Abstract
Increased exposure to environmental contaminants is a concern for local koala (Phascolarctos cinereus) populations where massive transformation or loss of habitat has occurred over the past century as a consequence of increased urbanisation and industrial and agricultural practices. The koala is a specialist eucalypt feeder that relies on an almost exclusive diet of eucalypt foliage. Eucalypts can accumulate elements to varying degrees. Chronic ingestion and bioaccumulation could potentially affect the metabolic pathways of koalas and consequently interfere with detoxification of eucalypt plant secondary metabolites, reduce food intake, and cause long-term health effects. This field study provides the first evidence of the significance of the environmental factor, land use, region and host factors, age and sex, on bioaccumulation of trace element and heavy metal (elements) in koalas. Data on observed ranges of element concentrations are provided from the hair of 328 koalas sampled across eight different regions of NSW and Victoria, Australia. Land use was shown to be the most significant factor affecting trace elements and heavy metal concentrations in koalas, followed by region. Associations between element concentrations and host factors, koala age and sex, were only seen for a small number of elements.
Similar content being viewed by others
Introduction
The majority of trace elements essential for major body functions act in very low concentrations and are maintained within physiological ranges via a variety of homeostatic mechanisms2. In contrast, heavy metals and non-essential trace elements can accumulate within body tissues and can cause adverse health effects20,37. All heavy metals and trace elements (from now on referred to as ‘elements’) occur in the earth’s crust and are more or less available for redistribution in the environment depending on their physicochemical form67. Anthropogenic activities, including those associated with metal smelting41coal combustion and mining activities7,85other industrial activities4and bushfire events10 can mobilise such elements and release them into the environment in high concentrations. The application of agricultural fertiliser in areas where natural soil quality is insufficient for cropping and pastoral activities can also result in localised accumulation of elements Cd and As12,39,77.
Once these elements enter the food chain, bioaccumulation can occur with uptake varying between species depending on dietary preferences and trophic level86.
There are few published studies of element concentrations in native Australian wildlife. Element accumulation has been reported in tissues of striped marsh frog tadpoles (Limnodynastes peronii) exposed to coalmine wastewater47 and elevated concentrations of elements have been reported in the tissues of Australian dugongs (Dugong dugon) and sea turtles (Chelonioidea spp.) in Queensland31,66. High levels of lead (Pb) were found in tissues of urban fruit bats (Pteropus spp.) in the Brisbane area, Queensland, Australia29and in grey-headed (Pteropus poliocephalus) and black flying foxes (P. alecto) sampled in the Sydney Basin (Pulscher et al., 2020) and in other bat species nationwide33. Considerably high mercury concentrations were also seen in the feathers of flesh-footed shearwaters (Puffinus carneipes) from Lord Howe Island and Western Australia8. Increased hair concentrations of cadmium (Cd) and Pb have been reported in native marsupial, the brown antechinus (Antechinus stuartii), and in the introduced black (Rattus rattus) and brown (Rattus norvegicus) rats sampled near a Pb/ zinc (Zn) smelter in NSW, and these data were significantly correlated with elevated soil element concentrations59. Manganese (Mn) was found to persist in the brain of northern quolls (Dasyurus hallucatus) living near a Mn mining site3 and excess fluoride intake of various Australian marsupial species through their natural diet was linked to degenerative joint disease (Death et al., 2018). Accumulation of aluminium (Al) was evident in the bones and kidneys of koalas (Phascolarctos cinereus) with acute renal failure sampled in the Adelaide Hills, South Australia32.
Eucalypt tree species, the most common genera of trees in Australia, accumulate elements in their roots and shoots23,69,79however these species also present a toxic cocktail of plant secondary metabolites (PSMs), which reduce palatability for herbivorous species63,81. Because of this low palatability to herbivorous species, eucalypt trees are often employed for landscape remediation to exploit their phyto-stabilisation capacities45,62. High concentrations of Cd, antimony (Sb), and Zn have been reported in the foliage of eucalypts used to remediate former Zn mining sites and phytoremediation treatment plants55,75. Eucalypt foliage in areas of alternative landfill remediation in Northern NSW had shown significantly higher concentrations of arsenic (As), silver (Ag), mercury (Hg), and Pb compared to foliage in control sites, leading to concerns for the local koala population79. Only few herbivorous species can use eucalypt leaves as a major feed source. The koala and the greater glider (Petauroides volans) are specialist feeders that rely on an almost exclusive diet of eucalypt leaves. The koala was listed as endangered under the Environment Protection and Biodiversity Conservation Act in 2022 as it has been deeply impacted by prolonged drought, the Black Summer bushfires and cumulative effects of disease, urbanisation and habitat loss. Increased exposure to anthropogenic environmental contamination has never been investigated in those species.
While increasing industrial, agricultural, and mining activities have the potential to distribute environmental contaminants, bio-monitoring of elements is rare, often due to a lack of samples. While organ tissues are considered the gold standard for determining the concentrations of elements, using hair as a sampling matrix facilitates greater sample sizes and therefore more robust statistical methods, and can be easily collected from live or deceased animals. Hair samples have previously been employed as a less invasive matrix to document exposure to elements in Australian terrestrial and marine mammals6,28,59,68. Hair follicles are in continuous contact with the blood and elements are incorporated into hair during growth via the binding affinity of cysteine sulfhydryl (thiol) groups6. Once incorporated, the concentrations of these elements remain unchanged65.
The aim of this study was to investigate the effect of land use and region on the concentration of Cd, Sb, Zn, As, Ag, Hg, Pb, Al, Mn, copper (Cu), iron (Fe), magnesium (Mg), cobalt (Co), nickel (Ni), selenium (Se), tin (Sn), titanium (Ti), chromium (Cr), and bismuth (Bi) in hair collected from free ranging koalas. Additionally, age-related variations in concentrations were evaluated to test for cumulative effects, and the effect of animal sex on element concentrations was also explored.
