Abstract
One anastomosis gastric bypass (OAGB) is a relatively novel bypass surgery variant, increasingly used as a primary surgical procedure. A total of 101 patients (mean age 44.7 ± 10.7 years, mean BMI 47.2 ± 6.6 kg/m2) underwent OAGB between 2014 and 2019 in a single institution. Results obtained 6,12 and 24 months postoperatively were compared with the pre-operative values using “Analyse-it software v5.40.2” and Graphpad Prism v9.3 for figures and tables. Data were tested for normality, described as Mean ± SD, compared using paired sample t-test with 5% p-value for significance and 95% confidence interval (CI). A significant body mass index (BMI), haemoglobin A1c (HbA1c) and low-density lipoprotein (LDL) reduction was recorded throughout follow-up period, with greatest improvement seen 2 years after surgery (47.6 ± 22 kg/m2 vs. 29.4 ± 6.4 kg/m2 ,6.7 ± 1.8% vs. 5.5 ± 0.2% and 3.2 ± 1 vs. 2.05 + 0.7 mmol/ l, p < 0.05). The number of glucose-lowering drugs decreased from 1.6 ± 0.9 to 0.3 ± 0.4 at 24-months, p < 0.001. The rates for zinc, ferritin, folate, B12 and vitamin D deficiency at 24-months were: 8.9%,4%, 5.9%, 0% and 3% respectively. OAGB can effectively downstage obesity disease and improve glucose and lipid homeostasis with a low risk for nutritional deficiencies. This study is one of few to report specifically on the frequency and type of nutritional deficiencies following OAGB surgery.
Similar content being viewed by others
Introduction
Obesity, defined as body mass index (BMI) ≥ 30 kg/m21, is a chronic metabolic disease of major public health importance2, affecting hundreds of millions of children, adolescents and adults all around the world3. The prevalence of obesity has been dramatically rising, with thrice as many people diagnosed with obesity in 2016 compared to 19753 and with incidences doubling in more than 70 countries between 1980 and 20154. With post-2000 trends remaining unchanged, about 18% of men and 21% of women are estimated to have obesity by 20255, while over 1 billion of individuals are projected to have obesity by 20306. The obesity pandemic is global in nature affecting high income and developing countries with the low socioeconomic classes shouldering most of its burden7.
Alterations in the universal food system encouraging population energy intake (production of more processed, inexpensive and effectively marketed foods), and reduction in physical activity and energy demands (as a result of urbanization, changes in transportation and active recreation opportunities) appear to be the leading factors in creating an obesogenic environment7.
Metabolic bariatric surgery (MBS) is superior to non-surgical methods in treating obesity and obesity-related co-morbidities8,9,10. It results in greater and longer-lasting weight loss and is more likely to lead to type 2 diabetes (T2D) remission, metabolic syndrome resolution and discontinuation of antihypertensive, antidiabetic and lipid lowering medications. MBS can lead to early, weight loss independent improvements in glycaemic control, due to the altered gastrointestinal hormone secretion such as glucagon like peptide 1 (GLP-1)8,9,10,11 However, careful patient selection and the expected benefits of surgery must be carefully assessed to mitigate the risks of any postoperative complications which can require readmission, postoperative interventions or reoperation, and can rarely even be fatal12.
Malabsorption of nutrients and trace elements as well as alterations of pharmacodynamics and pharmacokinetics is also expected following metabolic procedures, as they alter the anatomy and physiology of the gastrointestinal tract affecting food ingestion and absorption of certain medicines13. Nutritional deficiencies are more prevalent across metabolic procedures14 which bypass the duodenum and jejunum, the primary sites of nutrient absorption14 with biliopancreatic diversion (BPD) carrying a higher risk than Roux-en-Y gastric bypass (RYGB) and one anastomosis gastric bypass (OAGB). Non-adherence to recommended supplementation after the procedure is another important determinant of nutrient status among patients following metabolic surgery. Patients undergoing bariatric procedures should take vitamin supplementation for life and be closely monitored for vitamin deficiencies, thus noncompliance with the above could lead to serious complications15,16,17. It should be noted that despite adherence to supplementation, some patients particularly after BPD will develop nutritional deficiencies refractory to supplementation and this is a potential indication for revision.
