Abstract
This study examined the relationship between the Diet Balance Index (DBI) and bone mineral density (BMD) in preschool children, along with other influencing factors. DBI was calculated using the adjusted Chinese Diet Balance Index for preschoolers (DBI_C), incorporating High Bound Score (HBS), Low Bound Score (LBS), and Diet Quality Distance (DQD). BMD was assessed using Z-scores and categorized into insufficient BMD (IB) (BMD Z-score > 0) and normal BMD (NB) (BMD Z-score ≤ 0). A logistic regression model analyzed the link between DBI quartiles and BMD, while binary logistic regression identified other factors influencing BMD. The study involved 1,277 children (1,231 NB, 46 IB) aged 3–6 years. BMD levels varied significantly based on factors such as age, mother’s working status, chest circumference, waist circumference, hip circumference, HBS, salt score, and drinking water score. Results indicated that children with higher HBS in the second quartile had a significantly higher risk of BMD insufficiency. Chest circumference and drinking water score were identified as significant factors affecting BMD. This study underscores the importance of a balanced diet and regular BMD monitoring for promoting healthy bone development in preschool children.
Similar content being viewed by others
Introduction
Bone mineral density (BMD), which refers to the amount of minerals contained in a given volume of bone, is an essential predictor of osteoporosis. Preschool age is the age within the human lifespan when the levels of metabolism, growth, and bone development are highest1. Therefore, achieving high bone density during this period is crucial for promoting adult bone health and preventing osteoporosis in later life.
Numerous factors influence children’s BMD, including genetic factors2,3,4, environmental factors3, and among others. However, as the economy grows and living standards rise, an increasing number of people are starting to recognize that diet and nutrition are also crucial factors in influencing bone mineral density. For example, a systematic study found that frequent consumption of dairy products was linked to increased BMD in children5, additional research has investigated the relationships of intake of fruit and vegetables6,7, minerals8, and vitamins9with children’s bone health. In recent years, however, few researchers have focused on the effect of overall diet quality on BMD, ignoring the complex interactions between different foods.
Diet quality denotes how well the kinds, amounts, as well as shares of key foods and dietary elements conform to dietary guidelines or recognized healthy eating patterns10. Considering that dietary habits vary by country and region, and recognizing that young children have different nutritional requirements than adults — including specific intake recommendations and unique physiological features like progressive increases in weight and height, as well as limitations in chewing and digestive abilities — the overall diet quality in preschool-aged children (3 to 6 years) in this study was evaluated using the adjusted Chinese Diet Balance Index for Preschool Children (DBI_C)11. The validity of DBI_C in assessing dietary quality has been verified by multiple studies. For example, studies have found that higher DBI_C scores are associated with lower risks of diet-related diseases12. This index includes 10 food category metrics: cereals, vegetables and fruits, dairy, soybean and nuts, animal products, drinking water, salt, cooking oil, and food diversity. The DBI_C assesses children’s food intake from these 10 groups by using the food frequency method or dietary review, employing the groups as indicators and addressing the dietary habits and challenges specific to Chinese preschool children13, this index provides a rapid assessment of insufficient and excessive intake across food groups and enables visualization of dietary imbalance at the individual and population levels. Following the DBI_C scoring methodology, the children’s dietary intake within this research was measured to compute the scores for each distinct index of DBI_C. The HBS reflects the sum of all positive scores, showing the level of excessive dietary intake, with a value range from 0 to 36. The LBS represents the sum of all negative scores across indicators, indicating the degree of insufficient dietary intake, with values ranging from 0 to 72. The DQD is the total value of individual index scores, highlighting issues related to dietary balance, with a span from 0 to 84. Additionally, the DBI_C focuses on food groups without considering nutrients, simplifying the process of dietary analysis and rendering it efficient for individuals and groups12.
The goals of this study included evaluating the diet quality of preschoolers between 3 and 6 years old in Anhui Province, China; identifying the association between the BMD and diet quality in these children; exploring the other factors influencing BMD. This study suggests strategies to prevent bone loss, enhance the bone health of preschool children, and create a robust framework for proper bone development.
