Abstract
The elexacaftor/tezacaftor/ivacaftor (ETI) combination for cystic fibrosis transmembrane regulator modulators is a safe and effective treatment in both adults and children who are homozygous or compound heterozygous for the F508del variant. However, few cases involving a significant reduction in blood platelets and an increase in the alanine aminotransferase/platelet ratio have been described in adult and pediatric patients receiving ETI therapy. In the present study, we describe 272 people with cystic fibrosis (pwCF) (166 adult and 106 pediatric pwCF) who were independently followed at two centers; moreover, these individuals were homozygous or compound heterozygous for the F508del variant, were treated with ETI for at least one year, and exhibited monitored platelet and leukocyte counts (together with liver and inflammatory biochemical indices). As controls, 272 healthy subjects (HCs) matched for sex and age were evaluated. At baseline, both adult and pediatric pwCF demonstrated significantly (p < 0.01) greater blood platelet and leukocyte counts compared with HCs. One year of treatment significantly reduced blood platelet counts (adults: 248*103/mmc vs. 288*103/mmc, p < 0.01; children: 283*103/mmc vs. 320*103/mmc, p < 0.01) and leukocyte counts (adults: 6.5*103/mmc vs. 7.6*103/mmc, p < 0.01; children: 6.8*103/mmc vs. 7.9*103/mmc, p < 0.01). In addition, the serum C-reactive protein (CRP) level was significantly (p < 0.01) decreased after therapy, whereas the alanine aminotransferase (ALT) level and the ALT/platelet ratio were significantly increased (p < 0.01). After the second year of therapy, the laboratory parameters were not further altered in approximately half of the patients. The reduction in platelets was significantly correlated with a decrease in leukocytes (rs: 0.352, p < 0.001), serum CRP levels (rs: 0.392, p < 0.001) and exacerbations (oral antibiotic cycles, rs: 0.241, p = 0.002; intravenous antibiotic cycles, rs: 0.153, p = 0.049). These findings suggest that the normalization of platelets may be dependent on the reduction in systemic inflammation induced by ETI therapy.
Similar content being viewed by others
Introduction
Outcomes for people with cystic fibrosis (pwCF) have greatly improved in the last decade due to the use of modulator drugs, which increase the amount and potentiate the activity of the cystic fibrosis transmembrane conductance regulator (CFTR)-mutated protein1,2,3. Various combinations of modulators are available; moreover, in vitro and ex vivo models, such as organoids4 or nasal epithelial cells5,6,7, can be used to predict the responsiveness of patients harboring different CFTR genotypes to modulators. Thus, the number of pwCF that have access to novel therapies has increased, including those with at least one allele harboring the most frequent F508del variant8.
The combination of elexacaftor/tezacaftor/ivacaftor (ETI) enhances the amount and activity of the CFTR protein7,9. This specific treatment reduces sweat chloride (SC) levels and improves both lung function and imaging parameters10,11, as well as body mass index (BMI) and glucose metabolism12, in both adult13,14 and pediatric15,16 pwCF who are homozygous14 or heterozygous17,18 for F508del in trans with other variants.
Few severe side effects (such as liver injury) have been reported in adults and children treated with ETI19. However, mild effects such as hyperbilirubinemia20 and hypertransaminasemia21 are more commonly observed, particularly in adults22; additionally, these effects may be dependent on the accumulation of cholesterol in the liver, which triggers inflammation23. Although no trials focusing on ETI in adult13 or pediatric pwCF15,16,24,25,26 have reported a reduction in blood platelet count as a side effect, a reduction in platelet count was observed in 52 pwCF treated with ETI27, and a significant increase in the alanine aminotransferase (ALT)/platelet ratio after ETI therapy has also been described28. Furthermore, few cases of severe reductions in blood platelets in pediatric pwCF during ETI therapy have been reported29. Moreover, the critical role of platelets in inflammation and the immune response, especially regarding their abilities to form aggregates with leukocytes30 and secrete proinflammatory mediators31, has been highlighted. Additionally, the ALT/platelet ratio has been reported to be a noninvasive marker of liver disease32,33.
Therefore, the present dual-center study aimed to evaluate the effects of ETI therapy on platelets and leukocytes related to pulmonary exacerbations, as well as liver and inflammatory biochemical indices. Herein, we describe 272 pwCF (166 adult and 106 pediatric pwCF) who were homozygous or compound heterozygous for the F508del variant and who were treated with ETI.