The frequency of the different types of bariatric procedures has been changing with time. Gastric banding was the most frequent procedure in the years 2002–2008 but after 2009 there was a rapid decrease in the number of laparoscopic adjustable gastric banding (LAGB) vs. RYGB and laparoscopic vertical sleeve gastrectomy (LVSG) procedures18. The number of OAGB procedures has been increasing in more recent years. In 2016, OAGB was the third most frequently performed bariatric procedure worldwide following LVSG and RYGB, accounting for about 4.8% of the total bariatric procedures19. In 2018, the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) approved OAGB as a bariatric/metabolic procedure but also stated that there is a lack of long-term nutritional information associated with OAGB20. In a prospective multicentre study (YOMEGA trial) OAGB showed comparable (non-inferior) to RYGB weight reduction and metabolic improvements at 2 years postoperatively but warned of increased risk of nutritional deficiencies21,61.
The objectives of this present study were to evaluate the impact of weight loss on obesity associated complications, the development of nutritional deficiencies and common post-operative complications following OAGB. This study also aimed to identify sub-groups of patients exhibiting greater benefits from this procedure. To our knowledge, this is one of a few studies to date, to evaluate nutritional deficiencies 2 years after OAGB.
Methods
Study design and patient population
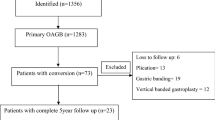
In this retrospective cohort study evaluating nutritional deficiencies developed following OAGB surgery, data of 101 adult patients who underwent laparoscopic OAGB surgery between 2014 and 2019 at a single institution were collected and analysed. Patients included herein were selected before the last IFSO/American Society for Metabolic and Bariatric Surgery (ASMBS) revision of surgical criteria made in 2022 as they were operated before this revision was made.
Patients who underwent surgery had baseline BMI ≥ 40 kg/m2 or a BMI ≥ 35 kg/m2 with obesity related medical complications that could improve with weight reduction. Patients had engaged in a structured non-surgical weight management programme under a specialist weight management service for a period between 6 and 12 months before surgery. The service comprised of a bariatric physician led multidisciplinary team that ensured patients who were referred to a service for bariatric surgery were medically optimized and had no medical, surgical, nutritional, psychological, psychiatric or social contraindications to the procedure22. None of the patients had previous history of MBS and all were offered OAGB as a primary procedure.
Surgical technique
Patients underwent a standardized OAGB procedure which was performed according to the following protocol: An afferent/biliopancreatic jejunal loop of 150 cm was anastomosed to about 15 cm narrow longitudinal gastric pouch which was created over 34 F bougie using Covidien tan stapler and crow’s foot as the landmark, leaving more than 300 cm measured common limb. The anastomosis was performed using 45 mm tan stapler of the side of the gastric pouch and the side of the small bowel, and the pathway was anticolic. Methylene blue test was performed to check for leaks. Haemostasis was secured and lavage was performed. Wounds were closed after being infiltrated with 40 ml of 0.5% Marcaine.
Definitions
Patients BMI is defined herein in kg/m2. In the results section, age is reported in years, weight in kg, BMI in kg/m2, EWL in %, and TWL in %.
Weight loss after surgery was calculated using both total weight loss (%TWL), and excess weight loss (%EWL). %TWL was obtained applying the formula: (initial weight – post-op weight) × 100/initial weight. %EWL was calculated by dividing the number of kilograms lost by pre-operative excess body weight (EBW), assuming a healthy BMI at 25 kg/m2.