Methods
Study pouplation
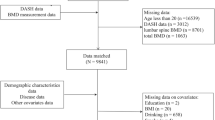
In the span of March to November 2021, whole-cluster sampling was used to randomly select 1,277 preschool children (ages 3 to 6 years) from seven kindergartens in the Longzihu Zone, Economic Development Zone and the Yuhui Zone of Bengbu City. The inclusion criteria were (1) having voluntarily provided informed consent; (2) being a healthy child aged 3–6 years; (3) not using prosthetic devices, surgical staples, or high-density materials at the test site; and (4) having provided complete survey data. The exclusion criteria were (1) being unwilling to undergo a physical examination; (2) having a joint injury or broken skin; (3) having a congenital, genetic, or chronic metabolic disease; and (4) having a psychological disorder. This study adhered to the Declaration of Helsinki and was approved by the Ethics Committee of Bengbu Medical University (No. [2021]102).
Data collection
Questionnaire survey
Data were collected using an author-designed preschool children’s health questionnaire, which was completed by the child participant’s primary caregiver. A pretest was conducted on 30 non-study participants to optimize the clarity and understandability of the questionnaire. The results showed good internal consistency (Cronbach’s α = 0.72–0.84, applicable to multi-item scales). The questionnaire focused on general demographic data (gender, age, residence, parents’ education level, parents’ employment status, monthly family income, only child status, left behind status, and current caregivers). Dietary data were collected through 3 non-consecutive 24-hour recalls (2 weekdays + 1 weekend day). During school hours: Collect children’s meal recipes from various kindergartens. Strictly trained investigative team will fill in the breakfast and lunch intake during school hours according to the intake amounts in the kindergarten recipes. During home hours: Distribute pre-coded survey forms to be completed by the child’s primary caregiver, who will retrospectively record the frequency, timing, types, and weights of foods consumed at home, as well as daily intake of fruits, beverages, and snacks. Tools such as dietary atlases, the plate model, the food photo collection, nutritional-component display cards, and food pyramid models were employed to assist caregivers in accurately recalling the types (cereals, vegetables and fruits, dairy, soybean and nuts, animal products, drinking water, salt, cooking oil) and quantities of the children’s food. For example, drinking water intake was based on caregivers’ recall, with approximately 200 milliliters per cup consumed per day.
Anthropometric measurements
Experienced professionals used a DMH-301 Automatic Weight and Height Meter (Heng Ding Technology DMH-301) to measure the weight and height of the child participants, after which they calculated their body mass index (BMI). In these measurements, the participant stood upright with their eyes forward, arms relaxed at their sides, heels together, toes pointing forward, and legs straight. Height was recorded in centimeters and weight in kilograms, with readings rounded to two decimal places. The average of two measurements was used, and the maximum error was 0.01 cm or kg. All measurements were conducted in accordance with the World Health Organization (WHO) Child Growth Standards, which specify standardized procedures for anthropometric assessment14. We utilized the Anthro and Anthro Plus software15,16 to assess the BMI-for-age Z-score (BAZ) of each participant.
Head circumference was measured by placing the lower edge of a soft ruler at the midpoint between the child’s eyebrows and circling horizontally to the highest point of the occipital bone. For measurements of chest circumference, the participant stood relaxed with their arms open; the surveyor faced the child, positioning the upper edge of a tape measure at the lower scapular angle and then circling it horizontally at the level of the nipples17. Waist circumference was measured 1 cm above the umbilicus, referencing the midpoint between the ilium’s upper edge and the 12th rib, and measurements were taken after quiet expiration. Hip circumference was measured by placing the soft ruler over the anterior pubic symphysis and the most prominent point of the gluteus maximus18. All circumferences were recorded in centimeters to one decimal place. The average of two measurements was used, with the measurement error less than 0.1 cm. All measurements were performed by trained professionals.
BMD measurement
An ultrasonic bone density detector (Nanjing WSP, Model: OSTEOKJ7000++) was employed to measure the BMD of the preschool children. The device employs quantitative ultrasound to precisely measure BMD. Quantitative ultrasound measurement is primarily applied to the calcaneus, radius, and finger regions. Due to its non-invasive, radiation-free, high-precision, and excellent pediatric compliance advantages, this technology is widely used in pediatric bone density testing19. A professionally trained investigator measured the distal end of the participant’s left radius, using the tip of the middle finger as the starting point and the elbow joint as the endpoint. A ruler was used to mark half of this length as the measurement site. The probe, coated with an ultrasound coupling agent, was placed above the marking line on the radius. The measurement was initiated, and results were read once the progress bar stabilized. The data recorded after automatic detection were the speed of sound, BMD Z-score, predicted bone age, and predicted height. In this study, groups were divided based on the Z-score of bone mineral density (BMD). Specifically, children with BMD Z-scores > 0 were assigned to the ‘IB group’ (i.e., insufficient bone density), while those with BMD Z-scores ≤ 0 were assigned to the ‘NB group’ (i.e., normal bone density).
