Abstract
Research has shown inconsistent links between triglyceride and glucose related indexes (TyG-related indexes) and metabolic dysfunction-associated fatty liver disease (MAFLD) across various groups and areas. Our research focuses on exploring these connections to improve early identification and prevention of MAFLD among rural residents in Xinjiang, China. A total of 10,703 rural residents were included in this cohort study. Cox regression modeling, receiver operating characteristic curves (ROC), restricted cubic spline (RCS) scores, subgroup analyses, and sensitivity analyses were used to conduct the study. Over a 5.7-year follow-up, 5.6% of participants developed MAFLD. Higher TyG-related indexes were linked to increased MAFLD risk after adjusting for confounders (P < 0.05). TyG-WHtR increased significantly in both sexes. The top quartile of TyG, TyG-BMI, TyG-WC, and TyG-WHtR had higher MAFLD risk compared to the bottom quartile, with TyG-BMI being the strongest predictor, particularly in unmarried individuals aged 18–30. Nonlinear relationships were observed for most TyG-related indexes and MAFLD risk, except for male TyG. ROC analysis confirmed the diagnostic value of TyG-related indexes for predicting MAFLD. TyG-related indexes were independently associated with MAFLD, with a stronger association observed in females. Furthermore, TyG-related indexes demonstrated predictive value for MAFLD, with TyG-BMI exhibiting the most robust predictive performance, particularly among unmarried individuals aged 18–30.
Similar content being viewed by others
Introduction
In 2024, the Hepatology Branch of the Chinese Medical Association officially proposed to rename non-alcoholic fatty liver disease (NAFLD) as metabolic dysfunction-associated fatty liver disease (MAFLD)1. MAFLD was the most prevalent liver-related disease globally, with various contributing factors such as insulin resistance, oxidative damage, genetic predisposition, and environmental influences2. Research indicated that MAFLD, unlike non-alcoholic fatty liver disease (NAFLD), encompasses both obesity and metabolic irregularities, making it a more accurate identifier of patients with complex fatty liver and liver fibrosis3,4,5,6,7. Additionally, MAFLD was viewed as the hepatic manifestation of metabolic syndrome (MS)8. The prevalence of MAFLD had risen notably in Asia due to lifestyle changes, reaching 30–40% in the general population9. Currently, the prevalence of MAFLD in adults was as high as 35.58%10, which has replaced viral hepatitis as the number one chronic liver disease in China and has become a significant public health issue. Therefore, the prevention and treatment of MAFLD was urgent.
Some studies suggested that fatty liver can be detected early using simple methods, such as the triglyceride and glucose index (TyG), which was cost-effective and suitable for large-scale screening. Crowd research had shown promising results11. At the same time, related indexes such as TyG-BMI, TyG-WC, and TyG-WHtR were also derived. In addition, various factors were related to the onset of MAFLD, and IR played a vital role in the onset of MAFLD. Pathological changes in the intestinal microbiota and increased IR in adipose tissue and skeletal muscle affect hepatic lipid metabolism to promote further hepatic fat accumulation and inflammation12. However, there were still inconsistent conclusions on the specific relationship between the TyG-related indexes and MAFLD, and the differences may be mainly reflected in aspects such as sex and population13. Some studies have reported a positive correlation between TyG index level and the risk of MAFLD14. Ru Zhang et al. found that there was a linear relationship between the TyG index and the risk of MAFLD in males and a non-linear relationship in females15. Other studies have found no correlation between the TyG index and the risk of MAFLD in females. Although there was a positive correlation between the TyG index and the risk of MAFLD in males, there was a saturation threshold effect. When the TyG index was ≥ 8.64, the male TyG index and MAFLD risk were irrelevant16. In addition, other TyG-related indexes also have similar differences regarding sex, population, and region17,18,19,20,21. Xinjiang is located on the northwest border of China. It was a multi-ethnic area with a unique natural environment and eating habits. The diet was mainly based on dairy products and high-calorie foods, which may differ from people in other areas. Previous research by this research found that the prevalence of fatty liver among rural people in Xinjiang reached 22.2%, and there were metabolic risk factors such as obesity, blood pressure, and blood lipid and blood glucose factors22. Moreover, research on the relationship between TyG-related indexes and the risk of MAFLD has not been reported in this region, so it was indispensable to use TyG-related indexes for early screening of MAFLD. Therefore, our study focused on the rural population in Xinjiang, collecting data from 2016 to 2022 using a prospective cohort study that included physical exams, blood tests, and questionnaires. The goal was to understand the relationship between TyG-related indexes and MAFLD, identify high-risk groups early, and provide valuable insights for developing comprehensive prevention strategies for MAFLD in Xinjiang’s rural areas.
