Abstract
Numerous issues with pharmacokinetics and water solubility of current anticancer drugs have hindered the development of chemotherapy, resulting in significant side effects and patient resistance to multiple therapies. Nanomedicine revolutionized cancer treatment by introducing nanocarriers to enhance drug delivery. This study investigates the use of benzimidazolone capsules as nanocarrier for drug delivery of flutamide (FLT) and gemcitabine (GB) (FLT@Cap and GB@Cap). Numerous structural and electrical parameters demonstrate that both drugs interact well with nanocapsules and are easily transported to the targeted site. Using complexation energy analysis, quantum theory of atoms in molecules (QTAIM), non-covalent interactions (NCI), natural bond orbital (NBO), frontier molecular orbital (FMO), and density of states (DOS) analyses, the drug delivery of flutamide (FLT) and gemcitabine (GB) via benzimidazolone capsule is described. FLT@Cap and GB@Cap exhibit strong drug-capsule interaction, with adsorption energies of -42.18 and − 51.59 kcal/mol, respectively. QTAIM and NCI analysis validate the non-covalent interactions between FLT, GB, and capsule. To comprehend the shifting of electron density from drug to surface, NBO analysis is utilized. After complexation, (EH−L) which describes the HOMO-LUMO energy gap is reduced, and the perturbations in the electronic parameters are estimated using FMO analysis. The results indicate a high degree of selectivity for FLT and GB on the surface of the benzimidazolone capsule.
Similar content being viewed by others
Introduction
One of the most serious types of diseases is cancer, which is caused by uncontrolled cell divisions and aberrant cell growths in tissues or organs1. Owing to its severe consequences, cancer ranks among the top global public health priorities and is a leading cause of mortality worldwide. According to the International Agency for Research on Cancer (IARC), approximately 20 million new cancer cases and 9.7 million cancer-related deaths occurred in 2022, accounting for 1 in 6 deaths globally (IARC, GLOBOCAN 2022)2,3. However, if detected early, the condition can often be effectively treated with radiation therapy, surgery, or chemotherapy4. Different nanoformulations with superior pharmacokinetic qualities such as enhanced tumor accumulation, prolonged systemic circulation, reduced off-target toxicity, and stimuli-responsive drug release and biocompatibility hold significant promise for the delivery of innovative anti-cancer medications as nanomedicine becomes more widely used5,6,7,8,9. The issues of unfavorable side effects and low water solubility during the therapeutic medication delivery agents are resolved by these nanocarriers. Additionally, these nanocarriers facilitate their use for tumor therapies and lengthen the therapeutic circulation period in blood for increased therapeutic and delivery efficacy10,11,12. Through the encapsulation of certain therapeutic chemicals, nanomedicines can be produced in nanocarriers to effectively target tumors through the effect-mediated passive targeting approach13.
Among different anti-cancerous drugs, the Flutamide (FLT), Gemcitabine (GB), (Fig. 1) are widely used14. Flutamide (FLT) is a unique antiandrogenic medication without a steroidal composition. Flutamide is a popular nonsteroidal antiandrogen medication used to treat prostate cancer, yet there have been isolated reports of hepatotoxicity in patients taking it. Its toxicity is thought to result from the bioactivation of FLT and its subsequent covalent attachment to cellular proteins. It is being utilized more frequently as a neoadjuvant treatment prior to radical prostatectomy and as part of total androgen ablation therapy15. Advanced prostate cancer is treated with flutamide, a drug that has no hormone agonist action16. DFT studies have also examined its interactions with several nanocarriers, which unveiled principal binding mechanisms and stability. For instance, a study on the complexation of flutamide with cucurbit[n]uril showed exothermic binding and spontaneous inclusion complex formation due to electrostatic interactions and hydrogen bonding17 .Another study explored the interaction of flutamide with COOH- and COCl-functionalized carbon nanotubes and found that COOH-functionalized nanotubes provided more desirable binding energies18. Graphitic carbon nitride (g-C3N4) was also examined as a flutamide carrier, with DFT verifying stable drug-nanocarrier interaction19. Zinc oxide nanosheets (ZnONS) also exhibited good adsorption behavior for flutamide, as verified by DFT calculations20.
A pyrimidine nucleoside antimetabolite, gemcitabine is one of the cytotoxic medications. Breast, pancreatic, bladder, and large cell lung cancer can all be treated with gemcitabine21,22,23. It is effective against ovarian cancer, according to recent studies. Nowadays, clinical trials are conducted to assess the role of gemcitabine in conjunction with novel targeted treatments24. Certain theoretical studies have also been evaluated their binding with different nanocarriers, including carbon nanotubes and fullerenes. Pd-doped carbon nanotubes have strong interaction with gemcitabine, indicating potential for effective drug delivery25. Further, interactions between gemcitabine and Mg@B40 or Ca@B40 fullerenes were shown to have strong adsorption energies, reflecting partial covalent interactions improving drug stability26. Another research identified pure B40 fullerene as a potential carrier for gemcitabine and concluded that DFT and QTAIM calculations were helpful in establishing its effectiveness27.
Even though these medications have therapeutic benefits, if taken in excess, they might harm healthy cells as well. Melanotic cells are destroyed along with healthy cells by standard chemotherapeutic, non-targeted approaches28,29. Therefore, it is necessary to regulate the plasma concentration of these anti-cancer medications using a targeted drug delivery system and to adjust the dosage based on the patient’s tolerance level and the severity of the disease30,31. Thus, new, and effective drug delivery systems with superior therapeutic characteristics are inevitable.