Calculation of adjusted DBI_C scores
The dietary balance index was calculated on the basis of the DBI_C developed by He in 202011. The DBI_C was adjusted to account for the age characteristics of the surveyed population and the dietary information collected. Given the limited food intake of preschoolers, accurately gauging their dietary consumption presents unique challenges. Thus, the eight indicators of DBI_C, adapted from DBI_16, cover cereals, vegetables & fruits, dairy & legumes, animal-derived foods, cooking oil, salt, food variety, and water. Specifically, animal-derived foods combine poultry/livestock meats, seafood (fish & shellfish), and eggs with a scoring range of −12 to 12 points; alcoholic beverages are excluded; cooking oil is scored 0–6 points; seasonings are limited to salt (0–6 points); and other food categories/score ranges remain identical to DBI_16. The DBI_C standard is specifically tailored for children aged 2 to 5 years, whereas the participants in this study were children aged 3 to 6 years. Given that the dietary requirements of 5- and 6-year-old children exhibit a high degree of similarity in terms of energy, macronutrients, and micronutrients, international organizations frequently categorize them together when formulating guidelines20,21. Consequently, we extended the standards applicable to 5-year-old children to 6-year-old children, as elaborated in Supplementary material Table 1.
The HBS, LBS, and DQD are further divided into five phases: “excellent” for a score of 0, “good” for scores between 1 and 7 for HBS, 1 and 14 for LBS, and 1 and 17 for DQD, “mild poor” for scores between 8 and 14 for HBS, 15 and 29 for LBS, and 18 and 34 for DQD, “moderately poor” for scores between 15 and 22 for HBS, 30 and 43 for LBS, and 35 and 50 for DQD, and “severely poor” for scores between 23 and 36 for HBS, 44 and 72 for LBS, and 51 and 84 for DQD.
Definitions
Left-behind children
Left-behind children are defined as those whose parents are employed away from their home area and who have been apart from one or both parents for a minimum of 6 months22.
Weight status
Body mass index (BMI) served as a general measure of obesity23, computed by the formula BMI (kg/m²) = weight (kg) ÷ height (m)². The physical growth of children was assessed using the WHO-recommended Z-score based on the reference data published by the World Health Organization in 200724. The BMI-for-age Z-score (BAZ) reflects children’s weight status in terms of Z-scores and standard deviations (SD). Obesity was classified as BAZ > + 2SD, overweight as BAZ > + 1SD, and underweight as BAZ <–2SD. Children with a normal weight were considered to be in between the overweight and underweight categories.
Statistical analysis
Epidata 3.1 was used to note demographic and eating behavior data. For data analysis, SPSS version 27.0 (IBM SPSS Inc., Chicago, USA) was utilized, while Nutrition Calculator V1.6 was employed to measure the children’s food and nutrient consumption. WHO Anthro and WHO Anthro Plus software was employed to calculate Z-scores for the children’s growth and development indices. Furthermore, graphs were created using GraphPad Prism 10.0 (GraphPad Software, San Diego, California, USA). The normality of the data was assessed using the Kolmogorov–Smirnov test, and the t-test was conducted to analyze the measurement data. Values that conformed to a normal distribution were represented as ( ̅x ± s), while skewed distribution measures were reported as M (P25, P75). The expression of categorical variables is in the form of counts and percentages. Variations in the children’s BMD levels on the basis of their nutritional status, parent literacy, parent employment, monthly family income, and other factors were evaluated using the χ2 test; the multivariate logistic regression analyzed the factors affecting BMD by including those that were significant in the univariate analyses. During analysis, the dietary balance index data were segmented into four groups, corresponding to the quartiles (Q1 – Q4). An examination of the link to the dietary balance index and BMD insufficiency across quartiles was conducted using logistic regression model. Variables with significance in the multivariate analysis were included in the stratified analysis and associated forest plots were drawn. Two-tailed tests were utilized for all inquiries, and the significance criterion determined to be P ≤ 0.05.
Results
Characteristics of the study subjects
The research encompassed 1,277 preschool-aged children, ranging from 3 to 6 years old, with their characteristics divided by BMD level, as presented in Table 1. The normal BMD group consisted of 1,231 children, and the insufficient BMD group included 46 children. The participants’ age distribution was as follows: 152 children (11.9%) were 3 years old, 335 (26.2%) were 4 years old, 521 (40.8%) were 5 years old, and 269 (21.1%) were 6 years old. The participants were predominantly lived in urban areas, and were mostly cared for by their parents. There were significant differences between the normal BMD and insufficient BMD groups in terms of age (P < 0.001), mother’s employment status (P = 0.006), and body circumference measurements including chest (P < 0.001), head (P < 0.001), hip (P < 0.001), and waist (P = 0.005).