Materials and methods
Study population
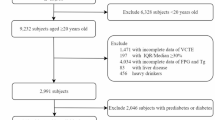
A cohort study in southern Xinjiang, China, used a typical sampling method to enroll 14,376 rural residents aged 18 or older from the 51 st Farm. From 2019 to 2022, follow-up surveys were conducted every year. After excluding 403 floating population and pregnant women, those who were unable to participate in this survey, 516 people with incomplete basic information, 239 people with missing ultrasound examination information, and 424 people with serious diseases, 12,794 participants were successfully followed up, with a follow-up rate of 88.9%. This study further excluded 2,091 individuals with baseline MAFLD and ultimately included 10,703 individuals (Fig. 1). The study followed the Declaration of Helsinki guidelines and was approved by the Institutional Ethics Review Board of the First Affiliated Hospital of Shihezi University School of Medicine (IERB no.: 2016–121-01).
Data collection
Clinical examinations and questionnaires were administered by trained healthcare professionals at the hospital’s physical examination center. In order to ensure the accuracy of the findings, medical insurance and hospitalization records from the years 2016 to 2022 were also reviewed. The clinical examination encompassed measurements of height, weight, BMI (body mass index), WC (waist circumference), blood pressure, systolic blood pressure (SBP), diastolic blood pressure (DBP), as well as the presence of Diabetes mellitus type 2 (T2DM), hypertension, dyslipidemia, or fatty liver. The final blood pressure reading was calculated as the average of three measurements. Chronic kidney injury was assessed using estimated glomerular filtration rate (eGFR)23. Diagnosis of T2DM and hypertension followed established criteria24,25. Obesity (BMI ≥ 30 kg/m2) was defined in accordance with the World Health Organization’s diagnostic guidelines for the adult population26. Dyslipidemia was identified by the presence of elevated levels of total cholesterol (TC) ≥ 6.22 mmol/L, triglycerides (TG) ≥ 2.26 mmol/L, low levels of high-density lipoprotein cholesterol (HDL-C) < 1.04 mmol/L, or high levels of low-density lipoprotein cholesterol (LDL-C) ≥ 4.14 mmol/L27. Abdominal ultrasonography was performed by a professional clinician.
Biochemical analysis
Participants fasted overnight for blood tests. Baseline lab tests included triglycerides, total cholesterol, HDL-C, LDL-C, fasting glucose, and serum creatinine. Blood was drawn after an 8-h fast, centrifuged to separate serum, and stored at −20 °C. All tests were conducted using an Olympus AU 2700 analyzer (Olympus Diagnostics, Hamburg, Germany) at the First Affiliated Hospital of Shihezi University School of Medicine’s lab.
Questionnaire survey
In the baseline and follow-up surveys, participants were interviewed in person using standardized questionnaires to collect demographic information, lifestyle habits, medical history, family background, and current medication use. Smoking was defined as having smoked ≥ 100 cigarettes or smoking continuously for ≥ 6 months28. Drinking was defined as consuming alcohol at least twice a month29.
Key definitions
MAFLD was defined as the presence of hepatic steatosis along with at least one of the following criteria: overweight/obesity (BMI ≥ 23.0 kg/m2 in Asian populations), type 2 diabetes mellitus (T2DM), or metabolic dysregulation30. However, our study did not assess the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) and plasma high-sensitivity C-reactive protein levels. The WHO recommends that adults engage in at least 150 min of moderate to vigorous physical activity (MVPA) per week, with each session lasting at least 10 minutes31. This study was conducted in rural areas. Most of the participants were farmers. Each farming session lasted for more than one hour. Therefore, activities such as lifting heavy objects, digging, and doing farm work were regarded as vigorous activities, while activities such as lifting light objects and doing housework were regarded as moderate-intensity activities. After referring to the suggestions of the WHO and combining the data of this questionnaire, the Movement were divided into no exercise, 1–3 times per week, and more than 4 times per week.
