Abstract
Patients with liver fibrosis and type 2 diabetes mellitus (T2DM) have an increased risk of cardiovascular events. However, long-term prognosis of liver fibrosis and T2DM after acute myocardial infarction (AMI) remain to be investigated. This study compared clinical characteristics and prognosis of AMI patients with T2DM and evidence of liver fibrosis. Patients were stratified into low, intermediate and high-risk for fibrosis, using serum-based non-invasive tests (NITs): Fibrosis-4 Index (FIB-4), Aspartate Aminotransferase to Platelet Ratio Index (APRI) and Non-alcoholic Fatty Liver Disease Fibrosis Score (NFS). The primary outcome was all-cause mortality, Kaplan-Meier curves were constructed for 5-year all-cause mortality. Cox regression analysis was used to determine the independent predictors of mortality, adjusting for confounders. Out of 3287 AMI patients, 1547 were stratified as high-risk by any NIT (mean follow-up duration 2.7 ± 2.3 years). A dose-response relationship was found with increasing mortality risk for higher APRI and NFS scores. High-risk FIB-4 also predicted mortality significantly (adjusted HR [aHR] 1.791, 95% CI 1.436–2.235, p < 0.001). High-risk FIB-4 and APRI independently predicted mortality regardless of T2DM status, while NFS only predicted mortality in T2DM patients. Following AMI, individuals stratified by FIB-4, APRI, NFS as high-risk for liver fibrosis were associated with excess long-term mortality (aHR 1.780, 95% CI 1.442–2.196, p < 0.001). Hence, readily available NITs may be beneficial in risk prognostication of AMI patients.
Similar content being viewed by others
Introduction
Acute myocardial infarction (AMI) is responsible for more than one-third of all deaths in developed nations1,2. AMI incidence is predicted to see a three-fold rise by 20503, and will become the leading driver of cardiovascular-related mortality4,5. Conventional risk stratification models such as the Global Registry of Acute Coronary Events (GRACE) Risk Score (GRS) have been used to estimate AMI prognosis and guide management6. However, current evidence suggests that risk stratification using GRS in AMI patients did not improve cardiovascular disease (CVD) events at 12 months compared to standard care7, and GRS predicts outcomes more poorly in patients with type 2 diabetes mellitus (T2DM)6. In the era of personalised medicine, enhanced risk stratification tools following AMI are needed8.
Previous studies have demonstrated that the presence of liver fibrosis is associated with increased liver and cardiovascular-related outcomes in the general population9, and is a key predictor of long-term mortality in the AMI population10,11,12,13,14. However, the gold standard for the diagnosis of hepatic fibrosis requires liver biopsy15, which is limited by procedural invasiveness and risk of bleeding, especially in the population with CVD requiring anti-platelet therapy16,17. As such, serum-based non-invasive tests (NITs) have been recommended in large-scale populations for the screening of hepatic fibrosis18, which include Fibrosis-4 Index (FIB-4), Aspartate Aminotransferase (AST) to Platelet Ratio Index (APRI) and Non-alcoholic Fatty Liver Disease Fibrosis Score (NFS)19. These NITs take advantage of readily available, low-cost serum-based biomarkers, and have been shown to add to the risk stratification of CVD risk in individuals with metabolic dysfunction-associated liver steatotic disease (MASLD). Studies have shown that FIB-4 ≥ 2.67 is associated with a 40% increase in cardiovascular mortality, and predicts major adverse cardiovascular events (MACE)17,20,21. Previous studies have shown that FIB-4 score is correlated with an increased risk of MACE and cerebrovascular events in patients following AMI, with or without the presence of T2DM19,22. However, other NITs such as APRI and NFS have not been investigated in a secondary AMI cohort.
The incorporation of NITs in conventional CVD risk prediction equations for the AMI population will require the examination of the performance of these liver indices in a secondary prevention population19,23. Given that more than one-third of people with concurrent T2DM and MASLD are affected by liver fibrosis24, there is a need to compare the utility of NITs in the population with and without T2DM. As such, this present study aims to fill the gap in the literature by performing head-to-head comparisons of NITs in the AMI cohort, stratified by the presence of T2DM25,26,27,28,29,30.
Patient and methods
In this retrospective observational cohort, we studied patients who presented with AMI between 1 January 2011 and 31 March 2021 at a tertiary hospital in Singapore. The academic centre is part of the Western network, consisting of one hub and two spoke hospitals, that provides percutaneous coronary intervention services to the western region of Singapore31. All patients were aged 18 years and older, presenting with either ST-elevation myocardial infarction (STEMI) or non-ST elevation myocardial infarction (NSTEMI), via the Emergency Department at the hub hospital or interhospital transfers from the two spoke hospitals. Patients with AMI were allocated into high, intermediate and low-risk groups using established NITs (FIB-4, APRI, and NFS) to evaluate liver fibrosis32,33. They were further stratified based on the presence of T2DM.
AMI diagnosis by attending cardiologists were based on clinical evidence of acute myocardial ischaemia, determined by a rise and/or fall of troponin values with at least one value above the 99th percentile upper reference limit coupled with at least one of the following: (1) symptoms of myocardial ischaemia, (2) new ischaemic electrocardiographic changes, (3) development of pathological Q waves, (4) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischaemic aetiology, or (5) identification of a coronary thrombus by angiography. These diagnoses fit the current universal definition of type 1 AMI34,35,36. Baseline demographic, past medical histories, clinical characteristics, angiographic and procedure characteristics and clinical outcomes were retrieved retrospectively from the electronic clinical records.