Recent years have seen a sharp advancement in nanotechnology, opening up a plethora of fascinating concepts and opportunities for the detection and management of disease32,33,34,35. As a result, nanocarriers’ focused assault on malignant cells has drawn a lot of interest. While protecting healthy cells, the medication delivery via nanocarriers in cancer treatment has demonstrated encouraging outcomes. Additionally, the use of nanoparticle-based drug delivery systems can address the problem of reduced absorption, demonstrating the remarkable potential of nanoparticle-based DDS in the treatment of cancer29. Examples of such nanocarriers that have been used for encapsulation and effective drug delivery including, micelles, nanoparticles, polymeric conjugates, dendrimers, liposomes, carbon nanotubes capsules and hydrogel28,30. When used as anticancer medication vehicles, these nanocarriers have demonstrated effective performance in reducing side effects36.
Literature reveals many structures which have been used as carriers such as, amphiphilic copolymers can be used to create nano self-assembled structures that function as effective delivery systems for anticancer medications like doxorubicin37.Moreover, a variety of near-IR light-responsive drug delivery vehicles have been effectively synthesized and described. When it comes to treating cancer cells, these drug delivery vehicles have remarkable outcomes. P. N. Samanta et al. described the use of fullerene (C60) nanocages as a drug delivery system for the transportation of temozolomide and carmustine38.They found that the increase in polarity of the C60 nanocage following adsorption of carmustine and temozolomide aids in the off-loading of these medications into biological systems. Additionally, it has been revealed that graphene and graphene oxide (GO) can also be used as nanostructured drug delivery vehicles. Because of their efficient loading, focused drug delivery, and regulated release, both have the potential as excellent options for the delivery of different medications39. To achieve considerable therapeutic efficacy and quick response, high sensitivity, selectivity, and minimal adverse effects, additional research must be done on novel nanocarriers40,41.
In this work, we examined the benzimidazolone capsule’s drug delivery capabilities for the drugs gemcitabine and flutamide. Benzimidazolone cavitand-based nanocapsule have not been the subject of extensive studies in literature. Weak noncovalent interactions are essential for the synthesis of more selective and active ligands in medicinal chemistry42. Dimeric nanocapsules based on deep benzimidazolone cavitands can be great hosts for tiny medicinal molecules. Various appropriate guests can be bound by the deep cavity of the benzimidazolone nanocapsule43. It also facilitates the bridging of hydrogen bonds to other surfaces through its waterlogged interface. The surface presents a viable contender for adsorption applications due to the cavity’s ability to enhance drug adsorption44. Theoretical investigations, especially those involving density functional theory (DFT), have shed light on their molecular interactions and efficiency. Investigations into benzimidazolone derivatives have been used in encapsulation, with emphasis on their structural and electronic properties. For example, DFT analysis of 1-(cyclohex-1-enyl)-1,3-dihydro-2 H-benzimidazolone (CHBI) showed its adsorption characteristics on copper surfaces, exhibiting an 86.49% rate of corrosion inhibition45. Benzimidazole derivatives have also been explored as anticancer agents, where DFT studies have optimized their geometric structures and correlated theoretical results with experimental cytotoxicity data46. In addition, benzothiazole-imidazolone hybrids were investigated using DFT for insight into their binding interactions with bacterial DNA gyrase, with emphasis on their antimicrobial activity47.
Despite extensive theoretical investigations on benzimidazolone capsules as promising drug carriers, their potential for the targeted delivery of flutamide and gemcitabine remains unexplored in the literature. Therefore, the current work uses DFT calculations to examine the adsorption mechanism of flutamide and gemcitabine on the surface of benzimidazolone capsules. To investigate electrical structure, the frontier molecular orbital (FMO) analysis is employed. Additionally, non-covalent interaction (NCI) and quantum theory of atom in molecule (QTAIM) investigations are carried out to determine the nature and degree of interaction.
Computational insights
The Guassian 09 program is used for analyzing all the calculations. The M06-2X /6-31G(d) level of theory is selected for geometry optimization. The hybrid functional M06-2X, which has twice as much non-local exchange, is considered ideal for studying noncovalent interactions48. M06-2X gives results with reasonable accuracy for systems exhibiting dispersion forces39,40,41,42. In literature, there are various examples reported, where complex systems have been frequently evaluated with a similar level of theory49,50,51,52. GaussView 5.0 and Chemcraft software53 were utilized to visualize the optimized geometries. Several drug@capsule orientations were investigated in order to determine the most stable configuration with the lowest energy. The equation used to investigate the energy of complex is:
In Eq. (1): Ecomplex, Ecapsule and Edrug represent the electronic energies of drugs-capsule complexes, isolated capsules and drug, respectively. Quantum chemical simulations involving fragments (interacting) are usually prone to BSSE (basis set superposition error). Therefore, it is imperative to add corrections for such types of errors. In this regard, BSSE is an efficient approach to overcome the error. The Counter poise method corrects this energy as shown given below.
where Eint.CP, Eint and EBSSE present the counterpoised interaction energy, raw complexation energy and energy after BSSE correction. To investigate the electronic changes Frontier molecular orbital (FMO) analysis is performed54. To find out charge transfer between drugs and capsule, natural bond orbital (NBO) and electron density differences (EDD) analyses was carried out55. EDD analysis provides a visual representation of the charge transfer between interacting atoms using colored surfaces, adding further to NBO results56. NBO charges are derived from DFT calculations by partitioning the electron density into localized “natural” orbitals in GaussView 5.0.
NCI analysis is used to visualize and determine the forces that interact between drugs and the capsule surface. To plot 2D RDG graphs and 3D-isosurfaces of drug@capsule complexes, Multiwfn 3.7 software is employed. In general, the non-covalent interaction index depends on two variables: electron density (ρ) and reduced density gradient (RDG), and the relationship between both of these variables is expressed in the following Eq.
