Abstract
Microdefects, including microcracks and resorption trenches, may be important contributors to bone fragility. 3D microdefect morphology was imaged using synchrotron micro-CT to develop a classification system for investigating the relationship with bone mechanics and hip-fractures. Femoral heads from ageing hip-fracture patients (n = 5, 74–82 years) were compared to ageing non-fracture controls (n = 5, 72–84 years). Two trabecular cores were prepared from the chiasma; one was imaged using synchrotron micro-CT to measure microdefects and one was mechanically tested to measure tensile strength. Morphological and mechanical data were compared and correlated using Mann Whitney U test and Pearson’s rank correlation. All the procedures performed were in accordance with the ethical standards of the Imperial College Tissue Bank (R13004) and the 1984 Declaration of Helsinki. Microdefects varied and were classified into four categories based on shape and measurable parameters. Hip-fracture donors exhibited significantly higher density of all microdefects (p < 0.05). Microdefect volume was strongly negatively correlated with ultimate tensile strength (p < 0.05) and stiffness (p < 0.05). Microdefects might contribute to loss of bone strength and fragility fracture via runaway resorption. Microcracks could promote focussed osteoclastic resorption and the formation of resorption pits which create stress risers leading to the re-formation of microcracks under continued load. CT-based classification methods should be used to explore the complex interaction between microdefects, metabolism, and bone fracture mechanics.
Similar content being viewed by others
Introduction
The role of 3D trabecular microarchitecture in bone mechanics and fragility has been extensively analysed1,2,3,4,5,6 but 3D microdefects, which could undermine architecture, have been less well studied5,7. Microdefects are damage that has been formed at the microscopic level of bone6,8,9, in the bone matrix6, and has been determined to be a normal physiological response to skeletal loading10,11,12,13,14. Microdefects have traditionally been assessed using 2D histomorphometry methods6,14,15,16,17,18 which do not reveal the 3D shape of defects for identification7,19. Benchtop micro-computed tomography (micro-CT) systems have also been used but specimens require radio-opaque staining which may not show all of the microcracks, and resolution is limited by noise7,20. Recent developments in Synchrotron Micro-CT allow for rapid scanning (approximately five to twenty minutes) at micron-level resolution due to low noise7. As such, it may be possible to investigate 3D microdefect morphology for biomedical research and address key questions about the role of microdefects in bone mechanics, health, and disease.
Microdefect types
Histomorphometry studies have identified three types of microdefects which are likely interrelated and form together. Microcracks are defined as fractures in bone tissue at the scale of 50–100 µm6,21. Diffuse damage consists of networks of small sub-lamellar size cracks (approximately 1 µm)22. Osteoclastic resorption pits23,24,25 usually appear as concavities in the bone surface or cylindrical tunnels in the tissue and are approximately 8–16 µm25 long. Within the bone matrix itself, there are many physiological discontinuities, not limited to vascular canals, canaliculi and osteocyte lacunae. The latter have been the subject of interest, also under the scrutiny of Micro-CT imaging26 Microcracks are a known stimulus for osteoclastic remodelling10,11,12,15,16,23,27,28 and microcracks undergoing targeted resorption have been observed in histology sectsion29. Osteoclastic resorption may also create stress risers that act as a stimulus and nucleation point for more cracks24,30. Therefore, the formation of microcracks and resorption pits are related and should be studied together.
Microdefects and mechanical properties
All three types of microdefects could undermine the microstructure of a bone and reduce the mechanical properties increasing the risk of a fracture during a bump or fall. Therefore, it is important to determine whether microcracks and resorption pits occur together in bone tissue or in isolation. The effect of microdefects on mechanical properties and fracture mechanics is likely complex, especially given that the two are interrelated because microcracks are resorbed by osteoclasts. Ultimately all fractures of the cortical and trabecular bone must begin as microscopic or sub-microscopic linear microcracks31,32 which propagate into a fracture, usually under a traumatic load8,18. However, microcracks are also thought to be a key component in dissipating energy and maintaining the mechanical properties of bone2,3,6,8,33,34,35,36. Therefore, microcrack formation is also considered a normal physiological process which reduces fracture risk by absorbing energy from fatigue or physiological loading4,10,11,20,37. When microcracks are removed and replaced with new tissue by the remodelling process there should be no long-lasting impairment of the tissue.