Non-invasive tests for advanced hepatic fibrosis
Serum-based NITs are simple and well-validated tools in evaluating advanced hepatic fibrosis for large-scale usage, given the prohibitive cost and availability of imaging33. Three serum-based NITs were used in this study. First, FIB-4 is an externally validated score using alanine aminotransferase (ALT), AST, age and platelet count. FIB-4 was calculated using FIB-4 = age [years] × AST [IU/L] / (platelet [× 109/L] × ALT [IU/L]1/2)37. Based on the established cut-offs, patients were allocated into high (FIB-4 ≥ 3.25), intermediate (1.3 ≤ FIB-4 < 3.25) and low-risk groups (FIB-4 < 1.3).34,38 High FIB-4 rules in advanced fibrosis with a positive predictive value of 65% and specificity of 97%.37 Low FIB-4 < 1.3 rules out advanced fibrosis with a 90% negative predictive value and sensitivity of 70%.37,37 An additional sensitivity analysis was done with a different cut-off of into high (FIB-4 ≥ 2.65), intermediate (1.30 ≤ FIB-4 < 2.65) and low-risk groups (FIB-4 < 1.30).40,41 Second, the APRI score was based on AST and platelet count, and calculated with the following formula: APRI = (AST [IU/L] / Upper normal limit of AST / platelet count [× 109/L]) × 100)32,42; Upper normal limit of AST = 56 IU/L.32 Patients were grouped into high (APRI ≥ 1.5), intermediate (0.3 < APRI < 1.5) and low-risk groups (APRI ≤ 0.3). High APRI ≥ 1.5 rules in significant fibrosis with a specificity of 91% and a sensitivity of 35%.32 Low APRI ≤ 0.3 rules out significant fibrosis with a specificity of 50%, sensitivity of 81%,32 and negative predictive value of 74%.43 Third, the NFS was calculated with NFS = -1.675 + (0.037 × age [years]) + (0.094 × body mass index (BMI) [kg/m2]) + (1.13 × hyperglycaemia) + (0.99 × AST [IU/L] / ALT [IU/L]) - (0.013 × platelet count [× 109/L]) - (0.66 × albumin [g/dL]).33,44 Patients were sorted into high (NFS > 0.676), intermediate (-1.455 ≤ NFS ≤ 0.676), and low-risk groups (NFS <-1.455).33,44 High NFS rules in significant fibrosis with a specificity of 96% and positive predictive value of 82%.45 Low NFS <-1.455 rules out significant fibrosis with a sensitivity of 77% and negative predictive value of 88%.45
Study outcomes
The primary outcome was long-term all-cause mortality. Secondary outcomes were cardiac arrest, heart failure, cardiogenic shock, stroke, 30-day cardiac and all-cause mortality. Outcomes were adjudicated according to prior AMI studies12,31,46,47,48. All-cause mortality was defined as any deaths regardless of the cause, while cardiac mortality was any deaths resulting from cardiovascular causes1. Cardiogenic shock was diagnosed as persistent hypotension, identified by a systolic blood pressure of less than 90 mmHg or an average arterial pressure that is 30 mmHg lower than the baseline value, cardiac index (< 1.8 L/min2 or < 2.2 L/min/m2 without and with haemodynamic support respectively), accompanied by adequate or elevated filling pressures (left ventricular end-diastolic pressure > 8 mmHg or right ventricular end-diastolic pressure > 10–15 mmHg)6. Heart failure was diagnosed based on clinical features and symptoms of congestion, along with structural and functional cardiac abnormalities6. Long-term mortality was retrieved from the mortality database that is accessible via the national integrated health information systems.
Statistical analysis
Statistical analysis was conducted on R 4.4.1 in R Studio. Continuous variables were presented as mean (standard deviation) for normally distributed data, and median (interquartile range) for non-normally distributed data. Categorical variables were presented as number (%). Continuous variables were compared by analysis of variance (ANOVA) for normally distributed data, and Kruskal-Wallis test for non-normally distributed data. Categorical variables were compared using Pearson chi-squared test. Kaplan-Meier (KM) curves were constructed for all-cause mortality for all three advanced fibrosis indices, and the high, intermediate and low-risk groups were compared using the log-rank test. Subgroup analysis was also performed based on T2DM status. A multivariable Cox proportional hazard regression model was designed to investigate the independent predictors of long-term all-cause mortality, which included the NITs (categorised into high, intermediate, and low-risk groups), age, sex, T2DM, previous AMI, STEMI, hyperlipidaemia and hypertension. These variables were adjudicated based on prior AMI studies13,46,47,49,50. To assess the association between continuous FIB-4, APRI and NFS scores with the clinical outcomes, a multivariable restricted cubic spline analysis was done using adjusted odds ratio. For further assessment of the association among NITs results, Kendall’s Coefficient of Concordance W (Kendall’s W) was used and corrected for ties. Kendall’s W quantifies the interrater reliability of ordinal ranking, which in this case is high, intermediate, and low-risk by FIB-4, APRI and NFS tests. Additionally, multivariable Cox proportional hazard regression model was fitted with the previously described variables to evaluate long-term all-cause mortality of patients who were categorised as high-risk by NITs. A p-value of < 0.05 was considered statistically significant.
The study was approved by the local institutional review committee in accordance with the revised Declaration of Helsinki (NHG Research—DSRB: 2021/00089-AMD0001). The institutional review board waived the need for written patient consent as this study involved a retrospective analysis of clinically acquired data.
Results
Baseline characteristics
A total of 3287 patients with available liver biomarkers to calculate APRI and FIB-4 were included from the AMI registry, of which only 2143 had data to calculate the NFS score. The mean follow-up duration of the study cohort was 2.7 ± 2.3 years. The mean age was 64 ± 13 years and 24.2% were female. Approximately half of the cohort presented with STEMI (51.3%). These baseline characteristics are summarised in Table 1.
Using FIB-4 score, the study cohort was stratified into 3 groups, with 455 (13.8%) patients in the low-risk, 1027 (31.2%) in the intermediate-risk and 1805 (54.9%) in the high-risk group. Patients with higher FIB-4 scores were significantly older (p < 0.001), had lower BMI (p < 0.001), with lower proportion of females (p < 0.001). Notably, T2DM was least prevalent in high FIB-4 group, compared to intermediate and low FIB-4 group (44.4% versus 57.5% versus 61.5% respectively, p < 0.001) (Table 1). The high-risk group was more likely to present with STEMI (65.5% versus 34.6% versus 32.5% respectively, p < 0.001), with lower LVEF (43 ± 13% versus 46 ± 14% versus 49 ± 14% respectively, p < 0.001), compared to intermediate and low-risk groups, based on FIB-4 (Supplementary Table S1, Supplementary Table S2).
The study cohort was also allocated into 3 groups using the APRI score – 685 (20.8%) were low-risk, 1369 (41.6%) were intermediate-risk and 1233 (37.5%) patients were high-risk. Patients with higher APRI scores were significantly younger (p < 0.001), with lower BMI (p = 0.011), and were less likely female (p < 0.001). The high-risk group had lowest rates of hypertension (56.4% versus 67.2% versus 78.4% respectively, p < 0.001) and T2DM (42.7% versus 51.2% versus 65.0% respectively, p < 0.001) compared to intermediate and low-risk groups (Table 1). The high-risk group was more likely to present with STEMI (76.1% versus 44.0% versus 21.2% respectively, p < 0.001) with lower LVEF (41 ± 13% versus 46 ± 14% versus 48 ± 14% respectively, p < 0.001), followed by intermediate and low-risk groups, based on APRI score (Supplementary Table S1, Supplementary Table S2).
The AMI cohort was stratified into 3 groups using NFS. A total of 161 (7.5%) patients were categorised as low-risk, 684 (31.9%) as intermediate-risk, and 1298 (60.6%) as high-risk. Patients with higher NFS tended to be female (p = 0.002), of older age (p < 0.001) and with higher BMI (p < 0.001). The high-risk group also had the highest rates of hypertension (74.3% versus 66.2% versus 52.2%, p < 0.001), T2DM (64.3% versus 44.2% versus 28.6%, p < 0.001), hyperlipidaemia (70.6% versus 63.6% versus 59.6%, p < 0.001), stroke (12.1% versus 10.1% versus 4.3%, p = 0.009) and previous AMI (31.6% versus 30.7% versus 19.3%, p = 0.006), followed by intermediate and low-risk groups (Table 1). The high-risk group experienced highest prevalence of STEMI (49.1% versus 42.5% versus 40.4% respectively, p = 0.006) and lowest LVEF (42 ± 14% versus 44 ± 14% versus 48 ± 15% respectively, p < 0.001) compared to intermediate and low-risk groups (Supplementary Table S1, Supplementary Table S2).
A comparison of the discharge medications are summarised in Supplementary Table S3.
