Abstract
Habitat loss and fragmentation are main drivers of biodiversity decline. However, disentangling their respective effects remains tricky and contrasting results have emerged in previous studies leading to a call for finely designed landscape-scale experiments that compare different levels of habitat fragmentation at fixed habitat loss, and that for different levels of habitat loss. Here we present early-stage results from MESOLAND, a new landscape-scale experiment designed to monitor the response of ground-dwelling arthropod communities to habitat loss and fragmentation, focusing on their short-term responses. Our experiment took place in a dry grassland in France, with natural stone cover representing the habitat and bare soil with stone cover manually removed being the matrix. We thus created experimental landscapes with nine levels of habitat loss (from 0 to 99%) combined with three levels of fragmentation (low, medium, high). Ground-dwelling arthropod communities were monitored using dry pitfall traps during six non-lethal capture sessions. In the months following habitat removal, we observed a drop in the number of captured arthropods with increasing habitat loss. Humidity-dependant groups such as woodlice and silverfish were affected. We observed no effect of habitat fragmentation at any level of habitat loss and no interactive effect with habitat amount. Early-stage results following the implementation of the MESOLAND experiment indicate that habitat loss has a greater effect than habitat fragmentation on ground-dwelling arthropods communities. We expect detecting further effects in the years to come, as most species will have completed several life cycles. The results of this experiment will contribute to the ongoing debate on habitat loss versus fragmentation, providing essential knowledge for applied spatial conservation.
Similar content being viewed by others
Introduction
Habitat loss and fragmentation are currently considered to be one of the main causes of terrestrial biodiversity decline. There is however a strong debate in the literature regarding the relative effects of habitat loss and fragmentation on this decline1,2. On the one hand, there is a consensus that habitat loss at the landscape scale has a strong impact on natural communities3. On the other hand, the effect of the spatial arrangement of habitats, and therefore their fragmentation, on biodiversity is still debated1,2,4,5. As habitat loss and fragmentation are not independent in a landscape - habitat fragmentation increases as the quantity of habitat decreases6 - it is not straightforward to assess the effects of habitat fragmentation independently of habitat loss, i.e. fragmentation per se7. This long-lasting scientific debate prevents coherent contributions between science and conservation applied to land planning8.
While many authors have found habitat fragmentation to be detrimental to biodiversity2,5,9 others have observed neutral10,11 or even positive effects12,13. These contrasting signals may be the result of limitations inherent to the methodology, three of which are highlighted below: (1) spatial scale of observation (e.g., habitat patch vs. landscape-scale), (2) study design, in particular the ranges of habitat loss and fragmentation level and how these are combined, and (3) study duration and how temporality is accounted for.
First, most fragmentation studies come from two different conceptual schools, which can lead to discrepancies in their results. On the one hand, studies relying on metapopulation theory focus on habitat patches and relate the biodiversity in the patch to its size, quality, and isolation14. On the other hand, studies relying on landscape ecology theory focus on landscape as a whole and relate biodiversity in the landscape with the amount and connectivity of habitats15,16. However, extrapolation from patch-scale to landscape-scale is not straightforward because a landscape is composed of many patches that differ in size and isolation15,17. Other mechanisms can also operate at landscape-scale (e.g. increased habitat diversity, landscape complementation) in addition to those operating at patch-scale (e.g. patch size, isolation)1 and processes and patterns are not necessarily consistent across spatial extents, meaning that multi-scale designs are necessary18,19. Developing a more comprehensive understanding of fragmentation effects on biodiversity thus requires further experiments at the landscape-scale1.
Second, landscape-scale experiments should be designed to discriminate the effects of both habitat quantity and fragmentation per se15. This can only be achieved by controlling for the amount of habitat, i.e. by investigating different levels of fragmentation at fixed habitat amount1. Even if previously conducted landscape-scale experiments have dealt with a variety of systems and biological models, such as terrestrial2,20 and marine arthropods21 or micro-mammals12 this type of experiment remains scarce16. So far, most of these studies have also used limited ranges of habitat quantity and fragmentation levels22. Few studies have explored the effects of more than two levels of fragmentation for a given habitat quantity12,23. These studies showed a strong trade-off between the range of habitat quantity and the levels of fragmentation investigated, limiting our in-depth understanding of their interactive and potentially non-linear effects6,24.
The third limitation relates to the lack of consideration of temporal aspects in landscape-scale experiments. After the loss and fragmentation of habitat, different facets of biodiversity (e.g., abundance, richness, composition) and different taxa (e.g., generalists vs. specialists, predator vs. prey) will respond differently through time. For instance, habitat specialists are expected to decline more quickly than generalists25. Transient effects can take place quickly after habitat loss, for instance animals can flee the degraded areas and colonize surrounding patches, temporarily increasing population densities in remnant habitat patches23. The response of organisms may also be delayed in time and therefore only observable in the long term, a phenomenon known as ‘extinction debt’26,27. The timing of observations will therefore largely influence conclusions on the effects of habitat loss and fragmentation.