Classifying and quantifying microdefects morphology
Studies investigating the relationship between bone fracture mechanics, fragility and crack morphology are required to determine whether microcracks, osteoclastic resorption pits, or both, are an important contributor to bone strength or fragility. The morphology of microdefects is complex and there is a scarcity of data on size, shape, and density. Two classifications of microdefects have been explored based on location within the bone9,18, and the number or density6,35,38,39 of microdefects. For example, Norman and Wang18 classified microdefects based on location relative to cement lines in both human femora and tibiae. This method of classification is useful with regards to the presence of linear microcracks in certain sections of the bone and the role of osteons but is not useful in exploring relationships between different types of microdefects and bone integrity. The criteria defined for linear microcracks and diffuse damage was limited to comparisons with other structures, for example linear microcracks were smaller than vascular channels but larger than the canaliculi9,38. Previous classification systems of microdefects relied on 2D images or computer simulations1,9,23,36, which have been unable to distinguish partially resorbed microdefects to the detail seen now. Previous simulations and models, e.g. the lamellar wood model, are oversimplified and therefore are not representative of how microdefects form4,40. Therefore, a system for classifying and quantifying microdefects is necessary to investigate the role of microcracks and resorption pits in bone health and disease.
Imaging microdefects
The use of synchrotron-CT is a relatively new modality which has already demonstrated its effectiveness in imaging microcracks5,7,33,41 but limited access to the technology has meant 2D histology images and 3D benchtop CT scans remain the most common methods of analysis. The advantage of synchrotron CT is that the combination of higher resolution and 3D imaging can be applied to image, classify, and measure microdefects and fully differentiate microcracks at different stages of remodelling and resorption pits. A few studies have already used the technique to image microdefects but there was no classification system for differentiating according to the shape and scale5,7,19,33,41.
Aims and objectives
The aim of this work was to investigate 3D microdefect morphology in human bone tissue from hip fracture patients versus ageing non-fracture controls: including microcracks and osteoclastic resorption pits. There were three objectives: (i) image microdefects using synchrotron X-ray micro-CT and investigate the range of variation of microdefect shape and scale, (ii) develop a classification system and (iii) test the application of this classification by comparing microdefects in trabecular bone from hip-fracture patients with non-fracture controls and correlating microdefects with mechanical properties such as strength and stiffness.
Materials and methods
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Health Research Authority (REC reference: 18/LO/0277 and IRAS project ID: 214772) and the institutional review board of the Imperial College Tissue Bank (R13004). Informed consent was obtained from all living donors.
Donors and sample preparation
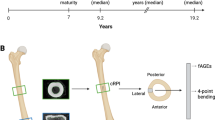
Trabecular bone cores were prepared from the femoral heads of two groups of donors: a hip-fracture group undergoing arthroplasty at Imperial College Healthcare NHS Trust and non-fracture cadaveric control group from the Vesalius Clinical Training Centre, UK19,41. The exclusion criteria included those with a history of primary or secondary bone disease or treatment with a bone metabolic medicine. In total, eight samples were taken from hip-fracture patients and five from non-fracture control donors. These samples were 10 mm in height and 7 mm in diameter and were taken from the primary compressive trabecular arcade of the femoral heads. The cores were stored at -80 C and remained in the storage facility unless directly being used for testing.
Synchrotron X-ray micro-CT
Imaging was carried out at Diamond Light Source, United Kingdom, with Synchrotron X-ray micro-CT using Beamline I1242 on only five cores from fracture patients and five cores from non-fracture controls, due to limited experiment time with the synchrotron. The setting parameters used were photon energy 53 keV, image volume 23.3 mm3, voxel resolution 1.38 μm3/voxel, 6400 projections, 180° rotation. Images were constructed in-house using GPU enabled Filtered Back Projection within the DAWN41 package, and using Titarenko’s ring suppression method43 and then linked to image analysis software ImageJ and VG Studio Max44 to allow for identification of microdamage and removal of ring artefacts.
