Abstract
Pathogenic bacterial infections represent a major threat to human health, which is worsened by the rise of antibiotic resistance stemming from misuse. Carbon dots (CCM-PBA-NH2) were synthesized and examined for their potential as photo-activated antimicrobial agents to address this issue. Various characterization methods were employed to investigate the structure and morphology of the CCM-PBA-NH2 carbon dots (CDs). Techniques including UV-VIS and fluorescence spectroscopy, FTIR, zeta potential analysis, Raman spectroscopy, XRD, SEM and TEM were utilized to assess their physicochemical properties, such as size, shape, surface functionalities and charge distribution. These carbon dots exhibited strong antibacterial activity against both Gram-positive and Gram-negative bacteria. They effectively prevented biofilm formation and disrupted preformed biofilms while displaying low cytotoxicity toward mammalian cells at concentrations of 0.1 mg/mL. The antibacterial properties of carbon dots were also evaluated on cellulose and oxidized cellulose fiber surfaces, where a significant reduction in bacterial growth was noted. CD-modified oxidized cellulose displayed strong adhesion, positioning carbon dots as a promising candidate for use in antimicrobial materials, including wound dressings and sterilization tools. Combining carbon dots with biocompatible carriers, like cellulose, presents a versatile and effective strategy for fighting bacterial infections.
Similar content being viewed by others
Introduction
Bacterial infections pose a major challenge to global healthcare systems, complicating treatment strategies and public health efforts. Gram-positive and Gram-negative bacteria employ distinct mechanisms to form biofilms, which contribute to their pathogenicity1. The World Health Organization (WHO) has recently emphasized the growing concerns regarding bacterial biofilms in water pollution and antibiotic resistance2,3. This highlights the urgent need for innovative and sensitive biofilm characterization techniques that can also be utilized for screening biofilm-targeting therapeutics4. Antibiotics have been instrumental in managing infections, but their misuse has led to the emergence of multidrug-resistant bacteria, complicating efforts to manage and treat bacterial infections effectively5,6,7,8,9,10,11,12.
Recent advances in nanoscience have led to the development of antimicrobial nanomaterials with high efficacy and low toxicity13,14,15,16,17. Among them, carbon dots (CDs) are especially promising due to their strong antimicrobial activity, along with their photoluminescence properties, biocompatibility and cost-effective synthesis18,19,20,21,22,23,24. CDs belong to a family of carbon nanomaterials known for their antibacterial effects, low toxicity, and reduced likelihood of inducing bacterial resistance, making them suitable for antimicrobial coatings25. CDs exhibit antimicrobial activity through mechanisms such as reactive oxygen species (ROS) production, membrane disruption, DNA fragmentation, and cytoplasmic leakage26,27,28,29. Light-activated CDs, which absorb wavelengths from near-UV to near-IR, have been shown to effectively eliminate bacteria by damaging cell walls, disrupting gene expression, and inducing oxidative stress30,31,32. Unlike conventional antimicrobial agents, CDs remain stable in aqueous solutions and exhibit excitation-dependent photoluminescence, enhancing their potential applications33,34.
CDs are synthesized using various methods, with hydrothermal treatment being preferred due to its simplicity, cost-effectiveness and eco-friendliness35. Structurally, CDs consist of a carbogenic core with surface functional groups that determine their charge, influencing their antibacterial efficacy. Oxygen-containing groups generally result in a negative charge, while nitrogen-containing groups yield a positive charge32. This charge variation plays a crucial role in their interactions with biological membranes and bacterial cells.
CD-polymer composites have gained attention in biomedical applications due to their biocompatibility and ease of preparation36,37,38. Besides, CD-based coatings provide a promising strategy for self-disinfecting surfaces, offering an effective means to eliminate bacteria and their biofilms and reduce the risk of contamination. Unlike traditional antibacterial coatings, which often rely on chemical agents that may degrade over time, CD coatings leverage photodynamic activation and ROS generation to kill bacteria efficiently32. Such material can enhance medical devices, wound dressings, and personal protective equipment (PPE) that play a crucial role in infection control, particularly for healthcare workers39,40,41. A recent study found bacterial or fungal contamination in 50% of tested PPE, particularly around the neckline, with persistent contamination over time42. This underscores the need for antimicrobial agents in PPE to reduce contamination risks.
In this study, carbon dots with amino and boronic acid groups on the surfaces were synthesized through a one-step hydrothermal process, using phenyl boronic acid, ethylenediamine, and curcumin as precursors. Curcumin, a polyphenol obtained from turmeric, is widely recognized for its therapeutic potential. As a green and non-toxic natural compound, curcumin holds great potential for applications in antioxidant, anti-inflammatory, anti-cancer and antibacterial therapies43. Moreover curcumin has been reported to suppress biofilm formation in Escherichia coli and Pseudomonas species44. Curcumin in its various forms has also demonstrated effects against Gram-positive bacterial and fungal biofilms45,46,47,48,49.
This study aimed to evaluate the antibacterial and antibiofilm efficacy of curcumin-based CDs and their composites with both oxidized and non-oxidized cellulose fibers. Specifically, the research focused on assessing the dual activity of these CDs against biofilms formed by Escherichia coli (EC) and Staphylococcus epidermidis (SE), including their ability to inhibit biofilm formation and eradicate preformed biofilms. Additionally, the study sought to investigate how the incorporation of CDs into cellulose fibers imparts antimicrobial properties to the material, and how partial oxidation of cellulose and exposure to simulated sunlight further enhance this activity. Cellulose is widely used in medical, fabrics, and PPE gear, and it is recognized for its strength, stability, and biodegradability50,51,52. The broader goal was to explore the potential of this approach in treatment for combating biofilm-associated infections and the development of materials and fabrics with self-disinfecting properties.
Materials and methods
Materials/bacterial strains/chemicals
Curcumin, phenyl boronic acid (PBA), phosphate-buffered saline (PBS) tablet capsules, ethylenediamine, crystal violet, sodium periodate, Terephthalic acid (TA) and 2,4-dinitrophenylhydrazine (2,4-DNP) were purchased from Sigma-Aldrich (Germany). 99.9% ethyl alcohol was obtained from Chemstock LLC (Dubai, UAE). The antibacterial activities of the CDs were evaluated against two bacterial strains: gram-negative Escherichia coli (MicroKwik culture 155065 A) and gram-positive Staphylococcus epidermidis (ATCC 12228). Microbiological growth media, including Tryptic Soy Agar (TSA), Mueller Hinton Broth (MHB) and Mueller Hinton Agar (MHA), and sterile 96 well plates used for bacterial assays were procured from Sigma Aldrich (Germany). Crystal violet used for biofilm assays, were obtained from Sigma Aldrich (Germany).
For cytotoxicity evaluations, human brain microvascular endothelial cells (HBMEC) from ScienCell Research Laboratories, (USA) (Cat. No. 1000) were cultivated in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% (v/v) Fetal Bovine Serum (FBS) with 10% (v/v) Pencillin-streptomycin, all purchased from Sigma Aldrich, (Germany). Dimethyl Sulfoxide (DMSO) and Thiazolyl Blue Tetrazolium Bromide (MTT) were also procured from Sigma Aldrich (Germany). Tissue culture well plates were obtained from Corning, (USA) were employed for experimental procedures. All chemicals and reagents were supplied by Labco LLC, United Arab Emirates.
