Abstract
Verbal fluency tests (VFTs) are commonly used tasks for analyzing frontal cortex activation imagined by functional near-infrared spectroscopy (fNIRS). Some new VFT versions have been developed to strengthen the test’s executive character. However, these modified tasks were not used to recognize whether they activate cortical areas significantly differently compared with typical tasks. We introduced a modified VFT version consisting of the necessity to produce words according to one phonemic criterion and exclude others according to another phonemic criterion to establish frontal cortex task-dependent hemodynamic activity for the proposed new task. The analysis was conducted by comparison with a typical initial letter fluency task in a group of 35 students. Behavioral results showed between-tasks differences in productivity during the initial phase of performance. Increased oxygenated hemoglobin (oxy-Hb) concentration in lateral and frontopolar regions was noted in both analyzed tasks, but oxy-Hb suppression has been observed in the middle/superior frontal cortex only for the new one. Such an arrangement of selective metabolic activation and inactivation suggests that the brain’s adaptation to increased processing demands may consist of restraining the oxygen intake of the neuronal region, which activity is not necessary to perform a task.
Similar content being viewed by others
Introduction
Task-dependent frontal reactivity has been extensively studied in various clinical populations1,2,3,4 and in many experimental studies on these regions’ engagement in numerous psychological processes5,6. The functional Magnetic Resonance Imaging (fMRI), with its high spatial resolution, seems to be a dominant neuroimaging modality used to monitor frontal cortex activation7,8; however, for at least two decades, the number of studies using functional near-infrared spectroscopy (fNIRS) to assess frontal hemodynamic response has also been increasing9. In short, the fNIRS emits near-infrared light penetrating through the scalp and skull into superficial cortical layers and subsequently receives a photonic signal informative of concentration of oxygenated (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb) within the cortical tissue’ microvasculature10. As documented by Strangman et al.11, the fNIRS signal is strongly correlated with fMRI-generated blood oxygenation level-dependent data (BOLD), and oxy-HB dynamics express at least satisfying stability over time12. The main limitation of fNIRS is the lack of subcortical nuclei metabolic activity visualization. On the other hand, it has many advantages, such as low cost of assessment, significant mobility of the device, lack of the necessity to place a testee within a narrow scanner, and the possibility of planning a study with greater sensitivity to ecological factors13.
In the studies encompassing various specific populations, one of the most common paradigms used to evaluate frontal areas’ hemodynamic response was the administration of a cognitive task, the performance of which should activate these structures14. As shown by reviews and meta-analyses, a verbal fluency test (VFT) was frequently combined with fNIRS in the studies encompassing populations diagnosed with schizophrenia15, depression16, and bipolar disorder17. The same applies to aging18, and dementia, especially when researchers studied word retrieval in these patients19. VFT performance consists of providing as many unique words from a specific category as possible within a restricted time range (e.g. 60 s). For example, the initial letter or phonemic fluency task consists of providing non-repeating words beginning with a given phoneme. The semantic fluency relates to the retrieval of words belonging to a certain category, such as animals or fruits, without restriction considering the initial phoneme. Initial letter fluency activates the left dorsolateral prefrontal cortex (DLPFC), supporting working memory and the controlled retrieval of lexical items20. Contrarily, semantic fluency activates the ventrolateral prefrontal cortex (VLPFC) and anterior temporal regions in service of categorical retrieval and semantic associative processes. Evidence from functional MRI (fMRI) and positron emission tomography (PET) studies shows left inferior frontal gyrus (IFG) activity using either task type, suggesting a role of this region in lexical access and cognitive control across both conditions21. The lesion literature further demonstrates structures in the left PFC contribute to phonemic fluency, while lesions that extend into temporal areas, will reliably produce a greater negative impact on semantic fluency than on phonemic fluency22.
The recurrent pairing of VFT with fNIRS is mainly due to the ease of this task’s administration, the lack of necessity to use specific equipment or space, and the short procedure duration23. However, the neuropsychological and linguistic specificity of VFT remains vague24. Generally, it has been shown that the initial letter task activates the frontal cortex more strongly than semantic VFT25. Bryant and co-workers26 suggested that VFT was predicted mainly by processing speed and verbal knowledge. Stolwyk et al.27 indicated the dominant impact of verbal intelligence and processing speed, but on the other hand, machine learning studies28,29 demonstrated major executive contribution to the scores obtained in VFT. Additionally, a set of various neurocognitive mechanisms leading to an underperformance of VFT in various clinical groups has been identified30,31,32,33. Research on clinical populations indicates that VFT scores are sensitive to the brain dysfunction itself, but have no power to localize that damage34. What is more, VFT outcomes are also strongly associated with education level35. Another issue is that some researchers analyzed VFT-dependent hemodynamic signals in 20 s intervals, from a trial lasting 60 s23. It leads to averaging segments from VFT intervals associated with divergent neurocognitive mechanisms regarding word finding. Although most words are given in the initial VFT phase36, it is mainly due to the use of easily available verbal associations. Only after exhausting this resource, a phase of more effortful, executively controlled processes is activated to engage strategic word retrieval37,38. Such dynamics are also reflected in the time-dependent changes in the semantic association structure of provided responses: in the initial phase, more typical and frequently used words dominate, and in the ending phase, words are less typical, and their stream presents weaker semantic similarity39.
Although the mentioned issues of VFTs are well-known, there were not many attempts to modify these tasks towards augmenting the involvement of the executive factor from the beginning of its performance. Such an adjustment should more firmly link task realization with a hemodynamic response of the frontal cortex. One example is alternating fluency when a subject is asked to switch between two or more-word categories (e.g. fruits and furniture)40,41. This arrangement places a greater burden on working memory and depends on cognitive flexibility, a typical executive function42. Another approach to VFT was presented by Crawford et al.43, who analyzed VFTs depending on how close the instruction is to the natural, semantic structure of verbal memory. Accordingly, a semantic VFT version is the closest, so its performance does not rely on executive effort, initial letter fluency would be more difficult, but the excluded letter (ELFT) version might be classified as most disparate from a semantic organization, and thus places heavier demands on inhibition and the executively-controlled retrieval25,44. ELFT consists of the necessity to produce as many words as possible that do not contain a given phoneme. Another attempt was presented by Macoir et al.45,46 with the idea of an extradimensional orthographic constraint semantic fluency test. These authors also tried to increase VFT difficulty by producing animal names whose written form did not contain the letter ‘A’. It was suggested that switching between two retrieval strategies (semantic and phonemic) activates executive functions. Although the performance of such a task demands more effort, a potentially poor outcome might be conditioned by executive or semantic difficulties due to mixed word retrieval mechanisms utilized during performance.