Different colors on 3D iso surfaces correspond to different kinds of forces. Typically, three distinct color schemes are displayed, each of which stands for a particular form of interaction. For example, the green color symbolizes weak forces and London dispersion. Strong forces like hydrogen bonds are represented by the blue color, whereas steric repulsion between interacting species is represented by the red color. The strength of a weak nonbonding contact is represented by the thickness of iso surfaces. The strength of contact is strongly correlated with its thickness. The 2D NCI graph represents the relationship visually between sign (λ2) ρ (au.) on the x-axis and RDG (reduced density gradient) on the y-axis. On this graph, hydrogen bonding, weak van der Waals forces, and repulsive forces are represented, respectively, by the blue, green, and red spikes56,57,58.
The QTAIM analysis is mainly used for studying noncovalent interaction. The various topological parameters are used in QTAIM analysis to evaluate bond critical point (BCPs). These topological parameters are electron density, total density and laplacian.
If the laplacian of electron density is greater than zero, it indicates weak intermolecular forces whereas, if the value of laplacian of electron density is less than zero it shows covalent bonding58,59,60,61. Multiwfn 3.7 is used for QTAIM analysis62.
Results and discussion
Geometric optimization
Two different drugs, gemcitabine and flutamide, have been selected for this investigation in order to examine their adsorption on benzimidazolone capsules as a drug delivery system. Chemical structure of both drugs is represented in Fig. 1. Figure 2 represents the optimized structure of the benzimidazolone capsule. Dimeric nanocapsules based on deep benzimidazolone cavitands are created from a resorcinarene core by covalent attachment of walls and adding additional characteristics like rigidified walls and intramolecular hydrogen bonding patterns in order to stabilize the malleable vase-like structures, are frequently used63. The dimeric nanocapsule based on benzimidazolone cavitands have symmetrical vase shape and hold C4v symmetry64.
The designed complexes are optimized to their most stable geometry using the M06-2X/6-31G(d, p) level of theory. In order to find the most suitable interaction site, all the possible orientations are studied whereas, the most stable geometries are selected for further analysis (see Fig. S1 of Supporting Information). Moreover, different possible conformations of drug molecules are also considered to get the most stable complex (see Fig. S2). The computed geometries of both GB and FLT complexes are presented in Fig. 3.
Two important factors for estimating interaction behavior are interaction distance and complexation energy (ΔE)65. As analyte approaches the surface (capsule) for complexation, bond distances decrease. Drug-capsule geometry interactions are determined by calculating complexation energies and the minimum interaction distances. In Table 1, we have presented the interaction distances and corresponding complexation energies for each complex (drugs@capsule).
The complexation energy (ΔE) values for both drugs@cap show that the drugs are physiosorbed on the capsule cavity; and the values are − 51.59 kcal/mol for GB@Cap and − 42.18 kcal/mol FLT@Cap complexes, respectively (see Table 1). With a complexation energy of -51.59 kcal/mol, the GB anticancer drug exhibits the strongest interaction then FLT@Cap complex.
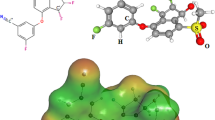
Non-covalent interactions, such as hydrogen bonds and weak van der Waals forces, are indicated by the interaction distances (Angstrom). The shortest interaction distance in the case of GB@Cap complex is calculated for O7—H8 with interaction distance of 1.83Å. The interaction distances observed in GB@capsule are 2.03Å, 2.46Å, and 2.73Å for O1—H2, H4—O3, H5—O6, respectively (See Fig. 3). The F-atoms of GB and the O-atoms of Capsule exhibit strong hydrogen bonding in the stable optimized geometry of GB@capsule. Moreover, in the case of FLT@cap, the interaction distances of H1—F2, H—O4, N5—H6 are 2.65Ǻ, 2.37Ǻ and 2.64 Ǻ (Fig. 3) confirming the presence of strong hydrogen bonds between the atoms of drug and capsule.
The complexation energies are also calculated by using dispersion-corrected functional (e.g., ωB97XD). The inclusion of dispersion-corrected ωB97XD functional slightly affects the complexation energy of studied complexes. The complexation energy with dispersion correction ωB97XD functional is higher than those without correction. However, the trend of complexation energy is the same with and without dispersion correction. The complexation energies of GB@Capsule, and FLT@Capsule complexes are quite comparable for both M06-2X (ωB97XD) functionals. and complexation energy values are − 51.59 kcal/mol (-54.67 kcal/mol) and − 42.18 kcal/mol (-45.82 kcal/mol), respectively (see Table 1). The complexation energy of FLT is lower as compared to GB. The higher complexation energy values display the higher stability of drugs@Capsule. Overall, gemcitabine drug shows slightly higher stability as compared to flutamide. This might be due to the reason that gemcitabine fits better in the capsule cavity than flutamide.
Moreover, the Counterpoise approach is also considered to calculate the BSSE (basis set superposition error) for the studied complexes. The complexation energies of GB@Capsule, and FLT@Capsule complexes are quite comparable with one another, and complexation energy values are − 51.59 kcal/mol (BSSE=-48.21 kcal/mol) and − 42.18 kcal/mol (BSSE=-40.32 kcal/mol), respectively (see Table 1). The same order of BSSE corrected energy and complexation energy values is observed in both the studied drug@Capsule complexes. The calculated complexation energy results reveal the same trend and comparable values of Counterpoise corrected interaction energy for both studied anticancer drugs (see Table 1).
Non-covalent interactions (NCI) analysis
NCI analysis is performed to obtain an in-depth understanding of the non-covalent interactions between the drugs@capsule complexes. 2D graphs and 3D iso surfaces are shown (Fig. 4) to examine the nature of attractive and repulsive forces between the drug and capsule complexes. It offers a clear description of non-covalent interactions using reduced density gradient (RDG) analysis, which is based on the second eigenvalue with electron density (ρ)66.