Anthropometric index calculation
Statistical analysis
The descriptive analysis presented continuous variables as mean ± SD or median (IQR), and categorical variables as proportions. The t-test and chi-square test were used to compare continuous and categorical variables, respectively. The TyG-related index was evaluated both as a continuous variable and in quartiles. The association between TyG-related indexes and MAFLD was examined using Cox proportional hazards models, quantified by HR and 95% CI. The predictive value of TyG-related indexes for MAFLD risk was assessed using ROC curves and AUC values, with AUC > 0.7 indicating acceptable prediction performance. Subgroup and sensitivity analyses were conducted to assess the stability of the association and explore interactions. RCS analysis was performed to investigate the dose–response relationship between TyG-related indexes and MAFLD risk, adjusting for covariates such as age, sex, TC, HDL-C, LDL, SCr, marital status, movement, smoking, and drinking. Statistical analyses were carried out using SPSS (version 25.0), MedCalc (Version 20.1.0), and R software (version 4.3.3), with statistical significance defined as a two-sided P-value < 0.05.
Results
Baseline characteristics
Baseline data indicated a MAFLD prevalence of 16.3% (2091/12,794 participants), with male at 15.7% and female at 17%. After excluding those with existing or past MAFLD, the cohort study comprised 10,703 participants, whose baseline characteristics were outlined in Table 1. Over a mean follow-up of 5.70 ± 0.44 years, 5.6% (596/10,703) developed MAFLD, higher among females at 6.7% (357/5332) vs. males. Participants’ mean age was 36.78 ± 14.08 years, with average TyG, TyG-BMI, TyG-WC, and TyG-WHtR values of 8.50 ± 0.70, 213.47 ± 43.20, 757.87 ± 144.72, and 4.66 ± 0.88, respectively. MAFLD patients had higher mean values for age, BMI, WC, WHtR, SBP, DBP, TC, LDL-C, and TyG-related indexes compared to non-MAFLD patients (all P < 0.05). The cumulative incidence of MAFLD was notably higher among those over 60 years of age, individuals with a primary school education or lower, widowed individuals, and those with dyslipidemia (all P < 0.05) (Table 1). Furthermore, females showed higher values for BMI, WHtR, DBP, HDL-C, and TyG-WHtR (all P < 0.05) (Table S1).
Association between TyG relevant index and the risk of incident MAFLD
After adjusting the complete model (Model 2), a significant increase in MAFLD risk was observed with elevated levels of TyG, TyG-BMI, TyG-WC, and TyG-WHtR (all P < 0.05). Notably, TyG-WHtR exhibited the highest risk, with a 43.8% increase in MAFLD risk per unit increase. Comparing the highest and lowest quartiles of TyG-related indexes, the risk of developing MAFLD was elevated by 1.363, 6.993, 2.540, and 2.541 times, respectively (Table 2). Sex-specific analysis revealed a stronger association between TyG-related indexes and MAFLD in males than in females. The risk of MAFLD associated with TyG-WHtR remained the highest for both sexes, with a 33.4% increase in males and a 44.6% increase in females per unit increase. Interestingly, a saturation threshold effect was observed in females, where TyG levels ≥ 8.86 showed a positive correlation with MAFLD risk, while levels < 8.86 did not. Notably, no significant association was found between male TyG levels and MAFLD risk (Table S2).
RCS analysis revealed a nonlinear relationship between TyG-related indexes and the risk of MAFLD in the general population after adjusting for the full model (all P-non-linear < 0.0001). However, upon sex stratification, the nonlinear relationship between male TyG and MAFLD was no longer evident (P-non-linear = 0.5856) (Fig. 2).
RCS analysis of TyG-related indexes and MAFLD risk. Adjusted age group, sex, TC, HDL-C, LDL-C, SCr, education, marital status, smoking, drinking, and movement. Male and female groups were not adjusted for sex. TyG, triglyceride and glucose index; BMI, body mass index; WC, waist circumference; WHtR, waist-to-Height Ratio.