To our knowledge, none of the discussed modified VFTs was used in an fNIRS study to evaluate whether such a task induces differently organized frontal cortex activity in regional and temporal dimensions, compared with typical tests, such as initial letter fluency. Considering former attempts to modify VFTs, and having in mind previous studies on relationships between VFT performance and fNIRS neuroimaging specificity, we would like to propose a new VFT version; the performance of this new VTF version requires primarily inhibitory control without the need to refer to the semantic organization of word retrieval. A combined initial-and-excluded phoneme fluency task consists of providing words beginning with a given letter, however, these words cannot contain a vowel indicated in the instruction. We believe that the performance of this task cannot rely on the easily accessible verbal association. Additionally, the necessity to engage both positive (initial letter) and negative (excluded letter) criteria in word retrieval absorbs more cognitive effort, and probably more executive control to avoid committing errors. The usage of a vowel in the negative criterion should maintain the task doable preventing extremely small verbal output. If the average subject provided, for example, only one or two correct words during an fNIRS study, the reliability of the hemodynamic signal associated with such substantially limited behavioral data would be problematic. There are more than twice fewer vowels as consonants, therefore, when retrieving words and checking their compliance with both criteria, applying the vowel-based elimination criterion makes the correct word identification feasible.
Considering the characteristics of the proposed combined letter fluency test (CLFT), this study aimed to analyze its performance specificity and the organization of the frontal cortex hemodynamic response by comparison with a typical initial letter fluency test’s (ILFT) behavioral output and associated fNIRS signals. At the behavioral level, we expect that during CLFT completion, subjects will provide a smaller number of unique words, commit more errors, and utilize an uneven number of clusters and switches. Analysis of the time-on-task effect will show a flatter curve of words given in successive intervals pointing at the inability to rely on easily accessible verbal associations in the initial phase of the task. Previous fNIRS studies on ILFT, apart from proving the left hemisphere predominance in task execution, showed that this task activates mainly the lateral PFC, superior and middle frontal gyri47,48. Considering the above, we formulate three hypotheses regarding potential between-task differences in the cortical oxygenation patterns: H1) CLFT, compared with ILFT, will be associated with increased HbO concentration in the prefrontal areas, due to greater executive control demands and increased task difficulty49; H2) compared with ILFT, CLFT performance will be associated with different regional activity organizations due to the task specificity requiring different word retrieval strategies; H3) the time-dependent oxygenation evolution will be different due to the necessity to engage in effortful processing from the very beginning of CLFT performance. Previous studies involving the analysis of time-dependent changes in word production confirmed that different cognitive functions are engaged in the initial and final phases of fluency task performance33. Verification of these hypotheses can determine to what extent VFT modification changes the arrangement of the frontal areas’ activity and whether fNIRS is sensitive enough to register the impact of potentially slight differences in the cognitive stimulation itself: both fluency tasks consist of word retrieval, and one of the retrieval criteria (initial letter) remains the same in both cases.
Results
Basic CLFT psychometric properties
The total number of correct words provided in CLFT performance correlated strongly and significantly with the total number of correct words provided in the performance of typical phonemic fluency task (ILFT): r = 0.70, p < 0.001, suggesting that CLFT elicits similar psychological processes as typical phonemic fluency.
To check for the CLFT internal consistency, correlations were calculated between the number of words given in the five separate trials and between individual trials and the sum of correct words provided in the entire task (Table 1S in Supplementary Materials). This analysis showed at least satisfactory internal consistency, all correlations were statistically significant and ranged between r = 0.40 and 0.80. Regarding the entire CLFT Cronbach’s α = 0.82, and in the case of individual trials it ranged between 0.76 and 0.80 confirming good task reliability.
CLFT and ILFT outcomes comparisons
As seen in Table 1A, during the CLFT performance participants provided significantly fewer correct words. Considering specific types of errors, the ILFT was associated with a significantly greater number of perseverative errors. However, normalized data comparison eliminated this difference. CLFT performance implemented the possibility of committing a unique type of instruction violation error regarding the eliminated letter. Only when the ‘eliminated letter errors’ number is added to the sum of all errors, the between-task difference regarding total errors is statistically significant. There were no significant correlations between the number of correct words and the number of any type of errors (p > 0.1).
Due to the possible impact of the number of words given in each fluency task on errors, clusters, and switches number, a comparison accounted for measures of analyzed parameters normalized by the total number of words given in each type of task. Part B of the Table 1 showed that after such an amendment there were no between-task differences in the number of clusters and switches, and the total amount of errors was still higher for the CLFT.
A mixed-model repeated-measures ANOVA (2 task types x four 15-second intervals) was applied to verify the assumption that CLFT and ILFT differ in performance dynamics analyzed as the number of correct words given in four consecutive time intervals (Fig. 1). The interaction effect was significant: F(3, 196) = 18.602, p < 0.0001, ηp2 = 0.22. The number of words given in the first half of the ILFT was almost twice as large as in the second half. This disproportion did not occur in the case of CLFT.
Next, the analysis of covariance (ANCOVA) was carried out to verify the assumption that clusters and switches used during tasks’ performance could account for the difference in the main outcome. However, before this analysis, potential correlations between the covariates were assessed. It was found that cluster types were significantly correlated (all r > 0.80), therefore both cluster types were summed. All types of switches were also significantly correlated and therefore were summed. As shown in Table 2, all included covariates were statistically significant but the difference between fluency tasks regarding the total number of correct words remained statistically significant.
fNIRS results
Outcomes regarding HbO concentration distribution related to task, time, lateralization, and interaction effects were computed for three separated regions: OFC, LF, and pre-SMA.
Orbital/frontopolar cortex
A three-way ANOVA with repeated measures showed a significant main effect of time: F(3, 29) = 4.89, p = 0.007, ηp2 = 0.34. Pairwise comparisons showed a significant increase of oxy-Hb in the interval 45–60 s (0.07 [SD ± 0.02]) compared to 0–15 s (-0.003 [SD ± 0.01]) (Fig. 2A).
Also, we found a significant main effect of lateralization: F(1, 31) = 4.22, p = 0.049, ηp2 = 0.12. Our results also showed a significant increase of oxy-Hb on the left side (0.04 [SD ± 0.02]) compared to the right side (0.02 [SD ± 0.02]) (Fig. 2B). The other main effects and interaction effects were statistically non-significant (ps > 0.05).
Lateral frontal cortex
A three-way ANOVA with repeated measures showed a significant main effect of time: F(3, 29) = 10.36, p < 0.001, ηp2 = 0.52. Pairwise comparisons showed a significant increase (p = 0.021) of oxy-Hb in the interval 45–60 s (0.14 [SD ± 0.03]) and in the interval 15–30 s (p < 0.001, 0.14 [SD ± 0.01]) compared to 0–15 s (0.06 [SD ± 0.01]). Additionally, the results revealed a significant increase (p = 0.018) of oxy-Hb in the interval 30–45 s (0.13 [SD ± 0.02]) compared to 0–15 s (0.06 [SD ± 0.01]) (Fig. 2C).
Also, we found a significant main effect of lateralization: F(1, 31) = 6.49, p = 0.016, ηp2 = 0.17. There was a significant increase of oxy-Hb on the left side (0.14 [SD ± 0.02]) compared to the right side (0.08 [SD ± 0.02]) (Fig. 2D). The other main effects and interaction effects were statistically non-significant (ps > 0.05).
Middle/Superior frontal cortex (pre-SMA)
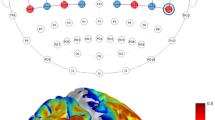
A three-way ANOVA with repeated measures showed a significant interaction between time vs. lateralization vs. task: F(3, 29) = 3.02, p = 0.046, ηp2 = 0.24. Pairwise comparisons showed a significant oxy-Hb decrease (p = 0.049) on the right side for 0–15 s time slot between CLFT (-0.04 [SD ± 0.01]) vs. ILFT (-0.36 [SD ± 0.01]) (Fig. 3).