The colored map in NCI analysis indicates the presence of weak van der Waals interactions between the drugs and the capsule surface, as seen by the appearance of a green and somewhat red isosurface. When looking at 3D iso-surfaces of GB@Cap, green patches between the drug molecule and the capsule surface indicate the presence of weak van der Waals interactions. The 2D map’s spikes, which are projected at the X-axis between 0.015 and − 0.015 a.u., also indicate weak van der Waals interactions. In the case of FLT@Cap, green spikes observed in 3D structures confirms the existence of non-covalent interactions between the drugs and molecules of the capsule. Furthermore, both complexes exhibit dispersed green spikes in 2D-RDG plots that range from − 0.015 a.u. to 0.005 a.u., validating weak van der Waals interactions. The steric repulsion that exists between some surface atoms is shown by the 3D red color patches (Fig. 4).
Similarly, strong electrostatic interactions are shown by the spikes on 2D NCI graphs at signλ2(ρ) > -0.01 (a.u.), whereas London dispersion forces exist below this negative value. Moreover, these results suggests that there are no covalent interactions between the drugs and the capsule, which facilitates the release of drug molecules at the targeted location.
Quantum theory of atoms in molecules (QTAIM) analysis
The strength and nature of interaction between drugs and capsule is characterized using QTAIM analysis. In QTAIM analysis, bond strength depends upon the (ρ) electronic density while the bond nature depends on the Laplacian of electronic density (∇2ρ) and sum of electron densities (H). In different molecular systems, Bader’s QTAIM analysis is often used to determine the non-covalent or nonbonding interactions67. According to topological evaluation, the bond critical points, which include electron density (ρ), Laplacian ∇2(ρ), and total electron density H(r), determine the type of nonbonding interactions. The sum of the local kinetic energy G(r) and potential energy V(r) determines the overall energy density H(r). The relationship can be shown as:
The topologies computed through the QTAIM analysis are given in Fig. 5, whereas the BCP parameters are presented in Table 2. The various topological parameters in QTAIM analysis are evaluated via BCPs (bond critical point), Laplacian of electronic density and total electronic values etc. .
The occurrence of weak intermolecular interactions is indicated by the Laplacian of the electron density (∇2ρ(r) > 0), whereas the existence of covalent bonding is indicated by the Laplacian of the electron density (∇2ρ(r) < 0)68. The link between the Laplacian of the electron density (∇2ρ(r)) and the density of kinetic energy (G(r)) and potential energy (V(r)) is demonstrated by equation;
H(r) for non-covalent bonds must either be larger than or equal to 0, and covalent bonds will occur if it is less than 0. Values > 0 for the energy density H(r) and Laplacian ∆2ρ(r) often suggest the presence of non-covalent interactions, whereas values < 0 generally imply covalent interactions. Table 2 lists the topological characteristics for each complex, and Fig. 5 displays the molecular graph of these features, which includes the bond critical point (BCP) and bond path.
The values of various parameters are evaluated through QTAIM analysis for drug@capsule complexes and are listed in Table 2, whereas 3D isosurfaces of drug@capsule are shown in Fig. 5. The values of Laplacian ∇2ρ and electron density (ρ) in Table 2 justified the noncovalent interactions in both studied drugs@capsule complexes. In QTAIM analysis, fifteen BCPs are found for FLT@cap (Table 2). The electronic density values of ρ(r) for these BCPs fall between 0.003 and 0.011 a.u. In contrast, H(r) and ∇2ρ(r) have values between 0.0004 and 0.0015 a.u. and 0.004 to0.05 a.u., respectively. The values of ∇2ρ, ρ(r), and H(r) indicate non-covalent contact that ranges from small to strong. The remaining two parameters, G(r) & −V(r)/G(r), exhibit non-covalent interactions.
Moreover, Table 2 shows fifteen BCPs according to the results obtained by the QTAIM analysis of GB@Cap complex. The ρ(r) electronic density values for these BCPs are determined to be in the range of 0.002–0.011a.u. The values of Laplacian electron density fall between 0.011 and 0.104 a.u. The values of topological parameters like G(r), H(r), V(r), and − V(r)/G(r) indicate non-covalent interactions. Overall, it can be evaluated that the strong non-covalent interactions are present.
Electronic properties
Natural bond orbital (NBO) analysis
NBO analysis is carried out to understand the charge transfer between drugs and the capsule surface. When examining the type of interaction that exists between drugs and the capsule surface, electronic properties are crucial. Table 3 shows the NBO charges upon the interaction of drugs and capsule surface. Charge transfer from the drug towards the surface is shown by the positive value whereas negative value shows that the charge is transferred from surface to drugs69.
The observed NBO charge transfer values are − 0.017 and 0.007 for (FLT@Capsule) and (GB@Capsule). Positive NBO values show that charge is being transferred from the capsule to the FLT, whereas negative NBO values indicate that charge is being transferred from the GB to the capsule. This opposite trend could possibly because of the flutamide having a relatively simple structure than gemcitabine. Flutamide has a more electron-rich region or donating groups such as the nitrogen of the amide, it might donate electron density to the benzimidazolone capsule, resulting in a negative charge transfer, i.e., − 0.017 in the case of (FLT@Cap). While, gemcitabine contains fluorine atoms and a sugar moiety, which could make the molecule more electronegative compared to flutamide. The fluorine atoms are highly electronegative, which may pull electron density away from the benzimidazolone capsule, leading to a positive charge transfer i.e., 0.007 in the case of (GB@Cap).