Synthesis of carbon dots
To synthesize CDs, 40 mL of distilled water and 10 mL of ethanol were mixed in a beaker and heated to 80 ºC for 20 min. After that, 0.3 g of curcumin was added to the mixture and stirred vigorously until completely dissolved. Next, 50 mg of ethylenediamine was introduced into the solution while continuously stirring at 80 ºC for 30 min, during which the color of the solution gradually changed to reddish. Afterward, 0.6 g of PBA was added to the solution. The mixture was then transferred to a hydrothermal reactor, degassed for 20 min, and heated in an oven at 200 ºC for 12 h. Once the reaction was complete, the reactor was allowed to cool. The resulting solution was centrifuged at 12,000 rpm to remove large precipitates, yielding the final carbon dots. The resulting carbon dot solution was then lyophilized and subsequently subjected to characterization studies and antibacterial assays.
Oxidation of cellulose papers
Oxidized cellulose fibers were prepared by treating Whatman filter papers with sodium periodate. Five filter papers, each measuring 10–15 cm in diameter, were submerged in 500 mL of distilled water in a conical flask containing a magnetic stir bar. The flask was placed on a hot plate, and stirring was initiated. Gradually, 7.5 g of sodium periodate was added to the solution while maintaining continuous stirring. To minimize light exposure, the flask was covered with aluminum foil. The solution was heated to a temperature of 55–60 °C and maintained at this temperature for three hours. After the reaction, the filter papers were removed and thoroughly washed with deionized water to eliminate any residual reactants. The washed papers were air-dried for one hour and then placed in an oven at 60 °C for an additional hour. Once dried, the papers were stored in sealed bags and refrigerated for later use in subsequent experiments.
After drying the oxidized samples, a drop of 2,4-dinitrophenylhydrazine (2,4-DNP) was added to each sample to test for the presence of carbonyl groups, including aldehydes and ketones. The reaction between 2,4-DNP and carbonyl compounds results in the formation of a colored precipitate, typically orange, depending on the concentration and type of carbonyl functional groups present.
Attachment of CDs to papers
To evaluate the binding of carbon dots to both non-oxidized and oxidized cellulose fibers, paper discs were used as a model. Discs measuring 6–7 mm in diameter were punched from Whatman filter paper and oxidized cellulose paper. These discs were sterilized by microwaving for 5 min before being treated with 20 µL of a CD solution (50 mg/mL in PBS at pH 7.4) in a well plate. The well plate was then incubated at room temperature for 24 h to promote binding. After incubation, the excess CD solution was removed, and the discs were washed three times with 20 µL of PBS (pH 7.4) to prepare them for further assays. The quantity of CDs attached to the paper discs was determined by measuring the absorbance difference at 341 nm of the CD solution before and after incubation with the discs using a Shimadzu UV-1800 spectrophotometer.
Characterization of carbon dots
The structural and fluorescence characteristics of carbon dots and their precursor materials were thoroughly investigated through various spectroscopic characterization techniques, applied to the CDs. The absorption behavior of carbon dots can vary depending on the carbon source used or the synthesis method applied. Despite these differences, CDs typically show strong absorption in the ultraviolet (UV) range (200–400 nm), with a gradual extension into the visible spectrum. One of the most interesting characteristics of CDs is their photoluminescence (PL), which is of considerable interest both from a fundamental research standpoint and for practical applications23. UV-Vis absorption spectra of the CDs were recorded using a Shimadzu UV-1800 spectrophotometer, while fluorescence emission profiles were obtained with a fluorescence spectrometer (Shimadzu 6000). Functional groups in the CDs were identified via Fourier transform infrared (FTIR) spectroscopy using a Perkin-Elmer FT-IR spectrometer. For FTIR analysis, the CDs and potassium bromide (KBr) were mixed in a 1:100 ratio, ground in an agate mortar, and pressed into pellets under high pressure for 2–3 min. The spectra were recorded, with the KBr signal serving as the background 53,54.
Microscopic and structural characterization was performed using different techniques. Transmission Electron Microscopy (TEM) is a fundamental characterization technique for CDs, providing high-resolution imaging to assess their structural and morphological properties at the nanoscale. TEM analysis was carried out using a JEOL JEM 2100 transmission electron microscope and a camera make GATAN ORIUS SC600A. Scanning electron microscopy (SEM) with a VEGA3 model from TESCAN was employed to analyze the surface morphology of the CDs. The elemental composition of the CDs was determined through energy-dispersive X-ray spectroscopy (EDS) using an Oxford system. Raman spectroscopy provided detailed insights into the chemical structure, polymorphism, and crystallinity of the CDs. The X-ray powder diffraction (XRD) technique was used to assess physical properties such as phase composition, crystal structure, and orientation in the powder form. Raman analysis was performed using a WITec Confocal Raman Microscope-α 300. For XRD, data were collected using a Panalytical X’pert 3 pro multipurpose diffractometer.
Antibacterial assays
The antibacterial efficacy was evaluated against 2 commonly used model organisms EC and SE selected for their rapid growth rates and ease of cultivation. EC is a gram negative rod shaped bacterium whereas SE is a gram positive coccus typically arranged in clusters. Both organisms were subjected to antibiotic susceptibility testing and exhibited distinct zones of inhibition against a panel of antibiotics including Gentamycin and tetracycline etc. Bacterial cultures were prepared in MHB medium and incubated overnight at 37 °C with shaking (180 rpm).
Bacterial inhibition assays
The assay was designed to assess the dose-dependent antibacterial activity of the samples, quantified in terms of IC₅₀ and IC₉₀ values, which correspond to the concentrations required to inhibit 50% and 90% of bacterial growth, respectively55. EC and SE cells, grown overnight, were diluted with MH broth to a concentration of 1 × 106 cells/mL. Bacterial growth was assessed in 96-well plates, where each well contained 50 µL of bacterial suspension with various concentrations of carbon dots, bringing the final volume to 100 µL and the final bacterial concentration to 1 × 105 CFU/mL. Triplicates for EC and SE were included for statistical analysis. One column served as a blank, containing only CDs and PBS, while the final column served as a negative control with only PBS and bacteria. One plate was exposed to simulated sunlight for 2 h under a Morelian Desk Lamp (Model No: B07W9KMXR8), equipped with white SMD 2835 LEDs that emit broad-spectrum white light. The lamp produces light through blue LED chips (peak emission: 450–470 nm) combined with a phosphor coating that emits in the yellow–orange range (500–650 nm), resulting in an overall visible spectrum spanning approximately 450–650 nm. The lamp has a color rendering index (CRI) greater than 70, indicating moderate color accuracy suitable for general lighting. The lamp was positioned 15 cm above the sample surface, and a nearby flame was maintained to prevent microbial contamination. The other plate was kept in the dark. After treatment, the bacterial samples were incubated overnight at 37 °C. The optical density (OD) at 600 nm was measured using a BioTek 800 TS Microplate Reader to assess bacterial growth. The inhibitory effect of the treatment was determined by comparing the OD600 values of treated samples to the untreated control, with a lower OD600 indicating greater inhibition. This data allowed the calculation of the bacterial cell viability and the antimicrobial efficacy of the CDs under both conditions.
Bacterial plate inhibition assay
A 40 mg/mL CD solution was initially prepared by dissolving powdered CDs in PBS (pH 7.4). The same procedure outlined in the bacterial inhibition assay above was followed up to the point of light treatment, which lasted for 2 h. After the incubation period, 5 µL of solution from each well was transferred to the adjacent well and diluted with 95 µL PBS. This process was repeated for each column, resulting in a final 40-fold dilution of the CD-bacterial solution. Subsequently, 10 µL from each well was plated onto MHA plates and incubated overnight at 37 °C. The colonies were counted to evaluate the bactericidal effect of the CDs under both light-exposed and dark conditions.