Materials and methods
Patients
We followed the same criteria and methodology of our previous studies focusing on pwCF treated with ETI21,22. This prospective study (2023–2025) was approved by the Ethics Committees of the CF Regional Centre of Tuscany (Ethics Clearance number: 63/2023) and Campania (Ethics Committee number: 77/2021). The inclusion criteria included pwCF with the F508del mutation (either homozygous or in trans with another CFTR variant) and with at least one year of treatment with ETI. The exclusion criteria included mechanical ventilation, CF liver disease (CFLD)34 and previous liver or lung transplantations. The patients were followed at the regional Centre of Campania and the regional Centre of Tuscany according to the CF standard of care35. The CFTR genotype was analyzed via CFTR gene sequencing36. Liver disease34 was evaluated via clinical, biochemical or ultrasonographic assessments conducted in two consecutive examinations within a 3-month period in the absence of other causes of congenital or acquired chronic liver disease. CFLD refers to experiencing one (or more) of the following conditions: nodular liver, advanced fibrosis (F4), multilobular cirrhosis with or without portal hypertension, or noncirrhotic portal hypertension34. Pulmonary exacerbations were reported as oral antibiotic cycles/year (OAC) and intravenous antibiotic cycles/year (IAC).
Biochemical parameters
Alanine aminotransferase (ALT) and C-reactive protein (CRP) levels were evaluated in the serum, whereas blood platelets and leukocytes were evaluated in whole blood by using EDTA; these parameters were measured within one hour of blood sampling via automated analyzers by using standard procedures. For all of the subjects, the samples were collected before the initiation of ETI therapy and after one or two years of ETI therapy, with a maximum difference of one month from the exact expected date being observed. No blood samples were collected during the occurrence of pulmonary exacerbations. Furthermore, the samples were analyzed in the same laboratory.
Statistical analysis
Continuous data are reported as medians and IQRs. The Shapiro–Wilk test was utilized to evaluate the normality of the distributions. Comparisons between two groups of independent samples were evaluated via the Mann–Whitney U test. Paired comparisons were performed via the Wilcoxon test (between two groups) and the Friedman test (between three groups). Categorical data are reported as frequencies (percentages) and were compared via the chi-square test. The correlations between variables were evaluated via Spearman correlation analysis. Statistical analyses were performed via SPSS (version 29, IBM SPSS Statistics). P values < 0.01 were considered to be statistically significant.
Results
Study population
We investigated 272 pwCF, 132 of whom were followed at the regional Centre of Campania, with 140 individuals being followed at the regional Centre of Tuscany (Table 1). Among the 272 pwCF, 166 adult patients (79 females [47.6%]; median age: 33 years, interquartile range [IQR]: 27–42) and 106 pediatric patients (58 females [54.7%]; median age: 14 years, IQR: 11–16) were identified. The 272 pwCF were treated with ETI for either one year (n = 175) or two years (n = 97). As a control group, we evaluated 272 healthy subjects (HCs) who were matched for sex and age with the 272 subjects with CF (Table 1).
Laboratory parameters in pwCF and HCs at baseline and after treatment with ETI
In adult pwCF, both the platelet and the leukocyte numbers at baseline were greater than those in HCs, although they were within normal limits (Table 2 A). After one year of treatment with ETI, the platelet and leukocyte counts of the pwCF were significantly lower (although they were still observed within normal limits) than those at baseline and in the HCs (Table 2 A). After one year of ETI therapy, most of the pwCF (including approximately 80% of the 166 adult pwCF and 75% of the 106 pediatric pwCF) demonstrated a blood platelet count reduction that was within 15% of the baseline values. However, the reduction in blood platelet count was observed to be so severe in one adult patient with CF that it caused an interruption in therapy (i.e., the blood platelet count decreased from 304*103/mmc before therapy to 22*103/mmc after one year of ETI). No significant differences in the serum ALT level or ALT/platelet ratio were observed between pwCF and HCs at baseline (Table 2 A). After one year of treatment with ETI, both of the aforementioned parameters were significantly greater in pwCF compared to both HCs and the parameter values of the pwCF at baseline (Table 2A).