Microdefect assessment
From a given bone sample, a central fiducial volume of dimensions 3.28 mm in diameter and 2.76 mm in height was taken, to reduce the likelihood of introducing artefacts from the cutting and drilling process. The volume of interest was segmented with an automated global threshold technique that was applied to identify the trabecular bone tissue (including the microdefects)5,45. Bone volume fraction (BVF) was calculated by dividing the total number of voxels that represented bone by the total number of voxels in the whole scan volume41,44. Subsequently, the microdefects within the bone were visually identified and segmented by masking the microcracks in VG Studio Max 2.2 (Volume Graphics)42 then inspected to qualify the morphology and measured quantitatively. There were, in total, 2000 continuous slices individually examined in three planes. Microdefect density per mm3 was calculated by normalising the number of defects to BVF. Microdefect volume was measured counting the segmented voxels. Microdefect length was established as the maximum axis of the bounding cuboid. Microdefect volume was calculated by converting the number of voxels into mm3 (1 voxel being the equivalent of 1.3 µm3).
Two step classification system for microdefects
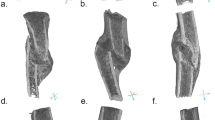
In the process of developing a classification system for microdefects based on shape and size, four defect types were consistently identifiable in synchrotron micro-CT images: resorption pits, microcracks, resorption trenches and partially resorbed microcracks (Fig. 1). Existing classification systems9 for microdefects do not account for partially resorbed microcracks visible in the synchrotron scans. Therefore, a two-step classification system was developed (Fig. 1) which incorporated the full range of variation in microdefects imaged. The first step involves categorising predominately by shape, which can be better appreciated in 3D, into three different groups. Parameters of length, width, depth, and volume were then used in the second step to differentiate within a class. These parameters are based on numerous analyses in the literature, with microcracks noted to be a length of 50–100 µm3,6,21 and resorption pits measured at 8–16 µm long23,30. This process is outlined in Fig. 1.
Histological validation
We acknowledge that classification was solely based on size and morphology derived from 3D synchrotron imaging. Histological co-registration was not performed and remains a limitation of this study.
Mechanical testing
Ten rectangular-shaped testing samples were harvested immediately adjacent to the region of the cylindrical cores7,41. The specimens were 11 mm in height, 2.8 mm in width, and 1 mm in depth. The ends of each sample were potted in bone cement, which served as clamps and stored at − 80 °C until testing. The length of the gripped part of the sample was 2 mm at each end. During tensile testing, the samples were kept hydrated in the fluid chamber built into the micromechanical device7,41. All specimens underwent displacement-controlled tensile tested at a fixed strain rate at 0.001 s−1 using a custom-built micromechanical tensile test rig, originally designed for previous projects7,41. The tests were conducted at room temperature. After loading, the stress–strain curves were examined to identify the Young’s modulus and ultimate tensile strength (UTS). Young’s modulus and ultimate tensile strength (UTS) were calculated from stress–strain curves and normalised by BV/TV. In this work, the same specimens and protocols as in previously cited work by Ma et al.41 were utilised and therefore, the mechanical properties and the outcomes from mechanical testing are shared between the works. The authors acknowledge that fabric tensor analysis was not included in this study due to scope and dataset limitations. The decision was made to load the samples under tension, rather than compression, to encourage the microdefects to open, rather than close under compression41. We acknowledge here that whilst microdamage from sample preparation is avoided in the synchrotron measurements as the outer sample was excluded from the data analysed, this was not the case with the mechanical testing samples.
Statistics
Statistical analyses were performed using IBM SPSS Statistics 23 (Armonk, New York) and the graphs were generated with GraphPad Prism 8 (San Diego, California). Donor groups were compared using parametric descriptive statistics with 2Way Anova. Microstructure and tissue mechanics were correlated using Pearson’s correlation co-efficient and coefficients of determination.
Results
3D images of microdefects were successfully visualised and reconstructed using synchrotron micro-CT scans revealing wide variation in morphology (Fig. 1). The morphology of the microdefects was consistent with linear microcracks, partially resorbed microcracks and osteoclastic resorption pits but not diffuse damage. The range of calculated dimensions of each microdefect sub-classification are documented in Table 1.