Materials and methods
Hair collection
Hair was collected from free-ranging (n = 328) koalas in NSW and Victoria, Australia from 2014 to 2016. The age of each individual was determined by reference to an ageing system for koalas based on tooth wear56grouping the koalas into the following age classes: juvenile (juv) 0–2 years; adult young (a/y) 2.5-5 years; adult mature (a/m) 5–10 years; and adult aged (a/a) > 10 years of age. The sex of each individual was also recorded. Battery-operated clippers were used to collect approximately 0.5 g of hair from each individual. In live koalas, hair was collected in conjunction with normal management activities (clipping of fur for blood sampling from cephalic vein where required), from animals in the field and licensed animal rehabilitation organisation. Hair was also collected from the antebrachium or shoulder area of deceased animals which were euthanised due to either injury or disease or that had died because of a road traffic accident. Individual hair samples were stored in envelopes at -20 °C until analysis. Experiments were approved by the Animal Ethics Committee of The University of Sydney, Protocol No. 565, and by the Office of Environment and Heritage, NSW, Scientific Licence number SL101290. We confirm that all experiments in this study were performed in accordance with the relevant guidelines and regulations. All procedures of the study are followed by the ARRIVE guidelines.
Effects of land use and region
Study animals were chosen based on access to samples from free-ranging koalas across regions of NSW and Victoria and to facilitate comparison of the impact of differing land uses on element concentrations. Regions are as follows: “Port Macquarie-Hastings Council”, NSW (n = 37); “Northern Rivers Region”, North Coast NSW (n = 195); “Gunnedah Shire”, North-West NSW (n = 62); “Newcastle area”, NSW (n = 5); “Southern Highlands”, NSW (n = 12); “Strzelecki Ranges”, Victoria (n = 10); and “Western Sydney region” (Campbelltown area and Upper Blue Mountains, n = 7).
Land use was determined in the three largest regions only “Northern Rivers Region”, “Gunnedah Shire”, and “Port Macquarie-Hastings Council” (Fig. 1) due to insufficient sample size for statistical analysis in all other regions. The mapping software ‘Nationalmap’ (Department of the Prime Minister and Cabinet, Department of Communications, and CSIRO Data 61 2014–2017, http://nationalmap.gov.au) was used, with the ‘Catchment Scale Land Use of Australia’ update of March 2020. This dataset was the most current available land use dataset at the time of data analysis, with a resolution of 50 m x 50 m. Land use categories are as follows: modified grazing pasture; horticulture; cropping and residential.
Agricultural primary productions in the Northern Rivers region are fruit and tree nut growing, sheep, beef cattle, grain, and other cropping industries74. Animals from Northern Rivers region (Fig. 2) were sampled in all 4 land use areas, modified grazing pasture; horticulture; cropping and residential (see map). Gunnedah Shire (Fig. 3) is located on the Liverpool Plains, one of the richest agricultural regions of Australia. Almost 90% of the shire’s land use is cropping (wheat, sunflowers, cotton, and sorghum, along with barley, canola, sunflowers, beans, chickpeas, corn, olives, and citrus) and most koalas are from cropping area. The Mid-North Coast region, including Port Macquarie-Hastings Council (Fig. 4) is mainly a residential area with some agricultural production in this region dominated by dairy, cattle, and poultry production and fruit and tree nut growing. The majority of animals included in this study are from residential areas. Table 1 presents the number of animals per land use in each region as well as the age and sex distribution of the three large regional populations.
main koala sampling sites: Northern River region, North Coast NSW (pink dots), Port Macquarie-Hastings Council, NSW (red dots), Gunnedah Shire North-West NSW (purple dots), http://nationalmap.gov.au.
Northern River region, North Coast NSW (pink dots are koala locations), http://nationalmap.gov.au.
Gunnedah Shire, North-West NSW (purple dots are koala locations), http://nationalmap.gov.au.
Port Macquarie-Hastings Council, NSW region (red dots are koala locations), http://nationalmap.gov.au.
Sample preparation
Hair samples were prepared and analysed at the Department of Chemical Pathology, Royal Prince Alfred Hospital, Camperdown, NSW, Australia as previously reported28with minor modifications. Briefly, hair was placed in clean 10 ml tubes (Polypropylene tubes, Sarstedt Australia, Ingle Farm, South Australia) and washed for 10 min on a vial roller, first in reagent grade acetone (AnalaR Acetone, Merck Pty Ltd, Kilsyth, Victoria, Australia), three times in double-distilled water, followed by a final wash in acetone. Following each wash, the liquid was decanted off with a single-use pipette. After washing, samples were freeze-dried to constant weight at 760mmHg (Edwards High Vacuum Ltd, Manor Royal, Crawley, Sussex, Model # EF03) for eight hours. After drying, duplicate hair samples of approximately 50 mg (48–52 mg) were weighed into individual 10 ml centrifuge tubes (polypropylene tubes, Sarstedt Australia, Ingle Farm, South Australia). Hair was digested in closed tubes in reagent-grade concentrated nitric acid (400 µl, Suprapur ® Nitric Acid 65% Merck, Darmstadt, Germany) in a sand bath at 80–100 °C for eight hours. Tubes were then centrifuged for three minutes to evaluate for sediments. If sediment was observed, a new 50 mg sample of washed and freeze-dried hair was digested as described in Gray, et al.28. Prior to analysis, 50 µl of sample was diluted with 4450 µl of double-distilled water. An inductively coupled plasma mass spectrometer (ICP-MS) (Agilent Technologies 7500ce inductively coupled mass spectroscope, Santa Clara, CA) was used for the analysis of the samples. The concentrations of Mg, Mn, Al, Co, Pb, Ni, Cu, Zn, As, Cd, Cr, Fe, Se, Sb, Hg, Sn, Ti, Bi, and Ag were determined for each hair sample. The limit of quantification (LOQ) for each element was 0.002ppm.
