Abstract
This study was designed to assess the associations between serum junctional adhesion molecule-like protein (JAML), nesfatin-1, and 25-hydroxy vitamin D (25(OH)D) and the incidence of diabetic kidney disease (DKD) in patients with type 2 diabetes mellitus (T2DM), as well as to explore their risk assessment value in DKD. Serum JAML, nesfatin-1, and 25(OH)D levels were measured in 227 patients with T2DM. All participants were categorized into tertiles based on their serum JAML, nesfatin-1, and 25(OH)D levels. For statistical analysis, multivariate logistic regression models and restricted cubic splines (RCS) were employed; additionally, receiver operating characteristic (ROC) curves and a nomogram were developed. Of the 227 patients with T2DM, 114 (50.2%) were diagnosed with DKD. The RCS analysis showed an S-shaped association between the serum JAML and DKD incidence and an L-shaped association of serum nesfatin-1 or 25(OH)D with the risk of DKD. Multivariate logistic regression revealed that, after controlling for confounders, individuals in the highest tertile of serum JAML level had a significantly greater risk of developing DKD than those in the lowest tertile (JAML: OR 5.70, 95% CI 2.66–12.22, P < 0.001). Conversely, those in the highest tertile of serum nesfatin-1 and 25(OH)D exhibited significantly reduced risks of DKD progression compared to those in the lowest tertile (nesfatin-1: OR 0.21, 95% CI 0.10–0.44, P < 0.001; 25(OH)D: OR 0.19, 95% CI 0.08–0.45, P < 0.001). The ROC curves showed that the serum JAML levels were better than nesfatin-1 or 25(OH)D at predicting DKD. Finally, a nomogram model based on the above three indicators combined with a history of hypertension, course of diabetes, and history of diabetic complications of retinopathy achieved 87.2% accuracy in assessing risk of DKD in patients with T2DM. Elevated serum JAML levels coupled with reduced nesfatin-1 and 25(OH)D concentrations were significantly associated with increased risk of DKD in patients with T2DM. The nomogram integrating these biomarkers demonstrated quantifiable advantages in risk assessment of DKD.
Similar content being viewed by others
Introduction
The incidence of diabetes has been rising quickly worldwide in recent years with the growth of living standards, and the International Diabetes Federation (IDF) projects that 643 million people will have the disease by 2030 and 784 million people by 20451. Chronic hyperglycemia with poor glycemic control over a long period can involve tissues or organs such as the heart, eyes, kidneys, and peripheral nerves, which can lead to a variety of complications, dysfunctions, and even organ failure2. Diabetic kidney disease (DKD) is one of the common microvascular complications of diabetes and also a key cause of end-stage renal disease (ESKD)3. According to statistics, over 40% of diabetes patients may present with DKD, which brings a significant healthcare burden4,5. The typical clinical symptoms of DKD are persistent proteinuria and gradual decrease of renal function, with which doctors carry out clinical diagnosis and therapeutic interventions in their clinical work at present6. Nevertheless, current conventional diagnostic methods are not sufficient to meet the clinical needs because of the early atypical clinical symptoms of DKD and the unnecessarily abnormal results of estimated glomerular filtration rate (eGFR) and albuminuria7. Early detection is critical, but current diagnostic tools lack sensitivity in early stages, highlighting the need for novel biomarkers.
Junctional adhesion molecule-like protein (JAML) is a secreted type I transmembrane glycoprotein characterized by junctional adhesion and belongs to the JAMs family found by Moog-Lutz et al. in 20038. Playing a role in mediating leukocyte adhesion and transendothelial migration, JAML is considered a new target for tumor immunotherapy, and the majority of research on JAML has focused on the field of oncology9,10. JAML was proved to be significantly upregulated in podocytes in a DKD mouse model, and its inhibition could enhance the silent information regulator 1 (SITR1) expression and the AMP-activated protein kinase α (AMPKα) activity, which in turn affected the sterol regulatory element binding protein-1 (SREBP1) and the downstream fatty acid and cholesterol synthesizing proteins, consequently improving lipid metabolism disorders in podocytes, reducing proteinuria, and alleviating kidney injury11. However, few JAML-related clinical studies have been reported so far.
Nesfatin-1 is a newly discovered satiety molecule with functions such as food intake control12 anti-inflammatory13 anti-oxidative stress14 and insulin resistance15. There has hitherto been no agreement on how serum nesfatin-1 levels fluctuate in patients with type 2 diabetes mellitus (T2DM)16,17. A meta-analysis claimed that serum nesfatin-1 levels were elevated in patients with newly diagnosed T2DM but decreased after drug treatment18. Moreover, to the best of our knowledge, few studies have focused on the variation of nesfatin-1 during the development of T2DM toward DKD, and the relationship between nesfatin-1 and DKD has not been elucidated yet.
25-hydroxy vitamin D (25(OH)D) is the major circulating form of vitamin D in the body that regulates bone and mineral metabolism19. 25(OH)D deficiency is highly prevalent among patients with T2DM, especially in the presence of DKD20. A number of studies manifested an association of low 25(OH)D level and albuminuria, indicating that 25(OH)D may be renoprotective in diabetes21,22 even though others disapproved of this association23.
Thus, this study sought to elucidate the associations of serum JAML, nesfatin-1, and 25(OH)D with DKD progression, evaluate their value for risk stratification of DKD, and ultimately enhance clinical management strategies for preventing or reducing the burden of DKD in individuals with T2DM.