The highest charge transfer is observed in the case of GB@Capsule. Furthermore, existence of weaker noncovalent interactions is revealed by the small NBO charge transfer values. The 3D iso-surfaces of the EDD study validate these quantitative results of the charge transfer.
Electron density differences (EDD)
To visualize the isosurfaces of the drug@capsule under study, EDD analysis is utilized. Figure 6 displays the drugs@capsule complex EDD graphs (Isovalue = 0.0004 a.u.). Multiwfn 3.7 software was used to obtain the EDD analysis results (isosurfaces). The interaction of the capsule and drug orbitals is represented by the purple and pink color isosurfaces that were produced using EDD analysis (Fig. 6). The purple color indicates the accumulation of electron density, whereas the pink color indicates the depletion of electrons.
Because of the electrostatic interactions between the H-atoms of the considered drugs and the N-atoms of the capsule surface, there is a greater accumulation of electron density between the drugs and the capsule surface, resulting in purple isosurfaces. Furthermore, in the case of the GB@capsule complex, these purple and pink surfaces confirm the transfer of charges toward dug from the capsule. For FLT@capsule complex, the charge transfer from drug to the capsules is validated through purple and pink isosurfaces. Figure 6 shows the pink isosurfaces, which indicate the depletion of electron density upon interaction. Electronic density distribution between the drugs and capsule surface is confirmed by EDD plots. The EDD results are further validated through NBO (charge transfer) analysis.
Frontier molecular orbital (FMO) analysis
FMO analysis is used to examine how the electrical properties of the capsule surface change when drug@capsule complex is formed. The FMO analysis is used to study the conductivity of selected system via H-L gap change. In general, as conductivity increases, the energy gap (HOMO-LUMO gap) decreases, but it decreases with a larger HOMO-LUMO gap70.
The energies of HOMO and LUMO, H-L gap and thier isosurfaces are presented in Table 3; Fig. 7, respectively. For the bare capsule surface, HOMO energy is − 6.88 eV and LUMO energy is − 0.22 eV, and the energy gap (EH−L) for bare capsule surface is − 6.66 eV. The calculated HOMO-LUMO (EH−L) energy gaps of FLT@Capsule complex is and GB@Capsule complexes are − 5.73 eV and − 6.54 eV, resepectively. For both studied drugs, upon adsorption of drugs on capsule surface appreciable reduction in energy gap (EH−L) is observed. Both drugs show almost similar behavior with minor variation in terms of variation HOMO-LUMO energy variation and their energy gap (EH−L). Upon FLT adsorption over capsule, energies of both HOMO and LUMO decrease which leads to reduction in EH−L gap of -5.73 eV, compared to -6.66 eV for isolated capsule. Whereas, the EH-L gap of GB@Complex is slightly decreased but is comparable to that of the bare capsule. This can be understood as, When gemcitabine binds to the capsule, the electron-withdrawing nature of gemcitabine may result in relatively weak electron donation to the capsule. This leads to a situation where the capsule’s electronic structure is not significantly altered, preserving the HOMO-LUMO gap in a range similar to the bare capsule.
In FLT@capsule complex, both HOMO and LUMO orbital density is present over drug, whereas in case of GB@capsule complex both HOMO and LUMO orbital density is located on the capsule surface. Overall, the FMO data clearly demonstrates that both drugs show a decrease in the EH−L gap as a result of complexation, indicating that the capsule surface exhibits greater sensitivity towards anticancer drugs.
Density of states (DOS) analysis
The graphical representation of energy states is provided by DOS analysis. The density of states analysis can be used to deduce the electronic change of the capsule upon drug adsorption71. Figure 8 shows the DOS spectra of the drugs@capsule and bare capsule. The DOS spectra can be used to visualize the differences in peak intensities between a capsule that is relatively bare and one that is loaded with GB and FA. The variations in conductivity following drug adsorption over the capsule surface are revealed by these shifts and intensities in the peaks.
Dipole moment (µ) analysis
The solubility of the drug molecule and its ease of release at the target site is determined by the change in dipole moment. Prior to drug adsorption, the bare capsule’s dipole moment (µ) is zero (0.004 D). Furthermore, isolated drug molecules have dipole moment values of 7.32 D for FLT and 5.44 D for GB. When the capsule interacts with the FLT and GB drugs, the dipole moment values in the resulting complexes shift to 5.79 D and 5.65 D, respectively. Strong interactions between the benzimidazolone capsule and the drugs, including hydrogen bonding, charge transfer, and electrostatic interactions, are responsible for the significant increase in dipole moment from 0.004 to 5.651 and 5.796 upon complexation of the capsule with gemcitabine and flutamide. Gemcitabine probably causes a large redistribution of electron density inside the capsule since it is a highly polar chemical with functional groups that can create strong interactions. As a result, the complex’s overall dipole moment is significantly higher than that of the bare capsule. The solubility of the complexes in aqueous media, which further aids in the mobility of drug molecules in a biological system, depends on their dipole moment. FLT’s dipole moment decreases slightly (from 7.32 D to 5.79 D), suggesting a weaker interaction, meaning FLT is likely to be released more easily. In contrast, GB’s dipole moment increases slightly (from 5.44 D to 5.65 D), indicating a stronger binding to the capsule, which would make GB harder to release. Thus, dipole moment analysis reveals that capsule can release FLT more effectively than GB on a target site.