Internal calibration standards for each element (concentrations – 0, 156, 313, 625, 1250, 2500, and 5000 µg/L) were analysed at the commencement of each analytical run to establish a standard calibration curve and accuracy was reassessed after every 50th sample as quality control. Blanks were analysed after every 10th duplicate sample. All quality assurance protocols were followed utilising quality control measures used in this commercial laboratory. Trace element concentrations were determined by ICP-MS (Varian Ultra-Mass Spectrometer System Varian Australia Pty Ltd Mulgrave, Victoria) and the signal intensities were compared to a standard calibration curve for each trace element. The accuracy and precision of the analytical procedure was verified by the analysis of replicates of standard material Normal Range Trace Element Serum Toxicology Control (UTAK Lot # 66816, UTAK Labs Inc., Valencia, Ca 91355;n = 10) and NIST (National Institute of Standards and Technology) Standard Reference Material 1577b Bovine Liver (U.S. Department of Commerce National Institute of Standards and Technology, Gaithersburg, MD; n = 9), comparison of observed and certified values (where available).
Statistical analysis
Medians and interquartile ranges were calculated using “Microsoft Excel” (Microsoft Corporation. 2018). Generalised linear models (GLMs) were fitted to evaluate element concentrations in the hair of free-ranging koalas against a set of intrinsic and extrinsic factors including region, land use, age, and sex. The statistical analysis focused on the three geographic regions with sufficient samples to support robust statistical modelling: Port Macquarie-Hastings Council, NSW (n = 37); Northern Rivers region, North Coast NSW (n = 195); and Gunnedah Shire, North-West NSW (n = 62). These data covered four land use categories (cropping, modified grazing pasture, horticulture, and residential), four age categories (Juv, A/y, A/m, and A/a), and both sexes. For some elements (Co, Cd, Sn, Ti, Cr, Bi, Ag) the data contained a large number of values below the limit of quantification (< LOQ) and were therefore excluded from the statistical analysis; descriptive statistics only are presented for these elements.
A Gaussian error distribution was assumed in the modelling and all element concentrations required log transformation. Outliers for each element were identified by visual inspection of the log-transformed sample distribution and the Q-Q plots of residuals. Forward selection using the Akaike Information Criterion (AIC) was employed to select parameters for each GLM model, starting with main effects (region, land use, age, and sex) and followed by first-order interaction terms. The significance of multi-level factors was assessed using Wald tests. Adequacy of the model fit was assessed using Q-Q plots and visual inspection of model residuals. When appropriate, Bonferroni-adjusted post hoc tests were used to assess the significance of differences between specific categories within a factor (for example, differences between specific age categories). Significance was established at p < 0.05 for all statistical analyses.
All statistical analyses were performed using R 2.14.1 software (R Development Core Team, Vienna, Austria). Model contrasts were evaluated using the multcomp package in the software35.
Results
Effects of region and land use
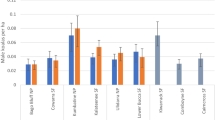
Table 2 provides a summary of the results of modelling the effect of land use, region, age, and sex on the concentrations of elements in the hair of free-ranging koalas, including the final fitted model for each element. Supplementary Figs. 1 and 2 display the results of the generalised linear modelling for differences in element concentrations for Cu, Pb, Hg, As, Sn, Al, Fe, Se, Mn and Zn with region, land use, age and gender.
Significant differences in element concentrations were observed for Sb, Al, Fe, Ni, and Se with region, while land use had significant effects on the concentration of Cu, Hg, As, Sb, Al, Fe, Se, Ni, Mg, and Pb. Concentrations in cropping areas in the Gunnedah Shire were significantly higher than in cropping areas in the Northern Rivers region for Sb (z = 5.04, p < 0.001), Al (z = 5.84, p < 0.001), Fe (z = 4.85, p < 0.001), Ni (z = 4.02, p < 0.001), and Se (z = 4.02, p < 0.001).
Copper
After controlling for the effect of age, Cu concentrations varied significantly with land use with concentrations in cropping areas significantly higher than those in modified grazing pasture (Bonferroni-adj. p = 0.02) but were comparable to other land use areas.
Mercury
Mercury concentrations varied significantly with land use (p < 0.01) with concentrations in cropping areas significantly higher than concentrations in other land use areas (z = 3.47, p < 0.001).
Arsenic
Arsenic concentrations differed significantly with land use (p < 0.001). While there was no significant difference in As concentrations between residential and cropping areas (p = 0.41), As concentrations in samples from cropping areas were significantly higher than in modified grazing pasture (p < 0.03) and in horticulture (p < 0.001). Arsenic concentrations were also significantly higher for residential use when compared to both modified grazing pasture (p < 0.001) and horticulture (p < 0.001).
Antimony, aluminium, iron, nickel, selenium
The concentrations of Sb, Al, Fe, Ni and Se differed significantly with land use (p < 0.001) and region (p < 0.001) with the highest concentrations seen in koalas sampled in cropping areas in the Gunnedah Shire. For all of these elements, posthoc comparisons indicated that concentrations in koalas within cropping land use areas in the Gunnedah Shire were significantly higher than those in other land use areas in the same region (p < 0.001) and were also significantly higher than concentrations in cropping areas in the Northern Rivers Region (p < 0.001).
Magnesium
After controlling for the effect of age and gender, significant differences in Mg concentrations were seen with land use (p < 0.001) with the concentrations of Mg in cropping areas significantly lower than those in other land use areas (z = 7.01, p < 0.001).
Manganese
After controlling for the effect of age, significant differences in Mn concentrations were seen with land use (p < 0.001) with cropping and residential areas having lower concentrations, across all age groups, compared to horticulture and grazing modified pasture.
Lead
Lead concentrations varied significantly with land use (p = 0.03) with Pb concentrations in residential locations significantly higher than concentrations in other land use areas (z = 3.00, p < 0.01) with the significant difference due primarily due to a difference between concentrations in cropping and residential land use.
Effect of host factors on element concentrations
Significant differences in element concentrations with age were seen for Cu, Mg, and Mn in the hair of free-ranging koalas (Table 2). Copper concentrations were significantly higher (p < 0.01) in younger koalas and decreased with increasing age. This pattern is clearly present for most land use areas. In contrast, concentrations of Mg and Mn increased significantly with age (p < 0.01).