Subgroup analysis was conducted based on various factors including age, education, marital status, and lifestyle habits. The TyG-related indexes were found to be associated with the risk of MAFLD, especially in unmarried individuals aged 18–30. Significant interactions were observed between TyG-related indexes and marital status, obesity, diabetes, and dyslipidemia (P < 0.001) (Fig. 3, Table S3). Sensitivity analyses were performed after handling individuals with a history of medication or family history of diabetes, hypertension, or hyperlipidemia, and the results were consistent (Table S4).
Subgroup analysis. A, Subgroup analysis of TyG; B, Subgroup analysis of TyG-BMI; C, Subgroup analysis of TyG-WC; D, Subgroup analysis of TyG-WHtR. Adjusted by age group, sex, TC, HDL-C, LDL-C, SCr, education, marital status, smoking, drinking, and movement. Variables related to subgroups were not adjusted during the analysis. For example, TC, HDL-C, and LDL-C were not adjusted for the dyslipidemia subgroup. TyG, triglyceride and glucose index; BMI, body mass index; WC, waist circumference; WHtR, waist-to-Height Ratio; T2DM, Diabetes mellitus type 2.
Diagnostic value of TyG relevant index for MAFLD
The results of ROC analysis demonstrated that the TyG-BMI index exhibited a robust predictive capability for detecting MAFLD across various subgroups within the total population, with statistically significant findings (P < 0.05). The Area Under the Curve (AUC) and Youden index reached optimal levels. The identified critical threshold for the TyG-BMI index was 218.91 (Table S5).
Discussion
MAFLD has emerged as a prevalent chronic liver disease worldwide, posing a significant threat to public health32. Numerous studies focused on identifying reliable, practical, and cost-effective predictors of MAFLD, addressing a critical need in clinical practice. Against this backdrop, we hypothesized a potential relationship between TyG-related indices and MAFLD, prompting our investigative analysis. Notably, this study represented a novel endeavor in rural Xinjiang, China. Our findings revealed a significant association between TyG-related indices and the risk of MAFLD across the general population and various subgroups. Furthermore, we observed a stronger correlation between TyG-related indices and MAFLD in females than males. TyG showed no significant association with MAFLD in the male cohort—a departure from previous research outcomes. Interestingly, we identified a non-linear relationship between TyG-related indices and MAFLD in the overall population, which was not observed in the male TyG-BMI subgroup, highlighting a unique disparity from prior studies.
In this study, the average values of TyG, TyG-BMI, TyG-WC, and TyG-WHtR were 8.50 ± 0.70, 213.47 ± 43.20, 757.87 ± 144.72, and 4.66 ± 0.88 respectively, which were higher than those in other studies19,21. Ru Zhang et al. found that the risk of occurrence of TyG in the highest quartile was twice that of the lowest quartile, which was higher than that in this study. The RCS analysis results showed that the relationship between TyG and the risk of MAFLD was linear in males (P-overall < 0.001, P-non-linear = 0.746) but non-linear in females (P-non-linear = 0.040)15. Males in rural Xinjiang, China, tend to engage in more high-intensity physical activities than females. On the other hand, females typically spend more time at home caring for children and doing household chores. This difference in physical activity levels may contribute to females having lower metabolic rates than males. Consequently, females may have a lower baseline BMI and higher WHtR than males, along with lower eGFR levels. These factors can increase the risk of conditions such as diabetic kidney disease (DKD)33 and obesity, among others. As a result, the relationship between TyG and MAFLD may vary significantly between different sexes. The results of another cohort study from Hebei, China, also showed that male TyG was positively correlated with the risk of MAFLD. However, the relationship was non-linear and had a saturation threshold effect. The inflection point was 8.64. When the TyG index was < 8.64, TyG was associated with the risk of MAFLD, and the risk was positively correlated. When the TyG index was ≥ 8.64, the two had no correlation (HR = 1.28, 95%CI: 0.81–2.03). In females, there was no correlation between TyG and the risk of MAFLD16. Our results were opposite and echo the RCS results of female TyG, which suggests that in our population, there may also be a saturation threshold effect on the risk of female TyG and MAFLD, and the inflection point is 8.86. This inflection point can be considered a female Monitoring point for MAFLD high-risk groups and can be verified through further studies.