Post-operative care
Postoperatively, all patients went through an 8-week post-operative staging diet, progressing from a liquid diet to a soft food diet, and eventually to solid foods. They were expected to attend follow up appointments with different members of the bariatric team within 2 weeks post op, and at 2, 3, 6, 12 and 24 months post operatively and have blood tests in advance of most follow-up appointments. Support and guidance for long-term weight loss and weight maintenance was offered. Patients were provided with nutritional supplementation (A-Z multivitamin supplement, vitamin D3 2000-4000IU daily, B12 IM 1 g 3/12 and once daily iron supplement) and underwent biochemical monitoring according to a national guideline on supplementation and biochemical investigations postoperatively26.
Data collection
Patients’ data were collected from the Electronic Patient Record (EPR) system at 4-time intervals: baseline before a liver-shrinking diet (2 weeks duration with 1200 kcal/day) and at 6, 12 and 24 months after the surgical procedure. Information gathered about patient demographics (date of birth, sex, race), smoking status, complications, medications (lipid-lowering, glucose-lowering and antihypertensive drugs), anthropometrics (weight, height), blood tests and compliance to postoperative supplementation.
Data collected from the blood test results included serum total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglycerides (TG), haemoglobin A1c (HbA1c), fasting blood glucose (FBG), random blood glucose (RBG), alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), albumin, bilirubin, total protein, globulin,, platelet count (PLT), thyroid stimulating hormone (TSH), free thyroxine (FT4), free triiodothyronine (FT3), parathyroid hormone (PTH), creatinine, estimated glomerular filtration rate (eGFR), corrected calcium, phosphate, vitamin A, vitamin B12, vitamin D, vitamin E, copper, ferritin, folate, magnesium, selenium and zinc.
Statistical analysis
Analyse-it v5.40.2 and GraphPad Prism softwares were used for all statistical analysis. Descriptive statistics were used to report clinical and laboratory data at baseline, and at 6-, 12- and 24-months postoperatively. Data were reported as mean ± standard deviation (SD) for continuous variables, and as percentage for categorical variables. Linear logistic regression was used to define predictive factors for lower BMI post operatively. Paired sample T test and independent sample T test were used for the comparison between parameters. Pearson correlation coefficient was used to determine relationships between variables. A p-value < 0.05 was considered statistically significant.
Ethical approval and study registration
Ethical approval from a Research Ethics Committee (REC) was not required as the data generated for the purposes of this project were fully anonymized, collected in line with standard of care protocols for OAGB surgery in our institution, and were processed and presented retrospectively. Furthermore, informed consent was deemed unnecessary for the purposes of this retrospective study. The study was discussed within Research Delivery Unit 6 (RDU6) meeting – Renal/Endo Research Group Board (RRGB) at our institution (Governance Arrangements for Research Ethics Committees (GafREC): Endo201).
Results
Baseline characteristics
Out of the 101 patients, 91 (90%) were female and 10 (10%) were male. Most patients were Caucasian white (59.4%), and for 36 patients (35.6%) race was not specified. The mean age was 44.7 ± 10.7 years, weight was 128.9 ± 22.5 kg and BMI was 47.2 ± 6.6 kg/m2. Ten patients (9%) were smokers and 13 (12%) were ex-smokers. Other baseline characteristics are shown in Table 1. The average number of complications among patients were 2.9 ± 1.8, with the number of complications ranging from 0 to 8. In this cohort, the most prevalent complications were osteoarthritis23 (49.5%), hyperlipidaemia (HL)24 (31.6%), anxiety and/or depression31 (30.6%), hypertension (HT)30 (29.7%), T2D23 (23%), prediabetes5 (14.8%), thyroid disease12(11.8%) (11 with hypothyroidism and 1 with thyrotoxicosis – all cases were medically controlled before surgery), Obstructive sleep Apnoea (OSA)25 of which 12 patients were on continuous positive airway pressure – CPAP) (16.8%) and polycystic ovary syndrome - PCOS26 (7.9%). On average, 1.6 ± 0.9 glucose-lowering, 1.5 ± 0.7 antihypertensive and 0.6 ± 0.5 lipid-lowering medications were used by patients with T2DM, hypertension and dyslipidaemia, respectively, at baseline27.