To distinguish between the effects of habitat loss and fragmentation per se, we designed an innovative landscape-scale experiment in which a wide range of habitat amount (9 levels from 0 to 99% loss) was combined with three levels of fragmentation. Our experiment, called MESOLAND, is set up in a formerly cultivated dry grassland in La Crau plain (southern France). This ecosystem is characterized by a high stone cover, that provides suitable micro-climatic conditions for ground-dwelling arthropods to thrive in a particularly sunny, windy, and dry area28,29. We have implemented our habitat loss and fragmentation design by experimentally creating contrasting habitat patches (intact stone cover) with a landscape matrix (bare soil obtained by removing stones). Here, we monitored the short-term response of ground-dwelling arthropod communities to habitat removal in the months following the experimental manipulation, using a multi-facet approach to measure biodiversity. Because of the idiosyncratic responses of taxa to habitat loss and fragmentation30 we used a multi-taxa approach, with a focus on the responses of the most frequently captured groups. Indeed, some taxa such as woodlice and silverfish may be more sensitive to desiccation and less mobile than others (e.g. spiders), and are therefore more vulnerable to habitat loss and fragmentation31,32.
We therefore sought to answer the following questions:
-
How do habitat loss and fragmentation, separately or in interaction, affect the number of captures (i.e., the activity-density), taxonomic richness, diversity, and evenness of arthropod communities in the short-term?
-
Are different taxa differently affected by habitat loss and fragmentation in the short-term and how?
Methods
Study area
The dry plain of La Crau (600 km2), in southern France, is the former delta of the Durance river and is covered with grasslands33. This region is characterised by a Mediterranean climate with high inter-annual variability, low rainfall (400–500 mm per year) mainly in spring and autumn, long and hot summers and mild winters (average annual temperature: 14 °C). The sun shines for an average of 3,000 h per year and the very strong prevailing wind (‘’mistral’’) blows from the northwest 334 days per year, increasing evapotranspiration34. More than 40% of the soil surface is covered with stones that were transported by the Durance river more than 30,000 years ago34. The harsh climate, the sheep grazing for millennia and the limited access to underground water (due to a layer of impermeable conglomerate bedrock) have led to a short and thick vegetation dominated by perennial stress-tolerant species, such as Brachypodium retusum (Pers.) P.Beauv., 1812 and Thymus vulgaris, L. 1753 33. The original steppe was degraded during the twentieth century by intensive cultivation of cereals and melons and the installation of industries, decreasing its area by more than 80% 33.
Design of the MESOLAND experiment
Between October 2021 and February 2022, we installed 21 mesocosms of 10 × 10 m in a part of La Crau plain (Fig. 1). These experimental landscapes formed a double gradient of habitat loss and fragmentation. Habitat was defined as areas with a non-manipulated stone cover. The habitat loss was achieved through the experimental removal of large stones by hand (approx. 60 tons). Ground surface was then raked from January to February 2022 to remove the remaining stones with a diameter of at least 3 cm, creating a matrix of bare soil (Fig. 1). Changes in stone cover is known to alter the composition of beetle communities in this study system29. We previously showed that apterous beetles from this study system mostly remained within 1–1.5 m from their release point over a 48 h period35. Therefore, our 10 × 10 m mesocosms, though of moderate size, are considered as experimental landscapes, with the remaining habitat areas within them referred to as habitat patches (falling within the range of extent for experimental landscapes14). The matrix can be seen more as a highly degraded habitat than as a strictly inhospitable one36.
Study design. (A) Aerial photo of the experimental site and landscapes (10 × 10 m each). (B) Experimental design forms a double gradient of habitat loss and fragmentation across all possibilities (grey surface). Selected levels are displayed as red and blue dots. ECA, the equivalent connected area is a measure of habitat reachability and is here calculated for a mean movement distance of 1 m. (C) Example of an experimental landscape with 75% habitat loss where patches and matrix can be seen, corresponds to blue dot in B.
We created nine levels of habitat loss: 0, 36, 51, 64, 75, 84, 91, 96 and 99%. These discrete levels were selected to cover the full range of potential loss, from 0 to 99%, with more levels representing intermediate to high losses, as these are the levels where the debate typically occurs37 (Fig. 1).