Significant differences between the sexes for element concentrations were only seen for Mg and Zn (Table 2, Supplementary Fig. 2). Concentrations of Mg were higher in males than in females in all geographical regions and land use areas (z = 5.08, p < 0.001). Zinc concentrations were higher in females than males across all regions and land use areas (p = 0.02).
Discussion
This study provides data on element concentrations in the hair of 328 koalas sampled across seven different regions of NSW and Victoria, and provides the first data on the significance of land use and region on element accumulation in this species. Land use was shown to be the most significant factor affecting element concentrations in koalas, followed by geographical region. Differences in element concentrations based on the host factors examined, koala age and sex, were limited to only a small number of elements.
Effects of land use and region
The results of this study particularly highlight the importance of land use for the bioaccumulation of elements in koala hair. For the majority of elements, cropping was associated with significantly higher element concentrations than all other land uses. Australia’s soils are ancient, naturally low in organic matter, and have a high clay content which results in low fertility36 that directly impacts agricultural output. Deficiencies in Cu and Se in grazing cattle and sheep were a common problem in some areas last century26,46and are managed using feed supplementation22,40,53,82 and the application of slow-release fertilisers to pastures83,84. The enhancement of soil nutrients through the application of superphosphate, trace elements, fertilisers, and pesticides has enabled an agricultural productivity revolution which has seen the development of Australian agriculture to its current capacity17,27,52,54,70,78. However with annual application of single super phosphates, lime and farmyard manure supplies a significant amount of heavy metal to soils1,51,58.
A detailed spatial analysis would be necessary to understand the interactions between region and land use but is beyond the scope of this study. Furthermore, a point-source of an element could be far removed from the area of accumulation, which makes understanding the role of region and land use even more challenging. For example, elements released into nearby streams by mining and processing80 can be carried up to 300 km into floodplains and coastal catchment areas5,80resulting in elevated element availability at a site remote from the point-source.
Significant regional differences in element concentrations were seen for seven elements analysed, with the koalas sampled in the Gunnedah Shire having significantly higher element concentrations compared to the other two regions analysed. This can be explained Agriculture and specifically, cropping is the main industry of the Gunnedah Shire region, constituting 88.5% of the Shire’s land area, which could explain significantly higher concentrations of most elements in this region through the use of fertilizers and pesticides. Many fertilizers contain trace elements investigated in this study such as zinc, manganese, copper, and molybdenum. They are essential for plant growth but can accumulate in the soil over time if applied in excess. Chemicals used for pest and weed control often contain metals and other trace elements as active ingredients or inert carriers. Repeated applications can lead to accumulation of elements in the soil .
In this study, significantly higher Pb concentrations were seen in koalas from residential areas than from other land use areas. With increasing urbanisation, the surface soils of roadsides, parks, and residential areas have been shown to be enriched with Hg, Cu, Zn, and Pb, the latter associated with roads and traffic30,72.
Host factors influencing element concentrations
Host factors such as animal age, sex, body size and weight, and dietary preferences affect the bioaccumulation of elements. In this study, sex differences for element concentrations in hair were only found for Mg and Zn, suggesting that sex is not a significant factor in the bioaccumulation of elements in the koala. Koalas are specialist eucalypt feeders and, as such, the primary source of ingested elements for both male and female koalas is Eucalyptus foliage.
As reported for humans, physiological differences, for example, pregnancy or other hormonal factors, could play a role in the sex-based differences in Mg and Zn concentrations9,11. Similarly, the effect of koala age was only significant for Cu (higher in younger age cohorts) and Mg and Mn (lower in younger age cohorts). In the only previous study of element concentrations in koalas, significantly higher Cu concentrations were also seen in the livers of young koalas compared to concentrations in older animals60which is consistent with the findings in hair samples from the current study. A likely explanation for the effect of age on Cu concentrations could relate to a higher specific requirement for, and therefore higher absorption of those elements, in younger animals compared to in adults9. Alternatively, the application of other elements in agriculture can result in copper deficiency in grazing animals and could have longer-term impacts on Cu bioavailability50 in koalas.
Concentrations of Mg and Mn were significantly higher in older animals than in younger animals, which could be related to a number of mechanisms. Both Mg and Mn are freely available in most areas of NSW as a result of soil acidity38,50so the potential for bioaccumulation with age could occur. Differences in these elements with age could also relate to calcium (Ca) intake. Intake of Ca is relatively higher in younger animals and Ca is antagonistic for Mg and Mn15,49. Additionally, Cu uptake is increased in younger animals and can antagonise and thereby reduce, Mg and Mn absorption25,44. Chronic renal disease can cause elevated blood concentrations of both Mg and Mn14,73. For this reason, accumulation of Mg and Mn in hair could become significant in animals with chronic renal insufficiency associated with oxalate nephrosis or chlamydia disease34,76.
Comparison of element concentrations in koalas and other herbivorous browsers
There are no published element concentrations in koala hair. Indeed, data on element concentrations in the hair of other herbivorous browsers are limited with no data published for native Australian herbivorous browsers. Table 3 presents data on concentrations of elements in the hair of free-ranging koalas from this study compared with concentrations of elements in the hair of other free-ranging browsers. Excepting Al, element concentrations in koala hair lie within or below values of other herbivorous browsing animals. Aluminium concentrations in the koala (median: 12.2/ IQ 6.2–22.5 ppm of hair) were considerably higher when compared to reported values in hares (Lepus spp.) and in roe deer (Capreolus capreolus)21. Unfortunately, there are limited data on Al concentrations in the hair of herbivore browsers but concentrations in the hair of guinea pigs (0.65 ppm; Katz, 1993) were similar to those reported in roe deer (Dugaszek and Kopczyski, 2014). Interestingly, high levels of Al were found in renal tissue and bones of koalas from the Adelaide Hills area, South Australia that presented with renal failure32. The finding of higher Al concentrations in koalas in this study compared to concentrations in other herbivorous browsers is of interest. Aluminium is normally bound by ligands or can occur as silicates and precipitates19. Farming practices, intensification of cropping, acid rain, and the increased use of nitrogenous fertilisers have all resulted in the acidification of soil in large areas of Australia42,57increasing Al availability in soils19,38.