Additionally, only in the CLFT in the time slot 30–45 s there was a significant difference between the left and right sides (p = 0.028). A significant decrease was registered on the left side (-0.8 [SD ± 0.03]) compared to the right side (0.15 [SD ± 0.2]) (Fig. 2E/F). The other main effects and interaction effects were statistically non-significant (ps > 0.05). Table 2S in the Supplementary Materials present results for all type of effects.
The difference in oxy-Hb for: Time (A) and Lateralization (B) in the Orbital/frontopolar cortex; Time (C) and Lateralization (D) in the Lateral frontal cortex; and initial letter fluency task (ILFT) and combined letter fluency task (CLFT) (E/F) in Middle/Superior frontal cortex (pre-SMA). All statistical significance levels adjusted for Bonferroni correction for multiple testing.
Discussion
Our study aimed to analyze the specificity of the newly introduced CLFT outputs and the related frontal cortex hemodynamic response. Analysis was handled by comparing CLFT-related data with those coming from the standard phonemic fluency task. We hypothesized that the new task would be more difficult as indicated by fewer correct words and more errors, and its completion would be less conditioned by the use of easily available verbal associations, especially in the initial phase of execution. Above all, it was presumed that CLFT would be associated with significantly different patterns of frontal activation regarding features such as oxy-Hb concentration level, and its regional or temporal organization.
The collected results confirmed these hypotheses. Behavioral outcomes showed a significantly smaller number of correct words while performing CLFT. Although the sum of all errors committed during CLFT performance was higher compared with ILFT, this sum consisted mainly of specific errors that could be committed only when performing CLFT. This suggests that higher CLFT difficulty stems primarily from the necessity to utilize a more demanding word retrieval strategy. Analyses concerning phonemic and semantic clusters and switches involving the correction for the total number of words did not corroborate the assumption that these linguistic mechanisms will significantly differ between tasks. It may potentially indicate that the performance of various types of verbal fluency tests is based on these universal dimensions of internal lexicon organization.
The most important result of the behavioral data analysis was establishing that the between-task difference in the total number of correct words was significantly conditioned by the verbal output provided in the initial phase of performance. The dynamic profile of the ILFT-related changes in word production showed a substantial concentration of words spoken in the initial stages of performance, which was not pronounced to such an extent in the case of CLFT. Presented findings confirm our presumptions, that CLFT execution from the beginning depends on a more effortful word retrieval mode.
The analysis of the oxygenated hemoglobin concentration in selected frontal areas showed that for both fluency tasks a significant HbO increase was observed, especially regarding orbitofrontal and lateral regions. This frontal hemodynamic activation was lateralized with a larger oxy-Hb increase on the left side. Besides the lateralization effect, in the orbital/frontopolar region, a statistically significant time-dependent gradual oxygenated hemoglobin increase was also noted. A slightly similar pattern was detectable in the lateral part of the frontal cortex, but here, the HbO increase occurred abruptly after the first-time interval.
The fNIRS-dependent indices that significantly differentiated the two fluency tasks concerned only the oxy-Hb decrease in the Middle/Superior frontal cortex area bordering the SMA. Oxygen metabolism decline during the CLFT execution concerned both left and right middle/superior PFC regions.
The right middle/superior region showed an additional significant time-dependent effect, with stronger hemodynamic suppression in the initial phase of CLFT performance compared with the ILFT. As illustrated in Fig. 2 (parts E and F), CLFT-related oxy-Hb concentration remained suppressed throughout the test span. In the case of ILFT execution, hemodynamic activity of mentioned region was also withdrawn, but gradually increased in the right part of this region.
Local deactivation of selected cortical regions as a mechanism improving information processing or behavior regulation has been shown in many previous studies. A series of rTMS (repetitive Transcranial Magnetic Stimulation) research can be included in this scope. For example, in brain-damaged patients with visuospatial neglect, Oliveri et al.50, utilizing the inhibitory effect of TMS on the undamaged hemisphere temporarily reduced the symptoms of the aforementioned hemineglect. Weiduschat et al.51, with the application of rTMS deactivated intact right hemisphere in patients with aphasia following left hemisphere damage, which paradoxically led to reduction of aphasic symptoms. Based on these outcomes Smirni et al.52 conducted a study including healthy individuals performing VFT. The administration of inhibitory rTMS stimulation regarding right hemisphere was found to significantly improve test performance. These inhibitory rTMS effects were interpreted as the elimination of interhemispheric inhibitory interaction or a specific functional conflict, moreover, deactivating functionally non-essential cortical areas might increase the excitability of unstimulated regions enhancing task execution52. From another point of neuroimaging research, there is a plethora of studies showing that the transition from easy to more demanding cognitive tasks is associated with decreased functional connectivity enabling selective activation of these sub-networks which are specified to process a given type of task, and simultaneously deactivate other functional network unrelated to such task, or which activity could interfere performance53,54,55.
Functional specificity of the middle/superior prefrontal area encompassing the SMA and pre-SMA regions was initially recognized in studies on lesioned patients presenting with a specific syndrome of an inability to trigger both motor and verbal behaviors56,57. Considering above, it was assumed that the basic function of these areas is to prompt behavior. Further fMRI research utilizing various experimental procedures confirmed this characteristic but additionally showed involvement into other cognitive domains such as response selection58, time perception59, or working memory60. Hertrich et al.61 in their seminal review of SMA/pre-SMA engagement in language conclude, that despite these regions’ functional heterogeneity, their fundamental role is to initiate speech and maintain its speed. SMA is primarily engaged in the motor aspect, the fluent articulation, while pre-SMA is involved in cognitive processes providing an optimal rate of word selection. Considering potential SMA/pre-SMA involvement in the verbal fluency test performance, it was argued that this region plays a crucial role in the “energization” mechanism defined as the initiation of responses62.
The CLFT performance was associated with increased activation of the lateral and orbital frontal cortex and decreased metabolism of the middle/superior frontal cortex. Since the task instruction impose mainly exclusion criteria regarding words to be spoken, and the behavioral outcomes confirm that it requires cautious word selection, cortical regions responsible just for the initiation and acceleration of responses can be withheld to optimize allocation of oxygenation-dependent metabolic resources to prefrontal regions more strictly associated with executive control, e.g. lateral PFC63. Previous studies show that the most uncontrolled word finding occurs mainly in the initial phase of typical fluency task performance33,36,37. During CLFT performance, pre-SMA region metabolic suppression was the strongest exactly in this initial interval (0–15 s), probably to limit uncontrolled associations and relocate efforts from the bare speed of word production to greater control over word retrieval.
Although our explanations regarding effects of the SMA/pre-SMA deactivation are to some extent speculative, the benefits of inhibiting brain areas whose activity is not crucial for a given task were already presented. Additionally, the researches on the so-called negative BOLD response also corroborate such conclusions (NBR64, –65). What seems also to be important, the NBR has neuronal, not only vascular origin66, suggesting that selective hemodynamic suppression while CLFT task represents an information processing-related phenomenon, not only a metabolic by-product of the fNIRS method67.