For each level of habitat loss, we designed alternative spatial configurations of the remaining habitat corresponding to three levels of habitat connectivity. To guide our selection, we used the equivalent connected area (hereafter ECA, Saura et al. 2011) as a connectivity index39,40. Here, the ECA was calculated based on the probability of reaching a habitat patch from another patch, following a negative exponential function of the distance among patches, with a decay parameter alpha = 1 m. This value was chosen because it corresponds to the distance that La Crau apterous beetles can cover in 48 h35. We then searched for habitat configurations (number of patches and inter-patch distances) that resulted in the highest, lowest and intermediate ECA values for each level of habitat loss, using a response surface sampling approach41. This ensures that our selection covers the entire field of possibilities and that the landscape-scale variables (loss and fragmentation) are as minimally correlated as possible42 (Fig. 1B). Hence, highly connected experimental landscapes contain a single habitat patch and for these, ECA is equal to habitat amount, i.e., it is maximal (for habitat loss levels: 0, 36, 51, 64, 75, 84, 91, 96 and 99%, Fig. 1B). In poorly connected experimental landscapes, ECA is minimal for a given habitat loss (for habitat loss levels: 36, 51, 64, 75, 84, 91 and 96%). ECA was intermediate in the other cases (for habitat loss levels: 36, 51, 64, 75 and 84%). Obviously, experimental landscapes with 0 and 99% loss could lead only to one configuration (minimal fragmentation), and high loss levels (96 and 91%) resulted in little variation in experimental landscape structures since habitat loss and fragmentation are known to be correlated6. Therefore, only two levels of fragmentation were chosen for these (maximal and minimal fragmentation, Fig. 1). Fragmentation was further considered as a three-level factor: minimal, intermediate, maximal, leading to two uncorrelated variables (Kruskal-Wallis chi-squared = 0.598, df = 2, p-value = 0.742).
Our experimental landscapes were enclosed by 14-cm high, semi-buried steel garden fences (solid and slick material, as difficult to climb for our study taxa as pitfall traps, Pers. Obs.), to prevent the entry or exit of species under investigation. To ensure that they all corresponded to the same species pool, experimental landscapes were located close to each other (min: 2 m, max: 65 m). Habitat loss and fragmentation levels have been spatially distributed so that two experimental landscapes with similar modalities are not systematically close in space.
Sampling methodology and data collection
In each experimental landscape, we set up 13 pitfall traps in a tessellation-like regular pattern that covers at best the area and minimizes the distance between traps (2 m max., Supplementary 1). We chose to apply the same trap grid for all experimental landscapes because the main goal here was to understand how arthropod communities differ between experimental landscapes with varying habitat amount and fragmentation, irrespective of habitat vs. matrix36. The only adaptation we did was to ensure that each trap is located either frankly in the habitat or in the matrix and not straddling the two (traps are located at least at one metre from the habitat edge). We used plastic circular pots with a diameter of 84 mm and a depth of 100 mm.
We carried out six sampling sessions: five in spring (ca. every three week) during the arthropod activity season (16–17 March, 5–8 April, 3–6 May, 31 May-1 June, 22–23 June), and one in early autumn (6–7 October), after the dry season. The pitfall traps remained active for 48 h at each session, with the exception of April and May - peak arthropod activity months - when we kept them active for 96 h. Pitfall traps were kept dry and were visited every day. Individuals were released immediately after determination. Data from all pitfall traps within an experimental landscape were then pooled to describe variations in communities and taxa (16 days x 13 traps) at the scale of the experimental landscapes.
We counted and identified all non-flying arthropods larger than 2 mm that could not leave our experimental landscapes. Alive animals were identified to the lowest possible taxonomic level (family, genus or species) in the field. Individuals identified at the species levels were those species that are unique within their genus at our study site (e.g., the beetles Asida sericea or Poecilus sericeus). Individuals that could not be identified in the field were photographed for later identification.
Data analysis
All analyses were performed with R version 4.1.1 43.
On the one hand, we calculated four complementary metrics to describe the arthropod communities in experimental landscapes : (i) the total number of captures which reflects the total activity-density, (ii) the taxonomic richness, (iii) the Shannon diversity, and (iv) the PIE evenness which is readily interpretable as the probability that two individuals drawn from an assemblage in our study are from different taxonomic groups, and is largely independent of the number of organisms in the sample44. The last three measures were calculated at the family level, the lowest common taxonomic level for all groups. On the other hand, we investigated the number of captures for the five most common taxonomic groups: Araneae (spiders), Isopoda (woodlice), Opiliones (harvestmen), Coleoptera (beetles), and Zygentoma (silverfish, Table 1). For Araneae and Coleoptera, we also calculated the taxonomic richness, Shannon diversity and the PIE evenness at the family and genus levels respectively.
To test the effect of habitat loss and fragmentation on the response variables, we used generalized linear models. We systematically checked for normality and homoscedasticity of the residuals and used Gaussian family models (see Supplementary 5 for details on models). We ran a model selection by comparing models with all combinations of landscape variables (habitat loss, fragmentation level and their interaction) and a null model with the Bayesian information criterion (BIC). Models were fitted with linear and quadratic terms for habitat loss. Only models with the lowest BIC and ΔBIC > 2 were reported.
To test the effect of the range of habitat loss on our results, and assess their robustness regarding the design (i.e., different numbers or fragmentation levels per habitat loss level), we also compared the full data set (all experimental landscapes, N = 21) to two data subsets: subset A = excluding the two extreme experimental landscapes (0 and 99% loss, N = 19), i.e. keeping habitat loss levels with 2 and 3 fragmentation levels, subset B = only experimental landscapes with three levels of fragmentation (i.e. experimental landscapes where habitat loss varies from 36 to 84%, N = 15).