pH effect
One of the most crucial steps is the release of the drug from the carrier surface to the target cell. The pH of a malignant cell environment is often lower than 6 than that of normal blood cells (7.35–7.45). We thus investigated the impact of pH on the GB@Capsule and FLT@Capsule complexes. In an acidic environment, we performed DFT simulations of drugs (gemcitabine and flutamide) loaded on benzimidazolone capsules (see Fig. 9). The flutamide and gemcitabine’s interacting sites (the N-atoms of the amide and amine groups) were protonated with H+ in an acidic medium, and the structure was then relaxed once again at the same theoretical level. When the GB@Capsule and FLT@Capsule complexes were compared in an acidic medium, the complexation energies of the two complexes drastically decreased, going from − 51.59 to -18.71 kcal/mole and from − 42.18 to -14.56 kcal/mole, respectively. In contrast, the GB@Capsule complex showed an increase in the interaction distances from 1.83 Å to 3.01 Å. This shows that, in contrast to GB, FLT can be easily offloaded from the carrier (benzimidazolone capsule) to the target location (Fig. 10). Numerous protons may also be affixed to the drug’s surface and other endpoints in an acidic environment. These protons might not have an impact on the drug’s release at the intended location, though.
Conclusion
Using DFT at M06-2X functional with 6-31G(d, p) basis set, the drug transport capability of porous material (capsule) is tested against anticancer drugs in the current investigation. Both of the investigated drugs exothermically adsorbed and the complexes are thermodynamically stable, according to the complexation energy (ΔE) values of most stable optimized geometries. The most stable complex, according to the results, is the GB@Capsule complex, which has the highest complexation energy at -51.59 kcal/mol. QTAIM analysis revealed that the strong electrostatic forces are responsible for the stability of the GB@capsule complex. Only noncovalent interactions between drugs and the capsule surface are found, according to the Laplacian and electron density values as well as the -V/G ratio of all complexes identified by QTAIM analysis. The presence of green spikes in noncovalent interaction (NCI) analyses indicated weak van der Waals contacts between interacting fragments. According to FMO analysis, the HOMO-LUMO energy gap (EH−L) decreased appreciably for all complexes under study. Given that malignant cells have an acidic pH in comparison to normal cells, the decrease in complexation energies obtained through DFT calculations suggest that anticancer drugs can be readily released at the targeted site. The key findings suggest that the capsule surface performs better when it comes to anticancer drug delivery.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Saini, A., Kumar, M., Bhatt, S., Saini, V. & Malik, A. Cancer causes and treatments. Int. J. Pharm. Sci. Res. 11, 3121–3134 (2020).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 68, 394–424 (2018).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 71, 209–249 (2021).
Cairns, J. The treatment of diseases and the war against cancer. Sci. Am. 253, 51–59 (1985).
Steichen, S. D., Caldorera-Moore, M. & Peppas, N. A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 48, 416–427 (2013).
De Koker, S., Hoogenboom, R. & De Geest, B. G. Polymeric multilayer capsules for drug delivery. Chem. Soc. Rev. 41, 2867–2884 (2012).
Tan, K. F., In, L. L. A. & Vijayaraj Kumar, P. Surface functionalization of gold nanoparticles for targeting the tumor microenvironment to improve antitumor efficiency. ACS Appl. Bio Mater. 6, 2944–2981 (2023).
Gabizon, A. A., Gabizon-Peretz, S., Modaresahmadi, S. & La-Beck, N. M. Thirty years from FDA approval of pegylated liposomal doxorubicin (Doxil/Caelyx): an updated analysis and future perspective. BMJ Oncol. 4, e000573 (2025).
Li, W. et al. Construction and in vitro evaluation of pH-sensitive nanoparticles to reverse drug resistance of breast cancer stem cells. Discover Oncol. 15, 21 (2024).
Karimi, M. et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 45, 1457–1501 (2016).
Ge, Z. & Liu, S. Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chem. Soc. Rev. 42, 7289–7325 (2013).
Cao, B. et al. Tailoring the d-band center of N-doped carbon nanotube arrays with Co4N nanoparticles and single-atom Co for a superior hydrogen evolution reaction. NPG Asia Mater. 13, 1 (2021).
Farhoudi, L., Kesharwani, P., Majeed, M., Johnston, T. P. & Sahebkar, A. Polymeric nanomicelles of curcumin: potential applications in cancer. Int. J. Pharm. 617, 121622 (2022).
Corrie, P. et al. Phase II study to evaluate combining gemcitabine with flutamide in advanced pancreatic cancer patients. Br. J. Cancer. 87, 716–719 (2002).
Mariappan, G. & Sundaraganesan, N. Spectral and structural studies of the anti-cancer drug flutamide by density functional theoretical method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 117, 604–613 (2014).
Labrie, F. Mechanism of action and pure antiandrogenic properties of flutamide. Cancer 72, 3816–3827 (1993).
Pandya, J. B., Shinde, S. M. & Jha, P. K. Theoretical study on the interaction of flutamide anticancer drug with cucurbit [n] uril (n = 5–8) as a drug delivery system. Int. J. Quantum Chem. 122, e26899 (2022).
Kamel, M., Morsali, A., Raissi, H. & Mohammadifard, K. Theoretical insights into the intermolecular and mechanisms of covalent interaction of flutamide drug with COOH and COCl functionalized carbon nanotubes: a DFT approach. Chem. Rev. Lett. 3, 23–37 (2020).
Rashid, A. et al. A DFT study of graphitic carbon nitride as drug delivery carrier for flutamide (anticancer drug). J. Comput. Biophys. Chem. 20, 347–358 (2021).
Niknam, P., Jamehbozorgi, S., Rezvani, M. & Izadkhah, V. Understanding delivery and adsorption of flutamide drug with ZnONS based on: Dispersion-corrected DFT calculations and MD simulations. Phys. E: Low-Dimensional Syst. Nanostruct. 135, 114937 (2022).