CD-modified cellulose fibers’ assay
Antibacterial activity of modified fibers on bacterial solutions
Non-oxidized and oxidized paper discs were treated with a CD solution as described above. Bacterial suspensions were adjusted to an OD600 of 0.22 (1 × 108 CFU/mL), and 10 µL of diluted suspension (1 × 106 CFU/mL) was added to CD-modified and unmodified discs (negative controls), along with 10 µL of PBS. The procedure was repeated for oxidized discs. Two plates were prepared: one exposed to simulated sunlight (Morelian Desk lamp emitting red and blue light, CRI > 70) at 15 cm for 2 h and the other kept in the dark. Following exposure, the discs were washed, and the resulting solution was appropriately diluted. Subsequently, 10 µL of the diluted solution was aseptically transferred onto MHA plates. The discs were then carefully placed onto the agar surface and incubated overnight at 37 °C. Colony counts were performed to assess bactericidal activity under light and dark conditions, comparing bacterial growth on CD-modified discs with control discs to evaluate their antibacterial effectiveness.
Antibacterial activity of modified fibers on bacterial lawn
Antibacterial activity was evaluated using a modified disc diffusion method based on the principles of the standard Kirby-Bauer assay. Bacterial cultures were grown in MHB, adjusted to a concentration of 1 × 106 CFU/mL, and uniformly spread on MHA plates using a pre-sterilized L-shaped glass spreader to ensure a consistent bacterial lawn. Instead of commercial antibiotic discs, sterile paper discs impregnated with the test CDs were placed onto the inoculated MHA plates. The plates were incubated overnight at 37 °C to allow for bacterial growth56.
AATTC 147 test
The antibacterial activity of the textile products was assessed qualitatively to evaluate the effectiveness of antimicrobial agents potentially diffusing from treated textile materials. Paper discs treated with CDs, measuring 1.2 × 2 cm, were placed across three streaks of bacteria to ensure adequate contact between the CDs and the test bacteria. A parallel experiment was conducted using untreated control discs for comparison. The plates were then incubated overnight at 37 °C to observe the antibacterial effect57,58,59.
Biofilm assays
Biofilm Inhibition assay
To check the effectiveness of CCM-PBA-NH2 in inhibiting biofilm formation, SE and EC bacterial cultures were exposed to varying concentrations of carbon dots (0.1 to 10 mg/mL). Following the protocol outlined in the bacterial inhibition assay above, the bacterial cultures and CDs were incubated in a 96-well plate for 48 h. Afterward, spectrophotometric analysis was conducted to quantify the total bacterial growth, growth in the planktonic phase, and bacterial biofilm formation60.
Total bio growth was quantified by measuring the optical density at 600 nm in each well post-incubation. To quantify the planktonic growth, the culture medium was transferred to a new 96-well plate, and its absorbance was measured at 600 nm. Biofilm formation was quantified by crystal violet staining, using a modified reported protocol61. The biofilm formed on the sides of well was washed with deionized water and stained with 100 µL of 0.1% crystal violet for 20 min, washed 2–3 times with deionized water to remove the excess crystal violet stain. The stained biofilm was then eluted with ethanol, providing a small agitation at 120 rpm for 10 min. The absorbance of the eluted solution was measured at 575 nm to quantify the biofilm growth.
Biofilm eradication assay
To check the effectiveness of CCM-PBA-NH2 to eradicate preformed bacterial biofilms, untreated cultures of SE and EC were grown in a 96-well plate for 72 h to develop mature biofilms. Following the incubation period, the planktonic bacteria were discarded, and fresh MHB media was added. The cultures were then exposed to treatment with different levels of CCM-PBA-NH2 (ranging from 0 to 10 mg/mL). The plates were incubated for an additional 72 h, after which the total, planktonic phase growth, and biofilm formation were measured using the crystal violet staining method as outlined above.
Cell culture and viability analysis
The MTT assay was employed to evaluate the cytotoxicity of CDs in human brain microvascular endothelial cells (HBMECs), providing insight into their biocompatibility. For this assay, 10,000 cells per well were seeded into 96-well plates and incubated overnight to ensure proper adhesion and growth under optimal conditions. Afterward, the medium was replaced with fresh media containing varying concentrations of CDs, and the cells were incubated for an additional 48 h to allow sufficient interaction with the test substance. Following the treatment period, MTT solution was added to each well, and the plates were incubated for 4 h, during which metabolically active cells reduced the MTT reagent into purple formazan crystals. After incubation, the medium was carefully removed to avoid disturbing the crystals, which were subsequently dissolved using DMSO to facilitate quantification. Absorbance was measured at 570 nm using a Biotek TS 800 microplate reader, with lower absorbance values indicating a reduction in cell viability due to cytotoxic effects62,63,64.
Cell viability was calculated as a percentage relative to untreated control cells, which served as a control for comparison. The experiment was conducted in triplicates and included three independent batches of CDs to ensure robustness, reproducibility, and statistical accuracy. Furthermore, the cytotoxicity of CD-modified paper discs was also assessed using the same MTT protocol. In this case, discs were exposed to HBMECs (50000 cells in 24 well plates) under identical conditions, and control discs without CD modification were included to provide a comparison. The results from this additional experiment highlighted the potential impact of CD incorporation into materials, such as paper discs, and provided further validation of the biocompatibility and safety of CDs in practical applications.
Statistical analysis
All statistical analyses were performed using GraphPad Prism, Version 6.05 (GraphPad Software, San Diego, CA, USA), available at https://www.graphpad.com/scientific-software/prism/. Both two-way and three-way ANOVA were employed for intragroup comparisons, with statistical significance established at p < 0.05. All experiments were performed in triplicate 3 times using three independent batches to ensure reproducibility and minimize variability. The results were expressed as mean ± standard deviation (SD), highlighting the consistency across experimental replicates. This robust analytical approach allowed for reliable interpretation of complex datasets and ensured the validity of the findings.
Results and discussion
Structural characterization of CDs
The photophysical properties of the synthesized CDs were evaluated using UV-Vis absorption and excitation-dependent photoluminescence (PL) spectroscopy. The UV-Vis spectrum of the diluted CD solution displayed two characteristic absorption features: a sharp peak at 245 nm and a broader band centered at 341 nm (Fig. SI-1), indicative of π–π* transitions and n–π* transitions associated with conjugated domains and surface functionalities. For PL analysis, emission spectra were recorded over an excitation range of 300–475 nm in 25 nm increments to assess excitation-dependent fluorescence behavior. The PL spectra of CCM-PBA-NH2 exhibited excitation-dependent emission with notable shifts in emission maxima and variations in intensity, which are characteristic of carbon-based nanomaterials and attributed to quantum confinement effects and surface state emissions65,66. These findings exhibit the tunable optical and photo physical properties of the CDs, as illustrated in Fig. 1.