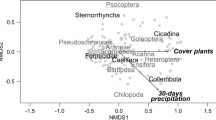
To test the effect of landscape-scale variables on the composition of arthropod communities, we performed a redundancy analysis45 and a Permutational multivariate analysis of variance46. Prior, we tested the distribution (linear or unimodal) of captures along the gradient of habitat loss, we standardized environmental variables and applied a Hellinger transformation of raw data to consider the presence of multiple 0 47.
Results
Community-level responses to habitat loss and fragmentation
Over the 16 days of surveys carried out in 2022 with 273 dry pitfall traps, we captured 2 862 individuals belonging to 27 families (2 713 individuals that could be identified to the family level, Supplementary 4). Spiders represented 51% of the captures, woodlice 19%, harvestmen 13%, beetles 7% and silverfish 6% (Table 1). For spiders, the dominant families where Lycosidae, Titanoecidae, and Gnaphosidae (Supplementary 3). For beetles, captures were dominated by Asida sericea (Olivier), Poecilus sericeus (Fischer von Walheim), and Amara sp. (Supplementary 3).
Some of the best models included habitat loss, but none included habitat fragmentation (Supplementary 5). Hereafter, estimates are provided for habitat loss in the best models. The number of captures in each landscape significantly decreased with increasing habitat loss (estimates = −0.32 ± 0.12, t-value = −2.75, p-value = 0.01, R2adj = 0.2, Fig. 2, Supplementary 5); both linear and quadratic relationships were found to be equivalent (ΔBIC < 2). This relationship remained significant when the two most extreme experimental landscapes (subset A) were removed from the analyses (estimates = −0.44 ± 0.15, t-value = −2.89, p-value = 0.01, R2adj = 0.25), but not when restricting the data to the intermediate levels of habitat loss (subset B, Supplementary 5). Habitat fragmentation levels had no effect on the total number of captures. Habitat loss also led to a significant increase in the number of encountered families (richness, estimates = 0.03 ± 0.01, t-value = 2.47, p-value = 0.02, R2adj = 0.16). This effect did not hold when the 0 and 99% loss experimental landscapes were removed (subset A), or when restricting the data to the intermediate levels of habitat loss (subset B). We found no other significant effects of habitat loss, fragmentation, or their interaction on the diversity, evenness, and composition of arthropod communities (Supplementary 5).
Effect of habitat loss on the number of captures (A) and the taxonomic richness (number of arthropod families, (B) at the community level. Relations are given for full dataset (black) and subset A (red). R2 display the adjusted R2 of all models, negative values corresponding to poor fit. Grey surfaces display the confidence intervals around models (Gaussian, 95%). Fragmentation levels are displayed as follows: circles = high fragmentation, triangles = intermediate fragmentation, squares = low fragmentation. * indicates models that are different from the null models.
Discrepancies among taxa
We found a significant linear decrease in the number of woodlice (estimate = −0.25 ± 0.07, t-value = −3.61, p-value = 0.002, R2adj = 0.34) and silverfish (est. = −0.06 ± 0.02, t-value = −3.04, p-value = 0.007, R2adj = 0.25) caught with increasing habitat loss (dropped by more than 50%), but no effect of habitat fragmentation (Fig. 3). The decrease in the number of woodlice captured with habitat loss remained significant, but weaker, when removing the two most extreme experimental landscapes from the dataset (subset A – woodlice: estimate = −0.28 ± 0.12, t-value = −2.24, p-value = 0.04, R2adj = 0.17) and when focusing on experimental landscapes with intermediate loss (subset B – woodlice: estimate = −0.24 ± 0.09, t-value = −2.48, p-value = 0.024, R2adj = 0.16). The relationship was lost for silverfish.
Effects of habitat loss on the number of captures of silverfish (top left), woodlice (top right), spiders (bottom left) and beetles (bottom right). In black, the relationships obtained with the full extent of habitat loss; in red, the relationships when removing extreme loss values, i.e. subset A. R2 display the adjusted R2 of all models, negative values corresponding to poor fit. Grey surfaces display the confidence intervals around models (Gaussian, 95%). Fragmentation levels are displayed as follows: circles = high fragmentation, triangles = intermediate fragmentation, squares = low fragmentation. * indicates models that are different from the null models.
The number of spiders, harvestmen and beetles caught in each experimental landscape was not influenced by our treatments. However, the number of spiders caught followed a significant quadratic relationship with habitat loss when focusing on experimental landscapes with intermediate loss (subset B: t-values = 2.8 & −2.8, p-values < 0.05, R2adj = 0.23). No other effect was found on the taxonomic richness, the diversity or the evenness calculated at the family or genus level for spiders and beetles (Supplementary 5).
Discussion
The objective of this study was to investigate the short-term effects of habitat loss and fragmentation per se on arthropod communities in the new landscape-scale experiment MESOLAND. Here, we observed (1) a rapid decline in the number of captures in response to habitat loss, driven by a decrease in the number of individuals captured for specialist arthropods (silverfish and woodlice), (2) an absence of short-term effects of habitat fragmentation on arthropod communities, and (3) a strong effect of the experimental design on the results, as we obtained contrasting responses to habitat loss when all the experimental landscapes were considered or when only a subset with intermediate habitat loss was selected.