The distribution of DBI_C indicators and components
As depicted in Fig. 1 (with detailed data provided in Table 2), there was a notable difference in the distribution of HBS between children with normal BMD and those with insufficient BMD. Children with normal BMD exhibited a higher percentage of excessive dietary intake (good: 12.8% vs. 8.7%, moderate poor: 24.0% vs. 6.5%) compared to children with insufficient BMD. As shown in Fig. 2 (with detailed data in Table 3), children with normal BMD had greater insufficiency in drinking water intake (− 2.4 ± 1.5 vs. −1.3 ± 1.5, P < 0.01) than children with insufficient BMD. On the other hand, children with insufficient BMD had a greater excessive salt intake (1.8 ± 0.4 vs. 1.6 ± 0.5, P < 0.01) than those with normal BMD. In general, the foods that were most frequently over-consumed included grains, animal-based foods, oils, and salt, while the foods that were most commonly under-consumed included vegetables, fruits, dairy products, legumes, and drinking water.
Logistic regression model of DBI_C quartiles and BMD
The data presented in Table 4 illustrate the relationships between the DBI_C quartiles and BMD deficiency in the preschool children. The BMD_Z-score values were divided into two groups: BMD Z-score > 0, indicating normal BMD, and BMD Z-score ≤ 0, indicating insufficient BMD. In the regression model, Q1 of DBI_C was used as the reference value (OR = 1.00). In Model 1, the occurrence of BMD deficiency was significantly greater in the Q2 group of HBS than in the Q1, Q3, and Q4 groups. (Q2: 2.84, 95% CI: 1.16–6.94; Q3: 1.60, 95% CI: 0.59–4.43; and Q4: 2.25, 95% CI: 0.88–5.70). The link to HBS and BMD insufficiency remained notable even after controlling for covariates in the multivariate models (Model 2 and Model 3).
Binary logistic regression analysis of the associated factors of BMD
Grounded in the findings of the univariate analysis, the dependent variable was categorized into two groups: insufficient BMD (BMD Z-score ≤ 0) and normal BMD (BMD Z-score > 0), with the normal BMD group serving as the control. To determine their individual contributions to BMD insufficiency, indicators with significant differences in the univariate analysis (age, mother working outside the home area, waist circumference, chest circumference, hip circumference, salt score, and drinking water score) were added to the multivariate logistic regression analysis. According to the data in Table 5, BMD insufficiency had a significant relationship with chest circumference (OR = 1.15, 95% CI: 1.01–1.31, P = 0.037) and drinking water score (OR = 2.68, 95% CI: 1.17–6.14, P = 0.020), whereas age (P = 0.133), mother not working outside the home area (P = 0.168), waist circumference (P = 0.164), hip circumference (P = 0.931), and salt score (P = 0.787) showed no significant associations.
Stratified analysis of BMD insufficiency in relation to chest circumference and drinking water scores
Figure 3 presents the stratified analysis of BMD insufficiency in relation to chest circumference and drinking water scores. The analysis revealed a significant association between BMD insufficiency and chest circumference across all gender, family monthly income, left-behind children status, and father’s employment status. Positive correlations between chest circumference and BMD insufficiency were observed in children living in urban areas, children who were not only children, children whose primary caregivers were their parents, children whose mothers worked outside the home, and children whose parents had an educational level of primary school or below, or junior high school or high school. Furthermore, a significant correlation between drinking water scores and BMD insufficiency was found across all gender, family income, and parents’ employment status. Positive associations between drinking water scores and BMD insufficiency were noted in children living in urban areas, children who were not left-behind, only children, children whose primary caregivers were their parents, children whose fathers’ education level was primary school or below, or junior high or high school, and children whose mothers’ education level was junior high school or high school, as well as those whose mothers had a college education or higher.
Discussion
This study revealed a significant difference in bone mineral density (BMD) between children with normal and insufficient BMD, with a higher HBS being linked to insufficient BMD in the Q2 group. Children with insufficient BMD were more likely to have excessive dietary intake than those with normal BMD. To the best of our knowledge, this is the first study to utilize the DBI_C tool to assess dietary quality and explore the relationship between DBI and BMD in Chinese preschool children. Furthermore, the study examined other factors, such as anthropometric and demographic factors, are associated with BMD, emphasizing the critical influence of these factors on BMD outcomes.