Results
Demographic and baseline characteristics of the study population
A total of 227 patients who were diagnosed with T2DM met the inclusion criteria and participated in the study. The median age of the population was 60.0 (52.5–68.0) years, and 81 (35.7%) of them were female. Of all the participants, 114 were diagnosed with DKD during hospitalization. Table 1 outlined the demographic and baseline characteristics of the participants, categorized by the presence or absence of DKD. The results showed that compared to patients without DKD, those with DKD tended to be older (P = 0.002), have higher systolic blood pressure (SBP) (P < 0.001), and slightly lower glycosylated hemoglobin (HbA1c) levels (P = 0.014). The prevalence of hypertension (P < 0.001), cardiovascular disease (P = 0.016), and complications of diabetic retinopathy (P < 0.001) was also significantly higher in the DKD group. Furthermore, individuals with DKD had lower eGFR levels and higher blood urea nitrogen (BUN), serum creatinine (Scr), uric acid (UA), urine microalbumin (UmALB), and urinary albumin creatine ratio (UACR) levels (each P < 0.001), indicating impaired renal function. Notably, patients with DKD exhibited higher serum JAML but lower nesfatin-1 and 25(OH)D than the non-DKD cases (each P < 0.001), with the results of JAML in the DKD, median 315.79 pg/mL (interquartile range (IQR): 266.83-443.71) vs. in the non-DKD, 242.19 pg/mL (IQR: 162.04-295.31); nesfatin-1 in the DKD, median 563.22 pg/mL (IQR: 419.45-668.57) vs. in the non-DKD, median 665.75 pg/mL (IQR: 579.62-751.59); and 25(OH)D in DKD, median 19.57 ng/mL (IQR: 13.09–25.09) vs. in the non-DKD, median 23.60 ng/mL (IQR: 19.63–27.76).
JAML and DKD
Spearman correlation analysis showed that serum JAML levels exhibited positive correlations with renal function indices of BUN (r = 0.20, P = 0.002), Scr (r = 0.29, P < 0.001), UmALB (r = 0.48, P < 0.001), and UACR (r = 0.44, P < 0.001), but negative correlations with HbA1c (r = -0.18, P = 0.005) and eGFR (r = -0.31, P < 0.001) (Fig. 1, Supplemental Table 1). Restricted cubic splines (RCSs) (Fig. 2A) revealed a non-linear association between JAML and the risk of DKD (P-Non-linear < 0.001); in contrast, the risk of DKD significantly increased with increasing serum JAML levels when the level was under 553 pg/mL, whereas an upward first then downward slope was observed when JAML was ≥ 553 pg/mL (Fig. 2A). Table 2 displayed the results of the three multivariate logistic regression models in evaluating the correlations between JAML and DKD incidence (model 1: OR 1.81, 95% CI 1.38–2.38, P < 0.001; model 2: OR 1.79, 95% CI 1.36–2.36, P < 0.001; and model 3: OR 1.65, 95% CI 1.24–2.19, P < 0.001). As illustrated in model 3, when using gender, age, BMI, SBP, hypertension, hyperlipidemia, and cardiovascular disease as covariates, each 100 pg/mL increase in JAML was associated with a 5% increase in the risk of DKD. For sensitivity analysis, we further transformed JAML from a continuous variable into an ordered categorical variable (tertiles). The results demonstrated that, compared with the first tertile, patients in the third tertile had more than a 5-fold increased risk of DKD, with statistical significance across models: model 1 (OR 6.45, 95% CI 3.18–13.09; P < 0.001), Model 2 (OR 6.47, 95% CI 3.15–13.28; P < 0.001), and model 3 (OR 5.70, 95% CI 2.66–12.22; P < 0.001). Moreover, compared with the first tertile, participants in the second tertile exhibited a 2.00-fold increased risk of DKD; however, this difference was statistically insignificant in model 3 (OR 2.00, 95% CI 0.97–4.14, P = 0.062). ROC curve analysis determined an optimal serum JAML cutoff of 289.47 pg/mL, yielding an AUC of 0.750 (95% CI 0.687–0.812) with sensitivity 64.0%, specificity 71.7%, positive predictive value 69.5%, and negative predictive value 66.4% (Fig. 3A).
Correlations of serum JAML, nesfatin-1, and 25(OH)D levels with DKD. Spearman correlation analysis was performed to disclose the correlation of serum JAML, nesfatin-1, and 25(OH)D levels with DKD. FBG, fasting blood glucose; 2 h-PG, 2 h plasma glucose; HbA1c, glycosylated hemoglobin; BUN, blood urea nitrogen; Scr, serum creatinine; UA, uric acid; eGFR, estimated glomerular filtration rate; UmALB, urine albumin; UACR, urine albumin creatine ratio; JAML, junctional adhesion molecule-like protein; 25(OH)D, 25-hydroxy vitamin D. * P < 0.05; ** P < 0.01; *** P < 0.001.
Nonlinear associations of the serum JAML (A), nesfatin-1 (B), and 25(OH)D (C) levels with different DKD in patients with T2DM. JAML, junctional adhesion mole-cule-like protein; 25(OH)D, 25-hydroxy vitamin D; T2DM, type 2 diabetes mellitus; DKD, diabetic kidney disease; OR, odds ratio; CI, confidence interval.