The chemical composition and functional groups of the CDs were examined using FTIR spectroscopy, with the recorded spectra shown in Fig. 2. The FTIR spectrum of curcumin displayed characteristic peaks at 3520 cm–1 and 1626 cm–1, corresponding to O–H and C = O functional groups, respectively, confirming its structural integrity67. In the spectrum of CCM-PBA-NH₂, a broad O–H stretching band and a shift in the C = O stretching band (1625–1650 cm–1) suggest interactions between PBA and curcumin. Additionally, the peak at 1030 cm–1, attributed to aryl oxygen (C–O–C) groups, confirms the successful incorporation of curcumin into the CDs68. Furthermore, zeta potential measurements of the CD solution in PBS (0.1 mg/mL) showed a near-neutral surface charge (0.6 mV). This neutrality may be due to the presence of both amino and boronic acid groups, which have opposite charges, on the surface of the CDs.
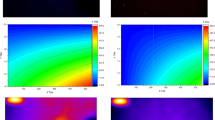
The structural properties and morphology of the synthesized CDs were investigated using Raman spectroscopy, X-ray diffraction, and HR-TEM. As seen in Fig. 3A, the Raman spectrum of CDs displays two characteristic peaks, denoted as D and G bands. The D band at 1331 cm− 1 is attributed to the breathing mode of the sp3-hybridized carbon atoms. It is associated with defects present in the structures, potentially stemming from vacancies, heteroatoms, edges, or amorphous domains. The G band, on the other hand, observed at 1576 cm− 1, arises from the in-plane stretching vibrations of sp2 hybridized carbons in graphitic domains69. The presence of both bands indicates the co-existence of ordered and disordered regions in the CDs. The intensity ratio (ID/IG) is commonly used to estimate the disorder-to-order ratio in carbonaceous materials. A lower ratio, meaning lower intensity of the D band, reflects a better graphitization process. In our case, ID/IG equaled 0.87, confirming thus a moderate graphitization and good crystallinity of the CDs70. The crystallinity was further corroborated by XRD data (Fig. SI-2), as evidenced by the sharp and well-defined peaks indicative of a high degree of structural order. Notably, the peaks noted at 2θ ≈ 28 and 42° reveal the lattice planes (002) and (100) of the graphitic carbons, respectively71.
The HR-TEM image (Fig. 3B) indicates a spherical shape of the synthesized CDs, with a mean diameter of 5.19 ± 1.48 nm and a narrow size distribution (Fig. 3B inset). Also, it displays clear lattice fringes with a measured interplanar spacing of 0.19 nm (Fig. 3C), which aligns with the (102) crystallographic plane of graphite72. Complementing this, the selected area electron diffraction (SAED) pattern shown in Fig. 3D reveals distinct diffraction rings, confirming the polycrystallinity of the CDs73. The presence of well-defined lattice fringes and the distinct diffraction spots together indicate the successful obtention of an ordered pattern of the CCM-PBA-NH272,73,74. As the HRTEM image (Fig. 3B and C) shows no visible amorphous regions, it is noteworthy that the D band observed in the Raman spectrum (Fig. 3A) could be attributed to the functionalization of CDs with amino-groups75.
Raman and TEM images of CCM-PBA-NH2. (A) Raman spectroscopic image at 10 X magnification (B) HR-TEM image of CCM-PBA-NH2 at 5 nm scale (Inset: histogram showing particle size distribution of CDs) (C) TEM image at 2 nm scale (D) lattice fringes indicating crystalline structure with visible interplanar spacing; SAED pattern.
The size, distribution, and elemental composition of the CDs were thoroughly analyzed using SEM/EDX. High-resolution SEM image (Fig. SI-3 A) revealed that the CDs were homogeneously dispersed with a consistent and well-defined size distribution. EDX analysis (Fig. SI-3 B) confirmed the presence of key elements, including carbon, boron, oxygen, and nitrogen, which are consistent with the expected composition, verifying the successful synthesis and elemental integration of the CDs. The presence of boron and nitrogen suggests successful doping and surface functionalization, while the detection of carbon and oxygen further supports the carbonaceous nature of the core structure. These elemental signals collectively verify the successful synthesis and incorporation of functional elements into the CDs, with uniform morphology and elemental composition which may contribute to their enhanced physicochemical and biological properties.
Antibacterial activity of the cds
Assessing inhibitory concentrations of cds
The antibacterial properties of CCM-PBA-NH₂ were systematically evaluated against EC and SE using broth microdilution assays to determine the half maximal inhibitory concentration (IC50) and 90% inhibitory concentration (IC90). IC50 and IC90 values were determined under dark and simulated sunlight conditions, and the results are summarized in Table 1. These results demonstrated concentration-dependent antibacterial activity, with enhanced effects observed under sunlight, probably due to the generation of reactive oxygen species (ROS). The observed trends were consistent across three independent experiments, each conducted in triplicate, confirming the reproducibility and reliability of the data, as indicated by the low standard deviation values presented in Table 1. Serial dilution assays revealed significant reductions in bacterial viability at CD concentrations of 1.25, 2.5, 5, and 10 mg/mL (Fig. 4). Complete bacterial growth inhibition was achieved at the highest concentration of 20 mg/mL for both EC and SE (Fig. 5/Table 2) while at concentrations of 5 mg/mL or below limited antibacterial effects were observed, irrespective of simulated sunlight exposure. At 10 mg/mL, however, a noticeable difference in bacterial growth was observed between the two conditions. Bacterial growth was significantly reduced in simulated sunlight compared to dark conditions, indicating an enhanced antibacterial effect and suggesting that light plays a critical role in augmenting the antibacterial action of CDs at this concentration. Additionally, these results suggest that the antibacterial activity of CDs is highly concentration-dependent, with lower concentrations insufficient to exert a bactericidal effect within the 2-hour experimental timeframe.
Biofilm growth Inhibition
The formation of bacterial biofilms and their strong resistance to antibiotics present significant challenges in the healthcare industry, particularly in chronic infections, dental implants, and medical device contamination4,76. Their complex polysaccharide matrix and modified microbial physiology make them difficult to eradicate77. Our research examined how effectively CCM-PBA-NH2 can prevent biofilm formation in EC and SE model organisms. We evaluated its impact on microbial growth and biofilm formation by applying varying concentrations of CCM-PBA-NH2 to bacterial cultures, as outlined in the methods section. Figure 6 displays the total and planktonic growth phases for both treated and control cells, with SE growth patterns shown in Fig. 6A(i) and EC data in Fig. 6B(i). Treatment with CCM-PBA-NH2 led to a statistically significant reduction in total bacterial growth at concentrations of 1 mg/mL or higher (p < 0.0001) (Fig. 6(i)). Approximately 50% of total growth inhibition was noticed at a 2.5 mg/mL concentration of CD solution. Additionally, planktonic growth progressively declined within the 1 mg/mL to 10 mg/mL range, with minimal growth observed at 5 mg/mL and 10 mg/mL, highlighting the strong inhibitory effect of CCM-PBA-NH2 on bacterial growth.
Biofilm development from bacterial culture was quantified using crystal violet staining, with the eluted stain measured at 575 nm and normalized to 100% for untreated bacteria (control). Treatment with 1 mg/mL CCM-PBA-NH2 solutions led to a gradual reduction in SE biofilm formation, reaching approximately 30% compared to the control bacterial culture (Fig. 6A(ii)). Significant inhibition was observed at 5 mg/mL or higher concentrations for both SE (Fig. 6A(ii)) and EC (Fig. 6B(ii)). At a CCM-PBA-NH2 concentration of 5 mg/mL, biofilm formation was significantly minimal (p < 0.0001), and at 10 mg/mL, no detectable biofilm formation was observed in either bacterial strain.