Zinc is an essential element and concentrations found in koala hair were low compared to the concentrations found in other browsing species21,24,61. Zinc deficiency is a common problem in orchards across all soil types in NSW16,50. However, Zn concentrations in animal hair are not considered to be accurate indicators of Zn status in some species Constable13.
Utility of hair as a sample matrix for determining element concentrations in koalas
Measuring element concentrations in eucalypt leaves collected from certain areas was not considered to be useful as a predictor of element accumulation in koalas. Foliage choice of koalas is inconsistent throughout the landscape but instead relies on complex leaf chemical composition48,63. Further the capacity of eucalypt species to accumulate elements vary significantly. For this reason, the focus in this study is to determine element concentrations in koala hair, and to investigate whether land use affects bioaccumulation of elements. Element concentrations in hair indicate that exposure has occurred during hair growth, when elements are incorporated into hair and then remain unchanged. Given that the time taken for growth of hair is unknown for the koala, it is not possible to determine the timeframe of accumulation represented by these values. Seasonal moulting is not discernible in this species. Instead, it is likely that koalas shed hair continuously, but in very low amounts, due to their reliance on their pelage for insulation18. External contamination of fur can be a risk to obscure interpretation of data as a measure of systemic exposure. Therefore, washing techniques are highly important in ecotoxicology studies. In this study, hair was washed extensively for 10 min on a vial roller, first in reagent grade acetone, three times in double-distilled water, followed by a final wash in acetone which is a highly effective method to reduce external contamination43.
As such, the element concentrations presented here can be used to provide empirical data for comparison with other populations when determining element concentrations in hair, as the bioaccumulation of elements differs between tissue matrices, e.g. liver, muscle. Although detecting element concentrations in hair indicates exposure has occurred, it does not reflect systemic exposure, nor the biological significance of these measured concentrations. Given there are no reference intervals for element concentrations established for this species to which the results of the present study can be compared, it is unclear if the concentration in koalas from cropping areas, which had the highest concentrations of many of the measured elements are of clinical significance. Despite this limitation, the results of this study address a key knowledge gap of element exposure in this threatened and iconic Australian native species and provide data for comparative studies across a range of geographical regions and land use.
Conclusions
The results of this study have shown that element bioaccumulation in the koala is significantly influenced by land use and region, with host factors such as age and sex determined to be of lesser importance. In this study, the highest concentrations of elements were found in koalas from cropping areas and, where regional differences were observed, the Gunnedah Shire had the highest element concentrations. Specific land uses were the most important factor in these differing concentrations, likely mediated by alterations in soil properties and therefore element bioavailability. Importantly, this study has established observed concentrations of 18 elements in the hair of koalas sampled across eight regions in Eastern Australia (Supplementary Table 1). Compared to data from other herbivorous browsers, only the concentrations of Al appear to be considerably higher in koalas, although comparative data is limited. The concentrations of these elements reported herein can be used to monitor the bioaccumulation of these elements in koala populations through repeated measurements over time.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adhikari, T., Gowda, R. C., Wanjari, R. H. & Singh, M. Impact of continuous fertilization on heavy metals content in soil and food grains under 25 years of long-term fertilizer experiment. Commun. Soil Sci. Plant Anal. 52, 389–405. https://doi.org/10.1080/00103624.2020.1854290 (2021).
Allain, P. & Leblondel, G. Endocrine regulation of trace element homeostasis in the rat. Biol. Trace Elem. Res. 32, 187–199 (1992).
Nasir, A. A. Manganese accumulates in the brain of Northern quolls (Dasyurus hallucatus) living near an active mine. Environ. Pollut. 233, 377–386. https://doi.org/10.1016/j.envpol.2017.10.088 (2018).
Angel, B. M. et al. Spatial variability of cadmium, copper, manganese, nickel and zinc in the Port Curtis estuary, queensland, Australia. Mar. Freshw. Res. 61, 170–183. https://doi.org/10.1071/MF09046 (2010).
Ashley, P. M., Graham, B. P., Tighe, M. K. & Wolfenden, B. J. Antimony and arsenic dispersion in the Macleay river catchment, new South wales: A study of the environmental geochemical consequences. Aust. J. Earth Sci. 54, 83–103. https://doi.org/10.1080/08120090600981467 (2007).
Beernaert, J., Scheirs, J., Leirs, H., Blust, R. & Verhagen, R. Non-destructive pollution exposure assessment by means of wood mice hair. Environ. Pollut. 145, 443–451. https://doi.org/10.1016/j.envpol.2006.04.025 (2007).
Birch, G., Siaka, M. & Owens, C. The source of anthropogenic heavy metals in fluvial sediments of a rural catchment: Coxs river, Australia. Water Air Soil Pollut. 126, 13–35. https://doi.org/10.1023/a:1005258123720 (2001).
Bond, A. & Lavers, J. Trace element concentrations in feathers of flesh-footed shearwaters (Puffinus carneipes) from across their breeding range. Arch. Environ. Contam. Toxicol. 61, 318–326. https://doi.org/10.1007/s00244-010-9605-3 (2011).
Burger, J., Diaz-Barriga, F., Marafante, E., Pounds, J. & Robson, M. Methodologies to examine the importance of host factors in bioavailability of metals. Ecotoxicol. Environ. Saf. 56, 20–31. https://doi.org/10.1016/S0147-6513(03)00047-2 (2003).
Campos, I., Vale, C., Abrantes, N., Keizer, J. J. & Pereira, P. Effects of wildfire on mercury mobilisation in Eucalypt and pine forests. Catena 131, 149–159. https://doi.org/10.1016/j.catena.2015.02.024 (2015).
Casteel, S. et al. Refining the risk assessment of metal-contaminated soils. Int. J. Hyg. Environ Health. 203, 473–474. https://doi.org/10.1078/1438-4639-00049 (2001).