In pediatric pwCF, the platelet number at baseline was significantly greater than that in HCs, although it was observed to be within normal limits (Table 2B). After one year of treatment with ETI, the platelet number significantly decreased compared with that at baseline and in the HCs (although this change was not statistically significant, Table 2B). At baseline, the leukocyte number was significantly greater (p < 0.01) than that in the HCs, although it was observed to be within normal limits (Table 2B). After one year of treatment with ETI, the leukocyte number was significantly lower than both the HC and pediatric pwCF values at baseline (Table 2B). Moreover, the serum ALT level was not significantly different between pediatric pwCF at baseline and HCs; additionally, it was not significantly altered by one year of treatment with ETI (Table 2B). Only one child discontinued therapy with ETI because of a severe increase in the ALT level (> 5 ×). The ALT/platelet ratio was not significantly different between pediatric pwCF at baseline and HCs, whereas it was significantly greater after one year of treatment than both HCs and pediatric pwCF at baseline (Table 2B). Finally, the serum CRP, OAC and IAC values (data not collected from the HCs) were significantly lower after one year of treatment compared to baseline values in both adult (Table 2A) and pediatric (Table 2B) pwCF.
Twenty-four adult and 73 pediatric pwCF completed two years of treatment with ETI. In adult pwCF, the platelet and leukocyte counts, as well as the serum CRP values and ALT/platelet ratios, after two years of treatment were not significantly different compared to the data obtained after one year of treatment, whereas the serum ALT values were significantly lower (although they were observed to be within normal limits) than those after one year of treatment (Supplementary Table 1). In pediatric pwCF, all of the data (including platelet numbers, leukocyte numbers, the serum ALT level, the ALT/platelet ratio and the CRP level after two years of treatment) were not significantly different compared to those obtained after one year of treatment with ETI (Supplementary Table 1).
Thus, we calculated the differences between the values obtained after one year of ETI therapy and the values at baseline for blood platelet, leukocyte, serum CRP, OAC and IAC values for all of the adult and pediatric pwCF (including delta-platelets, leukocytes, CRP, OAC and IAC). We correlated the values of delta-platelets with those of delta-leukocytes, delta-CRP, delta-OAC and delta-IAC in both adult (Table 3A) and pediatric (Table 3B) pwCF. Delta-platelets were significantly and positively correlated with delta-leukocytes, delta-CRP values, delta-OAC and delta-IAC in both adult and pediatric pwCF (except for delta-OAC in pediatric pwCF) (Table 3A and 3B).
The impact of the CFTR genotype and CFHBI on laboratory parameters
Our group of pwCF included both patients who were homozygous for the F508del variant and patients who were compound heterozygous for F508del in trans with another CFTR variant. Thus, we evaluated laboratory parameters at baseline and after one year of treatment with ETI in the two subgroups (Table 4). In adult pwCF (both at baseline and after one year of treatment), we observed significantly higher values of serum ALT and the ALT/platelet ratio (although they were observed within normal limits) and lower values of OAC in patients who were homozygous for the F508del variant compared to compound heterozygous pwCF (Table 4A). The other parameters (including platelet and leukocyte counts, as well as the serum CRP level) were not significantly different between the two subgroups at baseline or after one year of treatment with ETI (Table 4A). In pediatric pwCF (both at baseline and after one year of therapy with ETI), no parameters were significantly different between pwCF who were homozygous for F508del and pwCF who were compound heterozygous for F508del in trans with another CFTR variant (except for OAC, which was lower in patients who were homozygous for the F508del variant) (Table 4B).
Moreover, our group of pwCF included both patients with CFHBI at baseline and patients without this complication. Thus, we evaluated laboratory parameters at baseline and after one year of treatment with ETI in the two subgroups (Table 5). In both adult (Table 5A) and pediatric (Table 5B) pwCF, laboratory parameters were not significantly different at baseline or after one year of treatment with ETI (except for IAC, which was greater in adult pwCF with CFHBI) (Table 5A).
Discussion
One year of treatment with ETI in 166 adult and 106 pediatric pwCF who were either homozygous for F508del or heterozygous for F508del in trans with another CFTR variant resulted in a significant reduction in blood platelet counts, although these values were observed to be within normal limits. This reduction was significantly correlated with decreases in blood leukocyte counts, serum CRP levels and pulmonary exacerbations. Moreover, ETI treatment significantly increased the serum ALT level and ALT/platelet ratio.