The mean age of the non-fracture controls and hip fracture-donors was similar (p > 0.05, Table 2). Compared to the controls, the hip-fracture donor group exhibited a significantly greater density of microcracks, partially resorbed microcracks and resorption trenches (p < 0.05, Table 2).
The density of resorption pits was similar in the non-fracture controls and hip fracture donors (p > 0.05, Table 2).
Bone strength and stiffness were negatively correlated with the average volume of microdefect in the bone sample. Normalised UTS was strongly and negatively correlated with the average volume of microdefects (− r2 = 0.82, p < 0.05, Fig. 2), which is defined as the total volume of microdefects in a bone core divided by the frequency of microdefects in a bone core sample. Normalised Young’s modulus was negatively correlated with the average volume of microdefects (− r2 = 0.53, p > 0.05, Fig. 2) but was not significant.
Bone strength and stiffness are negatively correlated with the mean volume of defect in the bone sample. (A) UTS is significantly strongly and negatively correlated with the average volume of microscopic bone defects (r2 = 0.82, p < 0.05). (B) Young’s Modulus is negatively correlated with the average volume of microscopic bone defects (r2 = 0.53, p > 0.05). Spearman’s r-correlation with Welch’s t-test.
Negative regressions were calculated for each category of microdefect across both ageing fracture and non-fracture groups compared to average UTS. There was a significant negative regression between average UTS and microcracks (p < 0.05, r2 = 0.2915, Fig. 3). The average UTS compared to partially resorbed microcracks (p > 0.05, r2 = 0.1496), resorption pits (p > 0.05, r2 = 0.00085), and resorption trenches (p > 0.05, r2 = 0.1363) were found to not have a significant negative regression.
Discussion
The aim was to investigate 3D microdefect morphology in human bone tissue by comparing data between hip fracture donors versus ageing non-fracture controls. Microcracks and resorption trenches are similar in size but have divergent shapes: microcracks are fissures whilst resorption trenches are cylindrical (Fig. 1). Resorption trenches are approximately ten times longer than resorption pits, although both are cylindrical in shape (Fig. 1).
Hip-fracture donors exhibited a significantly higher density of microcracks, partially resorbed microcracks and resorption trenches compared to ageing controls (Table 2). Further, hip-fracture patients exhibited significantly lower tensile strength and showed lower stiffness (Fig. 2). Inferior mechanics of hip-fracture patients could be attributed to the increased density and volume of defects, which reduce the connectivity of the elements and the cross-sectional area for resisting loads, ultimately leading to failure of the trabecular architecture. The presence of a high proportion of partially resorbed microcracks in hip-fracture patients relative to controls suggests osteoclastic resorption of microcracks could be a cellular mechanism contributing to reduced strength, therefore contributing to age-related fragility fractures at the hip.
Age-related fractures are associated with accumulated microdefects
Synchrotron microdefect data support the theory that microdefects contribute to a reduction in trabecular bone strength and increase the risk of a hip-fracture after a bump or fall7,19,41. Microcrack density was five-fold greater in the fracture group and resorption trench density was 12-fold in the fracture group compared to non-fracture controls. There was no significant difference in the density of resorption pits between either group. There was a significant negative correlation in bone strength compared to average microdefect volume in bone tissue (− r2 = 0.82, p < 0.05, Fig. 2). There was also a negative correlation in bone stiffness compared to average microdefect volume in bone tissue (− r2 = 0.53, p > 0.05, Fig. 2), however this was not significant. This suggests stiffness is a mechanical property representative of the structure as a whole, hence why stiffness is typically affected by total mineral density and metabolic disease states14,46,47 and is only moderately reduced48 in this analysis. Comparatively, ultimate tensile strength is representative of the structure at a specific tested point, therefore an accumulation of microdefects could exceed the intrinsic repair capability of the bone, resulting in further damage and potentially failure of the bone, therefore strongly reduce bone material strength.
These conclusions support and are supported by the literature. Prendergast and Taylor suggest there is the presence of homeostatic microdefects and remodelling only occurs if a critical value is reached27. In a review paper, Seref-Ferlengez discussed how when bone tissue structure becomes compromised, microdefect accumulation is a significant contributor to future fracture risk4,40. Zioupos and colleagues determined that changes in mechanical properties were caused by microdefect occurrence, rather than microdefects being an artefactual consequence3. Yeh and Keaveny explored loading trabecular bone and found that extensive microdefects were primarily responsible for the loss in strength and stiffness49.