The concentration-dependent inhibition of biofilm formation in both SE (Fig. SI-4) and EC (Fig. 7) was further confirmed through the color bright field microscopy images of crystal violet-stained biofilms. At concentrations above 1 mg/mL of CCM-PBA-NH2, minimal biofilm growth remained compared to the untreated control, as clearly visible from Fig. SI-4(SE) and Fig. 7(EC). Concentrations of 5 mg/mL or higher led to complete biofilm inhibition. These results highlight the effectiveness of CCM-PBA-NH2 in preventing bacterial growth and biofilm development. The supplementary Fig. SI-5 shows the layout of the 96-well plate crystal violet staining assay, visually illustrating the progressive reduction in EC biofilm formation (color intensity) with increasing CCM-PBA-NH2 concentration.
Eradication of mature biofilms using carbon Dots
To confirm the biofilm eradication effectiveness of CCM-PBA-NH2, bacterial cultures were first grown in a 96-well plate for 72 h for mature biofilm development. After incubation, the liquid phase (planktonic cells) was gently removed. The wells were then treated with different concentrations of CCM-PBA-NH2 (0–10 mg/mL), as detailed in the methods section, and the total, planktonic, and biofilm were quantified.
The results indicate that CCM-PBA-NH₂ effectively disrupts pre-formed SE and EC mature biofilms at concentrations above 0.1 mg/mL, as shown by a significant reduction in % biofilm biomass compared to control cultures (Fig. 8A(ii) and 8B(ii)). Quantification of total bacterial growth and planktonic phases further confirmed a substantial decrease in viable SE (Fig. 8A(i)) and EC (Fig. 8B(i)) within biofilms at all tested concentrations above 0.1 mg/mL, highlighting its strong potential for biofilm eradication and bacterial growth inhibition.
Color bright-field microscopy confirmed the eradication of SE (Fig. SI-6) and EC (Fig. 9) biofilms and provided further insights into CCM-PBA-NH2 interaction with bacterial cells and the biofilm polysaccharide matrix. At concentrations of 2.5 mg/mL and above, treatment significantly suppressed planktonic bacterial growth and resulted in more than a 50% increase in SE biofilm eradication (Fig. 9A(ii)) and a 40% of EC biofilm disruption (Fig. 9B(ii)). The reduction in residual biofilm traces suggests that while CCM-PBA-NH2 primarily targets bacterial cells, it also disrupts the extracellular matrix components, preventing new biofilm formation while disrupting existing biofilm complex structures. The antibiofilm activity of carbon dots, including CCM-PBA-NH2, is likely mediated by mechanisms such as interference with bacterial adherence, quorum sensing disruption, and extracellular matrix degradation78,79. Given its efficacy against both gram-positive and gram-negative bacteria, CCM-PBA-NH2 presents a promising strategy for treating planktonic bacteria and biofilm-associated infections, a major challenge in the medical industry.
Antibacterial activity of CD-modified cellulose fibers
Preparation and characterization of modified cellulose fibers
We have previously reported that CDs with boronic acid groups on their surface can be readily anchored to cellulose fibers, presumably due to the covalent interaction between the boronic acid groups and the diol groups on the cellulose53,54. Since the CCM-PBA-NH2 contains an amino group on its surface, it is proposed that introducing aldehyde groups to the cellulose fiber will further enhance the binding and loading of the CDs onto the fiber’s surface due to the formation of imine bonds. This modification is expected to enhance the antibacterial efficacy of the fibers due to a high load of the CDs on the fiber surface. Thus, the cellulose fibers were partially oxidized (6–10%) with periodate anion80 to selectively cleave the C2-C3 bonds in the glucose rings of the cellulose backbone, resulting in the formation of aldehyde groups at these positions81,82,83,84,85,86. The successful oxidation of the cellulose surface was visually confirmed using the 2,4-dinitrophenylhydrazine (2,4-DNP) test, which produced a characteristic orange coloration on the oxidized paper discs (Fig. SI-7)87.
Cellulose paper discs were loaded with CDs by immersing them in a PBS solution containing CDs at a concentration of 50 mg/mL, allowing the fibers to absorb the material through exhaustion. UV spectrophotometry monitoring of the concentration of CDs in the exhausted solution revealed that approximately 164.96 ± 29 µg of CDs were loaded onto the oxidized paper discs, while only 28.9 ± 11 µg were loaded on the non-oxidized discs (2.0 ± 0.3 mg). This binding was also visually confirmed by a noticeable change in fluorescence under UV light, as illustrated in Fig SI-8.
Enhanced antibacterial activity with oxidized cellulose
The antibacterial activity of CD-modified cellulose discs was evaluated for the oxidized and non-oxidized paper discs and the unmodified controls. The paper discs were incubated with bacterial cultures for two hours with or without simulated sunlight, and then the discs were washed with PBS to remove loosely adherent bacteria. Diluted samples (1:50) of the wash solutions were plated onto MHA agar along with the discs and then incubated overnight at 37 °C to assess bacterial viability in both.
The CD-modified oxidized discs exhibited superior antibacterial performance compared to the non-oxidized ones. No bacterial growth was observed around the CD-modified oxidized discs (Fig. 10), indicating effective bacterial inhibition. In contrast, CD-modified non-oxidized discs showed reduced bacterial growth compared to controls but were less effective than CD-modified oxidized discs. These results suggest that both the oxidation state of fibers and the immobilization of CDs contribute to the antibacterial activity, with oxidized fibers providing enhanced performance likely due to improved CD binding and increased interaction with bacterial cells.
The generation of hydroxyl radicals (•OH), a key reactive oxygen species (ROS), was evaluated using the terephthalic acid (TA) fluorescence assay. Terephthalic acid is a non-fluorescent molecule that selectively reacts with hydroxyl radicals to produce 2-hydroxyterephthalic acid (HTA), a fluorescent compound with an emission peak around 425 nm upon excitation at 315 nm88,89. A significant increase in fluorescence intensity under light conditions compared to dark controls indicate the photo-induced generation of •OH by the CDs immobilized on the cellulose fibers (Fig. SI-9).
It is worth noting that the CDs remain attached to fibers during the incubation period. This was confirmed by the inhibition zone assay and the AATCC 147 assay57,58,59 on CD-modified paper discs using MHA agar plates. No zones of inhibition were observed around the CD-modified fibers (Figs. SI-10 and SI-11), indicating that the CDs remained firmly attached to the fibers and did not diffuse into the surrounding medium. The absence of diffusion of CDs from the fibers emphasizes their stability and indicates their suitability for applications requiring durable, non-leaching antimicrobial surfaces, such as medical textiles or protective fabrics.
Paper disc assay of CCM-PBA-NH2 (A) Escherichia coli and (B) Staphylococcus epidermidis (i) Oxidized discs (O) modified with CCM-PBA-NH2; (ii) non-oxidized discs (NO) modified with CCM-PBA-NH2; (iii) discs on MHA plates. Each plate contains non-modified control discs (C) and CCM-PBA-NH2 modified discs (P) from incubations in the presence (sun) and absence (dark) of simulated sunlight.