None of the previous large trials that evaluated the effects of ETI have reported changes in platelet number, including the first phase III trial13 and a long-term registry-based study conducted in adults37, as well as a phase III study conducted in children aged 6–11 years15, a larger study performed in children of the same age24 and a phase III trial conducted in children aged 2–5 years25. This lack of change in platelet number could be due to the fact that the platelet reduction that we observed in most of the patients (including approximately 80% of adult and 75% of pediatric pwCF) was within 15% of the baseline values. However, only one individual with CF demonstrated a reduction in platelet number that was sufficiently relevant to interrupt the treatment, and this result aligned with a previous study reporting of only one pwCF exhibiting a severe reduction in blood platelet count after ETI therapy29. Our data also matched the significant reduction in blood platelets reported in 52 pediatric pwCF treated with ETI27. Another study reported a significant reduction in the ALT/platelet ratio in 74 pwCF after one year of therapy with ETI28; however, in this study, the values of ALT and platelets were missing. We confirmed these data in our larger population of adult and pediatric pwCF, demonstrating that the increase in the ALT/platelet ratio was likely due to both the significant increase in ALT, which we previously described in adult pwCF after one year of therapy21,22, as well as due to a significant reduction in platelet number. The ALT/platelet ratio is a noninvasive biomarker of liver fibrosis, and it has been used to assess the severity of liver disease38 and liver fibrosis39 in pwCF by using carefully defined cutoff values34.
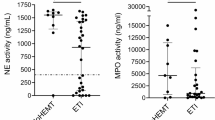
However, we excluded the possibility that the significant increase observed in the ALT/platelet ratio was dependent on liver fibrosis due to ETI therapy for various reasons. First, both adult and pediatric pwCF included in our study exhibited significantly greater numbers of platelets compared to HCs before treatment, whereas after one year of treatment, the number of platelets in pwCF was not significantly different from that of HCs. Furthermore, after the second year of treatment with ETI, the ALT/platelet ratio was not further altered compared with that in the first year of treatment, thus leading to the progression of liver fibrosis being an unlikely scenario, which aligned with the results of a previous study demonstrating a reduction in liver stiffness in pwCF treated with ETI40. Moreover, the reduction in blood platelets observed after one year of ETI therapy in our included pwCF was significantly correlated with a decrease in blood leukocytes and serum CRP levels (although these parameters were observed within normal limits), thereby suggesting that the reduction in blood platelets is related to reduced systemic inflammation in response to ETI. Moreover, treatment with ETI significantly reduces leukocyte and serum Ig levels41; moreover, it resulted in a reduction in inflammatory cytokines in the sputum of 76 adolescent pwCF after one month of ETI treatment42. Furthermore, ETI can reduce ATP/P2X7R-induced inflammasome activation in circulating monocytes43, along with significantly downregulating the expression of proinflammatory genes in airway epithelial cells44 and reducing platelet activation45. In addition, after one year of ETI therapy, we detected a significant reduction in pulmonary exacerbation, which aligns with the literature17 and is correlated with a decrease in the platelet count. It has been reported that an increase in the platelet count may be associated with an increase in inflammation markers during exacerbations in patients with chronic obstructive pulmonary disease46. Therefore, the decrease in platelet count observed in our cohort may be dependent on the reduction in systemic inflammation induced by ETI therapy. Finally, although pwCF with CFLD were excluded from treatment with ETI, 86 pwCF who were included in the present study (including 53 adult and 33 pediatric pwCF) had CFHBI, and no differences were observed for the platelet number or the ALT/platelet ratio between pwCF with and without CFHBI both before and after treatment with ETI.
One limitation of this study involves the reduction in platelet counts, leukocyte counts, ALT levels and CRP levels to within normal ranges, which represents an unclear result (if clinically meaningful). Furthermore, ALT fluctuations should be considered to occur over time (even during regular follow-ups) due to infections47, nutritional status48 and the use of other medications, such as antibiotics49.