Receiver operating characteristic curves showing the performance of serum JAML (A), nesfatin-1 (B), and 25(OH)D (C) for predicting DKD incidence. JAML, junctional adhesion molecule-like protein; 25(OH)D, 25-hydroxy vitamin D; AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Nesfatin-1 and DKD
In addition to JAML, Spearman correlation analysis concluded that serum nesfatin-1 had significantly positive correlations with HbA1c (r = 0.15, P = 0.027) and eGFR (r = 0.24, P < 0.001), but significantly negative correlations with renal function indicators including BUN (r = -0.14, P = 0.033), Cr (r = -0.19, P = 0.003), UA (r = -0.14, P = 0.038), UmALB (r = -0.28, P < 0.001), and UACR (r = -0.33, P < 0.001) (Fig. 1, Supplemental Table 2). Figure 2B demonstrated a distinct L-shaped association between nesfatin-1 levels and DKD incidence (P-Non-linear < 0.001). The risk of DKD decreased significantly with rising serum nesfatin-1 until levels reached 657 pg/mL, where the curve displayed a plateau effect. Multifactorial logistic regression analysis revealed that nesfatin-1, when treated as a continuous variable, was strongly negatively correlated with DKD (model 1: OR 0.86, 95% CI 0.75–0.98, P = 0.021; model 2: OR 0.88, 95% CI 0.77–0.99, P = 0.041; and model 3: OR 0.85, 95% CI 0.74–0.98, P = 0.029). When analyzed by tertiles, participants in the third tertile exhibited significantly reduced risk of DKD versus the first tertile (model 1: OR 0.23, 95% CI 0.11–0.45, P < 0.001; model 2: OR 0.24, 95% CI 0.12–0.48, P < 0.001; and model 3: OR 0.21, 95% CI 0.10–0.44, P < 0.001). Similarly, the second tertile group showed significantly lower risk than the first tertile. ROC analysis identified an optimal nesfatin-1 cutoff at 513.35 pg/mL (AUC 0.704; 95% CI 0.636–0.771), with sensitivity 41.2%, specificity 92.0%, positive predictive value 83.9%, and negative predictive value 60.8% (Fig. 3B).
25(OH)D and DKD
Beyond JAML and nesfatin-1, Spearman correlation analysis identified significant associations between serum 25(OH)D levels and DKD-related parameters. Specifically, positive correlations emerged with risk indicators of DKD: FBG (r = 0.14, P = 0.050), 2-PG (r = 0.20, P = 0.004), and eGFR (r = 0.16, P = 0.020). Conversely, negative correlations were observed with renal function markers: BUN (r = -0.25, P < 0.001), Scr (r = -0.24, P < 0.001), UA (r = -0.29, P < 0.001), UmALB (r = -0.39, P < 0.001), and UACR (r = -0.35, P < 0.001) (Fig. 1, Supplemental Table 3). The L-shaped RCS curve plotted for 25(OH)D (P-Non-linear = 0.008) was similar to that for nesfatin-1, depicting a significant reduction in the risk of DKD with 25(OH)D elevation, while the slope turned to be flat when 25(OH)D ≥ 21.5 ng/mL (Fig. 2C). Multifactorial logistic regression analysis explicated a strong negative correlation of 25(OH)D, when introduced as a continuous variable, with DKD (model 1: OR 0.94, 95% CI 0.91–0.98, P = 0.001; model 2: OR 0.91, 95% CI 0.87–0.95, P < 0.001; model 3 OR 0.92, 95% CI 0.87–0.96, P = 0.001). After adjusting for gender, age, BMI, SBP, hypertension, hyperlipidemia, and cardiovascular disease (model 3), each 1 ng/mL increase in 25(OH)D was associated with an 8% reduction in risk of DKD. Notably, when analyzed by tertiles, the third tertile group exhibited significantly lower risk of DKD versus first tertile (model 1: OR 0.26, 95% CI 0.13–0.53, P < 0.001; model 2: OR 0.16, 95% CI 0.07–0.37, P < 0.001; model 3: OR 0.19, 95% CI 0.08–0.45, P < 0.001). Participants in second tertile also exhibited a significantly reduced risk of DKD compared with those in first tertile. ROC analysis for 25(OH)D yielded an optimal cutoff at 18.93 ng/mL (AUC 0.638; 95% CI 0.562–0.714), with sensitivity 47.6%, specificity 80.4%, positive predictive value 71.4%, and negative predictive value 59.9% (Fig. 3C). Serum JAML demonstrated superior predictive value to 25(OH)D (DeLong’s test: JAML vs. 25(OH)D, P = 0.019); however, serum nesfatin-1 showed no significant difference compared with 25(OH)D (DeLong’s test: nesfatin-1 vs. 25(OH)D, P = 0.196).