Also, an alternative explanation of the CLFT results might be connected with wider differences in HbO activation on the right and left sides of the prefrontal cortex (PFC), which is worth considering. One might expect higher activation in the right PFC as domain-general inhibitory control68; in contrast, semantic control tends to be left-lateralized. Given that the CLFT requires participants to simultaneously consider both inclusionary (initial letter) and exclusionary (vowel exclusion) criteria, the left PFC may be primarily involved in the controlled retrieval and selection of appropriate words. At the same time, the right PFC plays a supporting role in inhibiting irrelevant responses. Thus, participants might prioritize word selection more than word inhibition processes. Apart from these potential explanations regarding pre-SMA metabolic inhibition during CLFT performance, it should be noted that our fNIRS outcomes presentation considers juxtaposition of two task-dependent HbO reaction patterns, not task-related HbO changes tracked against the baseline state. This specific approach to the fNIRS data analysis should also be considered while interpreting the obtained findings regarding SMA/pre-SMA deactivation.
Considering the above, one may state that modification of the verbal fluency test towards heightened demands associated with more selective word retrieval changed the arrangement of the frontal cortex regional hemodynamic response into more restrained, not locally increased. This pattern is different from that of brain reactions to increased cognitive load studied with working memory tasks when gradually higher oxygenation was noted49. Such difference may indicate that the brain copes with increasing task difficulty in various ways and that there are also other adaptive strategies than an increase in oxygen demand69. Even though the discussed findings demand verification, a potentially positive replication suggests that the combined letter fluency task can be useful in further research on cortical activity suppression mechanisms, including clinical samples burdened with neuronal inhibitory deficits70.
The results obtained regarding the CFLT indicate that its performance is more difficult compared to ILFT, inter alia, due to a smaller pool of acceptable responses. Generally, fewer words provided in one trial transfer to the limited output to analyze. Therefore, to increase it, it seems reasonable to administer more trials, e.g. five or more, and not three, as in some more typical fluency tests. It is also likely that a more difficult task may be non-feasible for a certain group of patients, e.g. with moderate-to-advanced dementia. On the other hand, cognitively challenging tasks are better at capturing subtle deficits and impairments typical of the early neurodegeneration stages71. CLFT may be also useful to administer in brain-injured subjects with substantial cognitive reserve enabling them to score normally on simple screening tests72. Considering the above, CFLT’s applicability in populations without advanced neurocognitive decline seems more justified. According to Ghisletta et al.73, commonly used fluency tasks have strong predictive and clinical utility because they rely on a broad profile of cognitive functions, including fluid abilities (e.g., executive control and fast information retrieval) and crystallized abilities (i.e., organized semantic knowledge). Standard fluency tasks have intermediate difficulty suitable for subjects with a wide range of neurocognitive impairments. In light of the above notions, the application of CLFT as an individual task of heightened difficulty and not involving the semantics dimension might be considered a drawback. This problem can be addressed by complementing CLFT with another assessment method, e.g. such as the Associative-Dissociative Retrieval Task (ADT) introduced by Marko and co-workers74,75. ADT is a semantically oriented task that contrasts automatic and dissociative word retrieval, and the latter depends on inhibitory control over verbal memory. Additionally, response-times analyses regarding associative and dissociative retrieval trials enable the calculation of the Inhibition Cost, a direct measurement of inhibition efficiency. We believe that CLFT and ADT have some similarities related to the involvement of inhibitory control, though differ in terms of the semantic factor contribution to their performance.
The presented study is not free from several limitations that should be addressed. Due to fNIRS specificity and utilized channels montage, not the entire cortex was evaluated, so we did not determine whether any unexamined cortical areas exhibited hemodynamic response differences regarding the two tasks. Although previous reports showed that initial letter fluency tasks elicit mainly frontal cortex76, it cannot be ruled out that the activity or deactivation of other regions could be differentiated by task specificity. Verbal production and associated cognitive processes depend on frontal-subcortical tracts which also have not been studied with the fNIRS method57. A more thorough recognition of brain mechanisms regulating behavior induced by newly introduced CLFT clearly requires further studies applying neuroimaging modalities not restricted to the cortical surface, such as fMRI. It should also be pointed out that some of the assumed features of the CLFT, e.g., its reliance on selectivity, may be verified in future studies involving clinical groups, especially those in which selective inhibition processes are known to be disrupted. Comparison of the results of such a group and the control group may allow for the verification of the indicated assumptions. The analysis performed did not take into account the correlation between behavioral data and HbO signal dynamics. Future studies should attempt to identify potential relationships between linguistic characteristics of the performed task and fNIRS data. We also draw attention to the arbitrary result in a 30–45–second window. The purpose of this study was to allow us to see the dynamics change during two tasks, but this result should be interpreted with caution. Additionally, it should be noted that the aim of our study was not to deliver full psychometric properties of the introduced cognitive task, nor to establish details of the task’s clinical feasibility and practical utility as a diagnostic method. However, considering obtained results it seems likely that it could be an interesting experimental procedure in studies focused on selection processes and related neuronal reactions, and therefore, it should be further explored.
Materials and methods
Participants
The study involved 35 participants. However, considering artifacts in the fNIRS signal, data from 3 participants were removed from the study; hence, data from 32 participants were eventually used in the analysis (19 females, age = 22.6 ± 2.8 years (mean ± standard deviation, SD); 13 males age = 25.0 ± 6.1 years. Whole sample mean age = 23.8 ± 4.45. Participants were students recruited from universities located in Lublin (Poland, EU), all Caucasians. None of them had any neurological diseases in the past or suffered from chronic pain caused by an illness. All participants were volunteers and received financial remuneration of 100 PLN (~ 24 $). Participants were informed that their participation was voluntary and that they may end the procedure anytime. A formal power analysis was not conducted prior to data collection. Due to the exploratory nature of this study and the limited availability of prior fNIRS data using this specific combined letter fluency task (CLFT). The sample size was determined based on previous fNIRS studies in verbal fluency77 HC simple size: 32;78 HC sample size: 31). The study was conducted in accordance with the Declaration of Helsinki and approved by the university bioethics committee (No: KE-0254/148/06/2022). All participants gave written informed consent to participate in the study.
Procedure
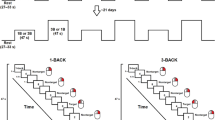
A computer and a functional near-infrared (fNIRS) were used in the experiment. The PsychoPy 2023.2.3v79 software has been applied to design the experimental procedure. For fNIRS acquisition, optical signals were recorded on a two-wavelength (760 and 850 nm) continuous-wave Photon Cap C20 system (Cortivision sp. z o.o., Lublin, Poland80), . Instructions and tasks for all participants were displayed on a 24-inch monitor. The monitor was positioned at the subjects’ eye level to limit the downward tilt of the head during the examination. Participants had two tasks: a typical initial letter fluency task (ILFT) and a combined letter fluency task (CLFT).