This study assessed the diet quality of children in Bengbu City by quantifying it using the modified dietary balance index for preschool children. The results indicated that the overall diet quality among the study population was unreasonable and imbalanced. This finding along with those of other studies focusing on Chinese children and adolescents. For example, Duan et al.25reported low dairy consumption among Chinese preschool children, A study conducted by Ji Zhang et al. on Chinese children spanning ages 4 to 17 found red meat and grain consumption to be higher than national recommendations for all groups26. This finding suggests that although the health status related to nutrition for children in China has seen significant improvements from rapid socioeconomic advancements27,28, unbalanced dietary patterns remain common in Chinese children29,30,31.
Many researchers and guidelines have emphasized the crucial role of dietary diversity32,33,34. A healthy, balanced diet is vital for preschool children because habits formed during this period can persist in the long term35. Dietary balance considerably influences BMD, bone mineral mass, and bone turnover. In the course of this investigation, the logistic regression model of DBI_C quartiles and BMD indicated that the chance of BMD deficiency rose notably with HBS for group Q2 (14.00–15.00). Excessive intake of cereals, animal foods, salt and oils (indicated by a high HBS) was identified as a key factor in a lower BMD Z-score.
Firstly, cereals contain high levels of starch and dietary fiber primarily from refined grains, which have low digestion rates and accumulate in the gut, producing triglycerides that induce abdominal fat accumulation and thereby affecting bone density36. However, a study conducted in Korea37 demonstrated that cereal intake was positively associated with BMD, a high intake of cereals or whole grains reduces the risk of low BMD. In the Dietary Guidelines for Chinese Residents, cereal consumption is highlighted as something to be enjoyed “in moderation.” Consequently, the Balanced Diet Pagoda’s recommended intake suggests that preschool children aged 2–3 years should consume 75–125 g of cereal daily, while those aged 4–6 years should aim for 100–150 g per day. Additionally, there is an emphasis on increasing the intake of roughage and fiber-rich cereals. Specifically, it is advisable to boost the consumption of coarse grains and fiber-rich cereals while reducing the intake of refined grains and starchy foods.
Secondly, a high intake of animal foods, particularly red meat, is linked to poor eating habits and boosts the potential for heart and vessel conditions. For preschool children, excessive meat consumption contributes to obesity and malnutrition. Meat contains a large amount of protein, excessive intake of meat will increase renal acid load and urinary calcium excretion, thus affecting bone health38. However, when adequate calcium, vitamin D, fruit and vegetables are consumed, contributing to a balanced intake of protein from animal sources will be beneficial in improving bone health39. Therefore, it is recommended that pre-school children consume an appropriate amount of animal food and increase the intake of high-quality proteins such as fish and eggs, with a minimum of 50 g of eggs and 50–75 g of poultry and fish per day in line with the recommended intake of the Balanced Diet Pagoda.
Thirdly, consuming cooking oil and salt in excess significantly raises the likelihood of cardiovascular disease and obesity40 and a light diet low in oil and salt reduces urinary calcium excretion, which is the consensus of most experts41. According to the recommended intake of the Balanced Diet Pagoda, the daily intake of oils for 2-3- year-old children is 10–20 g, while for 4-6- year-old children, it should be 20–25 g. Additionally, the daily salt consumption should be under 2 g for 2-3- year-old children and under 3 g for those aged 4 to 6 years.
In addition to dietary factors, we discuss other factors that affect bone density. Firstly, the demographic analysis uncovered significant differences between children with normal and insufficient BMD. The majority of the children resided in urban areas, mirroring the demographic trends observed in numerous Chinese cities. Age was identified as a key factor, with children aged 3–4 showing a higher likelihood of having insufficient BMD. This observation is consistent with previous studies42 indicating that BMD development improves with age, and that younger children are particularly vulnerable to dietary deficiencies and environmental factors that affect bone health. Additionally, the link between BMD and the mother’s employment status highlights the role of socio-economic factors and parental involvement43 in shaping children’s nutrition and overall health.
Secondly, the multivariate logistic regression analysis identified chest circumference and drinking water scores as significant predictors of BMD insufficiency. Specifically, children with smaller chest circumferences were more likely to have insufficient BMD. This association may reflect an underlying relationship between body size and bone density44, as smaller children may have less bone mass development. Furthermore, the drinking water score was positively correlated with BMD insufficiency. This suggests that low water intake could be an overlooked factor contributing to poor bone health in early childhood. Given the critical role of hydration in metabolic functions45 and nutrient absorption46, addressing water consumption could serve as an important intervention for improving BMD in young children.