Cytotoxicity evaluation of carbon dots and modified fibers
The cytotoxicity of the CDs and the CD-modified paper discs was assessed using human brain microvascular endothelial cells (HBMEC) (Fig. 11). At CD concentrations equal to 0.1 mg/mL, cell viability remained high, indicating minimal cytotoxic effects. However, at concentrations exceeding 0.1 mg/mL, a significant reduction in cell viability was observed, suggesting a dose-dependent toxic effect of the CDs. The cytotoxicity evaluation of the CD-modified paper discs revealed distinct patterns based on the type of modification. Non-oxidized CD-modified discs exhibited minimal toxicity, indicating their compatibility with the tested cells. In contrast, the oxidized paper discs, including those without CDs, displayed significant toxicity. This suggests that the oxidation process of the paper discs contributes to their inherent toxicity, independent of the presence of CDs. Sodium periodate oxidation modifies the cellulose structure by introducing aldehyde groups, which enhances its chemical reactivity. These aldehyde groups may interact with cellular components and potentially induce toxicity in mammalian cells. Although cytotoxicity was observed at 1 mg/mL in vitro, such concentrations are unlikely to be encountered in vivo or in practical applications and may not pose significant concern under realistic conditions90,91.
Conclusion
CDs prepared from curcumin and phenylboronic acid were successfully synthesized via a simple and efficient hydrothermal method, resulting in boronic acid-functionalized nanomaterials. Their structural and physicochemical properties were comprehensively characterized using FTIR, Raman, and fluorescence spectroscopy, along with XRD, SEM, EDX, and HR-TEM analyses. The CDs exhibited potent, broad-spectrum antibacterial activity against both Gram-positive and Gram-negative bacterial strains. They demonstrated potent inhibition of biofilm formation and effectively disrupted pre-formed biofilms of Escherichia coli and Staphylococcus epidermidis. Cytotoxicity assays further confirmed their biocompatibility, showing low toxicity toward mammalian cells at the tested concentrations.
Moreover, the CDs displayed strong binding affinity to cellulose and oxidized cellulose fibers, successfully imparting antimicrobial properties to these substrates. Upon exposure to simulated sunlight, the antibacterial performance of the CD-functionalized fibers was significantly enhanced, highlighting their potential utility in environments where natural or artificial light can aid disinfection. While the findings are promising, certain limitations should be noted. Antibacterial assessments were conducted in vitro, and additional in vivo studies are necessary to establish the safety, efficacy, and mechanistic insights of the CDs in biological systems. Despite these limitations, the study provides a valuable foundation for the future application of carbon dots in antimicrobial textiles, as well as in other fields such as water purification, wound care, and biomedical device sterilization. Continued research, focusing on in vivo validation, mechanistic understanding, and long-term safety, will be essential for advancing the practical deployment of these materials.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Das, S. et al. Piperine exhibits potential antibiofilm activity against Pseudomonas aeruginosa by accumulating reactive oxygen species, affecting cell surface hydrophobicity and quorum sensing. Appl. Biochem. Biotechnol. 195, 3229–3256 (2023).
Larsson, J. Background document to Guidance on wastewater and solid waste management for manufacturing of antibiotics Evidence synthesis for deriving PNECs for resistance selection (WHO, 2024).
Guidance on Wastewater and Solid Waste Management for Manufacturing of Antibiotics (WHO, 2024).
Funari, R. & Shen, A. Q. Detection and characterization of bacterial biofilms and Biofilm-Based sensors. ACS Sens. 7, 347–357 (2022).
Mishra, A., Lzaod, S., Dutta, T. & Bhattacharya, S. Selective bacterial growth inactivation by pH-Sensitive sulfanilamide functionalized carbon Dots. ACS Appl. Bio Mater. 7, 2752–2761 (2024).
Lin, F., Wang, Z. & Wu, F. G. Carbon Dots for killing microorganisms: an update since 2019. Pharmaceuticals 15, 1236 (2022).
Huang, S., Song, Y., Zhang, J., Chen, X. & Zhu, J. Antibacterial carbon Dots-Based composites. Small 19, 2207385 (2023).
Wang, Z. et al. Natural biomass-derived carbon Dots as potent antimicrobial agents against multidrug-resistant bacteria and their biofilms. Sustain. Mater. Technol. 36, e00584 (2023).
Urban-Chmiel, R. et al. Antibiotic resistance in Bacteria—a review. Antibiotics 11, 1079 (2022).
Martinez, J. L. General principles of antibiotic resistance in bacteria. Drug Discov Today Technol. 11, 33–39 (2014).
Van Duin, D. & Paterson, D. L. Multidrug-Resistant Bacteria in the community. Infect. Dis. Clin. North. Am. 30, 377–390 (2016).
Medina, E. & Pieper, D. H. Tackling threats and future problems of Multidrug-Resistant Bacteria. In How To Overcome the Antibiotic Crisis (eds. Stadler, M. & Dersch, P.) vol. 398 3–33 (Springer International Publishing, 2016).
Zhao, L., Ma, Y., Sun, Z., Zhang, X. & Liu, M. Boric Acid-Functionalized carbon Dots as a High-Performance antibacterial agent against Escherichia coli. Langmuir 39, 18302–18310 (2023).
Dong, X., Liang, W., Meziani, M. J., Sun, Y. P. & Yang, L. Carbon Dots as potent antimicrobial agents. Theranostics 10, 671–686 (2020).
Fang, M., Lin, L., Zheng, M., Liu, W. & Lin, R. Antibacterial functionalized carbon Dots and their application in bacterial infections and inflammation. J. Mater. Chem. B. 11, 9386–9403 (2023).
Lysenko, V. et al. Application of carbon Dots as antibacterial agents: a Mini review. BioNanoScience 14, 1819–1831 (2024).
Varghese, M. & Balachandran, M. Antibacterial efficiency of carbon Dots against Gram-positive and Gram-negative bacteria: a review. J. Environ. Chem. Eng. 9, 106821 (2021).
Li, H. et al. Degradable carbon Dots with Broad-Spectrum antibacterial activity. ACS Appl. Mater. Interfaces. 10, 26936–26946 (2018).
Ghirardello, M., Ramos-Soriano, J. & Galan, M. C. Carbon Dots as an emergent class of antimicrobial agents. Nanomaterials 11, 1877 (2021).
Liang, J. et al. Antibacterial activity and synergetic mechanism of carbon Dots against Gram-Positive and -Negative Bacteria. ACS Appl. Bio Mater. 4, 6937–6945 (2021).
Maruthapandi, M., Saravanan, A., Das, P., Luong, J. H. T. & Gedanken, A. Microbial Inhibition and biosensing with multifunctional carbon dots: progress and perspectives. Biotechnol. Adv. 53, 107843 (2021).
He, F. et al. Guanidinium-Functionalized carbon dots: an efficient antibacterial agent against Multidrug-Resistant ESKAPE pathogens. ACS Appl. Mater. Interfaces. 16, 65955–65969 (2024).
Liu, J., Li, R. & Yang, B. Carbon dots: a new type of Carbon-Based nanomaterial with wide applications. ACS Cent. Sci. 6, 2179–2195 (2020).
Riahi, Z., Rhim, J. W., Bagheri, R., Pircheraghi, G. & Lotfali, E. Carboxymethyl cellulose-based functional film integrated with chitosan-based carbon quantum Dots for active food packaging applications. Prog Org. Coat. 166, 106794 (2022).
Yang, L. et al. Thin-Film composite polyamide membranes decorated with photoactive carbon Dots for antimicrobial applications. ACS Appl. Nano Mater. 7, 1477–1490 (2024).
Du, X. et al. Size-dependent antibacterial of carbon Dots by selective absorption and differential oxidative stress of bacteria. J. Colloid Interface Sci. 634, 44–53 (2023).
Walia, S., Shukla, A. K., Sharma, C. & Acharya, A. Engineered bright Blue- and Red-Emitting carbon Dots facilitate synchronous imaging and Inhibition of bacterial and Cancer cell progression via1 O2 -Mediated DNA damage under photoirradiation. ACS Biomater. Sci. Eng. 5, 1987–2000 (2019).