Of these 2143 patients, 1547 were stratified as high-risk by any NIT. Among the individuals deemed high-risk of liver fibrosis, 488 (31.5%) were stratified as high-risk by two out of three NITs, and 656 (42.4%) were stratified as high-risk by all three NITs (Supplementary Fig. S1).
The risk score that identified the largest proportion of individuals at high-risk of liver fibrosis was NFS, regardless of obesity status and sex. NFS detected the highest proportion of high-risk individuals in the T2DM group, while FIB-4 detected the highest proportion of high-risk individuals in the non-T2DM group. The risk score that stratified the lowest proportion of individuals at high-risk of liver fibrosis was APRI across the T2DM, obesity and sex subgroups (Graphical Abstract). The association quantification of NITs using Kendall’s W reported a coefficient of 0.710, suggesting a substantial agreement among NITs rankings of high, intermediate and low-risks in the AMI population.
Study outcomes
FIB-4
Patients with high FIB-4 score experienced higher rates of in-hospital complications compared to those with intermediate and low FIB-4 scores. High FIB-4 group had higher rates of cardiac arrest (9.1% versus 2.6% versus 2.0% respectively, p < 0.001), heart failure (28.1% versus 24.4% versus 19.6% respectively, p < 0.001) and cardiogenic shock (20.1% versus 6.5% versus 5.7% respectively, p < 0.001) compared to intermediate and low FIB-4 groups. In addition, high FIB-4 group had the highest 30-day all-cause mortality (18.1% versus 6.7% versus 5.5% respectively, p < 0.001), and 30-day cardiac mortality (16.1% versus 5.6% versus 4.6% respectively, p < 0.001) compared to intermediate and low FIB-4 groups (Table 2). Our findings are similar in subgroup analyses of patients with and without T2DM (Supplementary Table S4). Sensitivity analysis using a FIB-4 cut-off of 1.30 and 2.67 revealed a similar finding of higher rates of cardiac arrest, heart failure and cardiogenic shock in the high FIB-4 group (Supplementary Table S5).
The KM curves demonstrated the divergence of survival following AMI sustained throughout the 5 years of follow-up, with excess mortality in the high-risk group, followed by the intermediate and low-risk groups (p < 0.0001). This trend was similar for patients with and without T2DM. Notably, in the T2DM group, there was a wider divergence in survival curves observed between the high-risk group and the other two groups (p < 0.0001) (Fig. 1). Cox regression analysis revealed that a high FIB-4 score was independently associated with higher long-term all-cause mortality (adjusted HR [aHR] 1.791, 95% CI 1.436–2.235, p < 0.001), but not the intermediate FIB-4 score (aHR 0.931, 95% CI 0.739–1.172, p = 0.541) after adjusting for important confounders (Table 3). A high FIB-4 score was also associated with higher all-cause mortality in patients with T2DM (aHR 1.737, 95% CI 1.351–2.234, p < 0.001) and without (aHR 2.314, 95%CI 1.411–3.793, p < 0.001) (Supplementary Table S6). Sensitivity analysis using a FIB-4 threshold of 1.30 and 2.67 revealed that high FIB-4 was associated with increased long-term all-cause mortality (aHR 1.594, 95% CI 1.280–1.985, p < 0.001) after adjusting for confounders. (Supplementary Table S7).
Kaplan-Meier survival curves of all-cause mortality in the AMI cohort stratified based on A) FIB-4 B) APRI and C) NFS. Subgroup analysis of all-cause mortality was performed in individuals without (center) and with T2DM (right). AMI, acute myocardial infarction; FIB-4, Fibrosis-4 index; APRI, Aspartate Aminotransferase (AST) to Platelet Ratio Index; NFS, Non-alcoholic Fatty Liver Disease Fibrosis Score; T2DM, type 2 diabetes mellitus.
The restricted cubic splines showed that FIB-4, as a continuous variable, had a positive linear relationship with all-cause mortality regardless of T2DM status (Supplementary Fig. S2). FIB-4 had a positive relationship with heart failure risk in patients without T2DM, and a non-linear relationship for cardiogenic shock in all patients, and cardiac arrest in patients with T2DM (Supplementary Fig. S3).
APRI
The high-risk group, based on APRI, had increased rates of cardiac arrest (13.4% versus 2.0% versus 1.3% respectively, p < 0.001), heart failure (28.2% versus 25.2% versus 22.5%, p = 0.025), cardiogenic shock (27.9% versus 6.5% versus 3.2%, p < 0.001) and stroke (5.5% versus 2.8% versus 2.9%, p < 0.001), compared to intermediate and low-risk groups. The high-risk group experienced least favourable 30-day all-cause mortality (22.8% versus 7.4% versus 5.5% respectively, p < 0.001), and 30-day cardiac mortality (21.0% versus 5.8% versus 4.5% respectively, p < 0.001), compared to intermediate and low-risk groups (Table 2). These trends were similar in the subgroup analyses of individuals with and without T2DM (Supplementary Table S4).
The KM curves demonstrated early excess mortality in the high-risk group, followed by the intermediate and low-risk groups based on APRI, with convergence of the survival curves at 5 years of follow-up (p < 0.001). Similar trends were observed in the subgroup analysis of individuals with and without T2DM (Fig. 1). After adjusting for confounders, Cox regression demonstrated that both high (aHR 2.666, 95% CI 2.221-3.200, p < 0.001) and intermediate-risk groups (aHR 1.284, 95% CI 1.085–1.519, p = 0.004) were independently associated with increased long-term all-cause mortality (Table 3). The high-risk group was associated with significantly higher all-cause mortality in patients with T2DM (aHR 2.863, 95% CI 2.292–3.576, p < 0.001) and without T2DM (aHR 2.486, 95% CI 1.798–3.437, p < 0.001). All-cause mortality of the intermediate-risk group was also higher in T2DM patients (aHR 1.312, 95% CI 1.072–1.604, p = 0.008). However, all-cause mortality for the intermediate-risk group did not achieve statistical significance in the non-T2DM patients (Supplementary Table S6).
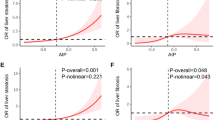
The restricted cubic splines showed that APRI, as a continuous variable, had a positive non-linear relationship with all-cause mortality risk in the overall AMI population, particularly in the non-T2DM group (Supplementary Fig. S2). On the other hand, the T2DM group demonstrated a positive linear relationship with APRI, as a continuous variable, with all-cause mortality risk. APRI had a positive linear relationship with stroke risk; while there was a positive non-linear relationship with heart failure (particularly in the non-T2DM cohort), cardiogenic shock, and cardiac arrest risks (Supplementary Fig. S4).
NFS
Based on NFS, the high-risk group was associated with increased rates of heart failure (35.2% versus 29.7% versus 14.3%, p < 0.001) and cardiogenic shock (19.0% versus 13.0% versus 3.1%, p < 0.001), in comparison to intermediate and low-risk groups. The high-risk group had the least favourable 30-day all-cause mortality (19.0% versus 9.2% versus 4.3%, p < 0.001), and 30-day cardiac mortality (16.4% versus 8.0% versus 3.1% respectively, p < 0.001), compared to the intermediate and low-risk groups (Table 2). These findings were similar in the subgroup analysis of patients without T2DM. In patients with T2DM, the high-risk group was associated with highest rates of cardiogenic shock, cardiac arrest, 30-day all-cause mortality, 30-day cardiac mortality and long-term all-cause mortality (Supplementary Table S4).