Interaction between resorption pits and microcracks causes runaway resorption
The synchrotron micro-CT images illustrate how microdefects exist on a spectrum and likely reflect the interaction in formation. For example, when a microcrack undergoes targeted remodelling by osteoclasts, this was frequently visible as microcracks undergoing active resorption by osteoclasts (Fig. 1). Burr and Allen initially proposed a theory that an age-associated failed feedback loop involving remodelling in response to cracks may precipitate microdefect accumulation (both microcracks and resorption pits), which reduce the strength and stiffness (see Fig. 2) and so promote bone fractures6,10,12,13.
When a microcrack forms as consequence of applied stress, generating tissue strain4, a targeted process of osteoclastic resorption would remove the crack. However, in the absence of osteoblastic bone formation, the cavity cannot be filled. Any unfilled resorption pits increase the area of stress concentration, leading to further microcracks, resorption and extension of the pits into a resorption trench. At a certain depth50,51, runaway resorption1,23,24,30,50 fundamentally undermines the architecture of the tissue1,50,51, compromising strength. This relationship has been addressed using computer simulations24,50, where in-silico models suggest that resorption trenches are formed from resorption of pits due to continuous remodelling of the same area of tissue, rather than a single resorption event24. The authors noted that pits greater than 32 μm in depth were associated with resorption trench formation because osteoblasts were not able to fill such a large space. Experimental and modelling studies are required in combination to create a platform for investigating the aetiology of fragility fractures with a classification system. Slyfield et al. demonstrated significant associations between the presence of microdefects and resorption pits, which is supported by the regression data in Fig. 3. As the only significant contributor to a significant reduction in ultimate tensile strength was the microcrack subcategory, this suggests that microcracks are the more pathological type of microdefect4,16. On this point, the study does recognise, however, the influence of potentially introduced damage due to the nature of the study design.
This study acknowledges several limitations that warrant consideration. First, while synchrotron micro-CT provides high resolution three-dimensional visualisation of microdefects, the classification lacks histological validation. Future work should incorporate co-registered histological sections to more definitively distinguish between microcracks and resorption trenches. Additionally, the presence of resorption trenches may not exclusively reflect stress-concentrated remodelling but also result from the resorption of large microcracks. Given the variability observed in the dimensions of microcracks and resorption trenches (Table 1), it is difficult to differentiate between the two aetiologies. Second, the current study did not include fabric tensor analysis. Although bone volume fraction (BV/TV) was used for normalisation, this does not account for anisotropy or trabecular orientation. Future studies should incorporate fabric-based parameters to enhance the predictive power of microstructural analysis. Third, the measurements on the microdefects were not site-matched with samples that underwent tensile measurements. Future work is planned to develop a loading rig which will work inside the synchrotron system to enable in-situ mechanical testing, which will allow for direct correlation between defect morphology and mechanical performance. Given the known impact of radiation levels on tissue mechanics, this future work will also endeavour to collect the mechanical data and imaging data separately to minimise artefactual influence. Expanding this work further to include a greater and more diverse cohort will allow for more refinement of the classification system. Despite these limitations, the findings underscore the importance of microdefect morphology and density as a major component of bone fragility, supporting the importance of risk factors outside of bone mineral density in predicting bone strength and fracture risk. The increased density of microcracks and the phenomena of runaway resorption of osteoclastic resorption pits could increase the potential risk for an age-related fragility fracture during a trip or fall.
Conclusion
This paper explored the morphology of microcracks and osteoclastic resorption pits in human bone tissue. Synchrotron micro-CT imaging successfully captured microdefects and distinguished microcracks, resorption pits and partially resorbed microcracks using a two-step classification system. The classification system presented in this paper has the potential to create a platform to study the role of microscopic defects in whole bone mechanics. Bone tissue samples from fracture patients exhibited significant greater densities of microcracks and resorption pits in hip-fracture patients than in controls. There is a likely interaction between microcracks and osteoclastic resorption pits, which may be integral to the development of fragility fractures. Microcracks likely act as a stimulus for resorption which can predispose to the generation of either physiological osteoclastic resorption pits and successful bone formation or runaway resorption leading to ultimate failure of bone. Future studies of the relationship between hierarchal bone structure and fracture mechanics need to take microdefects into account.