Dong, W. et al. Co-, N-doped carbon Dot nanozymes based on an untriggered ROS generation approach for anti-biofilm activities and in vivo anti-bacterial treatment. J. Mater. Chem. B. 12, 1052–1063 (2024).
Ristic, B. Z. et al. Photodynamic antibacterial effect of graphene quantum Dots. Biomaterials 35, 4428–4435 (2014).
Dong, X. et al. Antibacterial effects of carbon Dots in combination with other antimicrobial reagents. PLOS ONE. 12, e0185324 (2017).
Cui, F., Ye, Y., Ping, J. & Sun, X. Carbon dots: current advances in pathogenic bacteria monitoring and prospect applications. Biosens. Bioelectron. 156, 112085 (2020).
Hussen, N. H. et al. Carbon Dot based carbon nanoparticles as potent antimicrobial, antiviral, and anticancer agents. ACS Omega. 9, 9849–9864 (2024).
Yan, F. et al. Surface modification and chemical functionalization of carbon dots: a review. Microchim Acta. 185, 424 (2018).
Ðorđević, L., Arcudi, F., Cacioppo, M. & Prato, M. A multifunctional chemical toolbox to engineer carbon Dots for biomedical and energy applications. Nat. Nanotechnol. 17, 112–130 (2022).
Chaudhary, M., Singh, P., Singh, G. P. & Rathi, B. Structural features of carbon Dots and their agricultural potential. ACS Omega. 9, 4166–4185 (2024).
Adam, G. O., Sharker, S. M. & Ryu, J. H. Emerging biomedical applications of carbon Dot and polymer composite materials. Appl. Sci. 12, 10565 (2022).
Chen, X. et al. Recent progress on chiral carbon dots: synthetic strategies and biomedical applications. ACS Biomater. Sci. Eng. 9, 5548–5566 (2023).
Marković, Z. M. et al. Photoactive graphene quantum dots/bacterial cellulose hydrogels: structural, mechanical, and pro-oxidant study. J. Appl. Polym. Sci. 139, 51996 (2022).
Setia, R. & Kamra Verma, A. Review of coveralls, gowns, and their use as part of personal protective equipment (PPE KIT) for effective protection of healthcare workers in India in COVID-19. Integr. J. Med. Sci. https://doi.org/10.15342/ijms.2021.329 (2021).
Kang, J. et al. Minimizing contamination in the use of personal protective equipment: simulation results through tracking contamination and enhanced protocols. Am. J. Infect. Control. 49, 713–720 (2021).
Brandner, J. M. et al. Contamination of personal protective equipment during COVID-19 autopsies. Virchows Arch. 480, 519–528 (2022).
Balter, S., Rodriguez, M. A., Pike, J. A. & Kleiman, N. J. Microbial contamination risk and disinfection of radiation protective garments. Health Phys. 120, 123–130 (2021).
Liao, Y., Yao, Y., Yu, Y. & Zeng, Y. Enhanced antibacterial activity of Curcumin by combination with metal ions. Colloid Interface Sci. Commun. 25, 1–6 (2018).
Yun, D. G. & Lee, D. G. Antibacterial activity of Curcumin via apoptosis-like response in Escherichia coli. Appl. Microbiol. Biotechnol. 100, 5505–5514 (2016).
Reddy, K. K. Antifungal and antibiofilm action of triphenylphosphonium-conjugated Curcumin on Candida albicans: efficacy and activity mechanisms. Int. Biodeterior. Biodegrad. 189, 105751 (2024).
Neelakantan, P. et al. Effectiveness of Curcumin against Enterococcus faecalis biofilm. Acta Odontol. Scand. 71, 1453–1457 (2013).
Raorane, C. J. et al. Antibiofilm and antivirulence efficacies of flavonoids and Curcumin against Acinetobacter baumannii. Front. Microbiol. 10, 990 (2019).
Hamzah, H., Hertiani, T., Utami Tunjung Pratiwi, S. & Nuryastuti, T. Bayu murti, Y. The biofilm Inhibition and eradication activity of Curcumin Againts polymicrobial biofilm. BIO Web Conf. 28, 04001 (2020).
Dong, H. et al. Synergistic antifungal effects of Curcumin derivatives as fungal biofilm inhibitors with fluconazole. Chem. Biol. Drug Des. 97, 1079–1088 (2021).
Dolez, P. I., Marsha, S. & McQueen, R. H. Fibers and textiles for personal protective equipment: review of recent progress and perspectives on future developments. Textiles 2, 349–381 (2022).
Verma, J., Petru, M. & Goel, S. Cellulose based materials to accelerate the transition towards sustainability. Ind. Crops Prod. 210, 118078 (2024).
Varnaitė-Žuravliova, S., Baltušnikaitė-Guzaitienė, J. & Properties Production, and recycling of regenerated cellulose fibers: special medical applications. J. Funct. Biomater. 15, 348 (2024).
Ravindran, S. et al. Harnessing Piperine for enhanced antimicrobial activity of carbon dot-modified cellulose fibers. Discov Appl. Sci. 6, 490 (2024).
Radha, R., Makhlouf, Z., Diab, R. & Al-Sayah, M. H. Modifying cellulose fibres with carbon dots: a promising approach for the development of antimicrobial fibres. R Soc. Open. Sci. 11, 231755 (2024).
Sun, W. et al. Rapid antimicrobial susceptibility test for identification of new therapeutics and drug combinations against multidrug-resistant bacteria. Emerg. Microbes Infect. 5, 1–11 (2016).
Dusane, D. H. et al. Complete killing of agar lawn biofilms by systematic spacing of Antibiotic-Loaded calcium sulfate beads. Materials 12, 4052 (2019).
Erdem, K. & Yurudu, S. The evaluation of antibacterial activity of fabrics impregnated with dimethyltetradecyl (3-(Trimethoxysilyl) Propyl) ammonium chloride (2008).
Antibacterial activity assessment. Of woolen fabric treated with natural dyes and Chitosan. Agric. Nat. Resour. https://doi.org/10.34044/j.anres.2019.53.2.14 (2019).
Pachiyappan, K. M., Sampath, V. R. & Kumar, B. S. Comparative study of antimicrobial activity of quaternary ammonium component coated knitted fabrics (2025).
Radha, R. et al. Enhanced antimicrobial and Biofilm-Disrupting properties of Gallium-Doped carbon Dots. ACS Omega Acsomega. https://doi.org/10.1021/acsomega.5c03575 (2025).
Abraham, W. L. et al. Biofilm Inhibition and bacterial eradication by C-dots derived from polyethyleneimine-citric acid. Colloids Surf. B Biointerfaces. 217, 112704 (2022).
Grela, E., Kozłowska, J. & Grabowiecka, A. Current methodology of MTT assay in bacteria – a review. Acta Histochem. 120, 303–311 (2018).
Van Meerloo, J., Kaspers, G. J. L. & Cloos, J. Cell sensitivity assays: the MTT assay. In Cancer Cell Culture (ed. Cree, I. A.) 237–245 (Humana, 2011).
Tolosa, L., Donato, M. T. & Gómez-Lechón, M. J. General cytotoxicity assessment by means of the MTT assay. In Protocols in in Vitro Hepatocyte Research (eds. Vinken, M. & Rogiers, V.) 333–348 (Springer, 2015).