In conclusion, therapy with ETI causes a significant reduction in blood platelet count both in adult and pediatric pwCF who are homozygous or compound heterozygous for the F508del variant. However, rather than a reduction being demonstrated, the platelet count seems to normalize. Moreover, our included pwCF demonstrated a significantly greater number of platelets before treatment and after the treatment, and the values returned to levels that were comparable with those of healthy subjects. This reduction is correlated with a significant decrease in leukocyte counts (which were observed to be higher before treatment), serum CRP levels and pulmonary exacerbations. Thus, when considering the idea that few cases of severe thrombocytopenia are induced by treatment, we suggest monitoring the platelet count during ETI therapy, thereby extending our investigation to populations of pediatric and adolescent pwCF who are receiving treatment for more than two years.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CF:
-
Cystic fibrosis
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- ETI:
-
Elexacaftor/tezacaftor/ivacaftor
- SC:
-
Sweat chloride
- BMI:
-
Body mass index
- CFLD:
-
CF liver disease
- IQR:
-
Interquartile range
- LI:
-
Lumacaftor/ivacaftor
- HC:
-
Healthy control subjects
- ALT:
-
Alanine aminostransferase
- CRP:
-
C-reactive protein
- URL:
-
Upper reference limit
- CFHBI:
-
CF hepatobiliary involvement
- OAC:
-
Oral antibiotic cycles
- IAC:
-
Intravenous antibiotic cycles
References
Grasemann, H. CFTR modulator therapy for cystic fibrosis. N. Engl. J. Med. 377, 2085–2088 (2017).
Terlizzi, V. & Lopes-Pacheco, M. Cystic fibrosis: New challenges and perspectives beyond elexacaftor/tezacaftor/ivacaftor. Ther. Adv. Respir. Dis. 19, 17534666251323194 (2025).
Terlizzi, V. & Farrell, P. M. Update on advances in cystic fibrosis towards a cure and implications for primary care clinicians. Curr. Probl. Pediatr. Adolesc. Health Care. 54, 101637 (2024).
Kleinfelder, K. et al. Theratyping of the rare CFTR genotype A559T in rectal organoids and nasal cells reveals a relevant response to Elexacaftor (VX-445) and Tezacaftor (VX-661) combination. Int. J. Mol. Sci. 24, 10358 (2023).
Amato, F. et al. Two CFTR variants within codon 970 differently impact on the chloride channel functionality. Hum. Mutat. 40, 742–748 (2019).
Terlizzi, V. et al. Ex vivo model predicted in vivo efficacy of CFTR modulator therapy in a child with rare genotype. Mol. Genet. Genomic Med. 9, e1656 (2021).
Dreano, E. et al. Theratyping cystic fibrosis patients to guide elexacaftor/tezacaftor/ivacaftor out-of-label prescription. Eur. Respir. J. 62, 2300110 (2023).
Despotes, K. A. & Donaldson, S. H. Current state of CFTR modulators for treatment of cystic fibrosis. Curr. Opin. Pharmacol. 65, 10239 (2022).
Bacalhau, M. et al. Elexacaftor-Tezacaftor-Ivacaftor: a life-changing triple combination of CFTR modulator drugs for Cystic Fibrosis. Pharmaceuticals (Basel) 16, 410 (2023).
Graeber, S. Y. et al. Effects of elexacaftor/tezacaftor/ivacaftor therapy on lung clearance index and magnetic resonance imaging in patients with cystic fibrosis and one or two F508del alleles. Am. J. Respir. Crit. Care Med. 206, 311–320 (2022).
McBennett, K. et al. Magnetic resonance imaging of cystic fibrosis: multi-organ imaging in the age of CFTR modulator therapies. J. Cyst. Fibros. 21, e148–e157 (2022).
Steinack, C. et al. Improved glucose tolerance after initiation of Elexacaftor / Tezacaftor / Ivacaftor in adults with cystic fibrosis. J. Cyst. Fibros. 22, 722–729 (2023).
Heijerman, H. G. M. et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del variant: a double-blind, randomised, phase 3 trial. Lancet 394, 1940–1948 (2019).
Carnovale, V. et al. Elexacaftor/Tezacaftor/Ivacaftor in patients with Cystic Fibrosis homozygous for the F508del variant and advanced lung disease: a 48-week observational study. J. Clin. Med. 11, 1021 (2022).
Zemanick, E. T. et al. A phase 3 open-label study of elexacaftor/tezacaftor/ivacaftor in children 6 through 11 years of age with cystic fibrosis and at least one F508del allele. Am. J. Respir. Crit. Care Med. 203, 1522–1532 (2021).