Rapid decline of specialists in response to habitat loss
In the short-term, habitat loss had a negative impact on the total number of arthropods captured. The reduction in the number of captures with increasing habitat loss can be explained by two non-exclusive hypotheses. Firstly, several individuals may have died when their habitat was destroyed during the creation of the experimental landscapes. To generate a loss of habitat, we removed large stones by hand, then raked the surface of the ground to remove smaller stones. In doing so, we produced a major disturbance that probably directly caused the death of some individuals. Secondly, this loss was particularly driven by a decline in the number of habitat specialists (silverfish and woodlice) that may have died quickly after the disturbance, due to the loss of their habitat. In general, habitat specialists are more sensitive to changes in the area and quality of their habitat than generalist species, which can establish and persist in a wide range of habitats48. Silverfish31 and woodlice32 are known to be dependent on high humidity levels for their development and survival. Stone cover offers moister and cooler microclimatic conditions that are thus favourable to these taxa49. Contrary to these habitat specialists, spiders, harvestmen and beetles did not respond to habitat loss. For beetles, this lack of response contrasts with previous studies that observed more diverse beetle communities under the original stone cover29. For spiders, more abundant and diverse assemblages have also been observed in the presence of stones, but for them, stones may serve more as shelters especially for immature spiders or as food reservoirs50. They can also use the micro-topography offered by stones to build their web51 leading to different responses. It has also been suggested that short-term response of different taxa is dependent on their dispersal ability and mode52; and while our experimental landscapes are enclosed, some groups of spiders may also easily repopulate through ballooning (e.g. Gnaphosidae, Thomisidae, Salticidae are families in which ballooning has been observed53).
Importance of time since disturbance
Overall, the effects of habitat loss and fragmentation on arthropod communities were moderate to absent in the short-term. In particular, we observed no effects of habitat fragmentation at either community or group level. Unlike for habitat loss, even habitat specialists did not respond to fragmentation. These results are consistent with previous experimental studies in which the degree of fragmentation appears to have no effect on the number of captured arthropods in the first few months following habitat destruction10. This could mean that there is either no effect of habitat loss – on habitat generalists – and fragmentation – on all groups – in our experimental system, or that effects act mainly at the scale of a life cycle, meaning responses are delayed. Regarding the first hypothesis, this could be linked with the matrix we created which might still be largely permeable for the majority of arthropods54.
Regarding the second hypothesis, the short time that elapsed between the creation of the experimental landscapes and the arthropod survey may explain the limited responses we recorded. Abundance of organisms is ephemeral, especially after a disturbance, and may lead ultimately to species extinction54,55 a phenomenon known as extinction debt26. This concept refers to the number or proportion of focal habitat specialist species that are expected to eventually become extinct when the community reaches a new equilibrium after an environmental disturbance such as habitat loss56. Time to extinction may vary among species or groups according to three main factors: (1) Spatial extent and severity of environmental disturbance57. (2) Species life history and mobility traits58,59. (3) Availability of sufficiently large patches60. Monitoring our experimental communities over years will enable us to distinguish between no effects of fragmentation or simply delayed responses at the community level, in particular following a potential change that will occur in the vegetation cover and type61. We expect an extinction debt to be revealed in the coming years in our experimental set up because arthropods are short-lived58 and because our experimental landscapes are enclosed (limited colonisation of new individuals or species). In a previous (non-enclosed) stone removal experiment conducted in our study area a reduction in beetle species richness was recorded four years after habitat destruction29. In our experiment, we also expect quicker extinctions in the experimental landscapes with stronger habitat loss but strong discrepancies among taxonomic groups.
A third hypothesis may also relate to spatial scale. Previous studies have shown that fragmentation impacts are often observed at larger spatial scales compared to the impacts of habitat loss62,63,64. We designed the experimental landscapes on the basis of observations of the movements of three species of apterous beetles present in the experimental area (La Crau), which showed an average movement distance of 1–1.5 m in 48 h35. For these species, patches separated by a distance of up to 2 m should appear to be physically separate. However, this is probably not the case for more mobile species such as spiders. These, especially active hunters, are indeed known to be capable of covering large distances, meaning habitat patches in our experimental set-up do not appear isolated from their perspective65. This is confirmed by the observations of spiders and harvestmen moving quite easily between patches in the experimental landscapes. As mobility greatly varies among taxa, and each taxon may also use different modes of mobility (e.g., movements related to feeding, mate searching, or dispersal) designing experimental landscapes at the community-level remain a challenge because experimental landscapes will always be too small for some groups and too large for others to detect response patterns.
Importance of experimental design and observation methods
Several previous papers warned that incomplete experimental designs may lead to misleading interpretations regarding the relative effects of habitat loss and fragmentation1,16,22. The design of our experiment was meant to be particularly extensive in exploring the full range of habitat loss and fragmentation and their interaction by following a surface response sampling scheme41. As recommended by Fahrig et al. [2], we used replicated experimental landscapes with the same levels of habitat loss but varying levels of fragmentation. Our results confirm the necessity of using such an exhaustive design to disentangle the effects of habitat loss and fragmentation. Indeed, we observed different relationships between community-level variables and habitat loss when considering all our experimental landscapes or only a subset with intermediate habitat loss, or when removing both extreme loss levels (0% and 99%). For instance, the total number of captures did not respond to habitat loss when focusing only on 36–84% loss range. However, the relationship was maintained when removing only the two extremes (0 and 99% habitat loss).