Data availability
Raw data can be made available by RA on request.
References
Van Oers, R. F. M., Van Rietbergen, B., Ito, K., Huiskes, R. & Hilbers, P. A. J. Simulations of trabecular remodeling and fatigue: Is remodeling helpful or harmful?. Bone https://doi.org/10.1016/j.bone.2011.01.011 (2011).
Zioupos, P. & Currey, J. D. The extent of microcracking and the morphology of microcracks in damaged bone. J. Mater. Sci. https://doi.org/10.1007/BF00351420 (1994).
Zioupos, P., Hansen, U. & Currey, J. D. Microcracking damage and the fracture process in relation to strain rate in human cortical bone tensile failure. J. Biomech. https://doi.org/10.1016/j.jbiomech.2008.07.025 (2008).
Seref-Ferlengez, Z., Kennedy, O. D. & Schaffler, M. B. Bone microdamage, remodeling and bone fragility: How much damage is too much damage?. Bonekey Rep. https://doi.org/10.1038/bonekey.2015.11 (2015).
Larrue, A. et al. Synchrotron radiation micro-CT at the micrometer scale for the analysis of the three-dimensional morphology of microcracks in human trabecular bone. PLoS ONE https://doi.org/10.1371/journal.pone.0021297 (2011).
Burr, D. B. et al. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J. Bone Miner. Res. https://doi.org/10.1359/jbmr.1997.12.1.6 (1997).
Ma, S. et al. Synchrotron imaging assessment of bone quality. Clin. Rev. Bone Min. Metab. https://doi.org/10.1007/s12018-016-9223-3 (2016).
Burr, D. B. Why bones bend but don’t break. J. Musculoskelet. Neuronal Interact. 11, 270–285 (2011).
Dominguez, V. M. & Agnew, A. M. Microdamage as a bone quality component: Practical guidelines for the two-dimensional analysis of linear microcracks in human cortical bone. JBMR Plus https://doi.org/10.1002/jbm4.10203 (2019).
Allen, M. R. & Burr, D. B. Bone growth, modeling, and remodeling. Basic Appl. Bone Biol. https://doi.org/10.1016/b978-0-12-813259-3.00005-1 (2019).
Lee, T. C., Staines, A. & Taylor, D. Bone adaptation to load: Microdamage as a stimulus for bone remodelling. J. Anat. https://doi.org/10.1046/j.1469-7580.2002.00123.x (2002).
Allen, M. R. & Burr, D. B. Skeletal microdamage: Less about biomechanics and more about remodeling. Clin. Rev. Bone Miner. Metab. https://doi.org/10.1007/s12018-008-9015-5 (2008).
Allen, M. R., Burr, D. B. & Turner, C. H. The effect of age on material properties. In Osteoporosis in Men (2010). https://doi.org/10.1016/B978-0-12-374602-3.00018-3
Pagano, S. L. UKnowledge the effect of varying bisphosphonate treatment on changes in bone microdamage in osteoporotic women (2016).
O’Brien, F. J., Brennan, O., Kennedy, O. D. & Lee, T. C. Microcracks in cortical bone: how do they affect bone biology?. Curr. Osteoporos. Rep. https://doi.org/10.1007/s11914-005-0002-1 (2005).
Brandi, M. L. Microarchitecture, the key to bone quality. Rheumatol. (United Kingdom) https://doi.org/10.1093/rheumatology/kep273 (2009).
Donahue, S. W. & Galley, S. A. Microdamage in bone: Implications for fracture, repair, remodeling, and adaptation. Crit. Rev. Biomed. Eng. https://doi.org/10.1615/CritRevBiomedEng.v34.i3.20 (2006).
Wang, Z. & Norman, T. L. Microdamage of human cortical bone: Incidence and morphology in long bones. Bone https://doi.org/10.1016/S8756-3282(97)00004-5 (1997).