In the ILFT, participants had to generate as many words as possible out loud that started with a certain letter within 60 seconds. Five main trials were executed with the letters K, T, A, N, and F. Initial letters were presented in random order for every participant. Before the first trial, an example of the correct word was displayed on the screen with a description regarding performance correctness. Instruction was based on an initial letter other than those used in main trials. Each main trial starts with instruction displayed on the computer screen. Instructions were given in Polish, its content was as follows: ”Proszę powiedzieć jak najwięcej słów zaczynających się na literę, która zostanie wyświetlona. Podane słowa nie mogą się powtarzać i nie mogą być to nazwy własne, takie jak nazwy miast i imiona. Zadanie należy wykonać w ciągu minuty. Gotowy/a?” [Please, say as many words as you can that begin with a letter which will be shown on the screen. Do not say the same word more than once. Do not use words that are proper names, such as city or country or names. You will have a time limit of one minute. Are you ready?] After that, participants saw the letter (e.g. A) for 2 s. During the 60-second task slot, participants saw only a blank screen. Participants were informed that the 60-second was over when the message: “Koniec zadania” [The end of the task] was displayed on the computer screen.
The CLFT procedure looks similar, except that participants had to generate as many words as possible that start with a certain consonant (e.g., F) but without a specific vowel (e.g., A). The following instruction was displayed on the screen: “Proszę powiedzieć jak najwięcej słów zaczynających się na podaną literę, ale jednocześnie podane słowa nie mogą zawierać innej określonej litery. Początkowa i wykluczona litera zostanie wyświetlona na monitorze. Podane słowa nie mogą się powtarzać i nie mogą być to nazwy własne, takie jak nazwy miast i imiona. Zadanie należy wykonać w ciągu minuty. Gotowy/a?” [Please, say as many words as you can that begin with a given letter, but these words cannot involve a specific letter. Initial and excluded letters will be displayed on the computer screen. Do not say the same word more than once. Do not use words that are proper names, such as city or country or names. You will have a time limit of one minute. Are you ready?]. Main trials were preceded by an example of a correct word considering specific restrictions. Instruction was based on other initial and excluded letters than were used in main trials. There were also five initial letters (R, S, W, M, and L) and five vowels (I, A, E, O, and U). For every participant, letters, and vowels were displayed in random order. Between experimental fluency trials, participants completed a control task consisting of naming aloud digits from 1 to 10 displayed consecutively on a monitor.
Also, due to the cognitive load caused by the tasks, and to control the transfer effect, the study was divided into two separate sessions within a 7–8-day break. Half of the participants started with the ILFT, and the other half started with the CLFT. Both the initial letter fluency task (ILFT) and a combined letter fluency task (CLFT) took less than 15 min.
Fluency tests’ output analysis
Spoken words were digitally recorded. The analysis of verbal output was conducted manually, following established linguistic norms, including semantic, morphological, and phonetic rules. Said words were classified as correct if they met the criteria of compliance with the task instructions. The main output of tasks was the total number of correct words given in the entire task (sum of 5 trials). Accordingly, three types of errors were recognized: perseverations, words not complying with instruction (e.g. proper nouns, failure to apply to the initial letter, the so-called ‘other errors’), and specific errors consisting of noncompliance to eliminated vowel criterion in CLFT. Provided words were also counted in successive 15-second intervals (0–15 s, 15–30 s, 30–45 s, 45–60 s). If the articulation of a given word began in one interval (e.g. 15–30 s) and ended in another (30–45 s), it was included in the interval in which articulation began. Verbal production was also analyzed concerning clusters and switches. Clusters were sets of two or more words said one after another representing a common phonetic, or semantic feature81. Phonemic clusters were based on words’ sounding, not the spelling, for example, in the Polish language there is a digraph ‘sz’, which has a different pronunciation than the sound ‘s’. Semantic clusters included word groups with a common root or word-forming element and groups of words related to a superordinate category regarding lexical-semantic understanding (i.e., subordinate word groups relative to a single hypernym). Phonemic and semantic switches were considered shifts between the presented types of clusters82.
fNIRS acquisition and analysis
Optical signals were recorded on a two-wavelength (760 and 850 nm) continuous-wave Photon Cap C20 system (Cortivision sp. z o.o., Lublin, Poland) device with 32 channel montage covering forehead regions of participants’ heads (see Fig. 4). The data, sampled at 5 Hz, were analyzed using CortiPrism (v. 06.2023) software. fNIRS data were first converted from raw intensity to optical density via the application of the modified Beer-Lambert Law83. Randomly occurring movement artifacts were corrected using Temporal Derivative Distribution Repair (TDDR) algorithm84. As temporal filtering, a low pass filter with a cutoff frequency of 0.2 Hz was applied. The artifacts were also corrected using short channel correction. Next, optical density was transformed to the concentration of oxyhemoglobin (oxy-Hb) by applying the modified Beer-Lambert Law83. Finally, the Correlation-Based Signal Improvement (CBSI) method85 was used in order to effectively minimize noise from physiological sources.
Each epoch was 15 s long, and the baseline necessary for calculating optical density was registered from the course of the first 2 s preceding task-related recording. Four segments: 0 to 15, 15 to 30, 30 to 45, and 45 to 60 s were considered. The hemoglobin concentration data from the channels were grouped in Regions of Interest (RoI) by averaging them (see Fig. 1): 1, 2 and 4 - left orbital/frontopolar cortex (OFCl); 5, 20 and 21 - right orbital/frontopolar cortex (OFCr); 18, 6, 19, 8, 13, 14, and 9 - left lateral frontal cortex left (LFl); 22, 24, 23, 27, 32, 31 and 26 - right lateral frontal cortex (LFr); 7, 10, 15, 16, and 17 - left middle/superior frontal cortex (left pre-supplementary motor area, pre-SMAl); 12, 29, 28, 25 and 30 - right middle/superior frontal cortex (pre-SMAr). These RoIs were determined based on the proposal of Liu et al.86, but with a reduction in the regions number to simplify the analyses. As shown in the study of Singk et al.87, standard displacement of the fNIRS emitter and detectors enables to localize cortical structures mapped accordingly to the MNI anatomic template.
Statistical analysis
The CLFT internal consistency was checked using Pearson correlations involving the number of correct words provided in five separate trials, and CLFT reliability was verified using Cronbach’s α. Behavioral and fNIRS-related measures were analyzed regarding compliance with normal distribution using the Shapiro-Wilk test. Fluency tasks outcomes, such as the total number of correct words, number of errors, data on clusters and switches, were compared with the paired t-test and with the Wilcoxon Signed Rank test for variables deviating from a normal distribution. Next, a mixed-model repeated-measures ANOVA (2 task types x four consecutive 15-second intervals) was applied in order to verify the assumption that the performance of two analyzed fluency tasks differed regarding time-dependent changes in word production. The within-subject analysis of covariance (ANCOVA) with the total number of correct words as a dependent variable, clusters and switches as controlled covariates have been used to establish whether mentioned covariates could account for the between-tasks difference in the total number of correct words. To compare tasks-related measures of oxy-Hb concentration in distinguished frontal regions, a three-way repeated measures ANOVA with the lateralization (left vs. right), time (0–15 s vs. 15–30 s vs. 30–45 s vs. 45–60 s), and tasks (ILFT vs. CLFT) as within-participant factors were used. Separate analyses were conducted for the structures of the orbital/frontopolar cortex, lateral frontal cortex, and pre-SMA. All post hoc tests were further adjusted for multiple comparisons using Bonferroni correction when there was a significant interaction between factors. Regarding all used versions of variance analyses, the effect size was assessed using a partial eta square (ηp²) to reflect the F-tests.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Gur, R. E. & Gur, R. C. Functional magnetic resonance imaging in schizophrenia. Dialogues Clin. Neurosci. 12 (3), 333–343. https://doi.org/10.31887/DCNS.2010.12.3/rgur (2010).