Post-operative changes in anthropometric parameters
Mean weight and BMI decreased significantly at 6-, 12- and 24-months post operatively (p < 0.001), results are shown on Table 2. Both TWL and EWL increased progressively with time; mean TWL% was 28.2, 37.9, and 37.3 at 6, 12 and 24 months, respectively, while EWL% was 60.6, 79.8 and 80.8 at 6,12 and 24 months, respectively (Table 2). Even though TWL increased significantly from 6 months to 12 months (p < 0.001), and from 6 months to 24 months (p < 0,001), the increase observed between the 12th and 24th month post-op was non-significant (p = 0.121). EWL followed the same pattern, with significant increases seen between months 6 to 12 (p < 0.001) and 6 to 24 (p = 0.004), but not between months 12 to 24 (p = 0.152).
Following the forementioned changes, the distribution of individuals among the five weight categories (healthy weight, overweight and class I, II, III obesity) changed during the follow-up period (Fig. 1). Most patients had class III obesity at baseline (89%), class I obesity at 6 months (36%), and overweight at 12 (39%) and 24 (45.7%) months (Table 3). All procedures were deemed effective based on the cut-off values of EWL ≥ 50% and TWL ≥ 20% (Table 2).
Subgroup analysis using linear regression model plotting BMI at 24 months postoperatively with baseline patients’ characteristics listed in Table 1 showed that having lower baseline BMI and age < 46 years can predict a significant lower BMI at 24 months post-operatively (P < 0.05). There was no effect of other baseline characteristics on the final weight loss outcome. Furthermore, independent sample T test was used to compare TWL and EWL based on the age and the baseline BMI. Patients with baseline BMI < 50 kg/m2 experienced greater EWL than patients with BMI ≥ 50 kg/m2, with significant differences seen at 6 (p = 0.001) and 12 (p = 0.019) months. Although TWL was greater among those with a lower BMI at 6-, 12- and 24-months post-surgery, the differences observed were not statistically significant. The data are presented in Table 4.
Correlations between TWL and EWL (at 6-, 12- and 24-months post-op) with baseline BMI and age were further investigated using Pearson Correlation Coefficient (Table 4). Baseline BMI correlated positively with TWL (r = 0.25, p 0.02) and negatively with EWL (r – 0.36, p < 0.0005) at 24 months postoperatively (Fig. 2). Age has significant negative correlation with TWL (r-0.25, p 0.02) at 6 and 24 months post operatively (Table 4).
TWL% and EWL% were also assessed in the presence of obesity related complications, particularly T2D, prediabetes, HT, and HL. Analysis showed that weight loss was not significantly different between patients with and without the above conditions at 6-, 12- and 24-months post-operation (Table 5).
Effects on obesity related complications
Analysis of laboratory results demonstrated a significant decrease in TC, TG, LDL-C and HbA1c with a significant increase in HDL-C at 24 months, compared to baseline [4.1 ± 0.8 mmol/l vs. 5.3 ± 1 mmol/l for TC (n = 15), 2 ± 1.1 mmol/l vs. 1.1 ± 1.6 mmol/l (n = 10) for TG, 2 ± 0.7 mmol/l vs. 3.2 ± 1 mmol/l for LDL-C (n = 11), 5.5 ± 0.8% vs. 6.7 ± 1.8% for HbA1c (n = 16) and 1.6 ± 0.2 mmol/l vs. 1.3 ± 0.2 mmol/l for HDL-C (n = 15), p < 0.05 ) (Fig. 3).
There was a significant reduction in the number of glucose-lowering medications used among participants with T2D at 24 months from baseline (1.6 ± 0.9 vs. 0.4 ± 0.3 medications, p < 0.005, n = 16) (Fig. 3).