Jin, A. et al. The effect of long-term bisphosphonate therapy on trabecular bone strength and microcrack density. Bone Jt. Res. https://doi.org/10.1302/2046-3758.610.BJR-2016-0321.R1 (2017).
Wang, X. & Niebur, G. L. Microdamage propagation in trabecular bone due to changes in loading mode. J. Biomech. https://doi.org/10.1016/j.jbiomech.2005.02.007 (2006).
Burr, D. B. The complex relationship between bone remodeling and the physical and material properties of bone. Osteoporos. Int. https://doi.org/10.1007/s00198-014-2970-4 (2015).
Alvandi, L. M. Diffuse damage repair mechanism in bone (2019).
Mcnamara, L. M. & Prendergast, P. J. Perforation of cancellous bone trabeculae by damage-stimulated remodelling at resorption pits: A computational analysis. Eur. J. Morphol. https://doi.org/10.1080/09243860500096289 (2005).
Mulvihill, B. M., McNamara, L. M. & Prendergast, P. J. Loss of trabeculae by mechano-biological means may explain rapid bone loss in osteoporosis. J. R. Soc. Interface https://doi.org/10.1098/rsif.2007.1341 (2008).
Merrild, D. M. H. et al. Pit- and trench-forming osteoclasts: A distinction that matters. Bone Res. https://doi.org/10.1038/boneres.2015.32 (2015).
Dong, P. et al. 3D osteocyte lacunar morphometric properties and distributions in human femoral cortical bone using synchrotron radiation micro-CT images. Bone 60, 172–185 (2014).
Prendergast, P. J. & Taylor, D. Prediction of bone adaptation using damage accumulation. J. Biomech. https://doi.org/10.1016/0021-9290(94)90223-2 (1994).
Herman, B. C., Cardoso, L., Majeska, R. J., Jepsen, K. J. & Schaffler, M. B. Activation of bone remodeling after fatigue: Differential response to linear microcracks and diffuse damage. Bone https://doi.org/10.1016/j.bone.2010.07.006 (2010).
Warden, S. J., Davis, I. S. & Fredericson, M. Management and prevention of bone stress injuries in long-distance runners. J. Orthop. Sports Phys. Ther. https://doi.org/10.2519/jospt.2014.5334 (2014).
McNamara, L. M., Van Der Linden, J. C., Weinans, H. & Prendergast, P. J. Stress-concentrating effect of resorption lacunae in trabecular bone. J. Biomech. https://doi.org/10.1016/j.jbiomech.2004.12.027 (2006).
Reeve, J. Role of cortical bone in hip fracture. Bonekey Rep. https://doi.org/10.1038/bonekey.2016.82 (2017).
O’Brien, F. J. et al. The behaviour of microcracks in compact bone. Eur. J. Morphol. https://doi.org/10.1080/09243860500096131 (2005).
Zimmermann, E. A. et al. Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc. Natl. Acad. Sci. U. S. A. https://doi.org/10.1073/pnas.1107966108 (2011).
Ritchie, R. O. How does human bone resist fracture?. Ann. N. Y. Acad. Sci. https://doi.org/10.1111/j.1749-6632.2009.05232.x (2010).
Burr, D. B. Repair mechanisms for microdamage in bone. J. Bone Miner. Res. https://doi.org/10.1002/jbmr.2441 (2015).
Mohsin, S., O’brien, F. J. & Lee, T. C. Microcracks in compact bone: A three-dimensional view. J. Anat. https://doi.org/10.1111/j.1469-7580.2006.00554.x (2006).
Lee, T. C. et al. Detecting microdamage in bone. J. Anat. https://doi.org/10.1046/j.1469-7580.2003.00211.x (2003).
Burr, D. B. & Stafford, T. Validity of the bulk-staining technique to separate artifactual from in vivo bone microdamage. Clin. Orthop. Relat. Res. https://doi.org/10.1097/00003086-199011000-00047 (1990).
Diab, T. & Vashishth, D. Effects of damage morphology on cortical bone fragility. Bone https://doi.org/10.1016/j.bone.2005.03.014 (2005).