Nomogram model for risk assessment of DKD
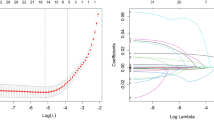
A nomogram model for risk assessment of DKD in the population with T2DM based on serum JAML, nesfatin-1, and 25(OH)D was built. The dataset was randomly split in the ratio of 7:3 to construct the training and validation cohorts, and by performing LASSO logistic regression analysis on the training cohorts, we screened seven risk factors (SBP, hypertension, course of diabetes, retinopathy, JAML, nesfatin-1, and 25(OH)D) (Fig. 4A,B, Supplementary Table 4). Participants with unstandardizable SBP data were excluded from modeling. The final nomogram is presented in Fig. 4C. ROC analysis yielded AUC values of 0.872 (training cohort) and 0.854 (validation cohort) (Fig. 4D,E). Calibration curves demonstrated close agreement between predicted and observed outcomes in both cohorts (Fig. 4F,G), indicating that the model predicted probability had a high degree of consistency with the actual outcome. Decision curve analysis (DCA) confirmed superior clinical utility of the nomogram for patients with T2DM in both the training and validation cohorts (Fig. 4H−J).
Nomogram model for predicting the risk of DKD in population with T2DM. A: LASSO regression cross-validation plot. B: LASSO regression coefficient path plot. C: Nomogram model for predicting the risk of DKD in the population with T2DM based on serum JAML, nesfatin-1, and 25(OH)D. D-E: Receiver operating characteristic curves of the nomogram prediction model for the training and internal test cohorts. F-G: Calibration curve of the nomogram prediction mode for the training and internal test cohorts. H-J: Decision curve analysis of the nomogram for the training and internal test cohorts. T2DM, type 2 diabetes mellitus; DKD, diabetic kidney disease; JAML, junctional adhesion molecule-like protein; 25(OH)D, 25-hydroxy vitamin D; LASSO, least absolute shrinkage and selection operator; AUC, area under the curve.
Discussion
Diabetes is a major disease that seriously endangers the health of the global population. In recent years, the incidence and prevalence of diabetes have sharply increased because of changes in diet and lifestyle, and there is a trend toward a younger age of onset24. As one of the most common complications of diabetes, DKD affects 30-50% of patients with T2DM and remains the leading cause of end-stage kidney disease, but its exact mechanism is not yet fully understood. It may be a combined result of damage to multiple kidney targets under hyperglycemic conditions25,26. Early detection, diagnosis, and treatment are the keys to improving the life quality and expectancy of patients with DKD27. eGFR and UACR are routinely measured in DKD diagnosis for clinical practitioners, but due to the compensatory function of the kidney, these conventional diagnostic indicators are not sufficient to meet the clinical needs7,28. Therefore, hunting for biomarkers that are more sensitive and efficient is urgent for the clinic. Our study establishes serum JAML as a novel risk promoter, while nesfatin-1 and 25(OH)D serve as protective biomarkers for DKD in patients with T2DM.
JAML was initially used as a specific co-stimulatory molecule for the activation of epithelial γδ T cells, activated by coxsackie virus-adenovirus receptor (CXADR)29. Its intracellular structural domain can mediate intracellular signaling through the PI3K/MAPK/Akt pathway, leading to cell activation, proliferation, and production of cytokines and growth factors30 which play roles in immune-inflammatory response and tissue homeostasis, as well as in transendothelial migration of neutrophils and monocytes31. In our study, patients with DKD had significantly higher serum JAML levels than those without DKD (P < 0.001). Serum JAML showed a significant positive correlation with the renal function indicators, including BUN, Scr, UmALB, and UACR, and a significant negative correlation with eGFR. The risk of DKD significantly increased with increasing serum JAML levels, and this growth slowed down when JAML levels were greater than 553 pg/mL (P-Non-linear = < 0.001). The association between the serum JAML level and the increased risk of DKD remained after further adjustment of the potential confounders. These findings suggest that serum JAML can be used as a monitoring indicator for DKD. In hyperglycemic and dyslipidemic environments, Fu et al. discovered that JAML expression was significantly upregulated in renal tissues and serum of patients with DKD, which is consistent with our findings. Further studies revealed that JAML could regulate lipid metabolism in podocytes through action on SREBP1, a transcription factor participating in the synthesis of fatty acids and cholesterol, interfering with downstream signaling pathways by regulating the expression of SITR1/AMPK11. Indeed, JAML was found to participate in macrophage phenotypic polarization and efferocytosis, which was pivotal to renal inflammatory and repair processes32. Therefore, it is reasonable to assume that increased serum JAML level in the development of DKD may be the result of the dual role of podocytes and inflammatory cells.
Nesfatin-1, an ingestion-regulating peptide derived from nucleobindin-2, attracted our attention for its regulatory role in food intake and glucose homeostasis33. Our previous studies disclosed downregulation of serum nesfatin-1 in prediabetes and patients with T2DM and its strong association with β-cell insulin secretion34,35. In this study, the patients with DKD exhibited significantly low changes in serum nesfatin-1 levels compared to the individuals with non-DKD (P < 0.001). Serum nesfatin-1 demonstrated a significant positive correlation with eGFR, meanwhile, a significant negative correlation with BUN, Scr, UA, UmALB, or UACR. Moreover, a distinctive L-shaped association was observed between the serum nesfatin-1 level and the risk of developing DKD (P-Non-linear = < 0.001). It suggested that nesfatin-1, as a diagnostic marker for DKD, performed excellently in the specificity, but the sensitivity needs to be further improved. Notably, another report gave evidence that serum nesfatin-1 levels were slightly higher in early DKD than in T2DM36 which was not consistent with our findings. Taken together, our results on the relationship of nesfatin-1 level with DKD conflict with prior work and deserve deep exploration.