Chen, W., Chang, A. C. & Wu, L. Assessing long-term environmental risks of trace elements in phosphate fertilizers. Ecotoxicol. Environ. Saf. 67, 48–58. https://doi.org/10.1016/j.ecoenv.2006.12.013 (2007).
Constable, H. K., Done, S. & Gruenberg, W. A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and GoatsVol. 11 (Saunders Ltd, 2016).
Cunningham, J., Rodríguez, M. & Messa, P. Magnesium in chronic kidney disease stages 3 and 4 and in dialysis patients. Clin. Kidney J. 5, i39–i51 (2012).
Dai, Q. et al. The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am. J. Clin. Nutr. 86, 743–751 (2007).
Dart, J. Vol. 396 (ed New South Wales Department of Primary Industries) (PRIMEFACT, (2007).
de Vries, W. & McLaughlin, M. J. Modeling the cadmium balance in Australian agricultural systems in view of potential impacts on food and water quality. Sci. Total Environ. 461–462, 240–257. https://doi.org/10.1016/j.scitotenv.2013.04.069 (2013).
Degabriele, R. & Dawson, T. J. Metabolism and heat balance in an arboreal marsupial, the Koala (Phascolarctos cinereus). J. Comp. Physiol. 134, 293–301. https://doi.org/10.1007/bf00709996 (1979).
Delhaize, E. & Ryan, P. R. Aluminum toxicity and tolerance in plants. Plant Physiol. 107, 315–321. https://doi.org/10.1104/pp.107.2.315 (1995).
Dolara, P. Occurrence, exposure, effects, recommended intake and possible dietary use of selected trace compounds (aluminium, bismuth, cobalt, gold, lithium, nickel, silver). Int. J. Food Sci. Nutr. 65, 911–924. https://doi.org/10.3109/09637486.2014.937801 (2014).
Dugaszek, M. & Kopczyski, K. Correlations between elements in the fur of wild animals. Bull. Environ Contam. Toxicol. 93, 25–30. https://doi.org/10.1007/s00128-014-1260-3 (2014).
Egan, A. Reproductive responses to supplemental zinc and manganese in grazing Dorset Horn Ewes. Aust. J. Exp. Agric. 12, 131–135 (1972).
Fine, P., Paresh, R., Beriozkin, A. & Hass, A. Chelant-enhanced heavy metal uptake by eucalyptus trees under controlled deficit irrigation. Sci. Total Environ. 493, 995–1005. https://doi.org/10.1016/j.scitotenv.2014.06.085 (2014).
Franzmann, A. W., Flynn, A. & Arneson, P. D. Alaskan moose hair element values and variability. Comp. Biochem. Physiol. 57, 299–306. https://doi.org/10.1016/0300-9629(77)90195-5 (1977).
Freeland-Graves, J. H. & Lin, P. H. Plasma uptake of manganese as affected by oral loads of manganese, calcium, milk, phosphorus, copper, and zinc. J. Am. Coll. Nutr. 10, 38–43 (1991).
Gartner, R., McLean, R., Little, D. & Winks, L. Trop. Grasslands 14 266–271 (1980).
Gifford, R. M. & Barson, M. M. Australia’s renewable resources: sustainability and global change (Bureau of Rural Resources & CSIRO Division of Plant Industry, 1992).
Gray, R., Canfield, P. & Rogers, T. Trace element analysis in the serum and hair of Antarctic Leopard seal, Hydrurga leptonyx, and Weddell seal, Leptonychotes weddellii. Sci. Total Environ. 399, 202–215. https://doi.org/10.1016/j.scitotenv.2008.03.039 (2008).
Hariono, B., Ng, J. & Sutton, R. Lead concentrations in tissues of fruit bats (Pteropus sp.) in urban and non-urban locations. Wildl. Res. 20, 315–319. https://doi.org/10.1071/WR9930315 (1993).
Harvey, P. J. et al. Geochemical sources, forms and phases of soil contamination in an industrial City. Sci. Total Environ. 584–585, 505–514. https://doi.org/10.1016/j.scitotenv.2017.01.053 (2017).
Haynes, D., Carter, S., Gaus, C., Müller, J. & Dennison, W. Organochlorine and heavy metal concentrations in blubber and liver tissue collected from Queensland (Australia) dugong (Dugong dugon). Mar. Pollut. Bull. 51, 361–369. https://doi.org/10.1016/j.marpolbul.2004.10.020 (2005).
Haynes, J. I., Askew, M. J. & Leigh, C. Dietary aluminium and renal failure in the Koala (Phascolarctos cinereus). Histol. Histopathol. 19, 777–784. https://doi.org/10.14670/HH-19.777 (2004).
Hernout, B. V. et al. A National level assessment of metal contamination in bats. Environ. Pollut. 214, 847–858. https://doi.org/10.1016/j.envpol.2016.04.079 (2016).
Higgins, D. P., Hemsley, S. & Canfield, P. J. Immuno-histochemical demonstration of the role of Chlamydiaceae in renal, uterine and salpingeal disease of the koala, and demonstration of Chlamydiaceae in novel sites. J. Comp. Pathol. 133, 164–174. https://doi.org/10.1016/j.jcpa.2005.04.005 (2005).
Hothorn, T., Bretz, F. & Westfall, P. Simultaneous inference in general parametric models. Biometrical Journal: J. Math. Methods Biosci. 50, 346–363 (2008).
Hubble, G., Isbell, R. & Northcote, K. in Soils: an Australian viewpoint Vol. Division of Soils, 17–47 (CSIRO: Melbourne/Academic Press: London, (1983).
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B. & Beeregowda, K. N. Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicol. 7, 60–72. https://doi.org/10.2478/intox-2014-0009 (2014).
Jenkins, B. & Morand, D. in Australian New Zealand Soils Conference (SuperSoil). 5–9.). 5–9. (2004).
Jiao, W., Chen, W., Chang, A. C. & Page, A. L. Environmental risks of trace elements associated with long-term phosphate fertilizers applications: A review. Environ. Pollut. 168, 44–53. https://doi.org/10.1016/j.envpol.2012.03.052 (2012).