Sciortino, A., Cannizzo, A. & Messina, F. Carbon nanodots: a review—from the current understanding of the fundamental photophysics to the full control of the optical response. C 4, 67 (2018).
Gan, Z., Xu, H. & Hao, Y. Mechanism for excitation-dependent photoluminescence from graphene quantum Dots and other graphene oxide derivates: consensus, debates and challenges. Nanoscale 8, 7794–7807 (2016).
Jabbar, A. et al. Improving Curcumin bactericidal potential against multi-drug resistant bacteria via its loading in polydopamine coated zinc-based metal–organic frameworks. Drug Deliv. 30, 2159587 (2023).
Liu, J., Li, L. & Xu, Z. P. Understanding of polydopamine encapsulation of hydrophobic Curcumin for pleiotropic drug nanoformulation. Part. Part. Syst. Charact. 40, 2200132 (2023).
Kazemifard, N., Ensafi, A. A. & Rezaei, B. Green synthesized carbon Dots embedded in silica molecularly imprinted polymers, characterization and application as a rapid and selective fluorimetric sensor for determination of thiabendazole in juices. Food Chem. 310, 125812 (2020).
Lee, S. J., Zheng, Y. Y., Chen, W. M. & Hsueh, Y. H. Nitrogen-doped carbon dots: a new powerful fluorescent dye with substantial effect on bacterial cell labeling. ACS Omega (2024). https://doi.org/10.1021/acsomega.4c04273.
Sharma, N., Sharma, I. & Bera, M. K. Microwave-Assisted green synthesis of carbon quantum Dots derived from Calotropis Gigantea as a fluorescent probe for bioimaging. J. Fluoresc. 32, 1039–1049 (2022).
Kunnath Parambil, N. S., Dasan, A., Premkumar, A. T., Renuka, N. K. & Raphael, S. J. Blue luminescent carbon quantum Dots derived from diverse banana peels for selective sensing of Fe(III) ions. Sens. Int. 6, 100301 (2025).
Kalaiselvi, K., Prabhu, S. & Muthu Mareeswaran, P. Construction of nickel oxide - carbon Dots Jasmine nano flowers derived from Sapindus Mukorossi for high performance supercapacitor applications. Diam. Relat. Mater. 155, 112259 (2025).
Zaca-Moran, O., Sánchez-Ramírez, J. F., Herrera-Pérez, J. L. & Díaz-Reyes, J. Electrospun polyacrylonitrile nanofibers as graphene oxide quantum Dot precursors with improved photoluminescent properties. Mater. Sci. Semicond. Process. 127, 105729 (2021).
Dager, A., Uchida, T., Maekawa, T. & Tachibana, M. Synthesis and characterization of Mono-disperse carbon quantum Dots from fennel seeds: photoluminescence analysis using machine learning. Sci. Rep. 9, 14004 (2019).
Liu, H. Y., Prentice, E. L. & Webber, M. A. Mechanisms of antimicrobial resistance in biofilms. Npj Antimicrob. Resist. 2, 27 (2024).
Pai, L., Patil, S., Liu, S. & Wen, F. A growing battlefield in the war against biofilm-induced antimicrobial resistance: insights from reviews on antibiotic resistance. Front. Cell. Infect. Microbiol. 13, 1327069 (2023).
Priyadarshini, E. et al. Biofilm Inhibition on medical devices and implants using carbon dots: an updated review. ACS Appl. Bio Mater. 7, 2604–2619 (2024).
Zhao, W. et al. Antibacterial carbon dots: mechanisms, design, and applications. Adv. Healthc. Mater. 12, 2300324 (2023).
Siller, M. et al. Effects of periodate oxidation on cellulose polymorphs. Cellulose 22, 2245–2261 (2015).
Fernández-Santos, J., Valls, C., Cusola, O. & Roncero, M. B. Periodate oxidation of nanofibrillated cellulose films for active packaging applications. Int. J. Biol. Macromol. 267, 131553 (2024).
Sultana, N., Edlund, U., Guria, C. & Westman, G. Kinetics of Periodate-Mediated oxidation of cellulose. Polymers 16, 381 (2024).
Plappert, S. F. et al. Transparent, flexible, and strong 2,3-Dialdehyde cellulose films with high oxygen barrier properties. Biomacromolecules 19, 2969–2978 (2018).
Lindh, J., Carlsson, D. O., Strømme, M. & Mihranyan, A. Convenient One-Pot formation of 2,3-Dialdehyde cellulose beads via periodate oxidation of cellulose in water. Biomacromolecules 15, 1928–1932 (2014).
Kim, U. J., Kuga, S., Wada, M., Okano, T. & Kondo, T. Periodate oxidation of crystalline cellulose. Biomacromolecules 1, 488–492 (2000).
Simon, J. et al. Debugging periodate oxidation of cellulose: why following the common protocol of quenching excess periodate with glycol is a bad Idea. Carbohydr. Polym. 310, 120691 (2023).
Frankel, E. N. Methods to determine extent of oxidation. In Lipid Oxidation 99–127 (Elsevier, 2012). https://doi.org/10.1533/9780857097927.99.
Montesinos, V. N., Sleiman, M., Cohn, S., Litter, M. I. & Destaillats, H. Detection and quantification of reactive oxygen species (ROS) in indoor air. Talanta 138, 20–27 (2015).
Villeneuve, L., Alberti, L., Steghens, J. P., Lancelin, J. M. & Mestas, J. L. Assay of hydroxyl radicals generated by focused ultrasound. Ultrason. Sonochem. 16, 339–344 (2009).
Lim, S. Y., Shen, W. & Gao, Z. Carbon quantum Dots and their applications. Chem. Soc. Rev. 44, 362–381 (2015).
Li, H. et al. Recent advances in carbon dots for bioimaging applications. Nanoscale Horiz. 5, 218–234 (2020).
Acknowledgements
The authors acknowledge the technical and logistical support of the BCE Department and Office of Research at the American University of Sharjah, UAE. The authors gratefully acknowledge Dr. Fatin Samara, Professor at the College of Arts and Sciences, American University of Sharjah, for her valuable instrumental support in conducting the microscopic analysis.
Funding
We acknowledge the financial support of the American University of Sharjah through grant # FRG23-C-S64, FRG24-C-S08, and CAS undergraduate grant (Khodja_A_CAS-URG23). The work in this paper was supported, in part, by the Open Access Program from the American University of Sharjah (OPFY25-3152-OC2530). This research work was also supported in part by Abu Dhabi National Oil Company (ADNOC), Emirates NBD, Sharjah Electricity Water & Gas Authority (SEWA), Technology Innovation Institute (TII), and GSK as sponsors of the 4th Forum for Women in Research (QUWA): Sustaining Women’s Empowerment in Research & Innovation at the University of Sharjah.
Author information
Authors and Affiliations
Contributions
S.R., R.R. and T.T.: data curation, formal analysis, investigation, methodology, writing—original draft; R.D. and A.K.: data curation, formal analysis, editing; M.H.A.-S.: conceptualization, project administration, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This work did not require ethical approval from a human subject or animal welfare committee.
Consent for publication
All authors accepted final approval for publication and agreed to publication. This paper represents the opinions of the authors and does not mean to represent the position or opinions of the American University of Sharjah.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ravindran, S., Radha, R., Terro, T. et al. Photoactivated carbon dots immobilized on cellulose for antibacterial activity and biofilm inhibition. Sci Rep 15, 27020 (2025). https://doi.org/10.1038/s41598-025-12317-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12317-8