Numerous studies have demonstrated that vitamin D insufficiency or deficiency is independently associated with an elevated risk of DKD events in diabetic patients37,38,39. A cross-sectional study uncovered a significant correlation between chronic kidney disease and vitamin D deficiency, where individuals with chronic kidney disease were 1.7 times more likely to have vitamin D deficiency than those without chronic kidney disease40. Serum 25(OH)D had a nonlinear connection with UACR, and a negative correlation between them was observed when 25(OH)D < 67 nmol/L, according to a recent retrospective study on patients with T2DM41. Interestingly, similar results were given in our study: Serum 25(OH)D in DKD was significantly lower than in non-DKD (P < 0.001); renal function indicators such as BUN, Scr, UA, UmALB, and UACR showed a significant negative connection with serum 25(OH)D but a significant positive correlation with eGFR. Furthermore, the RCS curve for 25(OH)D was very similar to that for nesfatin-1, where the risk of DKD significantly decreased with increasing serum 25(OH)D levels, and the slope of this change became flat to unchanged when 25(OH)D ≥ 21.5 ng/mL (P-Non-linear = 0.008). The serum 25(OH)D threshold for predicting DKD was 18.93 ng/mL, with a sensitivity of 47.6%, specificity of 80.4%, positive predictive value of 71.4%, and negative predictive value of 59.9%. The mechanisms regarding the reversal of DKD progression by vitamin D are multifaceted, which include the role of vitamin D in assisting glucose processing, protecting podocytes from apoptosis, reducing renin-angiotensin activation, attenuating fibrosis, and activating anti-inflammation42; nevertheless, more work needs to be done to clarify the mechanism and benefit the clinic.
Our nomogram transcends single-biomarker approaches by synergizing molecular and clinical factors for precise risk stratification of DKD. The combination of serum JAML, nesfatin-1, and 25(OH)D with easily obtainable records of history of hypertension, course of diabetes, and history of diabetic complications of retinopathy ultimately resulted in good performance of the nomogram model. Following internal validation, the model demonstrates high concordance between the predicted risk of DKD and actual incidence, thereby enhancing clinical management strategies. However, this study has some limitations. First, our study samples were all from Xiangya Hospital of Central South University, which lacks external validation with broad significance. Follow-up should be done to enlarge the sample size and conduct multicenter validation. Second, as a cross-sectional study, this study failed to observe the dynamic changes of JAML, nesfatin-1, and 25(OH)D levels during the disease progression process or determine their causal relationship with DKD. Third, since the clinical treatment data of the subjects were incomplete in this cross-sectional study, how the clinical strategy would affect serum JAML, nesfatin-1, and 25(OH)D in T2DM and DKD was unknown and deserves further exploration. Last but not least, although we revealed the associations of JAML, nesfatin-1, and 25(OH)D with UACR and eGFR and their potential risk assessment value for DKD, more efforts should be made to elucidate the mechanism by which they are involved in DKD progression.
Methods
Participants
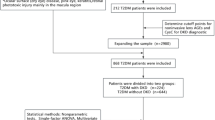
This cross-sectional study comprised 227 patients with T2DM who were aged between 18 and 85 years old, met the inclusion and exclusion criteria, attended Xiangya Hospital of Central South University from Apr. 2023 to Nov. 2024, and were admitted to the hospital for poor glycemic control or severe diabetic complications. According to the American Diabetes Association (ADA) guidelines for diabetes and studies43 T2DM status is defined as (1) a diagnosis previously reported by a healthcare professional, or (2) fasting blood glucose (FBG) ≥ 7.0 mmol/L, or (3) glycosylated hemoglobin (HbA1c) ≥ 6.5%, or (4) 2 h plasma glucose (2 h-PG) ≥ 11.1 mmol/L during the oral glucose tolerance test (OGTT), or (5) random blood glucose ≥ 11.1 mmol/L for patients with classic symptoms of hyperglycemia. Patients with type 1 diabetes or other specific types of diabetes, other causes of chronic kidney disease (interstitial nephritis and nephrosclerosis), acute infections or inflammatory conditions, pregnant or breastfeeding women, or missing essential laboratory data were excluded. The study adhered to the Declaration of Helsinki and received approval from the Xiangya Hospital of Central South University Ethics Review Committee (No. 2023020085). Appropriate written informed consent and assent were obtained from all participants.
Assessment of DKD and study variables
DKD was defined as (1) documented diabetes meeting ADA 2024 diagnostic standards; (2) renal function changes attributable to diabetes; (3) urinary albumin creatine ratio (UACR) ≥ 30 mg/g and/or eGFR < 60 mL/min/1.73 m² sustained for > 3 months; and (4) exclusion of other primary renal diseases6,43. UACR was calculated based on the ratio of urinary microalbumin to urinary creatinine, and eGFR was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for “Asian origin”.
Demographic characteristics included gender, age, height, weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), hypertension, hyperlipidemia, cardiovascular disease, smoking status, alcohol consumption, course of diabetes, and complications of diabetes. Body mass index (BMI) was computed by dividing the weight (kg) by the square of the height (m). Hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg on repeated examinations or taking antihypertensive medication. Hyperlipidemia was defined as total cholesterol (TC) ≥ 5.2 mmol/L and/or triglycerides (TG) ≥ 1.7 mmol/L, or taking lipid-lowering drugs. Cardiovascular diseases, including coronary heart disease, angina pectoris, and myocardial infarction, were obtained from participants’ medical history. Smoking status is categorized as never smoker (< 100 cigarettes before the study), former smoker (smoked ≥ 100 cigarettes before the study but quit before the study), and current smoker (smoked ≥ 100 cigarettes before the study and smoked during the study)44. Participants who consumed at least 12 drinks of any type of alcoholic beverage within the past year were classified as drinkers44.