Pilmeyer, J. et al. Functional MRI in major depressive disorder: A review of findings, limitations, and future prospects. J. Neuroimaging. 32 (4), 582–595. https://doi.org/10.1111/jon.13011 (2022).
Saldarini, F., Gottlieb, N. & Stokes, P. R. A. Neural correlates of working memory function in euthymic people with bipolar disorder compared to healthy controls: A systematic review and meta-analysis. J. Affect. Disord. 297, 610–622. https://doi.org/10.1016/j.jad.2021.10.084 (2022).
Sebastian, A. et al. Frontal dysfunctions of impulse control - a systematic review in borderline personality disorder and attention-deficit/hyperactivity disorder. Front. Hum. Neurosci. 8, 698. https://doi.org/10.3389/fnhum.2014.00698 (2014).
Alvarez, J. A. & Emory, E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol. Rev. 16 (1), 17–42. https://doi.org/10.1007/s11065-006-9002-x (2006).
Friedman, N. P. & Robbins, T. W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacol 47, 72–89. https://doi.org/10.1038/s41386-021-01132-0 (2022).
Cabeza, R. & Nyberg, L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 12, 1–47. https://doi.org/10.1162/08989290051137585 (2000).
Haber, S. N., Liu, H., Seidlitz, J. & Bullmore, E. Prefrontal connectomics: from anatomy to human imaging. Neuropsychopharmacology 47 (1), 20–40. https://doi.org/10.1038/s41386-021-01156-6 (2022).
Ren, Y. et al. The promising fNIRS: Uncovering the function of prefrontal working memory networks based on multi-cognitive tasks. Front. Psychiatry. 13, 985076. https://doi.org/10.3389/fpsyt.2022.985076 (2022).
Chen, W. L. et al. Functional Near-Infrared spectroscopy and its clinical application in the field of neuroscience: advances and future directions. Front. Neurosci. 14, 724. https://doi.org/10.3389/fnins.2020.00724 (2020).
Strangman, G., Culver, J. P., Thompson, J. H. & Boas, D. A. A quantitative comparison of simultaneous bold FMRI and NIRS recordings during functional brain activation. Neuroimage 17, 719–731. https://doi.org/10.1006/nimg.2002.1227 (2002).
Plichta, M. M. et al. Event-related functional near-infrared spectroscopy (FNIRS): are the measurements reliable? Neuroimage 31, 116–124. https://doi.org/10.1016/j.neuroimage.2005.12.008 (2006).
Doherty, E. J. et al. Interdisciplinary views of fNIRS: current advancements, equity challenges, and an agenda for future needs of a diverse fNIRS research community. Front. Integr. Neurosci. 17, 1059679. https://doi.org/10.3389/fnint.2023.1059679 (2023).
Pinti, P. et al. The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N Y Acad. Sci. 1464 (1), 5–29. https://doi.org/10.1111/nyas.13948 (2020).
Kumar, V. et al. Functional near infra-red spectroscopy (fNIRS) in schizophrenia: A review. Asian J. Psychiatr. 27, 18–31. https://doi.org/10.1016/j.ajp.2017.02.009 (2017).
Ho, C. S. H. et al. Diagnostic and predictive applications of functional Near-Infrared spectroscopy for major depressive disorder: A systematic review. Front. Psychiatry. 11, 378. https://doi.org/10.3389/fpsyt.2020.00378 (2020).
Tassi, E. et al. A scoping review of near infrared spectroscopy studies employing a verbal fluency task in bipolar disorder. J. Affect. Disord. 298(Pt A), 604–617. https://doi.org/10.1016/j.jad.2021.11.019 (2022).
Udina, C. et al. Functional Near-Infrared spectroscopy to study cerebral hemodynamics in older adults during cognitive and motor tasks: A review. Front. Aging Neurosci. 11, 367. https://doi.org/10.3389/fnagi.2019.00367 (2020).
Butters, E., Srinivasan, S., O’Brien, J. T., Su, L. & Bale, G. A promising tool to explore functional impairment in neurodegeneration: A systematic review of near-infrared spectroscopy in dementia. Ageing Res. Rev. 90, 101992. https://doi.org/10.1016/j.arr.2023.101992 (2023).
Birn, R. M. et al. Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. Neuroimage 49 (1), 1099–1107. https://doi.org/10.1016/j.neuroimage.2009.07.036 (2010).
Godefroy, O. et al. GRECogVASC study group. Functional architecture of executive processes: evidence from verbal fluency and lesion mapping in stroke patients. Cortex 164, 129–143. https://doi.org/10.1016/j.cortex.2023.03.013 (2023).
Ghanavati, E. et al. Differential role of prefrontal, Temporal and parietal cortices in verbal and figural fluency: implications for the supramodal contribution of executive functions. Sci. Rep. 9, 3700. https://doi.org/10.1038/s41598-019-40273-7 (2019).
Yeung, M. K. & Lin, J. Probing depression, schizophrenia, and other psychiatric disorders using fNIRS and the verbal fluency test: A systematic review and meta-analysis. J. Psychiatr Res. 140, 416–435. https://doi.org/10.1016/j.jpsychires.2021.06.015 (2021).
Shao, Z., Janse, E., Visser, K. & Meyer, A. S. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Fronti Psychol. 5, 772. https://doi.org/10.3389/fpsyg.2014.00772 (2014).
Onishi, A., Furutani, H., Hiroyasu, T. & Hiwa, S. An fNIRS study of brain state during letter and category fluency tasks. J. Robot Netw. Artif. Life. 5, 228–231. https://doi.org/10.2991/jrnal.k.190220.003 (2019).
Bryan, J., Luszcz, M. & Crawford, J. R. Verbal knowledge and speed of information processing as mediators of age differences in verbal fluency performance among older adults. Psychol. Aging. 12 (3), 473–478. https://doi.org/10.1037/0882-7974.12.3.473 (1997).
Stolwyk, R., Bannirchelvam, B., Kraan, C. & Simpson, K. The cognitive abilities associated with verbal fluency task performance differ across fluency variants and age groups in healthy young and old adults. J. Clin. Exp. Neuropsychol. 37 (1), 70–83. https://doi.org/10.1080/13803395.2014.988125 (2015).
Amunts, J., Camilleri, J. A., Eickhoff, S. B., Heim, S. & Weis, S. Executive functions predict verbal fluency scores in healthy participants. Sci. Rep. 10, 11141. https://doi.org/10.1038/s41598-020-65525-9 (2020).
Amunts, J. et al. Comprehensive verbal fluency features predict executive function performance. Sci. Rep. 11 (1), 6929. https://doi.org/10.1038/s41598-021-85981-1 (2021).
Delgado-Álvarez, A. et al. Cognitive processes underlying verbal fluency in multiple sclerosis. Front. Neurol. 11, 629183. https://doi.org/10.3389/fneur.2020.629183 (2021).
Fossati, P., Guillaume, B., Ergis, A. M. & Allilaire, J. F. Qualitative analysis of verbal fluency in depression. Psychiatry Res. 117 (1), 17–24. https://doi.org/10.1016/s0165-1781(02)00300-1 (2003).