An exhaustive design means there are fewer experimental landscapes representing the extreme conditions because the options to create experimental landscapes with similar loss and different fragmentation were restraint (2 levels of fragmentation for 96 and 91% loss and 1 level for 0 and 99% loss). This quest of exhaustiveness had also to be balanced by a lack of true replicates for each experimental landscape which was not logistically feasible66. Such an approach could indeed hinder other effects related to initial heterogeneity. In our case, local heterogeneity in stone cover or arthropod distribution could have been reinforced by past uses such as agriculture and pastoralism67. This could explain the counterintuitive increase in family richness we observed with increasing habitat loss. However, this effect disappeared when both extremes of the gradient (0 and 99% habitat loss) were removed and when focusing on intermediate levels of habitat loss (16 to 64%).
In conclusion, arthropod communities in our experimental landscapes showed little or no response to habitat loss and fragmentation during the first months following habitat destruction, i.e. in the very short-term. Consistent with theoretical concepts and empirical data, habitat specialists are the first to be affected, and only by habitat loss, suggesting that this is the main driver of community change in the short-term and at small spatial scales1,68. In the coming years, the MESOLAND experiment will enable us to closely monitor the response dynamics of arthropod communities over the medium and long term, which will provide essential data for distinguishing the effects of habitat loss and fragmentation.
Data availability
Data are provided in the supplementary materials.
References
Fahrig, L. et al. Is habitat fragmentation bad for biodiversity? Biol. Conserv. 230, 179–186 (2019).
Fletcher, R. J. et al. Is habitat fragmentation good for biodiversity? Biol. Conserv. 226, 9–15 (2018).
Pimm SL et al (2014) The biodiversity of species and their rates of extinction, distribution, and protection. Sci 344:1246752
Chase, J. M., Blowes, S. A., Knight, T. M., Gerstner, K. & May, F. Ecosystem decay exacerbates biodiversity loss with habitat loss. Nat. 2020 5847820. 584, 238–243 (2020).
Gonçalves-Souza, T. et al. Species turnover does not rescue biodiversity in fragmented landscapes. Nat. 2025 https://doi.org/10.1038/s41586-025-08688-7 (2025).
Villard, M. A., Metzger, J. P. & REVIEW. Beyond the fragmentation debate: a conceptual model to predict when habitat configuration really matters. J. Appl. Ecol. 51, 309–318 (2014).
Fahrig, L. Ecological responses to habitat fragmentation per se. Annu. Rev. Ecol. Evol. Syst. 48, 1–23 (2017).
Arroyo-Rodríguez, V. et al. Designing optimal human-modified landscapes for forest biodiversity conservation. Ecol. Lett. 23, 1404–1420 (2020).
Haddad, N. M. et al. Experimental evidence does not support the habitat amount hypothesis. Ecography (Cop). 40, 48–55 (2017).
Haynes, K. J. & Crist, T. O. Insect herbivory in an experimental agroecosystem: the relative importance of habitat area, fragmentation, and the matrix. Oikos 118, 1477–1486 (2009).
De Camargo, R. X., Boucher-Lalonde, V. & Currie, D. J. At the landscape level, birds respond strongly to habitat amount but weakly to fragmentation. Divers. Distrib. 24, 629–639 (2018).
Collins, R. J. & Barrett, G. W. Effects of habitat fragmentation on meadow vole (Microtus pennsylvanicus) population dynamics in experiment landscape patches. Landsc. Ecol. 12, 63–76 (1997).
Caley, M. J., Buckley, K. A. & Jones, G. P. Separating ecological effects of habitet fragmentation, degradation, and loss on coral commensals. Ecology 82, 3435–3448 (2001).
Haddad NM et al (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:e150005
Fahrig, L. Rethinking patch size and isolation effects: the habitat amount hypothesis. J. Biogeogr. 40, 1649–1663 (2013).
Fletcher, R. J., Smith, T. A. H., Kortessis, N., Bruna, E. M. & Holt, R. D. Landscape experiments unlock relationships among habitat loss, fragmentation, and patch-size effects. Ecology 104, e4037 (2023).
Saura, S., Bodin, Ö. & Fortin, M. Stepping stones are crucial for species’ long-distance dispersal and range expansion through habitat networks. J. Appl. Ecol. 51, 171–182 (2014).
Bosco, L., Wan, H. Y., Cushman, S. A., Arlettaz, R. & Jacot, A. Separating the effects of habitat amount and fragmentation on invertebrate abundance using a multi-scale framework. Landsc. Ecol. 34, 105–117 (2019).