Samimi, S., Ardestani, M. S. & Dorkoosh, F. A. Preparation of carbon quantum dots-quinic acid for drug delivery of gemcitabine to breast cancer cells. J. Drug Deliv. Sci. Technol. 61, 102287 (2021).
Mohamad Saimi, N. I., Salim, N., Ahmad, N., Abdulmalek, E. & Abdul Rahman, M. B. Aerosolized niosome formulation containing gemcitabine and cisplatin for lung cancer treatment: optimization, characterization and in vitro evaluation. Pharmaceutics 13, 59 (2021).
Sarvepalli, D. et al. Gemcitabine: a review of chemoresistance in pancreatic cancer. Crit. Reviews™ Oncog. 24 (2019).
Paroha, S. et al. Recent advances and prospects in gemcitabine drug delivery systems. Int. J. Pharm. 592, 120043 (2021).
Ajeel, F. N., Bardan, K. H., Kareem, S. H. & Khudhair, A. M. Pd doped carbon nanotubes as a drug carrier for gemcitabine anticancer drug: DFT studies. Chem. Phys. Impact. 7, 100298 (2023).
Sukumar, A. N., Paul, S., Gopalan, P. M. & Abiram, P. Therapeutic potential of M@ B40 (M = Mg and Ca) fullerene as a drug delivery system for gemcitabine Anti-Lung cancer drug: A DFT approach. ChemistrySelect 10, e202403076 (2025).
Sukumar, A. N., Duraisamy, P. D., Paul. S, P. M., Gopalan, P. & Angamuthu, A. Pristine B40 fullerene as a potential gemcitabine drug carrier for anti-lung cancer properties: a DFT and QTAIM study. J. Biomol. Struct. Dyn. 1–14 (2024).
Jiang, L. et al. Overcoming drug-resistant lung cancer by paclitaxel loaded dual-functional liposomes with mitochondria targeting and pH-response. Biomaterials 52, 126–139 (2015).
Fayyaz, F., Yar, M., Gulzar, A. & Ayub, K. First principles calculations of the adsorption of fluorouracil and nitrosourea on CTF-0; organic frameworks as drug delivery systems for cancer treatment. J. Mol. Liq. 356, 118941 (2022).
Zhou, Y. et al. Engineering a photosensitizer nanoplatform for amplified photodynamic immunotherapy via tumor microenvironment modulation. Nanoscale Horizons. 6, 120–131 (2021).
Yang, G. et al. Two-dimensional magnetic WS2@ Fe3O4 nanocomposite with mesoporous silica coating for drug delivery and imaging-guided therapy of cancer. Biomaterials 60, 62–71 (2015).
Liu, W. & Huang, Y. Cell membrane-engineered nanoparticles for cancer therapy. J. Mater. Chem. B. 10, 7161–7172 (2022).
Malik, S., Lodhi, M. A., Ayaz, S. & Ullah, Z. Unlocking potential diabetes therapeutics: insights into alpha-glucosidase Inhibition. J. Mol. Liq. 400, 124572 (2024).
Ullah, Z. et al. Unlocking the potential of ovalene: a dual-purpose sensor and drug enhancer. J. Mol. Liq. 377, 121540 (2023).
Ullah, Z. et al. A foundational theoreticalAl12E12 (E = N, P) adsorption and quinolone Docking study: cage–quinolone pairs, optics and possible therapeutic and diagnostic applications. J. Biomol. Struct. Dynamics. 41, 3630–3646 (2023).
Gardikis, K. et al. New drug delivery nanosystem combining liposomal and dendrimeric technology (liposomal locked-in dendrimers) for cancer therapy. J. Pharm. Sci. 99, 3561–3571 (2010).
Oh, Y. K. & Park, T. G. SiRNA delivery systems for cancer treatment. Adv. Drug Deliv. Rev. 61, 850–862 (2009).
Samanta, P. N. & Das, K. K. Noncovalent interaction assisted fullerene for the transportation of some brain anticancer drugs: a theoretical study. J. Mol. Graph. Model. 72, 187–200 (2017).
Liu, J., Cui, L. & Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 9, 9243–9257 (2013).
Lee, J. H. & Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 125, 75–84 (2015).
Szefler, B. Nanotechnology, from quantum mechanical calculations up to drug delivery. Int. J. Nanomed. 6143–6176 (2018).
Dumele, O., Trapp, N. & Diederich, F. Halogen bonding molecular capsules. Angew. Chem. Int. Ed. 54, 12339–12344 (2015).
Yang, J. M., Chen, Y. Q., Yu, Y., Ballester, P. & Rebek, J. Jr Rigidified cavitand hosts in water: bent guests, shape selectivity, and encapsulation. J. Am. Chem. Soc. 143, 19517–19524 (2021).
Guan, H. W. et al. Recognition of hydrophilic molecules in deep cavitand hosts with water-mediated hydrogen bonds. Chem. Commun. 57, 8147–8150 (2021).
Adardour, M. et al. Exploring the efficacy of benzimidazolone derivative as corrosion inhibitors for copper in a 3.5 wt.% NaCl solution: a comprehensive experimental and theoretical investigation. Molecules 28, 6948 (2023).
Zouaghi, M. O. et al. Benzimidazole derivatives as a new scaffold of anticancer agents: synthesis, optical properties, crystal structure and DFT calculations. Heliyon. 10 (2024).
Desai, N. et al. Benzothiazole clubbed imidazolone derivatives: synthesis, molecular docking, DFT studies, and antimicrobial studies. Curr. Comput.-Aided Drug Design. 19, 123–136 (2023).
Wang, Y., Verma, P., Jin, X., Truhlar, D. G. & He, X. Revised M06 density functional for main-group and transition-metal chemistry. Proc. Natl. Acad. Sci. 115, 10257–10262 (2018).
