJ-06)), key project of Hubei Provincial Department of Education (D20211404, T2022011).
Author information
Authors and Affiliations
Contributions
Tongtong Zhang, Jun Yu, Methodology, Writing—Original Draft. Zhongde Zhao, Writing—Review & Editing. Chunlei Yang, Conceptualization, Writing—Review & Editing. Xiong Chen, Formal analysis, Writing—Review & Editing, Project administration, Funding acquisition. Lan Yao, Formal analysis, Writing—Review & Editing.
Corresponding authors
Ethics declarations
Competing interests
Jun Yu and Chunlei Yang were employed by Tobacco Research Institute of Hubei Province (Wuhan, China). Neither the Tobacco Research Institute of Hubei Province, nor China Tobacco Corporation, have any influence in the study design, reporting, and manuscript writing. All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, T., Yu, J., Zhao, Z. et al. Fermentation quality improvement of cigar wrapper inoculated with exogenous strain Staphylococcus capitis S1. Sci Rep 15, 29396 (2025). https://doi.org/10.1038/s41598-025-12260-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12260-8