Laboratory investigations were performed in the Laboratory Department of Xiangya Hospital of Central South University. 10 mL of venous blood were drawn from each participant after fasting for more than 8 h. 2 mL of the blood were placed in a K3 EDTA vacutainer for HbA1c estimation by high-pressure liquid chromatograph (ARKRAY, Kyoto, Japan); another 4 mL were put into a plain tube for serum separation at 3000 rpm for 10 min, used for the determination of FBG, 2-h PG, TG, TC, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), blood urea nitrogen (BUN), creatinine, and uric acid (UA) on an AU5800 automatic biochemical analyzer (Beckman Coulter, CA, USA), as well as 25(OH)D determination on a chemiluminescence autoanalyzer; and the last 4 mL were prepared for serum JAML and nesfatin-1 measurement using enzyme-linked immunosorbent assay (ELISA) kits (Jiangsu Meimian Industrial Co., Ltd, Jiangsu, China) with a detection range of 15–550 pg/mL and 30–800 pg/mL, respectively. Simultaneously, morning urine samples were collected and immediately centrifuged upon collection. Urine microalbumin (UmALB) and creatinine (Ucr) levels were determined by AU680, a fully automated biochemical analyzer (Beckman Coulter, CA, USA), and UACR (mg/g) was calculated.
Statistical analysis
Normality was tested using the Kolmogorov-Smirnov test. Continuous variables with a normal distribution were presented as the mean ± standard deviation, non-normally distributed continuous variables were expressed as the median and interquartile range (IQR), and categorical variables were expressed as frequency and percentage. The t-test, Mann-Whitney U-test, and chi-square test were utilized to assess parameter differences between the patients with DKD and non-DKD. Spearman correlation analysis was employed to assess the correlation of serum JAML, nesfatin-1, and 25(OH)D with risk indicators of DKD. Participants were categorized into tertiles based on the levels of JAML (T1 < 240, T2 240–318, T3 > 318), nesfatin-1 (T1 < 551, T2 551–682, T3 > 682) or 25(OH)D (T1 < 18.9, T2 18.9–25, T3 > 25). Multivariate logistic regression models with three progressive stages of adjustment were used to evaluate the associations between JAML, nesfatin-1 or 25(OH)D tertiles, and DKD incidence. Model 1 was unadjusted for any covariates; model 2 was adjusted for age and sex; and model 3 was adjusted for variables in model 2 plus BMI, SBP, hypertension, hyperlipidemia, and history of cardiovascular disease. Trend tests were conducted by introducing the JAML, nesfatin-1, and 25(OH)D tertiles in the model as ordered categorical variables and calculating the Wald statistic. Additionally, restricted cubic splines (RCSs) analyses were performed to examine the nonlinear relationship of JAML, nesfatin-1, or 25(OH)D with DKD incidence. The knots between 3 and 7 were tested respectively, and the model with the lowest Akaike information criterion value was selected for RCS. Finally, we used five knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles for RCS analysis between JAML and the risk of developing DKD. Three knots were placed at the 10th, 50th, and 90th percentiles for RCS analysis between nesfatin-1 or 25(OH)D and the risk of developing DKD. The diagnostic performance of serum JAML, nesfatin-1, and 25(OH)D for DKD was assessed by the receiver operating characteristic (ROC) curve. Note that these cutoff values represent preliminary findings; clinical application requires validation in larger multicenter cohorts. The areas under the curve (AUC) of the three ROC curves were compared using DeLong’s method, and Youden’s index identified the optimal cutoff values of the index. Finally, the dataset of this study was randomly divided into training and validation cohorts. In the training cohort, the least absolute shrinkage and selection operator (LASSO) logistic regression analysis was operated for multivariate analysis to screen the independent risk factors and build a nomogram model to assess the risk of DKD in the T2DM population. Furthermore, the performance and clinical benefit of the nomogram were assessed using the ROC curve, calibration curve, and decision curve analysis (DCA) in the training and validation cohorts, respectively. All statistical analyses were performed using the R software (version 4.2.2). Results with P < 0.05 (two-tailed) were considered statistically significant.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sun, H. et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. https://doi.org/10.1016/j.diabres.2021.109119 (2022).
Mao, W., Yip, C. W. & Chen, W. Complications of diabetes in china: health system and economic implications. BMC Public. Health. 19, 269. https://doi.org/10.1186/s12889-019-6569-8 (2019).
Mauricio, D., Gratacòs, M. & Franch-Nadal, J. Diabetic microvascular disease in non-classical beds: the hidden impact beyond the retina, the kidney, and the peripheral nerves. Cardiovasc. Diabetol. 22, 314. https://doi.org/10.1186/s12933-023-02056-3 (2023).
Alicic, R. Z., Rooney, M. T. & Tuttle, K. R. Diabetic kidney disease: challenges, progress, and possibilities. Clin. J. Am. Soc. Nephrol. 12, 2032–2045. https://doi.org/10.2215/cjn.11491116 (2017).