Johnson, R., Bhandary, P. R., Guddatu, V., Kamath, C. & John, S. Comparison of verbal fluency performance in Kannada-speaking adults with and without euthymic bipolar disorder type 1. Appl. Neuropsychol. Adult. 20, 1–11. https://doi.org/10.1080/23279095.2023.2289550 (2023).
Krukow, P. et al. Ineffective initiation contributes to deficient verbal and non-verbal fluency in patients with schizophrenia. Cogn. Neuropsychiatry. 22 (5), 391–406. https://doi.org/10.1080/13546805.2017.1356710 (2017).
Henderson, S. K., Peterson, K. A., Patterson, K., Lambon Ralph, M. A. & Rowe, J. B. Verbal fluency tests assess global cognitive status but have limited diagnostic differentiation: evidence from a large-scale examination of six neurodegenerative diseases. Brain Commun. 5 (2), fcad042. https://doi.org/10.1093/braincomms/fcad042 (2023).
Fichman, H. C. et al. Age and educational level effects on the performance of normal elderly on category verbal fluency tasks. Dement. Neuropsychol. 3 (1), 49–54. https://doi.org/10.1590/S1980-57642009DN30100010 (2009).
Crowe, S. F. Decrease in performance on the verbal fluency test as a function of time: evaluation in a young healthy sample. J. Clin. Experimental Neuropsychology. 20 (3), 391–401. https://doi.org/10.1076/jcen.20.3.391.810 (1998).
Hurks, P. P. et al. Developmental changes in semantic verbal fluency: analyses of word productivity as a function of time, clustering, and switching. Child. Neuropsychol. 16 (4), 366–387. https://doi.org/10.1080/09297041003671184 (2010).
Lehtinen, N., Kautto, A. & Renvall, K. Frequent native Language use supports phonemic and semantic verbal fluency in L1 and L2: an extended analysis of verbal fluency task performance in an L1 Language attrition population. Int. J. Biling. 28 (5), 884–906. https://doi.org/10.1177/13670069231193727 (2024).
Michalko, D., Marko, M. & Riecansky, I. Executive functioning moderates the decline of retrieval fluency in time. Psychol. Res. 87, 1–13. https://doi.org/10.1007/s00426-022-01680-0 (2022).
Diamond, A. Executive functions. Annu. Rev. Psychol. 64, 135–168. https://doi.org/10.1146/annurev-psych-113011-143750 (2013).
Delis, D. C., Kaplan, E. & Kramer, J. H. Delis-Kaplan Executive Function System (D-KEFS) Technical Manual (The Psychological Corporation, 2021).
de Paula, J. J., Paiva, G. C. C. & Costa, D. S. Use of a modified version of the switching verbal fluency test for the assessment of cognitive flexibility. Dement. Neuropsychol. 9 (3), 258–264. https://doi.org/10.1590/1980-57642015DN93000008 (2015).
Crawford, J. R., Wright, R. & Bate, A. Verbal, figural and ideational fluency in CHI. J. Int. Neuropsychol. Soc. 1, 321 (1995).
Hughes, D. L. & Bryan, J. Adult age differences in strategy use during verbal fluency performance. J. Clin. Exp. Neuropsychol. 24 (5), 642–654. https://doi.org/10.1076/jcen.24.5.642.1002 (2002).
Macoir, J., Tremblay, P. & Hudon, C. The use of executive fluency tasks to detect cognitive impairment in individuals with subjective cognitive decline. Behav. Sci. (Basel). 12 (12), 491. https://doi.org/10.3390/bs12120491 (2022).
Macoir, J. & Hudon, C. Normative data for the alternating and orthographic constraint semantic fluency tests in the adult French-Quebec population and validation study in mild cognitive impairment and alzheimer’s disease. Arch. Clin. Neuropsychol. 4, acad065. https://doi.org/10.1093/arclin/acad065 (2023).
Tung, H. et al. Left frontotemporal region plays a key role in letter fluency Task-Evoked activation and functional connectivity in normal subjects: A functional Near-Infrared spectroscopy study. Front. Psychiatry. 13, 810685. https://doi.org/10.3389/fpsyt.2022.810685 (2022).
Tupak, S. V. et al. Differential prefrontal and frontotemporal oxygenation patterns during phonemic and semantic verbal fluency. Neuropsychologia 50 (7), 1565–1569. https://doi.org/10.1016/j.neuropsychologia.2012.03.009 (2012).
Lucas, I., Urieta, P., Balada, F., Blanco, E. & Aluja, A. Differences in prefrontal cortex activity based on difficulty in a working memory task using near-infrared spectroscopy. Behav. Brain Res. 392, 112722. https://doi.org/10.1016/j.bbr.2020.112722 (2020).
Oliveri, M. et al. rTMS of the unaffected hemisphere transiently reduces contralesional visuospatial Hemineglect. Neurology 57 (7), 1338–1340. https://doi.org/10.1212/wnl.57.7.1338 (2001).
Weiduschat, N. et al. Effects of repetitive transcranial magnetic stimulation in aphasic stroke: a randomized controlled pilot study. Stroke 42 (2), 409–415. https://doi.org/10.1161/STROKEAHA.110.597864 (2011).
Smirni, D. et al. Modulating phonemic fluency performance in healthy subjects with transcranial magnetic stimulation over the left or right lateral frontal cortex. Neuropsychologia 102, 109–115. https://doi.org/10.1016/j.neuropsychologia.2017.06.006 (2017).
Elton, A. & Gao, W. Task-related modulation of functional connectivity variability and its behavioral correlations. Hum. Brain Mapp. 36 (8), 3260–3272. https://doi.org/10.1002/hbm.22847 (2015).
Shine, J. M. The dynamics of functional brain networks: integrated network States during cognitive task performance. Neuron 92 (2), 544–554. https://doi.org/10.1016/j.neuron.2016.09.018 (2016).
Kaposzta, Z., Stylianou, O., Mukli, P., Eke, A. & Racz, F. S. Decreased connection density and modularity of functional brain networks during n-back working memory paradigm. Brain Behav. 11 (1), e01932. https://doi.org/10.1002/brb3.1932 (2021).
Krainik, A. et al. Postoperative speech disorder after medial frontal surgery: role of the supplementary motor area. Neurology 60, 587–594. https://doi.org/10.1212/01.WNL.0000048206.07837.59 (2003).
Kinoshita, M. et al. Role of fronto-striatal tract and frontal Aslant tract in movement and speech: an axonal mapping study. Brain Struct. Funct. 220 (6), 3399–3412. https://doi.org/10.1007/s00429-014-0863-0 (2015).
Tremblay, P. & Gracco, V. L. On the selection of words and oral motor responses: evidence of a response-independent fronto-parietal network. Cortex 46 (1), 15–28. https://doi.org/10.1016/j.cortex.2009.03.003 (2010).
Pastor, M. A., Macaluso, E., Day, B. L. & Frackowiak, R. S. The neural basis of Temporal auditory discrimination. NeuroImage 30, 512–520. https://doi.org/10.1016/j.neuroimage.2005.09.053 (2006).
Pollmann, S. & von Cramon, D. Y. Object working memory and visuospatial processing: functional neuroanatomy analyzed by event-related fMRI. Exp. Brain Res. 133, 12–22. https://doi.org/10.1007/s002210000396 (2000).