Finally, the stratified analysis of BMD insufficiency, based on factors such as gender, family income, and parental education, revealed consistent associations across various subgroups. For example, children from urban areas and those whose mothers had lower levels of education were more likely to have insufficient BMD, despite being cared for by their parents. This highlights the multifactorial nature of bone health, where both environmental (urban living) and educational factors may influence access to nutritional resources or health education. Importantly, the analysis also showed that the positive correlation between chest circumference and BMD insufficiency was present across all subgroups, suggesting that body size remains a universal factor in BMD status44, irrespective of socio-economic background.
While this study provides valuable insights, several limitations should be acknowledged. The study’s cross-sectional design restricts the ability to draw causal conclusions, and further longitudinal studies are needed to establish causality between dietary and hydration habits and BMD development. Additionally, other potential factors influencing BMD, such as physical activity, genetic predispositions, and environmental exposures, were not fully explored in this study. We also emphasize that, due to the lack of adjustment for these factors, our study results should be interpreted with caution, and the results should be considered as associations rather than causal relationships. Future research should aim to include a broader range of variables and consider longitudinal tracking of children’s health over time to better understand the development of BMD and related conditions. As an aside, the questionnaire used was self-designed and has not yet undergone external validation against a gold standard measurement method, which may affect its generalizability. And, dietary data may be subject to recall bias.
In conclusion, this study underscores the significant impact of dietary habits, hydration, and physical growth indicators on the bone health of preschool-aged children. Imbalanced diets, insufficient water intake, and certain physical growth parameters, such as chest circumference, were found to be closely associated with BMD insufficiency. These findings highlight potential risk factors for pediatric bone health, but it is crucial to note that the cross - sectional design limits causal inference. BMD is influenced by multifactorial determinants, including genetic predispositions, physical activity, and vitamin D status—variables not controlled for in this analysis. Thus, the observed associations should be interpreted as exploratory rather than definitive. Future research is warranted to adopt longitudinal designs and integrative approaches that evaluate lifestyle, biological, and environmental factors simultaneously.
Data availability
The datasets analyzed during the current study are not publicly available due to the personal privacy but are available from the corresponding author on reasonable request.
References
Smith, G. C. et al. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J. Clin. Endocrinol. Metab. 87, 1762–1767. https://doi.org/10.1210/jcem.87.4.8430 (2002).
Choi, H. S., Park, J. H., Kim, S. H., Shin, S. & Park, M. J. Strong Familial association of bone mineral density between parents and offspring: KNHANES 2008–2011. Osteoporos. Int. 28, 955–964. https://doi.org/10.1007/s00198-016-3806-1 (2017).
Cvijetić Avdagić, S., Colić Barić, I., Keser, I., Rumbak, I. & Šatalić, Z. Influence of heredity and environment on peak bone density: a review of studies in Croatia. Arh Hig Rada Toksikol. 63 (Suppl 1), 11–16. https://doi.org/10.2478/10004-1254-63-2012-2130 (2012).
Hou, R. et al. Genetic variants affecting bone mineral density and bone mineral content at multiple skeletal sites in Hispanic children. Bone 132, 115175. https://doi.org/10.1016/j.bone.2019.115175 (2020).
de Lamas, C. et al. Effects of dairy product consumption on height and bone mineral content in children: A systematic review of controlled trials. Adv. Nutr. 10, S88–s96. https://doi.org/10.1093/advances/nmy096 (2019).
Darling, A. L. et al. Dietary protein and bone health across the life-course: an updated systematic review and meta-analysis over 40 years. Osteoporos. Int. 30, 741–761. https://doi.org/10.1007/s00198-019-04933-8 (2019).
Rondanelli, M. et al. An update on magnesium and bone health. Biometals 34, 715–736. https://doi.org/10.1007/s10534-021-00305-0 (2021).
Rizzoli, R. et al. Benefits and safety of dietary protein for bone health-an expert consensus paper endorsed by the European society for clinical and economical aspects of osteopororosis, osteoarthritis, and musculoskeletal diseases and by the international osteoporosis foundation. Osteoporos. Int. 29, 1933–1948. https://doi.org/10.1007/s00198-018-4534-5 (2018).