ROC analysis showed that TyG-BMI showed good predictive value for MAFLD, which was consistent with the study of Mingxing Chang et al.20. However, Zhi Liu et al.’s study in Sichuan reported that the predictive value of TyG for MAFLD was higher than that of TyG-BMI17. After comparing Sichuan MAFLD patients and non-MAFLD patients, the BMI was lower than that in this study, which may be caused by differences in BMI levels. The reason may be that the living and eating habits of rural people in Xinjiang differ from those of other ethnic groups. Xinjiang people eat mostly dairy products and high-calorie foods. It may also be related to Xinjiang’s climate and geographical location. Some studies have shown that arid climate and geographical conditions may lead to the occurrence of hypertension-related diseases34, and high BMI itself is closely related to BP. Moreover, Lin Ning’s study also found that TC and TG levels were positively correlated with indexes such as latitude and temperature difference and negatively correlated with indexes such as annual average temperature, annual average relative humidity, and annual precipitation35. Rural areas in Xinjiang, China, have lower humidity, dry climate, frequent sandstorms, lower annual rainfall, and higher latitudes than Sichuan, China. This may lead to higher TC and TG levels in residents, thereby increasing the level of BMI. Secondly, some studies have reported that TyG-WC and TyG-WHtR in Americans had better predictive value for MAFLD than TyG-BMI19,21. This may be due to heterogeneity caused by country and sample size13. Some studies have shown that the genetic background of the Uighurs in Xinjiang was relatively complex, with European and Asian ancestry36, which may lead to differences in the identical indexes between different countries and ethnic groups. In addition, subgroup analysis results showed that TyG-BMI had the highest AUC95%CI in the 18–30-year-old and unmarried subgroups, reaching 0.786 (0.774, 0.799) and 0.862 (0.775, 0.949) respectively. On the one hand, it may be because the average age of our population is relatively low; on the other hand, it may be because getting married will reduce the possibility of depression and anxiety37, thereby reducing the BMI level and the risk of metabolic syndrome38,39. Therefore, it may cause unmarried people to compare with married and older people. Other marital statuses are associated with higher risk of MAFLD. It showed that TyG had a crucial predictive value for MAFLD in this subgroup of people. It was also rarely reported in previous studies, showing the differences in the risk association between TyG-related indexes and MAFLD at different ages. However, expanding the sample size or conducting multi-center studies was still necessary to verify this inference. In summary, maintaining good health awareness, maintaining a healthy diet, and improving the living environment were of great significance to the prevention and control of MAFLD, and it was necessary to select appropriate MAFLD prediction indexes according to different regions and groups.
Strengths and limitations
The study examined the link between TyG-related indexes and MAFLD risk, focusing on rural residents in Xinjiang, China. It provided epidemiological insights for early MAFLD screening in rural areas. However, the results may not apply to other populations, and the diagnosis was based on imaging, not histology. Ultrasound, though, is a sensitive and practical tool for large-scale fatty liver diagnosis40. The study’s findings were reliable, but potential confounders were not controlled for, and the impact of time on TyG-related indexes was not considered. Future research will explore these aspects. TyG, TyG-BMI, TyG-WC, and TyG-WHtR were independently associated with MAFLD, indicating the need for early detection and intervention to manage high-risk groups effectively.
Conclusion
TyG, TyG-BMI, TyG-WC, and TyG-WHtR were independently linked to MAFLD, with a stronger link in females. TyG did not associate with MAFLD in males. TyG-related indexes predict MAFLD, especially TyG-BMI in unmarried people aged 18–30. These findings are important for early management and prevention of MAFLD in rural China.
Data availability
The data that support the findings of this study are available from the corresponding author, Jia He, upon reasonable request.
References
Association, H. B. O. C. M. Guidelines for the Prevention and Treatment of Metabolism-related (Non-alcoholic) Fatty Liver Disease (2024 Edition). Chin. J. Hepatol. 32(5), 418–434 (2024).
Tarantino, G., Citro, V. & Capone, D. Nonalcoholic Fatty Liver Disease: A Challenge from Mechanisms to Therapy. J. Clin. Med. 9(1), 15 (2019).
Wang, Y. et al. Cardiovascular and renal burdens among patients with MAFLD and NAFLD in China. Front. Endocrinol. (Lausanne) 13, 968766 (2022).