Hertrich, I., Dietrich, S. & Ackermann, H. The role of the supplementary motor area for speech and Language processing. Neurosci. Biobehav Rev. 68, 602–610. https://doi.org/10.1016/j.neubiorev.2016.06.030 (2016).
Stuss, D. T. & Alexander, M. P. Is there a dysexecutive syndrome? Philos. Trans. R Soc. B. 362 (1481), 901–915. https://doi.org/10.1098/rstb.2007.2096 (2007).
Hertrich, I., Dietrich, S., Blum, C. & Ackermann, H. The role of the dorsolateral prefrontal cortex for speech and Language processing. Front. Hum. Neurosci. 15, 645209. https://doi.org/10.3389/fnhum.2021.645209 (2021).
Huber, L. et al. Investigation of the neurovascular coupling in positive and negative BOLD responses in human brain at 7 T. Neuroimage, 97, 349 – 62. (2014). https://doi.org/10.1016/j.neuroimage.2014.04.022
Wilson, R., Thomas, A. & Mayhew, S. D. Spatially congruent negative BOLD responses to different stimuli do not summate in visual cortex. Neuroimage 218, 116891. https://doi.org/10.1016/j.neuroimage.2020.116891 (2020).
Mullinger, K. J., Mayhew, S. D., Bagshaw, A. P., Bowtell, R. & Francis, S. T. Evidence that the negative BOLD response is neuronal in origin: a simultaneous EEG-BOLD-CBF study in humans. Neuroimage 94, 263–274. https://doi.org/10.1016/j.neuroimage.2014.02.029 (2014).
Maggioni, E. et al. Investigation of negative BOLD responses in human brain through NIRS technique. A visual stimulation study. Neuroimage 108, 410–422. https://doi.org/10.1016/j.neuroimage.2014.12.074 (2015).
Wessel, J. R. & Anderson, M. C. Neural mechanisms of domain-general inhibitory control. Trends Cogn. Sci. 26, S1364–66132300258. https://doi.org/10.1016/j.tics.2023.09.008 (2023).
Dräger, B. et al. How does the brain accommodate to increased task difficulty in word finding? A functional MRI study. Neuroimage 23 (3), 1152–1160. https://doi.org/10.1016/j.neuroimage.2004.07.005 (2004).
Mehta, U. M. et al. A transdiagnostic evaluation of cortical Inhibition in severe mental disorders using transcranial magnetic stimulation. J Psychiatr Res. 143, 364–369. https://doi.org/10.1016/j.jpsychires.2021.09.049 (2021).
Curiel Cid, R. E., Matias-Guiu, J. A. & Loewenstein, D. A. A review of novel cognitive challenge tests for the assessment of preclinical alzheimer’s disease. Neuropsychology 37 (6), 661–672. https://doi.org/10.1037/neu0000883 (2023).
Elkana, O. et al. Sensitivity of neuropsychological tests to identify cognitive decline in highly educated elderly individuals: 12 months follow up. J. Alzheimers Dis. 49 (3), 607–616. https://doi.org/10.3233/JAD-150562 (2016).
Ghisletta, P., Aichele, S., Gerstorf, D., Carollo, A. & Lindenberger, U. Verbal fluency selectively predicts survival in old and very old age. Psychol. Sci. 36 (2), 87–101. https://doi.org/10.1177/09567976241311923 (2025).
Marko, M. et al. Assessment of automatic and controlled retrieval using verbal fluency tasks. Assessment 30 (7), 2198–2211. https://doi.org/10.1177/10731911221117512 (2023).
Marko, M., Michalko, D., Kubinec, A. & Riečanský, I. Measuring semantic memory using associative and dissociative retrieval tasks. R Soc. Open. Sci. 11 (2), 231208. https://doi.org/10.1098/rsos.231208 (2024).
Vonk, J. M. J. et al. Letter and category fluency performance correlates with distinct patterns of cortical thickness in older adults. Cereb. Cortex. 29 (6), 2694–2700. https://doi.org/10.1093/cercor/bhy138 (2019).
Qiao, Y. et al. Neurological activation during verbal fluency task and resting-state functional connectivity abnormalities in obsessive-compulsive disorder: a functional near-infrared spectroscopy study. Front. Psychiatry. 15, 1416810. https://doi.org/10.3389/fpsyt.2024.1416810 (2024).
Wu, P. et al. Prefrontal cortex functional connectivity changes during verbal fluency test in adults with short-term insomnia disorder: a functional near-infrared spectroscopy study. Front. Neurosci. 17, 1277690. https://doi.org/10.3389/fnins.2023.1277690 (2023).
Peirce, J. PsychoPy2: experiments in behavior made easy. Behav. Res. Methods. 51 (1), 195–203. https://doi.org/10.3758/s13428-018-01193-y (2019).
Zapała, D., Augustynowicz, P. & Tokovarov, M. Recognition of attentional States in VR environment: an fNIRS study. Sensors 22 (9), 3133. https://doi.org/10.3390/s22093133 (2022).
Mayr, U. On the dissociation between clustering and switching in verbal fluency: comment on troyer, moscovitch, winocur, Alexander and Stuss. Neuropsychologia 40 (5), 562–556. https://doi.org/10.1016/S0028-3932(01)00132-4 (2002).
Troyer, A., Moscovitch, M. & Winocur, G. Clustering and switching as two components of verbal fluency: evidence from younger and older healthy adults. Neuropsychology 11, 138–146. https://doi.org/10.1037/0894-4105.11.1.138 (1997).
Kocsis, L., Herman, P. & Eke, A. The modified Beer–Lambert law revisited. Phys. Med. Biol. 51 (5), N91. https://doi.org/10.1088/0031-9155/51/5/N02 (2006).
Fishburn, F. A., Ludlum, R. S., Vaidya, C. J. & Medvedev, A. V. Temporal derivative distribution repair (TDDR): a motion correction method for fNIRS. Neuroimage 184, 171–179. https://doi.org/10.1016/j.neuroimage.2018.09.025 (2019).
Cui, X., Bray, S. & Reiss, A. L. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. Neuroimage 49 (4), 3039–3046. https://doi.org/10.1016/j.neuroimage.2009.11.050 (2010).
Liu, Y. et al. Brain activation patterns in patients with post-stroke cognitive impairment during working memory task: a functional near-infrared spectroscopy study. Front. Neurol. 15, 1419128. https://doi.org/10.3389/fneur.2024.1419128 (2024).
Singh, A. K., Okamoto, M., Dan, H., Jurcak, V. & Dan, I. Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. NeuroImage 27 (4), 842–851. https://doi.org/10.1016/j.neuroimage.2005.05.019 (2005).
Author information
Authors and Affiliations
Contributions
P.K. and N.K-P. wrote the main manuscript and conducted the study; M.M. - data analysis; V.R., C.G., and J.P. verified the analytical methods and reviewed the original manuscript. All authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Krukow, P., Kopiś-Posiej, N., Rodríguez-González, V. et al. Effects of the combined letter fluency task on frontal cortex regional and dynamic oxygenation patterns. Sci Rep 15, 26468 (2025). https://doi.org/10.1038/s41598-025-12558-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-12558-7