Seref-Ferlengez, Z., Basta-Pljakic, J., Kennedy, O. D., Philemon, C. J. & Schaffler, M. B. Structural and mechanical repair of diffuse damage in cortical bone in vivo. J. Bone Miner. Res. https://doi.org/10.1002/jbmr.2309 (2014).
Ma, S. et al. Long-term effects of bisphosphonate therapy: Perforations, microcracks and mechanical properties. Sci. Rep. https://doi.org/10.1038/srep43399 (2017).
Drakopoulos, M. et al. I12: The joint engineering, environment and processing (JEEP) beamline at Diamond Light Source. J. Synchrotron Radiat. https://doi.org/10.1107/S1600577515003513 (2015).
Titarenko, S., Withers, P. J. & Yagola, A. An analytical formula for ring artefact suppression in X-ray tomography. Appl. Math. Lett. 23, 1489–1495 (2010).
Abel, R. L., Laurini, C. R. & Richter, M. A palaeobiologist’s guide to ‘virtual’ micro-CT preparation. Palaeontol. Electron https://palaeo-electronica.org/content/issue-2-2012-technical-articles/223-micro-CT-workflow (2012).
Yan, L. et al. A method for fracture toughness measurement in trabecular bone using computed tomography, image correlation and finite element methods. J. Mech. Behav. Biomed. Mater. 109, 1–7 (2020).
Feng, X. & McDonald, J. M. Disorders of bone remodeling. Annu. Rev. Pathol. Mech. Dis. https://doi.org/10.1146/annurev-pathol-011110-130203 (2011).
Van Der Linden, J. C., Homminga, J., Verhaar, J. A. N. & Weinans, H. Mechanical consequences of bone loss in cancellous bone. J. Bone Miner. Res. https://doi.org/10.1359/jbmr.2001.16.3.457 (2001).
Reilly, G. C. & Currey, J. D. The effects of damage and microcracking on the impact strength of bone. J. Biomech. https://doi.org/10.1016/S0021-9290(99)00167-0 (2000).
Yeh, O. C. & Keaveny, T. M. Relative roles of microdamage and microfracture in the mechanical behavior of trabecular bone. J. Orthop. Res. https://doi.org/10.1016/S0736-0266(01)00053-5 (2001).
van Oers, R. F. M. Simulations of bone remodeling at the cellular scale. Technol. Univ. Endoven https://doi.org/10.6100/IR675320 (2010).
Allen, M. R. & Burr, D. B. Bone modeling and remodeling. Basic Appl. Bone Biol. https://doi.org/10.1016/B978-0-12-416015-6.00004-6 (2013).
Acknowledgements
We wish to thank the direct care teams at St Mary’s Hospital for consenting patients and collecting tissue samples. The authors would also like to thank the patients who consented to donate tissue for research and the Imperial College Healthcare National Health Service (NHS) staff and Imperial Tissue Bank staff who helped with the collection of the samples. All the procedures performed were in accordance with the ethical standards of the Imperial College Tissue Bank (R13004) and the 1984 Declaration of Helsinki. The research was funded by the Wellcome Trust and Engineering and Physical Sciences Research Council (EPSRC) Osteoarthritis Centre of Excellence (088844/Z/09/Z), the Michael Uren Foundation and the Science and Technology Facilities Council (STFC) Impact Acceleration Grant. The Diamond Light Source funded beam time allocations (EE0852, EE9811, SM10458, EE11204, SM13337). The authors are very grateful for the help of the Diamond Light Source staff at Harwell, UK.
Author information
Authors and Affiliations
Contributions
The authors declare no competing financial interests. Author Contributions Study Design: JPC, UH and RLA. Study conduct: MG. Data Collection: MG, OB, CR, NTV, RA. Data Analysis: MG, UH, RLA. Data Interpretation: MG, UH, CW, RLA. Drafting Manuscript: MG, JPC, UH, CW, RS, RLA. Approving final version of manuscript: All. MG takes responsibility for the integrity of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gray, M., Boughton, O., Wiles, C. et al. Runaway resorption of microcracks contributes to age-related hip-fracture patients. Sci Rep 15, 28894 (2025). https://doi.org/10.1038/s41598-025-12494-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-12494-6