Bosco L et al (2023) Habitat area and local habitat conditions outweigh fragmentation effects on insect communities in vineyards. Ecol. Solut. Evid 4:e12193
With, K. A. & Pavuk, D. M. Habitat area Trumps fragmentation effects on arthropods in an experimental landscape system. Landsc. Ecol. 26, 1035–1048 (2011).
Lefcheck, J. S., Marion, S. R., Lombana, A. V. & Orth, R. J. Faunal communities are invariant to fragmentation in experimental seagrass landscapes. PLoS One. 11, e0156550 (2016).
Bestion, E., Cote, J., Jacob, S., Winandy, L. & Legrand, D. Habitat fragmentation experiments on arthropods: what to do next? Curr. Opin. Insect Sci. 35, 117–122 (2019).
Grez, A., Zaviezo, T., Tischendorf, L. & Fahrig, L. A transient, positive effect of habitat fragmentation on insect population densities. Oecologia 141, 444–451 (2004).
With, K. A., Pavuk, D. M., Worchuck, J. L., Oates, R. K. & Fisher, J. L. Threshold effects of landscape structure on biological control in agroecosystems. Ecol. Appl. 12, 52–65 (2002).
Öckinger, E. et al. Life-history traits predict species responses to habitat area and isolation: a cross-continental synthesis. Ecol. Lett. 13, 969–979 (2010).
Tilman, D., May, R. M., Lehman, C. L. & Nowak, M. A. Habitat destruction and the extinction debt. Nat. 1994 3716492. 371, 65–66 (1994).
Vellend, M. et al. Extinction debt of forest plants persists for more than a century following habitat fragmentation. Ecology 87, 542–548 (2006).
Lamb, J. & Chapman, J. E. Effect of surface stones on erosion, evaporation, soil temperature, and soil Moisture1. Agron. J. 35, 567–578 (1943).
Blight, O. et al. Using stone cover patches and grazing exclusion to restore ground-active beetle communities in a degraded pseudo-steppe. J Insect Conserv (2011).
Betts, M. G. et al. A species-centered approach for Uncovering generalities in organism responses to habitat loss and fragmentation. Ecography (Cop). 37, 517–527 (2014).
Sweetman, H. L. Responses of the silverfish, Lepisma saccharina L., to its physical environment. J. Econ. Entomol. 32, 698–700 (1939).
Warburg, M. R. The response of isopods towards temperature, humidity and light. Anim Behav 12, (1964).
Buisson, E. & Dutoit, T. Creation of the natural reserve of La crau: implications for the creation and management of protected areas. J Environ. Manage (2006).
Devaux, J. P., Archiloque, A., BorelL, L. & Bourrelly, M. & Louis-Palluel. Notice de La Carte phyto-écologique de La Crau (Bouches du Rhône). Biol Écologie Méditerranéenne (1983).
Blight, O., Geslin, B., Mottet, L. & Albert, C. H. Potential of RFID telemetry for monitoring ground-dwelling beetle movements: A mediterranean dry grassland study. Front. Ecol. Evol. 11, 1040931 (2023).
Brudvig, L. A. et al. Evaluating conceptual models of landscape change. Ecography (Cop). 40, 74–84 (2017).
Villard, M. & Metzger, J. P. Beyond the fragmentation debate: a conceptual model to predict when habitat configuration really matters. J. Appl. Ecol. 51, 309–318 (2014).
Saura, S., Estreguil, C., Mouton, C. & Rodríguez-Freire, M. Network analysis to assess landscape connectivity trends: application to European forests (1990–2000). Ecol. Indic. 11, 407–416 (2011).
Awade, M., Boscolo, D. & Metzger, J. P. Using binary and probabilistic habitat availability indices derived from graph theory to model bird occurrence in fragmented forests. Landsc. Ecol. 27, 185–198 (2012).
Poli, C., Hightower, J. & Fletcher, R. J. Validating network connectivity with observed movement in experimental landscapes undergoing habitat destruction. J. Appl. Ecol. 57, 1426–1437 (2020).
Albert, C. H. et al. Sampling in ecology and evolution - bridging the gap between theory and practice. Ecography (Cop). 33, 1028–1037 (2010).
Pasher, J. et al. Optimizing landscape selection for estimating relative effects of landscape variables on ecological responses. Landsc. Ecol. 28, 371–383 (2013).
R Core Team. R: A Language and Environment for Statistical Computing. (2023).
Hurlbert, S. H. The nonconcept of species diversity: A critique and alternative parameters. Ecology 52, 577–586 (1971).
Legendre P, Anderson MJ (1999) Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments.Ecol. Monogr. 69 1–24
Anderson MJ (2011) Permutation tests for univariate or multivariate analysis of variance and regression. canadian. J. Fish. Aquat. Sci. 58 (3) 626–639
Legendre, P. & Gallagher, E. D. Ecologically meaningful transformations for ordination of species data. Oecologia 129, 271–280 (2001).
Zurita, G. A., Pe’er, G. & Bellocq, M. I. Bird responses to forest loss are influence by habitat specialization. Divers. Distrib. 23, 650–655 (2017).