Daccò, V. et al. Effectiveness and safety of elexacaftor/tezacaftor/ivacaftor in children aged 6–11 years with cystic fibrosis in a real-world setting. Pediatr. Pulmunol. 59, 2792–2799 (2024).
Middleton, P. G. et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a single Phe508del allele. N. Engl. J. Med. 381, 1809–1819 (2019).
Carnovale, V. et al. Cystic Fibrosis patients with F508del/minimal function genotype: laboratory and nutritional evaluations after one year of Elexacaftor/Tezacaftor/Ivacaftor treatment. J. Clin. Med. 11, 6900 (2022).
Lowry, S., Mogayzel, P. J., Oshima, K. & Karnsakul, W. Drug-induced liver injury from elexacaftor/ivacaftor/tezacaftor. J. Cyst. Fibros. 21, e99–e101 (2022).
Terlizzi, V. et al. Hyperbilirubinemia and Gilbert’s syndrome in Cystic Fibrosis patients treated with elexacaftor/tezacaftor/ivacaftor. J. Cyst. Fibros. 22, 1130–1132 (2023).
Castaldo, A. et al. One year of treatment with elexacaftor/tezacaftor/ivacaftor in patients with cystic fibrosis homozygous for the F508del variant causes a significant increase in liver biochemical indexes. Front. Mol. Biosci. 10, 1327958 (2024).
Castaldo, A. et al. Liver biochemical indexes and cholesterol metabolism in cystic fibrosis patients with F508del/CFTR variant genotype after elexacaftor/tezacaftor/Ivacaftor treatment. Sci. Rep. 14, 17422 (2024).
Amato, F. et al. Impaired cholesterol metabolism in the mouse model of cystic fibrosis. A Preliminary study. PLoS One 16, e0245302 (2021).
Mall, M. A. et al. Efficacy and safety of elexacaftor/tezacaftor/ivacaftor in children 6 through 11 years of age with cystic fibrosis heterozygous for F508del and a minimal function mutation: a phase 3b, randomized, placebo-controlled study. Am. J. Respir. Crit. Care Med. 206, 1361–1369 (2022).
Goralski, J. L. et al. Phase 3 open label clinical trial of elexacaftor/tezacaftor/ivacaftor in children aged 2–5 years with cystic fibrosis and at least one F508del allele. Am. J. Respir. Crit. Care Med. 208, 59–67 (2023).
Terlizzi, V. et al. Reported adverse events in a multicenter cohort of patients ages 6–18 years with cystic fibrosis and at least one F508del allele receiving elexacaftor/tezacaftor/ivacaftor. J. Pediatr. 274, 114176 (2024).
Levitte, S., Fuchs, Y., Wise, R. & Sellers, Z. M. Effects of CFTR modulators on serum biomarkers of liver fibrosis in children with cystic fibrosis. Hepatol. Commun. 7, e0010 (2023).
Tewkesbury, D. H., Athwal, V., Bright-Thomas, R. J., Jones, A. M. & Barry, P. J. Longitudinal effects of elexacaftor/tezacaftor/ivacaftor on liver tests at a large single adult cystic fibrosis centre. J. Cyst. Fibros. 22, 256–262 (2023).
Manciulli, T. et al. Prevalence of adverse events in cystic fibrosis patients treated with elexacaftor/tezacaftor/ivacaftor: experience of the regional center in Tuscany. Italy. Ped. Pulmunol. 58, 3626–3629 (2023).
Thomas, M. R. & Storey, R. F. The role of platelets in inflammation. Thromb. Haemost. 114, 449–458 (2015).
Margraf, A. & Zarbock, A. Platelets in inflammation and resolution. J. Immunol. 203, 2357–2367 (2019).
Alempijevic, T. et al. Biochemical markers for non-invasive assessment of disease stage in patients with primary biliary cirrhosis. World J. Gastroenterol. 15, 591–594 (2009).
Avihingsanon, A. et al. Advanced liver fibrosis by transient elastography, fibrosis 4, and alanine aminotransferase/platelet ratio index among asian hepatitis C with and without human immunodeficiency virus infection: Role of vitamin D levels. J. Gastroenterol. Hepatol. 29, 1706–1714 (2014).