Notably, the KM curves revealed an early divergence in mortality following AMI, with excess mortality found in the high and intermediate NFS groups, compared to the low NFS group (p < 0.001). This trend was similar for groups with or without T2DM (Fig. 1). Cox regression demonstrated that high (aHR 2.127, 95% CI 1.399–3.232, p < 0.001) and intermediate (aHR 1.889, 95% CI 1.237–2.886, p = 0.003) NFS scores were independently associated with higher all-cause mortality (Table 3). However, subgroup analyses showed that high (aHR 3.462, 95% CI 1.630–7.350, p = 0.001) and intermediate NFS scores (aHR 2.929, 95% CI 1.365–6.289, p = 0.006) were significant predictors of mortality in patients with T2DM, but not in patients without T2DM (Supplementary Table S6). The restricted cubic splines showed that NFS, as a continuous variable, had a positive non-linear relationship with all-cause mortality risk in all AMI patients (Supplementary Fig. S2).
On the other hand, NFS demonstrated positive linear relationship with all-cause mortality risk in AMI individuals with T2DM. NFS had a positive linear relationship with heart failure risk in the non-T2DM group, and a positive non-linear relationship with the cardiogenic shock and cardiac arrest risk (particularly in the T2DM group) (Supplementary Fig. S5).
Number of high-risk NIT scores
Furthermore, Cox regression revealed that compared to individuals stratified as high-risk by only one out of three NITs, individuals stratified as high-risk by two NITs (aHR 1.460, 95% CI 1.189–1.794, p < 0.001) and individuals scored as high-risk by all three NITs (aHR 1.780, 95% CI 1.442–2.196, p < 0.001) were both independently associated with higher all-cause mortality (Supplementary Table S8). These findings were similar in the subgroup of patients with T2DM, however in patients without T2DM, such differences in all-cause mortality were not statistically significant (Supplementary Table S9).
Discussion
Previous studies have shown that FIB-4 score is correlated with an increased risk of MACE and cerebrovascular events in patients following AMI, with or without the presence of T2DM19,22. This study adds to the growing evidence that individuals at high-risk of liver fibrosis, scored based on NITs, had unfavourable survival following AMI. The key findings highlighted that (1) there was stepwise incremental risk in AMI-related complications including, cardiac arrest, heart failure, cardiogenic shock and stroke, with increasing risk scores for FIB-4, APRI and NFS, independent of T2DM status; (2) Cox regression showed that high FIB-4, intermediate and high APRI, and intermediate and high NFS risk groups were independent predictors of long-term mortality following AMI; (3) Although FIB-4 and APRI high-risk groups independently predicted mortality regardless of T2DM status, NFS only predicted mortality in the T2DM group; (4) A positive linear relationship with all three risk scores (as a continuous variable) and mortality risk following AMI was observed (Graphical Abstract).
Liver disease exists as a continuous spectrum from simple hepatic steatosis to steatohepatitis, that may progress to advanced fibrosis and cirrhosis13. Increasing liver fibrosis severity has been associated with higher incidence of cardiometabolic diseases and greater CVD severity51,52,53,54,55. Several studies have shown the increasingly adverse metabolic-inflammatory burden with higher degrees of liver fibrosis, including increased hepatic insulin resistance, dysregulated lipid and glucose metabolism, altered secretion of coagulation factors, systemic low-grade inflammation, and endothelial dysfunction2,27,56,57,58,59. Furthermore, this dose-response relationship between the severity of liver disease and adverse cardiovascular outcomes has been described in the AMI population, with individuals with hepatic fibrosis at significantly higher mortality risk in contrast to those without12,13. Our study highlights the prognostic value of the three liver indices given the stepwise incremental associations with AMI-related outcomes such as cardiac arrest, heart failure, cardiogenic shock, stroke, and mortality rates, from the low, intermediate to high-risk groups. As such, the use of liver indices for the assessment of hepatic fibrosis and its degree of severity holds promising potential in the role as both predictive and prognostic biomarkers following the AMI event12,23,59.
Several studies have examined each of the liver indices in silos, and have shown that high FIB-460, NFS61 and APRI62 score were significantly associated with elevated risk of MACE and all-cause mortality in patients with AMI. Our head-to-head comparison of the various NITs highlight that the high-risk category across all 3 liver NITs have important long-term prognostic value in risk stratifying the population presenting with AMI. Furthermore, this dose-response relationship from the low to intermediate and high-risk categories, associated with incremental rise in mortality risk, was evident in the APRI and NFS risk stratification, but not in the FIB-4. The current practice guidelines have identified FIB-4 as an effective tool for stratification of liver fibrosis risk, and our study extends the literature demonstrating that a high FIB-4 is also an effective predictor of all-cause mortality in the AMI cohort19,33,41. However, as intermediate FIB-4 did not predict a higher mortality risk compared to the low FIB-4 score, this highlights the need for a nuanced understanding of its use in mortality risk stratification following AMI63,64,65.
Our study also compared the use of different cut-offs of FIB-4 in predicting outcomes following AMI. The higher cut-off of 3.25 has been well validated in studies for ruling in severe fibrosis with a high specificity of 92.6% but a lower sensitivity of 41.4%.66,67 In an effort to improve sensitivity, recent studies have also proposed a lower cut-off of 2.67 to identify those at high risk for advanced fibrosis, with positive predictive values ranging from 60 to 80%, and a sensitivity of 70.7% and specificity of 79.1%.40,41,68,69 As such, the lower FIB-4 score of 2.67 has been recommended for initial screening while the higher FIB-4 score of 3.25 has been recommended for ruling in severe fibrosis66,69. Our sensitivity analysis demonstrated that both FIB-4 score of ≥ 2.67 and ≥ 3.25 independently predicted poorer long-term survival in AMI patients.
T2DM is a key driver of MASLD progression, with more than a third of individuals with concurrent T2DM and MASLD progressing to hepatic fibrosis24. Moreover, the associations between hepatic fibrosis and cardiometabolic risk factors are often bidirectional70,71, with the presence of fibrosis conferring an increased risk of incident T2DM72,73,74. Notably, prevalence of T2DM was higher in low and intermediate-risk versus high-risk groups when stratified by FIB-4 and APRI. Although more studies are needed to examine this finding, existing evidence suggests that MASLD without elevated serum aminotransferase is prevalent in individuals with concurrent T2DM, leading to falsely low FIB-4 and APRI scores75,76. While previous studies have examined the performance of serum-based scoring systems in patients with T2DM77, these scoring systems have yet to be evaluated in an AMI cohort stratified by T2DM12,22. Our study highlights the key finding that the high-risk groups determined by all three liver indices were predictive of mortality in the AMI population with T2DM. However, NFS was not predictive of mortality in patients without T2DM, in line with previous evidence which suggests limited sensitivity of NFS in this subgroup78. As the presence of hyperglycemia is one of the key components within the NFS formula, the risk of hepatic fibrosis will be underestimated in patients without T2DM78,79. While existing CVD risk-prediction models may underestimate the cardiovascular risk in the population with liver fibrosis – given that these models do not incorporate fibrosis-related risk factors such as insulin resistance, obesity, and hypertriglyceridemia74,80,81,82,83,84,85– the goal of improving the discriminant value in these models must be met with cautious integration of NITs with the understanding of the limitations of specific NITs in certain population groups (in particular, the NFS in the non-diabetic cohort).