Out of the 12 patients who were using CPAP for OSA, 6 patients stopped using CPAP postoperatively as symptoms improved, 3 patients continued using the machine and there was no information about CPAP use for the last 3.
Out of the 15 patients who were taking treatment for HL, 6 patients did not need medications post operatively, 5 patients continued and for 4 patients, information was not available.
Effects on nutrients and trace elements
Total protein decreased significantly from 71.8 ± 4.2 g/l at baseline to 67 ± 4.6 g/l at 24 months (p < 0.05). Similarly, Globulin and corrected Calcium decreased from 28 ± 3.5 g/l and 2.35 ± 1mmol/l at baseline to 24 ± 3.5 g/l and 2.25 ± 0.07mmol/l at 24 months respectively (p < 0.05). Baseline albumin (44 ± 2.3 g/L) and baseline platelet count (275 ± 66 10^9 g/L) decreased at 12 months to 42 ± 3 g/L and increased 284 ± 45 10^9 g/L respectively (p < 0.005), however the decrease was not statistically significant at 24 months (p = 0.12) and not clinically significant at any time interval. ALP showed a statistically significant increase (81.5 ± 22.1 IU/L at baseline vs. 92 ± 27.4 IU/L at 24 months, p < 0.005).
Compliance with multivitamin supplements after the procedure was reported by patients and assessed using information in follow up clinic letters. Most of the patients were compliant with their supplements (Fig. 4).
Baseline nutrition data were obtained for some of the patients. Vitamin D and Ferritin deficiencies were the most commonly identified nutritional deficiencies preoperatively (30.7% and 6.9% of total patients respectively) (Table 6).
Postoperatively, even though calcium levels decreased to a statistically significant level, no patient had calcium concentration below the normal reference range at 24 months after surgery. None of the patients had vitamin B12 deficiency. The rate for vitamin D, ferritin and folate deficiency at 24 months were 3%, 4% and 5.9% respectively. Zinc was most the common deficient trace element (8.9% at 24 months). The number of patients tested and the percentage of each nutrition marker deficiency at 6, 12 and 24 months are listed in Table 6.
Adverse outcomes reported by patients after surgery included; hair loss (15 patients), late dumping syndrome (4 patients), gastroesophageal reflux disease (5 patients) not requiring adjustment of the surgical procedure, loose stool (4 patients), cholelithiasis (4 patients), incisional hernia (1 patient) and nephrocalcinosis (1 patient). Two patients reported excessive alcohol intake after surgery and another 2 described eating habits consistent with eating disorder. One patient required laparoscopic jejunojejunostomy due to persistent nausea.
Discussion
MBS is superior to non-surgical interventions in achieving greater and more sustainable weight reduction and in resolution of medical complications. However, as most of the patients who are eligible for MBS have severe obesity or suffer from significant weight related complications, they usually present a higher peri-operative surgical risk and are prone to a wide array of intra and post-operative complications. The risk of these complications can increase with the complexity and invasiveness of a surgical intervention (RYGB has a higher risk profile than LVSG). With the steep rise of obesity in both developed and developing countries, there is a significant demand for bariatric procedures producing significant weight loss results, albeit with low risk of adverse outcomes.
In this study we aimed to retrospectively examine the outcome of a relatively novel, but increasingly popular variant of bypass surgery; the OAGB in terms of weights loss, improvement of medical complications, changes in biochemical and nutritional markers and the incidence of post-operative complications. OAGB achieved excellent weight loss results. Comparing our study with Theodoropoulos et al.29, which examined the effect of OAGB on 94 patients over 36 months, our mean EWL% at 6, 12 was similar and slightly lower at 24 months at 60.6%, 79.8%, 80.8% vs. of 66.1%, 83.6%, 98.8%. The percentage of patients who achieved good response to surgery at 24 months (based on the cut off value of EWL ≥ 50%) and patients who had BMI < 35 kg /m2 at 12 months were also comparable at 94% vs. 98.8% and 79% vs. 83% respectively. This variance might be explained by the slightly larger number of patients included in the analysis at 24 months in Theodoropoulos et al. (94 vs. 82 in our study), the difference in patient baseline characteristics (mean age 44.7 vs. 41.5 years), gender (91% vs. 72% females) and length of biliopancreatic limb (200 to 300 cm vs. 150 cm in our study). EWL % was also higher in 2 other studies reporting 89%+ 16.5 EWL% at 12 months27,30,31. One of these studies reported minimum BP length of 250 cm55 and the other did not report the BP length32.