Sellers, Z. M. et al. Cystic fibrosis screening, evaluation and management of hepatobiliary disease consensus recommendations. Hepatology 79, 1220–1238 (2024).
Southern, K. W. et al. Standards for the care of people with CF. J. Cyst. Fibros. 22, 961–962 (2023).
Bergougnoux, A. et al. Multicenter validation study for the certification of a CFTR gene scanning method using next generation sequencing technology. Clin. Chem. Lab. Med. 56, 1046–1053 (2018).
Bower, J. K. et al. Real-world safety and effectiveness of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis: Interim results of a long-term registry-based study. J. Cyst. Fibros. 22, 730–737 (2023).
Karnsakul, W. et al. A longitudinal assessment of non-invasive biomarkers to diagnose and predict cystic fibrosis-associated liver disease. J. Cyst. Fibros. 19, 546–552 (2020).
Scott, J. A. et al. Improving detection of cystic fibrosis related liver disease using liver fibrosis assessment tools. Heliyon 9, e21861 (2023).
Terlizzi, V. et al. Effect of elexacaftor-tezacaftor-ivacaftor on liver transient elastography, fibrosis indices and blood tests in children with cystic fibrosis. J. Cyst. Fibros. https://doi.org/10.1016/j.jcf.2024.12.010 (2025).
Pepe, A. et al. Elexacaftor/Tezacaftor/Ivacaftor and inflammation in children and adolescents with cystic fibrosis: a retrospective dual center cohort study. Ther. Adv. Chron. Dis. https://doi.org/10.1177/17534666251314706 (2025).
Lepissier, A. et al. Moving the dial on airway inflammation in response to Trikafta in adolescents with cystic fibrosis. Am. J. Respir. Crit. Care Med. 207, 792–795 (2023).
Gabillard-Lefort, C. et al. Trikafta Rescues CFTR and lowers monocyte P2X7R-induced inflammasome activation in cystic fibrosis. Am. J. Respir. Crit. Care Med. 205, 783–794 (2022).
Hampton, T. H. et al. Gene expression responses of CF airway epithelial cells exposed to elexacaftor/tezacaftor/ivacaftor suggest benefits beyond improved CFTR channel function. Am. J. Physiol. Lung Cell. Mol. Physiol. 327, L905–L916 (2024).
Schmidt, H. et al. Multimodal analysis of granulocytes, monocytes, and platelets in patients with cystic fibrosis before and after elexacaftor-tezacaftor-ivacaftor treatment. Front. Immunol. 14, 1180282 (2023).
Muñoz-Esquerre, M. et al. Impact of acute exacerbations on platelet reactivity in chronic obstructive pulmonary disease patients. Int. J. Chron. Obstruct. Pulmon. Dis. 13, 141–148 (2017).
Aitokari, L., Hiltunen, P., Huhtala, H., Kurppa, K. & Kivelä, L. Measurement practices of alanine aminotransferase in children: Temporal changes and etiology for increased values. J. Pediatr. Gastroenterol. Nutr. 78, 1383–1388 (2024).
Maeda, D. et al. Relation of Aspartate Aminotransferase to Alanine Aminotransferase Ratio to Nutritional Status and Prognosis in Patients With Acute Heart Failure. Am. J. Cardiol. 139, 64–70 (2021).
Andrade, R. J. & Tulkens, P. M. Hepatic safety of antibiotics used in primary care. J. Antimicrob. Chemother. 66, 1431–1446 (2011).
Author information
Authors and Affiliations
Contributions
Design of the work: VC, VR and VT; Methodology, investigation and data analysis: AC, CC, CF, PI, MG, AS and AT; Manuscript writing and validation: AC, MG and AT. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical committee of the CF regional Centre of Tuscany (Ethics Clearance number 63/2023) and of Campania (Ethics Committee number 77/2021). Informed consent was obtained from all participants or the legal guardian/next relative of the participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Castaldo, A., Cimbalo, C., Fevola, C. et al. Blood platelet reduction after elexacaftor/tezacaftor/ivacaftor treatment in people with cystic fibrosis may depend on systemic inflammation reduction. Sci Rep 15, 27571 (2025). https://doi.org/10.1038/s41598-025-12333-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12333-8
Keywords
This article is cited by
-
Elexacaftor/Ivacaftor/tezacaftor
Reactions Weekly (2025)