Judson, G. J. Trace element supplements for sheep at pasture. ACIAR Monogr. Ser. 37, 57–80 (1996).
Kachenko, A. G. & Singh, B. Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollut. 169, 101–123. https://doi.org/10.1007/s11270-006-2027-1 (2006).
Kennedy, I. R. Acid Soil and Acid Rain: the Impact on the Environment of Nitrogen and Sulphur Cycling (Research Studies, 1986).
Keute, J., Rizzo, J., Giunta, F. & Hernout, B. V. Evaluating washing techniques to eliminate external contamination of trace elements in Bat fur and bird feathers. Ecotoxicol. Environ. Saf. 283, 116819. https://doi.org/10.1016/j.ecoenv.2024.116819 (2024).
Kies, C. & Harms, J. M. in Copper Bioavailability and Metabolism 45–58 Springer, (1989).
King, D. J., Doronila, A. I., Feenstra, C., Baker, A. J. M. & Woodrow, I. E. Phytostabilisation of arsenical gold mine tailings using four Eucalyptus species: Growth, arsenic uptake and availability after five years. Sci. Total Environ. 406, 35–42. https://doi.org/10.1016/j.scitotenv.2008.07.054 (2008).
Koh, T., Bansemer, P. & Melnyczyn, K. in JS Davies Beef Research Forum. 1–10.
Lanctôt, C. et al. Behaviour, development and metal accumulation in striped marsh frog tadpoles (Limnodynastes peronii) exposed to coal mine wastewater. Aquat. Toxicol. 173, 218–227. https://doi.org/10.1016/j.aquatox.2016.01.014 (2016).
Lawler, I. R., Foley, W. J. & Eschler, B. M. Foliar concentration of a single toxin creates habitat patchiness for a marsupial folivore. Ecology 81, 1327–1338 (2000).
Levine, B. S. & Coburn, J. W. (Mass Medical Soc, (1984).
Lines-Kelly, R. Vol. Agdex 531 (ed Wollongbar Agricultural Institute) (CaLM and NSW Agriculture, North Coast region, Soil Sense leaflet 8/92., (1992).
Loganathan, P. et al. Fertiliser contaminants in New Zealand grazed pasture with special reference to cadmium and fluorine: A review. Aust. J. Soil Res. 41, 501 (2003).
Lottermoser, B. G. Trace metal enrichment in sugarcane soils due to the long-term application of fertilisers, North Queensland, Australia: geochemical and Pb, Sr, and U isotopic compositions. Australian Journal of Soil Research 47, 311+ (2009).
MacPherson, A. Oral treatment of trace element deficiencies in ruminant livestock. BSAP Occasional Publication. 7, 93–103. https://doi.org/10.1017/S0263967X00030123 (2018).
Mann, S. S., Rate, A. W. & Gilkes, R. J. Cadmium accumulation in agricultural soils in Western Australia. Water Air Soil Pollut. 141, 281–297. https://doi.org/10.1023/a:1021300228019 (2002).
Marchiol, L. et al. Gentle remediation at the former Pertusola Sud zinc smelter: evaluation of native species for phytoremediation purposes. Ecol. Eng. 53, 343–353. https://doi.org/10.1016/j.ecoleng.2012.12.072 (2013).
Martin, R. W. Age-specific fertility in three populations of the koala, Phascolarctos cinereus (Goldfuss), in Victoria. Wildl. Res. 8, 275–283. https://doi.org/10.1071/WR9810275 (1981).
McKenzie, N. H. et al. Mark. Priorities for Improving Soil Condition across Australia’s Agricultural Landscapes (Australian Government Department of Agriculture and Water Resources, 2017).
Mclaughlin, M., Tiller, K., Naidu, R. & Stevens, D. Review: the behaviour and environmental impact of contaminants in fertilizers. Soil. Res. 34, 1–54. https://doi.org/10.1071/SR9960001 (1996).
McLean, C. M., Koller, C. E., Rodger, J. C. & MacFarlane, G. R. Mammalian hair as an accumulative bioindicator of metal bioavailability in Australian terrestrial environments. Sci. Total Environ. 407, 3588–3596. https://doi.org/10.1016/j.scitotenv.2009.01.038 (2009).
McOrist, S. & Thomas, K. W. Levels of trace elements in the liver and diet of free-living koalas, Phascolarctos cinereus (Goldfuss). J. Wildl. Dis. 20, 220–225. https://doi.org/10.7589/0090-3558-20.3.220 (1984).
Medvedev, N. Levels of heavy metals in Karelian wildlife, 1989–91. Environ. Monit. Assess. 56, 177–193. https://doi.org/10.1023/A:1005988511058 (1999).
Mok, H. F. et al. Native Australian species are effective in extracting multiple heavy metals from biosolids. Int. J. Phytoremediation. 15, 615–632. https://doi.org/10.1080/15226514.2012.723063 (2013).
Moore, B. D., Lawler, I. R., Wallis, I. R., Beale, C. M. & Foley, W. J. Palatability mapping: a koala’s eye view of Spatial variation in habitat quality. Ecology 91, 3165–3176. https://doi.org/10.1890/09-1714.1 (2010).
O’Hara, T. M. et al. Mineral and heavy metal status as related to a mortality event and poor recruitment in a moose population in Alaska. J. Wildl. Dis. 37, 509–522. https://doi.org/10.7589/0090-3558-37.3.509 (2001).
Phelps, R. W., Clarkson, T. W., Kershaw, T. G. & Wheatley, B. Interrelationships of blood and hair mercury concentrations in a North American population exposed to Methylmercury. Arch. Environ. Health. 35, 161–168 (1980).
Pople, A. R., Gordon, A. N. & Ng, J. Trace metal concentrations in livers and kidneys of sea turtles from south-eastern queensland, Australia. Mar. Freshw. Res. 49, 409–414. https://doi.org/10.1071/MF97266 (1998).
Prokop, Z. et al. Mobility, bioavailability, and toxic effects of cadmium in soil samples. Environ. Res. 91, 119–126. https://doi.org/10.1016/S0013-9351(02)00012-9 (2003).