The study incorporated continuous FIB-4, APRI and NFS scores into the restricted cubic splines to examine the correlation between these indices and cardiovascular events. The findings demonstrate a positive linear relationship with mortality risk for FIB-4, and a significant non-linear relationship for APRI and NFS following AMI. However, NFS did not demonstrate a significant relationship with mortality risk for non-T2DM patients. Notably, many of these liver biomarkers have been implemented in relation to a cross-sectional, binary assessment of categorical risk scores, which do not reflect the continuous and dynamic nature of liver fibrogenesis86. The integration of the levels of liver biomarkers as a continuum should be considered when developing future risk stratification tools.
Clinical implications
While current hepatology guidelines recommend the use of these NITs in the risk stratification of hepatic fibrosis within the primary care setting, little attention has been given to the prognostic utility in a secondary preventative AMI cohort33. The dose-response relationship of increasing mortality risk from low to intermediate and high-risk groups was only evident in the APRI and NFS risk stratification, but not in the FIB-4. Nevertheless, all three risk scores could identify high-risk individuals following AMI over a 5-year duration; although the utility of the NFS in risk stratification was not observed in the non-T2DM cohort. However, clinicians need to be cognisant that APRI identified the lowest proportion of individuals at high-risk of liver fibrosis, while NFS had the propensity to detect the highest proportion of high-risk individuals except in those without T2DM. The FIB-4 might be the most suitable risk stratification tool in the T2DM cohort19. The comorbidity of liver fibrosis, T2DM and AMI leads to the complex situation in which risk stratification is important72,73. While it was reassuring that more than two-thirds of AMI patients were concordantly stratified as high-risk by at least two out of three NITs (73.9%), our analysis further depicts the significant increasing mortality risk associated with higher number of concordant high-risk NITs (i.e. from those with one, to two, to all three high-risk NITs) within the AMI population.
Limitation
This study has its limitations. First, the gold standard liver biopsy for the identification and staging of liver fibrosis and ultrasound-based techniques such as elastography were not used. Nonetheless, the use of serum-based liver indices have been shown to be well-validated and feasible in the larger scale-screening of hepatic fibrosis, especially in an ‘all-comers’ AMI cohort12,33,70. In addition, as both imaging and biopsy are not feasible in a large-scale population screening, the use of readily available, low-cost serum based indicators may be important in identifying high risk patients87. Second, serum transaminases may be deranged during the acute phase of AMI. Most NITs used in hepatology practice incorporate AST. However, AST serves as a biomarker not only for hepatic conditions but also for cardiac and muscular disorders. In acute clinical settings, reliance on these indicators may lead to misinterpretation, as cardiac injuries disproportionately elevate AST levels compared to ALT. Therefore, it is essential to consider these limitations when interpreting results to avoid diagnostic confusion. Nevertheless, this confounder was mitigated by using serum transaminases measured at discharge as a more precise indicator of liver enzyme derangement88. Third, the study is limited by its retrospective, monocentric design, which may affect the generalizability of the findings. Given the varied prevalence of T2DM and MASLD across different populations, the generalisability and interpretation of the findings from this multi-ethnic Asian cohort should be made with caution, especially when applying these findings to other demographic cohorts89.
Conclusion
In conclusion, individuals at high risk of advanced hepatic fibrosis, staged by high FIB-4, high APRI, and high NFS scores, could predict all-cause mortality following AMI. Both FIB-4 and APRI were helpful risk stratification tools, irrespective of T2DM status; but this discrimination value was not observed with NFS in the non-T2DM cohort. The stepwise incremental risk of mortality and AMI-related complications, as well as the positive relationship with the liver risk scores and AMI-related mortality risk, suggest that these readily available tools can be beneficial for risk stratification of patients presenting with AMI, irrespective of the presence of T2DM. This may guide timely identification and management of high risk patients with AMI, with the concerted goal of improving cardiovascular prognosis. The study findings should be validated against future large prospective, non-selective AMI cohorts with systematic concomitant evaluation of hepatic fibrosis, preferably by liver elastography (short of biopsy descriptors), to better understand and assess the reproducibility of hepatic fibrosis biomarkers as a predictor of mortality and cardiovascular-related outcomes.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACEI:
-
Angiotensin Converting Enzyme Inhibitor
- aHR:
-
Adjusted Hazard Ratio
- ALT:
-
Alanine Aminotransferase
- AMI:
-
Acute Myocardial Infarction
- ANOVA:
-
Analysis of Variance
- APRI:
-
Aspartate Aminotransferase to Platelet Ratio Index
- ARB:
-
Angiotensin Receptor Blockers
- AST:
-
Aspartate Aminotransferase
- BMI:
-
Body Mass Index
- CI:
-
Confidence Interval
- CVD:
-
Cardiovascular Disease
- FIB-4:
-
Fibrosis-4 Index
- GRACE:
-
Global Registry of Acute Coronary Events
- GRS:
-
Global Registry of Acute Coronary Events Risk Score
- HDL:
-
High-density Lipoprotein
- HR:
-
Hazard Ratio
- Kendall’s W:
-
Kendall’s Coefficient of Concordance W
- KM:
-
Kaplan-Meier
- LDL:
-
Low-density Lipoprotein
- LVEF:
-
Left-ventricular Ejection Fraction
- MACE:
-
Major Adverse Cardiovascular Events
- MASLD:
-
Metabolic Dysfunction-associated Liver Steatotic Disease
- NFS:
-
Non-alcoholic Fatty Liver Disease Fibrosis Score
- NIT:
-
Serum-based Non-invasive Test
- NSTEMI:
-
Non-ST Elevation Myocardial Infarction
- STEMI:
-
ST-Elevation Myocardial Infarction
- T2DM:
-
Type 2 Diabetes Mellitus
References
Di Cesare, M. et al. The heart of the world. Glob Heart. 19, 11. https://doi.org/10.5334/gh.1288 (2024).
Gutierrez-Cuevas, J., Santos, A. & Armendariz-Borunda, J. Pathophysiological molecular mechanisms of obesity: A link between MAFLD and NASH with cardiovascular diseases. Int. J. Mol. Sci. 22 https://doi.org/10.3390/ijms222111629 (2021).
Chew, N. W. S. et al. Trends and predictions of metabolic risk factors for acute myocardial infarction: findings from a multiethnic nationwide cohort. Lancet Reg. Health West. Pac. 37, 100803. https://doi.org/10.1016/j.lanwpc.2023.100803 (2023).