Di Marco, M. et al. Cardiovascular risk and renal injury profile in subjects with type 2 diabetes and non-albuminuric diabetic kidney disease. Cardiovasc. Diabetol. 22, 344. https://doi.org/10.1186/s12933-023-02065-2 (2023).
Committee, A. D. A. P. P. Chronic kidney disease and risk management: standards of care in Diabetes-2024. Diabetes Care. 47, 219–s230. https://doi.org/10.2337/dc24-S011 (2024).
Vučić Lovrenčić, M. & Božičević, S. Smirčić duvnjak, L. Diagnostic challenges of diabetic kidney disease. Biochem. Med. (Zagreb). 33, 030501. https://doi.org/10.11613/bm.2023.030501 (2023).
Moog-Lutz, C. et al. JAML, a novel protein with characteristics of a junctional adhesion molecule, is induced during differentiation of myeloid leukemia cells. Blood 102, 3371–3378. https://doi.org/10.1182/blood-2002-11-3462 (2003).
Luissint, A. C., Lutz, P. G., Calderwood, D. A., Couraud, P. O. & Bourdoulous, S. JAM-L-mediated leukocyte adhesion to endothelial cells is regulated in cis by alpha4beta1 integrin activation. J. Cell. Biol. 183, 1159–1173. https://doi.org/10.1083/jcb.200805061 (2008).
Roh, S. E., Jeong, Y., Kang, M. H. & Bae, Y. S. Junctional adhesion molecules mediate transendothelial migration of dendritic cell vaccine in cancer immunotherapy. Cancer Lett. 434, 196–205. https://doi.org/10.1016/j.canlet.2018.07.029 (2018).
Fu, Y. et al. Elevation of JAML promotes diabetic kidney disease by modulating podocyte lipid metabolism. Cell. Metab. 32, 1052–1062e1058. https://doi.org/10.1016/j.cmet.2020.10.019 (2020).
Stengel, A., Mori, M. & Taché, Y. The role of nesfatin-1 in the regulation of food intake and body weight: recent developments and future endeavors. Obes. Rev. 14, 859–870. https://doi.org/10.1111/obr.12063 (2013).
Meng, Q. et al. Nesfatin-1 inhibits free fatty acid (FFA)-induced endothelial inflammation via Gfi1/NF-κB signaling. Biosci. Biotechnol. Biochem. 86, 47–55. https://doi.org/10.1093/bbb/zbab186 (2021).
Yang, X. et al. Nesfatin-1 alleviates hyperoxia-induced lung injury in newborn mice by inhibiting oxidative stress through regulating SIRT1/PGC-1α pathway. Cytokine 169, 156239. https://doi.org/10.1016/j.cyto.2023.156239 (2023).
Yin, C. et al. Copeptin and Nesfatin-1 are interrelated biomarkers with roles in the pathogenesis of insulin resistance in Chinese children with obesity. Ann. Nutr. Metab. 76, 223–232. https://doi.org/10.1159/000508883 (2020).
Guo, Y., Liao, Y., Fang, G., Dong, J. & Li, Z. Increased nucleobindin-2 (NUCB2) transcriptional activity links the regulation of insulin sensitivity in type 2 diabetes mellitus. J. Endocrinol. Invest. 36, 883–888. https://doi.org/10.3275/9000 (2013).
Algul, S., Ozkan, Y. & Ozcelik, O. Serum nesfatin-1 levels in patients with different glucose tolerance levels. Physiol. Res. 65, 979–985. https://doi.org/10.33549/physiolres.933186 (2016).
Zhai, T. et al. Circulating nesfatin-1 levels and type 2 diabetes: A systematic review and meta-analysis. J. Diabetes Res. 2017 7687098. https://doi.org/10.1155/2017/7687098 (2017).
Xu, M. Z. et al. Association between serum uric acid levels and bone mineral density in patients with osteoporosis: a cross-sectional study. BMC Musculoskelet. Disord. 24, 306. https://doi.org/10.1186/s12891-023-06414-w (2023).
Ucak, S. et al. Evaluation of the relationship between microalbuminuria and 25-(OH) vitamin D levels in patients with type 2 diabetes mellitus. Aging Male. 22, 116–120. https://doi.org/10.1080/13685538.2018.1479385 (2019).
Dall’Agnol, A., Brondani, L. A., Cancelier, V. A., Camargo, E. G. & Silveiro, S. P. Lower serum 25-hydroxyvitamin D levels are associated with impaired glomerular filtration rate in type 2 diabetes patients. Therapeutic Adv. Endocrinol. Metabolism. 11, 2042018820930904. https://doi.org/10.1177/2042018820930904 (2020).
Felício, J. S. et al. Association between 25(OH)Vitamin D, HbA1c and albuminuria in diabetes mellitus: data from a population-based study (VIDAMAZON). Front. Endocrinol. 12 (2021).
Joergensen, C. et al. levels, microvascular complications, and mortality in type 1 diabetes. Diabetes Care. 34, 1081–1085. https://doi.org/10.2337/dc10-2459 (2011).
Zheng, Y., Ley, S. H. & Hu, F. B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 14, 88–98. https://doi.org/10.1038/nrendo.2017.151 (2018).