Pulscher, L. A. et al. Investigation into the utility of flying foxes as bioindicators for environmental metal pollution reveals evidence of diminished lead but significant cadmium exposure. Chemosphere, 254, 126839 (2020).
Pyatt, F. B. Copper and lead bioaccumulation by Acacia retinoides and Eucalyptus torquata in sites contaminated as a consequence of extensive ancient mining activities in Cyprus. Ecotoxicol. Environ. Saf. 50, 60–64. https://doi.org/10.1006/eesa.2001.2087 (2001).
Radcliffe, J. C. Pesticide Use in Australia. Report No. 1875618694 (Australian Academy of Technological Sciences and Engineering, 2002).
Roug, A. et al. Comparison of trace mineral concentrations in tail hair, body hair, blood, and liver of mule deer (Odocoileus hemionus) in California. J. Vet. Diagn. Invest. 27, 295–305. https://doi.org/10.1177/1040638715577826 (2015).
Rouillon, M., Gore, D. B. & Taylor, M. P. The nature and distribution of cu, zn, hg, and Pb in urban soils of a regional city: lithgow, Australia. Appl. Geochem. 36, 83–91. https://doi.org/10.1016/j.apgeochem.2013.06.015 (2013).
Sánchez-González, C. et al. Association of plasma manganese levels with chronic renal failure. J. Trace Elem. Med Biol. 31, 78–84 (2015).
SGS. in Regional Development Australia (ed SGS Economics and Planning Pty Ltd). RDA Northern Rivers, (2009).
Shukla, O. P., Juwarkar, A. A., Singh, S. K., Khan, S. & Rai, U. N. Growth responses and metal accumulation capabilities of woody plants during the phytoremediation of tannery sludge. Waste Manage. (New York N Y). 31, 115–123. https://doi.org/10.1016/j.wasman.2010.08.022 (2011).
Speight, K. N. et al. Plasma biochemistry and Urinalysis variables of Koalas (Phascolarctos cinereus) with and without oxalate nephrosis. Vet. Clin. Pathol. 43, 244–254. https://doi.org/10.1111/vcp.12145 (2014).
Stacey, S., McLaughlin, M. & Hettiarachchi, G. in In Trace Elements in Soils. 135–154 (eds Hooda, P. S.) (Blackwell Publishing Ltd, 2010).
Stephens, C. G. & Donald, C. M. in In Advances in Agronomy. Vol. 10, 167–256 (eds Normax, A. G.) (Academic, 1959).
Summer, K. & Reichelt-Brushett, A. Trace element contaminant uptake in phytocap vegetation and implications for Koala habitat, lismore, Australia. Environ. Sci. Pollut. Res. 25, 24281–24292. https://doi.org/10.1007/s11356-018-2441-0 (2018).
Tighe, M., Ashley, P., Lockwood, P. & Wilson, S. Soil, water, and pasture enrichment of antimony and arsenic within a coastal floodplain system. Sci. Total Environ. 347, 175–186. https://doi.org/10.1016/j.scitotenv.2004.12.008 (2005).
Wallis, I. R. et al. A chemical perspective on the evolution of variation in Eucalyptus globulus. Perspect. Plant. Ecol. Evol. Syst. 13, 305–318. https://doi.org/10.1016/j.ppees.2011.05.005 (2011).
Wesley-Smith, R. & Schlink, A. in Australian Soc. Anim. Production 428–431 .
Whelan, B. & Barrow, N. Slow-release selenium fertilizers to correct selenium deficiency in grazing sheep in Western Australia. Fertilizer Res. 38, 183–188 (1994).
Whelan, B., Peter, D. & Barrow, N. Selenium fertilizers for pastures grazed by sheep. 1. Selenium concentrations in whole blood and plasma. Aust. J. Agric. Res. 45, 863–875. https://doi.org/10.1071/AR9940863 (1994).
Xie, R., Seip, H. M., Wibetoe, G., Nori, S. & McLeod, C. W. Heavy coal combustion as the dominant source of particulate pollution in Taiyuan, China, corroborated by high concentrations of arsenic and selenium in PM10. Sci. Total Environ. 370, 409–415. https://doi.org/10.1016/j.scitotenv.2006.07.004 (2006).
Zaccaroni, A. et al. Trace metal concentration in wild avian species from Campania, Italy. Cent. Eur. J. Chem. 9, 86–93. https://doi.org/10.2478/s11532-010-0119-7 (2011).
Acknowledgements
We appreciate the support of Aileen Wing-Simpson and David Lee at the Department of Chemical Pathology, Trace and Toxic Element Unit at the Royal Prince Alfred Hospital Camperdown, NSW, Australia, for their support in the lab during sample analysis. We would like to thank individual groups for their support in fur sample collection: Prof. Mathew Crowther at the University of Sydney and landholders in the Liverpool Plains region, Cheyne Flanagan and her team at the Port Macquarie Koala Hospital, the team of “Friends of the Koalas” at Lismore, Prof. David Phalen at the University of Sydney and Lachlan Willmott at the Office of Environment and Heritage (OEH).
Funding
Betty Rosalie Richards and Grace Mary Mitchell Bequest Sydney School of Veterinary Science, Faculty of Science, The University of Sydney.
Author information
Authors and Affiliations
Contributions
CM: Conceptualization, Study design, Sample collection, Sample analysis, Data curation, Writing- original draft, VisualizationRG: Conceptualization, Supervision, Methodology, Recourses, Validation, Writing- review and editingMT: Statistical analysis, Writing- review and editingDH: Conceptualization, Supervision, Writing- review and editingRQ: Resources, MethodologyMK: Conceptualization, Supervision, Recourses, Methodology, Writing- review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Marschner, C., Gray, R., Terkildsen, M. et al. Effect of land use on the concentrations of trace elements and heavy metals in the hair of the koala (Phascolarctos cinereus). Sci Rep 15, 27920 (2025). https://doi.org/10.1038/s41598-025-12702-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12702-3