Goh, R. S. J. et al. The burden of cardiovascular disease in Asia from 2025 to 2050: a forecast analysis for East asia, South asia, South-East asia, central asia, and high-income Asia Pacific regions. Lancet Reg. Health West. Pac. 49, 101138. https://doi.org/10.1016/j.lanwpc.2024.101138 (2024).
Chong, B. et al. Global burden of cardiovascular diseases: projections from 2025 to 2050. Eur. J. Prev. Cardiol. https://doi.org/10.1093/eurjpc/zwae281 (2024).
Gale, C. P. et al. Evaluation of risk scores for risk stratification of acute coronary syndromes in the myocardial infarction National audit project (MINAP) database. Heart 95, 221–227. https://doi.org/10.1136/hrt.2008.144022 (2009).
Gale, C. P. et al. Effectiveness of GRACE risk score in patients admitted to hospital with non-ST elevation acute coronary syndrome (UKGRIS): parallel group cluster randomised controlled trial. BMJ 381, e073843. https://doi.org/10.1136/bmj-2022-073843 (2023).
Figtree, G. A. et al. Clinical pathway for coronary atherosclerosis in patients without conventional modifiable risk factors: JACC State-of-the-Art review. J. Am. Coll. Cardiol. 82, 1343–1359. https://doi.org/10.1016/j.jacc.2023.06.045 (2023).
Sanyal, A. J. et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl. J. Med. 385, 1559–1569. https://doi.org/10.1056/NEJMoa2029349 (2021).
Fu, C. E. et al. A Meta-analysis on associated risk of mortality in nonalcoholic fatty liver disease. Endocr. Pract. 29, 33–39. https://doi.org/10.1016/j.eprac.2022.10.007 (2023).
Fu, C. E. et al. The prognostic value of including non-alcoholic fatty liver disease in the definition of metabolic syndrome. Aliment. Pharmacol. Ther. 57, 979–987. https://doi.org/10.1111/apt.17397 (2023).
Kong, G. et al. The prognostic value of metabolic dysfunction-associated steatotic liver disease in acute myocardial infarction: A propensity score-matched analysis. Diabetes Obes. Metab. https://doi.org/10.1111/dom.15660 (2024).
Chin, Y. et al. Hepatic steatosis and advanced hepatic fibrosis are independent predictors of long-term mortality in acute myocardial infarction. Diabetes Obes. Metab. 25, 1032–1044. https://doi.org/10.1111/dom.14950 (2023).
Kong, G. et al. A sex-disaggregated analysis of the prognostic value of lean type 2 diabetes mellitus in the adult population with acute myocardial infarction. Cardiovasc. Diabetol. 24, 59. https://doi.org/10.1186/s12933-024-02552-0 (2025).
Bravo, A. A., Sheth, S. G. & Chopra, S. Liver biopsy. N Engl. J. Med. 344, 495–500. https://doi.org/10.1056/NEJM200102153440706 (2001).
Ratziu, V. et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 128, 1898–1906. https://doi.org/10.1053/j.gastro.2005.03.084 (2005).
Enomoto, H., Bando, Y., Nakamura, H., Nishiguchi, S. & Koga, M. Liver fibrosis markers of nonalcoholic steatohepatitis. World J. Gastroenterol. 21, 7427–7435. https://doi.org/10.3748/wjg.v21.i24.7427 (2015).
Stahl, E. P. et al. Nonalcoholic fatty liver disease and the heart: JACC State-of-the-Art review. J. Am. Coll. Cardiol. 73, 948–963. https://doi.org/10.1016/j.jacc.2018.11.050 (2019).
Chew, N. W. S. et al. Cardiovascular-Liver-Metabolic health: recommendations in screening, diagnosis, and management of metabolic Dysfunction-Associated steatotic liver disease in cardiovascular disease via modified Delphi approach. Circulation 151, 98–119. https://doi.org/10.1161/CIRCULATIONAHA.124.070535 (2025).
Yong, J. N. et al. Non-alcoholic fatty liver disease association with structural heart, systolic and diastolic dysfunction: a meta-analysis. Hepatol. Int. 16, 269–281. https://doi.org/10.1007/s12072-022-10319-6 (2022).
Pouwels, S. et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 22, 63. https://doi.org/10.1186/s12902-022-00980-1 (2022).
Koh, J. et al. Long-term all-cause mortality of metabolic-dysfunction associated steatotic liver disease based on body weight phenotypes following acute myocardial infarction: A retrospective cohort study. Diabetes Obes. Metab. 27, 683–696. https://doi.org/10.1111/dom.16062 (2025).
Chew, N. W. S. et al. FIB-4 predicts MACE and cardiovascular mortality in patients with nonalcoholic fatty liver disease. Can. J. Cardiol. 38, 1779–1780. https://doi.org/10.1016/j.cjca.2022.07.016 (2022).
Qi, X., Li, J., Caussy, C., Teng, G. J. & Loomba, R. Epidemiology, screening, and co-management of type 2 diabetes mellitus and metabolic dysfunction-associated steatotic liver disease. Hepatology https://doi.org/10.1097/HEP.0000000000000913 (2024).
Muthiah, M. et al. Type 2 diabetes mellitus in metabolic-associated fatty liver disease vs. type 2 diabetes mellitus non-alcoholic fatty liver disease: a longitudinal cohort analysis. Ann. Hepatol. 28, 100762. https://doi.org/10.1016/j.aohep.2022.100762 (2023).
Kueh, M. T. W., Chew, N. W. S., Al-Ozairi, E. & le Roux, C. W. The emergence of obesity in type 1 diabetes. Int. J. Obes. (Lond). 48, 289–301. https://doi.org/10.1038/s41366-023-01429-8 (2024).
Yaow, C. Y. L. et al. Higher risk of adverse cardiovascular outcomes in females with type 2 diabetes mellitus: an umbrella review of systematic reviews. Eur. J. Prev. Cardiol. 30, 1227–1235. https://doi.org/10.1093/eurjpc/zwad133 (2023).
Chan, K. E. et al. Longitudinal Outcomes Associated With Metabolic Dysfunction-Associated Steatotic Liver Disease: A Meta-analysis of 129 Studies. Clin. Gastroenterol. Hepatol. 22, 488–498 e414 (2024). https://doi.org/10.1016/j.cgh.2023.09.018
Lee, E. C. Z. et al. The global epidemic of metabolic fatty liver disease. Curr. Cardiol. Rep. 26, 199–210. https://doi.org/10.1007/s11886-024-02025-6 (2024).
Anand, V. V. et al. General public’s knowledge, awareness, and perception of cardiometabolic diseases: data from a Singapore study population. Front. Med. (Lausanne). 10, 1193829. https://doi.org/10.3389/fmed.2023.1193829 (2023).
Chin, Y., Lim, J. & Kong, G. & et.al. Hepatic steatosis and advanced hepatic fibrosis are independent predictors of long-term mortality in acute myocardial infarction. Diabetes Obes. Metab 25, 1032–1044 https://doi.org/10.1111/dom.14950
Loaeza-del-Castillo, A., Paz-Pineda, F., Oviedo-Cardenas, E., Sanchez-Avila, F. & Vargas-Vorackova, F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann. Hepatol. 7, 350–357 (2008).