Rahbar, M., Morsali, A., Bozorgmehr, M. R. & Beyramabadi, S. A. Quantum chemical studies of chitosan nanoparticles as effective drug delivery systems for 5-fluorouracil anticancer drug. J. Mol. Liq. 302, 112495 (2020).
Shahi, M., Falahati, D. & Ashtari, F. Density functional theory study of interaction between ibuprofen and alginic acid for targeted drug delivery. Lett. Org. Chem. 21, 804–820 (2024).
Moumivand, A., Naderi, F., Moradi, O. & Makiabadi, B. Smart drug delivery: a DFT study of C 24 fullerene and doped analogs for Pyrazinamide. Nanoscale Adv. (2025).
Najafi, M., Morsali, A. & Bozorgmehr, M. R. DFT study of SiO2 nanoparticles as a drug delivery system: structural and mechanistic aspects. Struct. Chem. 30, 715–726 (2019).
Khan, S. et al. First-principles study for exploring the adsorption behavior of G-series nerve agents on graphdyine surface. Comput. Theor. Chem. 1191, 113043 (2020).
Sajid, H., Ayub, K. & Mahmood, T. A comprehensive DFT study on the sensing abilities of cyclic oligothiophenes (n CTs). New J. Chem. 43, 14120–14133 (2019).
Hussain, R. et al. Density functional theory study of palladium cluster adsorption on a graphene support. RSC Adv. 10, 20595–20607 (2020).
Jadoon, T., Mahmood, T. & Ayub, K. Silver-graphene quantum dots based electrochemical sensor for trinitrotoluene and p-nitrophenol. J. Mol. Liq. 306, 112878 (2020).
Yar, M., Hashmi, M. A. & Ayub, K. The C 2 N surface as a highly selective sensor for the detection of nitrogen iodide from a mixture of NX 3 (X = Cl, br, I) explosives. RSC Adv. 10, 31997–32010 (2020).
Yar, M., Shah, A. B., Hashmi, M. A. & Ayub, K. Selective detection and removal of picric acid by C 2 N surface from a mixture of nitro-explosives. New J. Chem. 44, 18646–18655 (2020).
Farooqi, B. A., Yar, M., Ashraf, A., Farooq, U. & Ayub, K. Graphene-polyaniline composite as superior electrochemical sensor for detection of cyano explosives. Eur. Polymer J. 138, 109981 (2020).
Khan, A. A., Ahmad, R. & Ahmad, I. Density functional theory study of emerging pollutants removal from water by covalent triazine based framework. J. Mol. Liq. 309, 113008 (2020).
Khan, S., Sajid, H., Ayub, K. & Mahmood, T. High sensitivity of graphdiyne nanoflake toward detection of phosgene, thiophosgene and phosogenoxime; a first-principles study. J. Mol. Graph. Model. 100, 107658 (2020).
Lu, T. & Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Sarfaraz, S., Yar, M., Sohaib, M., Ashraf, M. U. & Ayub, K. Cavitands based nanocapsule as smart and highly effective vehicle for 5-fluorouracil anti-cancer drug delivery: DFT insights. J. Mol. Liq. 399, 124436 (2024).
Rahman, F. U. et al. Chalcogen bonding and hydrophobic effects force molecules into small spaces. J. Am. Chem. Soc. 142, 5876–5883 (2020).
Sajid, H., Mahmood, T. & Ayub, K. An accurate comparative theoretical study of the interaction of furan, pyrrole, and thiophene with various gaseous analytes. J. Mol. Model. 23, 1–18 (2017).
Sajid, H., Ullah, F., Ayub, K. & Mahmood, T. Cyclic versus straight chain oligofuran as sensor: A detailed DFT study. J. Mol. Graph. Model. 97, 107569 (2020).
Mata, I., Alkorta, I., Espinosa, E. & Molins, E. Relationships between interaction energy, intermolecular distance and electron density properties in hydrogen bonded complexes under external electric fields. Chem. Phys. Lett. 507, 185–189 (2011).
Cremer, D. & Kraka, E. Chemical bonds without bonding electron density—does the difference electron-density analysis suffice for a description of the chemical bond? Angewandte Chemie Int. Ed. Engl. 23, 627–628 (1984).
Rad, A. S. & Ayub, K. O3 and SO2 sensing concept on extended surface of B12N12 nanocages modified by nickel decoration: a comprehensive DFT study. Solid State Sci. 69, 22–30 (2017).
Arshad, M. N., Faidallah, H. M., Asiri, A. M., Kosar, N. & Mahmood, T. Structural, spectroscopic and nonlinear optical properties of sulfonamide derivatives; experimental and theoretical study. J. Mol. Struct. 1202, 127393 (2020).
Toriyama, M. Y. et al. How to analyse a density of states. Mater. Today Electron. 1, 100002 (2022).
Acknowledgements
AcknowledgementsThe authors thank the Universiti Brunei Darussalam for the research grant (UBD/RSCH/1.4/FICBF/2024/067) and EVPVA fund (UBD/OAVCR/EVPVA/LA/Jan24-27).
Author information
Authors and Affiliations
Contributions
K.A.: Methodology, Conceptualization, Investigation, Writing-original draft. S.S.: Software, Methodology, Writing-original draft. M.Y.: Conceptualization, validation, Funding. M.H.S.A.H: Methodology, Visualization, Funding. N.S.S.: Funding acquisition, Validation, Investigation. K.A.: Supervision, Writing-review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Akhtar, K., Sarfaraz, S., Yar, M. et al. DFT investigation of therapeutic potential of benzimidazolone capsule as a drug delivery vehicle for anticancer drug. Sci Rep 15, 28383 (2025). https://doi.org/10.1038/s41598-025-12817-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-12817-7