Lee, H. et al. Metabolic Dysfunction-Associated Fatty Liver Disease and Incident Cardiovascular Disease Risk: A Nationwide Cohort Study. Clin. Gastroenterol. Hepatol. 19(10), 2138-2147.e10 (2021).
Sakura Y, Mohammed E, Takumi K, et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD[J]. Liver International, (2020).
Lim, S., Kim, J. W. & Targher, G. Links between metabolic syndrome and metabolic dysfunction-associated fatty liver disease. Trends Endocrinol. Metab. 32(7), 500–514 (2021).
Lin, S. et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 40(9), 2082–2089 (2020).
Opazo-Ríos, L. et al. Meta-Inflammation and De Novo Lipogenesis Markers Are Involved in Metabolic Associated Fatty Liver Disease Progression in BTBR ob/ob Mice. Int. J. Mol. Sci. 23(7), 3965 (2022).
Lim, G. E. H. et al. An Observational Data Meta-analysis on the Differences in Prevalence and Risk Factors Between MAFLD vs NAFLD. Clin. Gastroenterol. Hepatol. 21(3), 619-629.e7 (2023).
Lei, F. et al. The prevalence of MAFLD and its association with atrial fibrillation in a nationwide health check-up population in China. Front. Endocrinol. (Lausanne) 13, 1007171 (2022).
Simental-Mendía, L. E., Rodríguez-Morán, M. & Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 6(4), 299–304 (2008).
Sakurai, Y. et al. Role of Insulin Resistance in MAFLD. Int. J. Mol. Sci. 22(8), 4156 (2021).
Wang, J. et al. The Diagnostic and Prognostic Value of the Triglyceride-Glucose Index in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD): A Systematic Review and Meta-Analysis. Nutrients 14(23), 4969 (2022).
Xie Rongai, Z. J. & Xiaobin, G. Correlation analysis between TyG and metabolism-related fatty liver disease in non-diabetic people. J. Nanchang Univ. (Med. Ed.) 62(03), 48–52 (2022).
Zhang, R. et al. Association Between Triglyceride-Glucose Index and Risk of Metabolic Dysfunction-Associated Fatty Liver Disease: A Cohort Study. Diabetes Metab. Syndr. Obes. 15, 3167–3179 (2022).
Xiaoli L. Study on the correlation between triglyceride-glucose index and risk of metabolic diseases. (2022).
Liu, Z. et al. Comparison of the diagnostic value between triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio in metabolic-associated fatty liver disease patients: a retrospective cross-sectional study. Lipids Health Dis. 21(1), 55 (2022).
Yang, Z. et al. Comparison of the prognostic value of a comprehensive set of predictors in identifying risk of metabolic-associated fatty liver disease among employed adults. BMC Public Health 23(1), 584 (2023).
Peng, H. et al. Prediction of MAFLD and NAFLD using different screening indexes: A cross-sectional study in US adults. Front. Endocrinol. (Lausanne) 14, 1083032 (2023).
Chang, M., Shao, Z. & Shen, G. Association between triglyceride glucose-related markers and the risk of metabolic-associated fatty liver disease: A cross-sectional study in healthy Chinese participants. BMJ Open 13(5), e070189 (2023).
Zou, H. et al. Comparison of the diagnostic performance of twelve noninvasive scores of metabolic dysfunction-associated fatty liver disease. Lipids Health Dis. 22(1), 145 (2023).
Yu Ren, S. G., Guo, H. & Rulin, M. Construction of a risk prediction model for coronary atherosclerotic heart disease among rural residents in Xinjiang based on metabolic syndrome factors. Chin. Public Health 39(10), 1255–1262 (2023).
Ke, G. et al. Comparison of 6 equations for estimating glomerular filtration rate in a Chinese benign hypertensive nephrosclerosis population. Med. (Baltimore) 100(51), e28318 (2021).
Executive summary: Standards of medical care in diabetes. Diabetes Care, 35 Suppl 1(Suppl 1): S4-s10. (2012)
Hypertension, W. G. O. C. G. F. T. M. O. 2018 Chinese guidelines for the management of hypertension. Chin. J. Cardiovasc. 24, 24–56 (2019).