European Association for the Study of the Liver. Electronic address, e. e. e., Clinical Practice Guideline & Chair, P. representative, E. G. B. & Panel, m. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J. Hepatol. 75, 659–689 (2021). https://doi.org/10.1016/j.jhep.2021.05.025
Thygesen, K. et al. Fourth Universal Definition of Myocardial Infarction Circulation 138, e618-e651 (2018). (2018). https://doi.org/10.1161/CIR.0000000000000617
Chew, N. W. S. et al. Meta-Analysis of percutaneous coronary intervention versus coronary artery bypass grafting for left main narrowing. Am. J. Cardiol. 173, 39–47. https://doi.org/10.1016/j.amjcard.2022.02.044 (2022).
Chew, N. W. S. et al. Coronary artery bypass grafting versus percutaneous coronary intervention for multivessel coronary artery disease: A One-Stage Meta-Analysis. Front. Cardiovasc. Med. 9, 822228. https://doi.org/10.3389/fcvm.2022.822228 (2022).
Sterling, R. K. et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43, 1317–1325. https://doi.org/10.1002/hep.21178 (2006).
Song, J. et al. Fibrosis-4 stage of liver fibrosis predicts cardiovascular outcomes in acute coronary syndrome patients with and without type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 195, 110206. https://doi.org/10.1016/j.diabres.2022.110206 (2023).
Shah, A. G. et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 7, 1104–1112. https://doi.org/10.1016/j.cgh.2009.05.033 (2009).
Liver, E. A. & Obesity, E. A. f. t. S. o. t., Diabetes, E. A. f. t. S. o. f. t. S. o. EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). Obesity Facts 17, 374–443 (2024).
Rinella, M. E. et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 77, 1797–1835 (2023).
Wai, C. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38, 518–526. https://doi.org/10.1053/jhep.2003.50346 (2003).
Sripongpun, P. et al. Evaluation of aspartate aminotransferase to platelet ratio index and fibrosis 4 scores for hepatic fibrosis assessment compared with transient elastography in chronic hepatitis C patients. JGH Open. 4, 69–74. https://doi.org/10.1002/jgh3.12219 (2020).
J, M. & Zając Nafld fibrosis score calculator, https://www.omnicalculator.com/health/nafld-fibrosis-score (.
Angulo, P. et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45, 846–854. https://doi.org/10.1002/hep.21496 (2007).
Kong, G. et al. Prognostic outcomes in acute myocardial infarction patients without standard modifiable risk factors: A multiethnic study of 8,680 Asian patients. Front. Cardiovasc. Med. 9, 869168. https://doi.org/10.3389/fcvm.2022.869168 (2022).
Chew, N. W. S. et al. Long-Term prognosis of acute myocardial infarction associated with metabolic health and obesity status. Endocr. Pract. 28, 802–810. https://doi.org/10.1016/j.eprac.2022.05.007 (2022).
Chew, N. W. S. et al. Long-term prognosis in patients with concomitant acute coronary syndrome and aortic stenosis. Can. J. Cardiol. 38, 1220–1227. https://doi.org/10.1016/j.cjca.2022.03.010 (2022).
Kong, G. et al. Long-term outcomes in acute coronary syndrome patients without standard modifiable risk factors: a multi-ethnic retrospective cohort study of 5400 Asian patients. J. Thromb. Thrombolysis. 54, 569–578. https://doi.org/10.1007/s11239-022-02704-7 (2022).
Kong, G. et al. Long-Term prognosis of patients with coexisting obesity and malnutrition after acute myocardial infarction: A cohort study. Circ. Cardiovasc. Qual. Outcomes. 16, e009340. https://doi.org/10.1161/CIRCOUTCOMES.122.009340 (2023).
Lonardo, A., Nascimbeni, F., Mantovani, A. & Targher, G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J. Hepatol. 68, 335–352. https://doi.org/10.1016/j.jhep.2017.09.021 (2018).
Ng, C. H. et al. Living in the non-alcoholic fatty liver disease silent epidemic: a qualitative systematic review of patients’ perspectives. Aliment. Pharmacol. Ther. 56, 570–579. https://doi.org/10.1111/apt.17121 (2022).
Kong, G. et al. A two-decade population-based study on the effect of hypertension in the general population with obesity in the united States. Obes. (Silver Spring). 31, 832–840. https://doi.org/10.1002/oby.23658 (2023).
Anand, V. V. et al. Barriers and facilitators to engagement with a weight management intervention in Asian patients with overweight or obesity: A systematic review. Endocr. Pract. 29, 398–407. https://doi.org/10.1016/j.eprac.2022.10.006 (2023).
Ng, C. H. et al. Hypertension is prevalent in non-alcoholic fatty liver disease and increases all-cause and cardiovascular mortality. Front. Cardiovasc. Med. 9, 942753. https://doi.org/10.3389/fcvm.2022.942753 (2022).
Targher, G., Tilg, H. & Byrne, C. D. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 6, 578–588. https://doi.org/10.1016/S2468-1253(21)00020-0 (2021).
Chong, B. et al. Epicardial adipose tissue assessed by computed tomography and echocardiography are associated with adverse cardiovascular outcomes: A systematic review and Meta-Analysis. Circ. Cardiovasc. Imaging. 16, e015159. https://doi.org/10.1161/CIRCIMAGING.122.015159 (2023).
Chew, N. W. S. et al. The genetic interactions between non-alcoholic fatty liver disease and cardiovascular diseases. Front. Genet. 13, 971484. https://doi.org/10.3389/fgene.2022.971484 (2022).
Chew, N. W. S., Ng, C. H., Muthiah, M. D. & Sanyal, A. J. Comprehensive review and updates on holistic approach towards Non-Alcoholic fatty liver disease management with cardiovascular disease. Curr. Atheroscler Rep. 24, 515–532. https://doi.org/10.1007/s11883-022-01027-5 (2022).
Cao, M., Li, T., Li, Z., Gong, F. & Chen, Z. A retrospective study on the relationship between fibrosis–4 index and all–cause mortality in patients with acute myocardial infarction. Exp. Ther. Med. 24, 643. https://doi.org/10.3892/etm.2022.11580 (2022).
He, H. M. et al. A comparison of the prognostic value of liver fibrosis scores in acute myocardial infarction patients with and without type 2 diabetes. Angiology 75, 219–230. https://doi.org/10.1177/00033197231197230 (2024).
Biccire, F. G. et al. Relationship between non-invasively detected liver fibrosis and in-hospital outcomes in patients with acute coronary syndrome undergoing PCI. Clin. Res. Cardiol. 112, 236–246. https://doi.org/10.1007/s00392-022-02078-z (2023).
Kumari, B. et al. Diagnostic accuracy of FIB-4 and FIB-5 scores as compared to fibroscan for assessment of liver fibrosis in patients with Non-Alcoholic fatty liver disease. Cureus 13, e17622. https://doi.org/10.7759/cureus.17622 (2021).
Xu, X. L. et al. The role of fibrosis index FIB-4 in predicting liver fibrosis stage and clinical prognosis: A diagnostic or screening tool? J. Formos. Med. Assoc. 121, 454–466. https://doi.org/10.1016/j.jfma.2021.07.013 (2022).