Malmir, H., Shab-Bidar, S. & Djafarian, K. Vitamin C intake in relation to bone mineral density and risk of hip fracture and osteoporosis: a systematic review and meta-analysis of observational studies. Br. J. Nutr. 119, 847–858. https://doi.org/10.1017/s0007114518000430 (2018).
Brát, J., Vrablík, M. & Herber, O. [Dietary changes in relationship to risk factors and coronary heart disease mortality]. Vnitr Lek. 61, 815–820 (2015).
Fang, Y. H., He, Y. N. & Li, C. L. [Evaluation of dietary quality of Chinese preschool children based on Chinese diet balance index for preschool children]. Zhonghua Yu Fang Yi Xue Za Zhi. 54, 662–667. https://doi.org/10.3760/cma.j.cn112150-20190909-00719 (2020).
Wang, X. et al. Association between early childhood caries and diet quality among Chinese children aged 2–5 years. Front. Public. Health. 10, 974419. https://doi.org/10.3389/fpubh.2022.974419 (2022).
Wang, Y. et al. Unfavorable dietary quality contributes to elevated risk of ischemic stroke among residents in Southwest china: based on the Chinese diet balance index 2016 (DBI-16). Nutrients 14 https://doi.org/10.3390/nu14030694 (2022).
WHO. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age. WHO. (2006).
Growth reference data for 5–19 years, https://www.who.int/tools/growth-reference-data-for-5to19-years/application-tools (2022).
Organization, W. H. Child growth standards, https://www.who.int/tools/child-growth-standards/software (2019).
Stewart, A. D., Marfell-Jones, M., Olds, T. & Ridder, H. D. International Standards for Anthropometric Assessment (International Standards for Anthropometric Assessment, 2011).
Organization, W. H. Waist circumference and waist-hip ratio: report of a WHO expert consultation, geneva, 8–11 December 2008. World Health Organization (2011).
Nwogu, U. B. et al. Bone mineral density in an urban and a rural children population-A comparative, population-based study in Enugu state. Nigeria Bone. 127, 44–48. https://doi.org/10.1016/j.bone.2019.05.028 (2019).
Organization, W. H. O. F. a. A. Vitamin and Mineral Requirements in Human Nutrition 2nd edn. 341 (2004).
Torun, B. Energy requirements of children and adolescents. Public. Health Nutr. 8, 968–993. https://doi.org/10.1079/phn2005791 (2005).
Wen, Y. J. et al. The neglect of Left-Behind children in china: A Meta-Analysis. Trauma. Violence Abuse. 22, 1326–1338. https://doi.org/10.1177/1524838020916837 (2021).
Popkin, B. M., Corvalan, C. & Grummer-Strawn, L. M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 395, 65–74. https://doi.org/10.1016/s0140-6736(19)32497-3 (2020).
de Onis, M. et al. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 85, 660–667. https://doi.org/10.2471/BLT.07.043497 (2007).
Duan, Y. et al. Association between dairy intake and linear growth in Chinese Pre-School children. Nutrients 12 https://doi.org/10.3390/nu12092576 (2020).
Zhang, J. et al. Urban-Rural disparities in energy intake and contribution of fat and animal source foods in Chinese children aged 4–17 years. Nutrients 9 https://doi.org/10.3390/nu9050526 (2017).
Dearth-Wesley, T., Wang, H. & Popkin, B. M. Under- and overnutrition dynamics in Chinese children and adults (1991–2004). Eur. J. Clin. Nutr. 62, 1302–1307. https://doi.org/10.1038/sj.ejcn.1602853 (2008).
Tzioumis, E. & Adair, L. S. Childhood dual burden of under- and overnutrition in low- and middle-income countries: a critical review. Food Nutr. Bull. 35, 230–243. https://doi.org/10.1177/156482651403500210 (2014).
Meng, L. et al. Dietary diversity and food variety in Chinese children aged 3⁻17 years: are they negatively associated with dietary micronutrient inadequacy?? Nutrients 10 https://doi.org/10.3390/nu10111674 (2018).
Wang, H. et al. Do Chin. Child. Get. Enough Micronutrients? Nutrients 9, doi:https://doi.org/10.3390/nu9040397 (2017).
Zhao, W. et al. Dietary diversity scores: an indicator of micronutrient inadequacy Ins tead of obesity for Chinese children. BMC Public. Health 17, 440, https://doi.org/10.1186/s12889-017-4381-x
Azadbakht, L. & Esmaillzadeh, A. Dietary energy density is favorably associated with dietary diversity score among female university students in Isfahan. Nutrition 28, 991–995. https://doi.org/10.1016/j.nut.2011.12.017 (2012).