Stanley J U. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. WHO Technical Report Series 894. Pp. 252. (World Health Organization, Geneva, 2000.) SFr 56.00, ISBN 92–4–120894–5, paperback[J]. Journal of Biosocial Science, (2003).
Adults C J C O R O G F P a T O D I. Guidelines for the Prevention and Treatment of Dyslipidemia in Adults in China (Revised Edition in 2016). Chinese Journal of Circulation, 26(31) 937–953. (2016)
From the Centers for Disease Control and Prevention. Cigarette smoking among adults–United States, 1992, and changes in definition of smoking. JAMA 272(1), 14–16 (1994).
Sun, K. et al. Alcohol consumption and risk of metabolic syndrome: a meta-analysis of prospective studies. Clin. Nutr. 33(4), 596–602 (2014).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73(1), 202–209 (2020).
Haskell, W. L. et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 39(8), 1423–1434 (2007).
Tan, Y. et al. Perilipin 5 Protects against Cellular Oxidative Stress by Enhancing Mitochondrial Function in HepG2 Cells. Cells 8(10), 1241 (2019).
Bae, J. & Lee, B. W. Significance of Diabetic Kidney Disease Biomarkers in Predicting Metabolic-Associated Fatty Liver Disease. Biomedicines 11(7), 1928 (2023).
Lin Xue S Y, Ma Li. Study on the relationship between environmental factor and climacteric hypertension:research on the background of Northwest dryness syndrome[J]. China Journal of Traditional Chinese Medicine and Pharmacy, (02): 125–127. (2008).
Ning, L. Relationship between reference values of serum total cholesterol and triglyceride and geographical factors in healthy Chinese adults (Shaanxi Normal University, 2019).
Feng, Q. et al. Genetic History of Xinjiang’s Uyghurs Suggests Bronze Age Multiple-Way Contacts in Eurasia. Mol. Biol. Evol. 34(10), 2572–2582 (2017).
Donato, K. M., Caron, L. & Hamilton, E. Migration and Mental Health in Mexico: Domestic Migrants, Return U.S. Migrants, and Non-Migrants. Front. Psychiatr. 10, 970 (2019).
Duan, H. et al. Research on sleep status, body mass index, anxiety and depression of college students during the post-pandemic era in Wuhan, China. J. Affect. Disord. 301, 189–192 (2022).
Lemche, A. V., Chaban, O. S. & Lemche, E. Depression contributing to dyslipidemic cardiovascular risk in the metabolic syndrome. J. Endocrinol. Invest. 40(5), 539–546 (2017).
Hernaez, R. et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 54(3), 1082–1090 (2011).
Acknowledgements
We sincerely thank the participants from rural areas who participated in this study for their willingness to participate in this study.
Funding
This research was supported by the National Natural Science Foundation of China (82460661), the Shihezi University High-level Talent Scientific Research Startup Project (RCZK202367), the Key science and technology project of Xinjiang Production and Construction Corps (2018AB022, 2021AB030) fund.
Author information
Authors and Affiliations
Contributions
(I) Conception and design: Shipeng Gao, Shuxia Guo, and Heng Guo, Jia He; (II) Administrative support: Xinyu Peng, Zhang Shijie, Jing Yang, Shuxia Guo, Heng Guo, and Jia He; (III) Provision of study materials or patients: Xinyu Peng, Zhang Shijie, Jing Yang, Shuxia Guo, Jia He and Heng Guo; (IV) Collection and assembly of data: Shipeng Gao, Xinyu Peng, Zhang Shijie, Jing Yang, Shuxia Guo, Yu Li, Rulin Ma, Yunhua Hu, Keerman Mulatibieke, Xianghui Zhang, Xinping Wang, Jia He and Heng Guo; (V) Data analysis and interpretation: Shipeng Gao, Keerman Mulatibieke, Jia He and Heng Guo; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Institutional review board
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Review Board (IERB) of the First Affiliated Hospital of Shihezi University School of Medicine (IERB no.: 2016–121-01).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, S., Peng, X., Zhang, S. et al. Relationship between triglyceride and glucose related indexes and MAFLD in rural populations of northwest China. Sci Rep 15, 30665 (2025). https://doi.org/10.1038/s41598-025-12658-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-12658-4