Roh, Y. H., Kang, B. K., Jun, D. W., Lee, C. M. & Kim, M. Role of FIB-4 for reassessment of hepatic fibrosis burden in referral center. Sci. Rep. 11, 13616. https://doi.org/10.1038/s41598-021-93038-6 (2021).
Huttman, M. et al. Liver fibrosis stage based on the four factors (FIB-4) score or forns index in adults with chronic hepatitis C. Cochrane Database Syst. Rev. 8, CD011929 (2024).
Vallet-Pichard, A. et al. FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 46, 32–36 (2007).
Kanwal, F. et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 161, 1657–1669 (2021).
Roh, Y. H., Kang, B. K., Jun, D. W., Lee, C. & Kim, M. Role of FIB-4 for reassessment of hepatic fibrosis burden in referral center. Sci. Rep. 11, 13616 (2021).
Jain, V. et al. Immune activation mediates the association of advanced hepatic fibrosis with adverse outcomes in patients with coronary artery disease. J. Am. Heart Assoc. 12, e031230. https://doi.org/10.1161/JAHA.123.031230 (2023).
Lee, T. B. Jr. et al. Biomarkers of hepatic dysfunction and cardiovascular risk. Curr. Cardiol. Rep. 25, 1783–1795. https://doi.org/10.1007/s11886-023-01993-5 (2023).
Dyson, J. et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 60, 110–117. https://doi.org/10.1016/j.jhep.2013.08.011 (2014).
Zoppini, G. et al. Mortality from chronic liver diseases in diabetes. Am. J. Gastroenterol. 109, 1020–1025. https://doi.org/10.1038/ajg.2014.132 (2014).
Chew, N. W. S. et al. Type 2 diabetes mellitus and cardiometabolic outcomes in metabolic dysfunction-associated steatotic liver disease population. Diabetes Res. Clin. Pract. 211, 111652. https://doi.org/10.1016/j.diabres.2024.111652 (2024).
Singh, J. et al. Lower FIB-4 threshold in patients with diabetes improves diagnostic accuracy of the test in a Hispanic population. Transl Gastroenterol. Hepatol. 9, 16. https://doi.org/10.21037/tgh-23-62 (2024).
Vigano, M. et al. Accuracy of FIB-4 to detect elevated liver stiffness measurements in patients with Non-Alcoholic fatty liver disease: A Cross-Sectional study in referral centers. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms232012489 (2022).
Anstee, Q. M. et al. Prognostic utility of Fibrosis-4 index for risk of subsequent liver and cardiovascular events, and all-cause mortality in individuals with obesity and/or type 2 diabetes: a longitudinal cohort study. Lancet Reg. Health Eur. 36, 100780. https://doi.org/10.1016/j.lanepe.2023.100780 (2024).
Goh, G. B. et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 3, 141–145. https://doi.org/10.1016/j.bbacli.2014.09.001 (2015).
Yoneda, M. et al. Noninvasive scoring systems in patients with nonalcoholic fatty liver disease with normal Alanine aminotransferase levels. J. Gastroenterol. 48, 1051–1060. https://doi.org/10.1007/s00535-012-0704-y (2013).
Lin, C. et al. Mortality, cardiovascular, and medication outcomes in patients with myocardial infarction and underweight in a Meta-Analysis of 6.3 million patients. Am. J. Cardiol. 196, 1–10. https://doi.org/10.1016/j.amjcard.2023.02.023 (2023).
Chin, Y. H. et al. The placebo response rate and Nocebo events in obesity Pharmacological trials. A systematic review and meta-analysis. EClinicalMedicine 54, 101685. https://doi.org/10.1016/j.eclinm.2022.101685 (2022).
Chin, Y. H. et al. Meta-analysis of the placebo and Nocebo effects associated with placebo treatment in randomized trials of lipid-lowering therapies. Eur. Heart J. Qual. Care Clin. Outcomes. 9, 511–519. https://doi.org/10.1093/ehjqcco/qcac060 (2023).
Pan, X. H. et al. Efficacy and safety of tirzepatide, GLP-1 receptor agonists, and other weight loss drugs in overweight and obesity: a network meta-analysis. Obes. (Silver Spring). 32, 840–856. https://doi.org/10.1002/oby.24002 (2024).
Chew, H. S. J. et al. Anthropometric and cardiometabolic effects of polyphenols in people with overweight and obesity: an umbrella review. Nutr. Rev. 82, 1556–1593. https://doi.org/10.1093/nutrit/nuad163 (2024).
Anand, V. V. et al. Sex differences in survival following acute coronary syndrome with and without standard modifiable risk factors. Clin. Res. Cardiol. https://doi.org/10.1007/s00392-024-02563-7 (2024).
Patel, K. & Sebastiani, G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2, 100067. https://doi.org/10.1016/j.jhepr.2020.100067 (2020).
Canivet, C. M. & Boursier, J. Screening for liver fibrosis in the general population: where do we stand in 2022? Diagnostics (Basel). 13. https://doi.org/10.3390/diagnostics13010091 (2022).
Gowda, S. et al. A review on laboratory liver function tests. Pan Afr. Med. J. 3, 17 (2009).
Collaborators, G. B. D. C. The global, regional, and National burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol. Hepatol. 5, 245–266. https://doi.org/10.1016/S2468-1253(19)30349-8 (2020).
Acknowledgements
Not applicable.
Funding
This was supported by the CSDU Clinician-Scientist Grant, and the National Medical Research Council Transition Award (TA24jul-0008), and the CArdiovascular DiseasE National Collaborative Enterprise (CADENCE) National Clinical Translational Program (MOH-001277-01). N.W.S.C has received research grant support from NUHS Seed Fund (NUHSRO/2022/RO5 + 6/Seed-Mar/03), National Medical Research Council Research Training Fellowship (MH 095:003/008-303), National University of Singapore Yong Loo Lin School of Medicine’s Academic Fellowship Scheme, the NUHS Clinician Scientist Program (NCSP2.0/2024/NUHS/NCWS), CSDU Clinician-Scientist Grant, and the National Medical Research Council Transition Award (TA24jul-0008). M.Y.C. receives speaker’s fees and research grants from Astra Zeneca, Abbott Technologies, and Boston Scientific.
Author information
Authors and Affiliations
Contributions
G.K, J.C and N.W.S.C conceived and designed the study. J.C was involved in data acquisition and statistical analyses. E.W, Y.C, and G.K were involved in interpretation of data. E.W, Y.C, G.K, J.C, A.Z, A.M, J.J, B.C, G.C, B.N, M.Y.C, M.M, P.L, M.A.M and N.W.S.C drafted the manuscript. N.W.S.C provided supervision and mentorship. All authors reviewed and revised the manuscript, gave final approval, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the local institutional review committee in accordance with the revised Declaration of Helsinki (NHG Research—DSRB: 2021/00089-AMD0001). The institutional review board waived the need for written patient consent as this study involved a retrospective analysis of clinically acquired data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wong, E., Chen, Y., Kong, G. et al. Non-invasive scoring systems of liver fibrosis predict prognosis in the cohort with myocardial infarction. Sci Rep 15, 26499 (2025). https://doi.org/10.1038/s41598-025-12583-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12583-6