Liu, T. et al. Dietary diversity and dietary patterns in School-Aged children in Western kenya: A latent class analysis. Int. J. Environ. Res. Public. Health. 19 https://doi.org/10.3390/ijerph19159130 (2022).
Mozaffari, H., Hosseini, Z., Lafrenière, J. & Conklin, A. I. The role of dietary diversity in preventing metabolic-related outcomes: findings from a systematic review. Obes. Rev. 22, e13174. https://doi.org/10.1111/obr.13174 (2021).
Jarman, M. et al. How do mothers manage their preschool children’s eating habits and does this change as children grow older? A longitudinal analysis. Appetite 95, 466–474. https://doi.org/10.1016/j.appet.2015.08.008 (2015).
Feingold, K. et al. R. in Endotext (eds K. R. Feingold (MDText.com, Inc. Copyright © 2000–2024, MDText.com, Inc., (2000).
Shin, S., Kim, S. H., Joung, H. & Park, M. J. Milk-cereal and whole-grain dietary patterns protect against low bone mineral density among male adolescents and young adults. Eur. J. Clin. Nutr. 71, 1101–1107. https://doi.org/10.1038/ejcn.2017.81 (2017).
Cao, J. J. & Nielsen, F. H. Acid diet (high-meat protein) effects on calcium metabolism and bone health. Curr. Opin. Clin. Nutr. Metab. Care. 13, 698–702. https://doi.org/10.1097/MCO.0b013e32833df691 (2010).
Yang, X. et al. Meat and fish intake and type 2 diabetes: Dose-response meta-analysis of prospective cohort studies. Diabetes Metab. 46, 345–352. https://doi.org/10.1016/j.diabet.2020.03.004 (2020).
Han, Y., Fan, Z. & Li, T. Oil/Salt Use Assessment of Chinese-Style Canteens Based on Consumers’ Perception of the Nutrition Environment. Nutrients 15 https://doi.org/10.3390/nu15204321 (2023).
Watson, N. A. et al. Comparison of two low-fat diets, differing in protein and carbohydrate, on psychological wellbeing in adults with obesity and type 2 diabetes: a randomised clinical trial. Nutr. J. 17, 62. https://doi.org/10.1186/s12937-018-0367-5 (2018).
Xu, H. et al. Bone mineral density of the spine in 11,898 Chinese infants and young children: a cross-sectional study. PLoS One. 8, e82098. https://doi.org/10.1371/journal.pone.0082098 (2013).
Shu, L. et al. Nutritional status of children left behind by migrant parents: evidence from a survey in rural anhui, Eastern China. Nutr. Bull. 49, 146–156. https://doi.org/10.1111/nbu.12666 (2024).
Binney, N. Reference-Class problems are real: Health-Adjusted reference classes and low bone mineral density. J. Med. Philos. 49, 128–146. https://doi.org/10.1093/jmp/jhae005 (2024).
Chycki, J., Zając, T., Maszczyk, A. & Kurylas, A. The effect of mineral-based alkaline water on hydration status and the metabolic response to short-term anaerobic exercise. Biol. Sport. 34, 255–261. https://doi.org/10.5114/biolsport.2017.66003 (2017).
Vannucci, L. et al. Calcium Intake in Bone Health: A Focus on Calcium-Rich Mineral Waters. Nutrients 10,https://doi.org/10.3390/nu10121930 (2018).
Acknowledgements
We are grateful to all staff professionals and participants.
Funding
This study was financed by the Ministry of education of Humanities and Social Science project (number: 23YJC840016), the “512” talent development program of Bengbu Medical University (number: by51202301), the Humanity and Social Science Research Project of Anhui Educational Committee (number: 2022AH051402), Graduate Research and Innovation Program of Bengbu Medical University (number: Byycxz23016 and Byycx23045).
Author information
Authors and Affiliations
Contributions
The study was designed by L.Z. and L.S.; L.Z., W.X., R.L., X.L. and W.S. participated in the methods section and collected the research data. L.Z. analyzes and interprets the collected research data. L.Z. and W.X. drafted the manuscript. L.S. and L.Z. critically revised the finished manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Contest to participate
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from their parents to publish this paper.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Bengbu Medical University (No. [2023] 261 and [2024] 143).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, L., Xue, W., Sun, C. et al. Cross sectional analysis of bone mineral density and diet balance index in preschool children in Bengbu, Anhui, China. Sci Rep 15, 28237 (2025). https://doi.org/10.1038/s41598-025-12874-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12874-y