Nobel, P. S., Miller, P. M. & Graham, E. A. Influence of rocks on soil temperature, soil water potential, and rooting patterns for desert succulents. Oecologia 1992. 921 (92), 90–96 (1992).
Benhadi-Marín, J., Pereira, J. A., Barrientos, J. A., Sousa, J. P. & Santos, S. A. P. Stones on the ground in olive groves promote the presence of spiders (Araneae). (2018). http://www.eje.cz/doi/10.14411/eje.2018.037.html 115, 372–379.
Růžička V, Spiders in rocky habitats in central bohemia (2000) J. Archanology 28:217–222. https://doi.org/10.1636/0161-8202
Nardi, D., Giannone, F. & Marini, L. Short-term response of ground-dwelling arthropods to storm-related disturbances is mediated by topography and dispersal. Basic. Appl. Ecol. 65, 86–95 (2022).
Bell, J. R., Bohan, D. A., Shaw, E. M. & Weyman, G. S. Ballooning dispersal using silk: world fauna, phylogenies, genetics and models. Bull. Entomol. Res. 95, 69–114 (2005).
Evans, M. J. et al. Short- and long-term effects of habitat fragmentation differ but are predicted by response to the matrix. Ecology 98, 807–819 (2017).
Alofs, K. M., Gonzalez, A. V. & Fowler, N. L. Local native plant diversity responds to habitat loss and fragmentation over different time spans and Spatial scales. Plant. Ecol. 215, 1139–1152 (2014).
Kuussaari, M. et al. Extinction debt: a challenge for biodiversity conservation. Trends Ecol. Evol. 24, 564–571 (2009).
Cousins, S. A. O. Extinction debt in fragmented grasslands: paid or not? J. Veg. Sci. 20, 3–7 (2009).
Deák, B. et al. Different extinction debts among plants and arthropods after loss of grassland amount and connectivity. Biol. Conserv. 264, 109372 (2021).
Bird, J. P. et al. Generation lengths of the world’s birds and their implications for extinction risk. Conserv. Biol. 34, 1252–1261 (2020).
Storck-Tonon, D. et al. Habitat patch size and isolation drive the near-complete collapse of Amazonian Dung beetle assemblages in a 30-year-old forest Archipelago. Biodivers. Conserv. 29, 2419–2438 (2020).
Meloni, F., Civieta, F., Zaragoza, B. A., Lourdes Moraza, J., Bautista, S. & M. & Vegetation pattern modulates ground arthropod diversity in Semi-Arid mediterranean steppes. Insects 11, 59 (2020).
Fletcher, R. J., Reichert, B. E. & Holmes, K. The negative effects of habitat fragmentation operate at the scale of dispersal. Ecology 99, 2176–2186 (2018).
Gelber S et al (2025) Geometric and demographic effects explain contrasting fragmentation-biodiversity relationships across scales. Oikos https://doi.org/10.1111/oik.10778
Cattarino, L., McAlpine, C. A. & Rhodes, J. R. Spatial scale and movement behaviour traits control the impacts of habitat fragmentation on individual fitness. J. Anim. Ecol. 85, 168–177 (2016).
Baatrup, E., Rasmussen, A. O. & Toft, S. Spontaneous movement behaviour in spiders (Araneae) with different hunting strategies. Biol. J. Linn. Soc. 125, 184–193 (2018).
Marshall DJ (2024) Principles of experimental design for ecology and evolution. Ecol. Lett. 27(4) e14400
Uroy, L., Ernoult, A., Alignier, A. & Mony, C. Unveiling the ghosts of landscapes past: changes in landscape connectivity over the last decades are still shaping current woodland plant assemblages. J. Ecol. 111, 1063–1078 (2023).
Fahrig, L. et al. Resolving the SLOSS dilemma for biodiversity conservation: a research agenda. Biol. Rev. 97, 99–114 (2022).
Acknowledgements
The authors thank all the people who helped preparing the experimental landscapes and collect the data: Lola Mottet, Mailys Queru, Karolina Argote, Mathieu Santonja, François Hamonic, Maëlle Bourgeois, Arun Martin, Bérengère Leys, Marie-Christine Cochet, Dahvya Belkacem, Tiffanie Stromboni, Valeria Romano, Yoann Pinguet, Romane Blaya, Emile Melloul, Clémentine Mutillot, Léo Rocher, Alexandre Millon, and Colin Moffa. Christian Marschal took the drone picture. Élise Buisson, Julien Tchilinguiran, and Christophe Mazzia helped with the identifications.
Funding
This study was supported by the European Research Council, project ERC STG SCALED no. 949812.
Author information
Authors and Affiliations
Contributions
CA, OB and BG conceived the ideas and designed the experiment. All authors collected the data. NH and CA analysed the data. NH, CA, OB wrote the first draft. All authors contributed to drafts and revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Albert, C.H., Blight, O., Geslin, B. et al. Short-term responses of ground-dwelling arthropod communities to habitat loss and fragmentation in experimental mesoscale landscapes. Sci Rep 15, 27937 (2025). https://doi.org/10.1038/s41598-025-12961-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12961-0