Kohsaka, S., Morita, N., Okami, S., Kidani, Y. & Yajima, T. Current trends in diabetes mellitus database research in Japan. Diabetes Obes. Metab. 23 (Suppl 2), 3–18. https://doi.org/10.1111/dom.14325 (2021).
Mohandes, S. et al. Molecular pathways that drive diabetic kidney disease. J. Clin. Invest. 133, e165654. https://doi.org/10.1172/jci165654 (2023).
McGrath, K. & Edi, R. Diabetic kidney disease: diagnosis, treatment, and prevention. Am. Fam Physician. 99, 751–759 (2019).
Selby, N. M. & Taal, M. W. An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes. Metab. 22 (Suppl 1), 3–15. https://doi.org/10.1111/dom.14007 (2020).
Witherden, D. A. et al. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science 329, 1205–1210. https://doi.org/10.1126/science.1192698 (2010).
Verdino, P., Witherden, D. A., Havran, W. L. & Wilson, I. A. The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3K. Science 329, 1210–1214. https://doi.org/10.1126/science.1187996 (2010).
Guo, Y. L. et al. Role of junctional adhesion molecule-like protein in mediating monocyte transendothelial migration. Arterioscler. Thromb. Vasc Biol. 29, 75–83. https://doi.org/10.1161/atvbaha.108.177717 (2009).
Huang, W. et al. JAML promotes acute kidney injury mainly through a macrophage-dependent mechanism. JCI Insight. 7, e158571. https://doi.org/10.1172/jci.insight.158571 (2022).
Öztürk Özkan, G. Effects of Nesfatin-1 on food intake and hyperglycemia. J. Am. Coll. Nutr. 39, 345–351. https://doi.org/10.1080/07315724.2019.1646678 (2020).
Huang, K. et al. The variation and correlation of serum adiponectin, Nesfatin-1, IL-6, and TNF-α levels in prediabetes. Front. Endocrinol. (Lausanne). 13, 774272. https://doi.org/10.3389/fendo.2022.774272 (2022).
Huang, K. et al. Influence of Circulating nesfatin-1, GSH and SOD on insulin secretion in the development of T2DM. Front. Public. Health. 10, 882686. https://doi.org/10.3389/fpubh.2022.882686 (2022).
Irannejad, A. et al. Association of peripheral nesfatin-1 with early stage diabetic nephropathy. Pathophysiology 24, 17–22. https://doi.org/10.1016/j.pathophys.2016.12.001 (2017).
Hong, S. H. et al. Association of vitamin D deficiency with diabetic nephropathy. Endocrinol. Metab. (Seoul). 36, 106–113. https://doi.org/10.3803/EnM.2020.826 (2021).
Senyigit, A. The association between 25-hydroxy vitamin D deficiency and diabetic complications in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. 13, 1381–1386. https://doi.org/10.1016/j.dsx.2019.01.043 (2019).
Xie, S. et al. Association between serum 25-hydroxyvitamin D and diabetic kidney disease in Chinese patients with type 2 diabetes. PLoS One. 14, e0214728. https://doi.org/10.1371/journal.pone.0214728 (2019).
Jamal Shahwan, M., Hassan, N. A. G. & Shaheen, R. A. Assessment of kidney function and associated risk factors among type 2 diabetic patients. Diabetes Metab. Syndr. 13, 2661–2665. https://doi.org/10.1016/j.dsx.2019.07.025 (2019).
Liang, Q. et al. A nonlinear relationship between serum 25-Hydroxyvitamin D and urine albumin to creatinine ratio in type 2 diabetes: A Cross-Sectional study in China. Diabetes Metab. Syndr. Obes. 14, 2581–2593. https://doi.org/10.2147/dmso.S308390 (2021).
Huang, H. Y., Lin, T. W., Hong, Z. X. & Lim, L. M. Vitamin D and diabetic kidney disease. Int. J. Mol. Sci. 24, 3751 (2023).
Committee, A. D. A. P. P. Diagnosis and classification of diabetes: standards of care in Diabetes-2024. Diabetes Care. 47, S20–s42. https://doi.org/10.2337/dc24-S002 (2024).
Guo, H., Wan, C., Zhu, J., Jiang, X. & Li, S. Association of systemic immune-inflammation index with insulin resistance and prediabetes: a cross-sectional study. Front. Endocrinol. 15 (2024).
Acknowledgements
We would like to thank all the investigators and subjects who participated in this project and The Clinical Mass Spectrometry Platform of the Clinical Laboratory of Xiangya Hospital.
Funding
This study was supported by the Hunan Natural Science Foundation (2023JJ40949, 2023JJ40971, 2023JJ30965 and 2023JJ40962).
Author information
Authors and Affiliations
Contributions
QH and KH performed the majority of the experiments and wrote the main manuscript draft, YL and WY analyzed the data, LL prepared the figures, WK prepared the tables, and BY amended the first draft, supervised the whole study and approved the final version. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study adhered to the Declaration of Helsinki and received authorization from the Xiangya Hospital of Central South University Ethics Review Committee (No. 2023020085). Appropriate informed consent and assent were obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hou, Q., Huang, K., Liang, Y. et al. Associations between serum JAML, nesfatin-1, and 25(OH)D and the risk of diabetic kidney disease in patients with type 2 diabetes. Sci Rep 15, 27438 (2025). https://doi.org/10.1038/s41598-025-12941-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12941-4