EWL% at 12 months in our cohort was in consensus with EWL% reported in the study by Rutledge and Walsh30 which examined the effect of OAGB in 2,410 patients (79.8 vs. 80%) and TWL% at 24 months in our cohort was similar to their study32.
BMI for our patients decreased by 26% at 6 months, 35% at 12 months and 37% at 24 months. Similar rates of reduction have been reported in the literature33,34. There was a statistically significant negative correlation between the baseline BMI and EWL% at all-time intervals, a finding which was also replicated in other studies35,36. Our regression model also showed that having higher baseline BMI predicted higher BMI values at 24-months post operatively. In contrast, TWL % at 24 months was positively correlated with baseline BMI (r: 0.25, p < 0.05). Although patients who are within class 3 obesity can lose more weight, they are less likely to achieve a healthy BMI value than patients at a lower baseline obesity class. However, even < 5% TWL at 6 months in patients with class 3 obesity can result in significant cardiometabolic improvements in blood pressure and glycaemic control37.
In this study, maximum mean EWL and TWL% were reached at 24-month post op and were comparable to other studies38,39. Another study by Jiménez et al. reported maximum weight loss achieved earlier at 12 rather than 24 months postoperatively40. This could be attributed to the lower baseline BMI in comparison with our cohort, as only 57% of their patients had class 3 obesity in comparison to 89% in our study.
OAGB has achieved higher weight reduction than LVSG in published literature41,42,43. A systematic review and meta-analysis of three clinical trials published in 2020 reported higher weight loss results with extended length biliopancreatic OAGB compared to RYGB, however this study acknowledged the low-quality evidence of the included studies44. An additional advantage of OAGB compared to other bypass surgery variants is the briefer time required to perform this type of surgery leading to improved cost-effectiveness.
Significant improvement in HbA1c and lipids profile following OAGB has been reported by multiple studies45,46,47,48. The mean LDL-C and TG concentrations at 12 months in our cohort decreased by 32% and 44% from baseline respectively. This was higher than the rates reported from 2 studies49,50 at the same time interval. Six of our patients achieved complete resolution of HL and stopped taking statins. Five patients continued having statin postoperatively. This is higher than Theodoropoulos et al.27 who reported complete resolution of dyslipidaemia, however 1 patient in our cohort had familial hypercholesterolemia and another patient was on statin for secondary prevention with no scope for discontinuing statins in both cases. Postprandial hypertriglyceridemia is caused by impaired lipolysis of TG-rich lipoprotein due to reduced activity of lipoprotein lipase enzyme a manifestation of insulin resistance in central obesity39. HL improvement can be partially explained by weight reduction, but the magnitude of improvement following bariatric procedures is higher compared to non-surgical interventions. This might suggest that the anatomical alteration in bypass surgery, with the resulting increase in incretin hormones, play an important role in increasing insulin sensitivity and improving dyslipidaemia independent of weight reduction51. Others report that compared to LVSG, OAGB achieved greater reduction in TG, TC and LDL-C when age, sex and BMI was statistically adjusted for52 and non-inferior results, when compared with RYGB52.
In our study, HbA1c level was reduced by 22% from baseline at 12 months postoperatively which allowed the significant reduction in the number and / or the doses of antidiabetic medications or discontinuation of medications in some cases. In published literature, the reported rates of HbA1c reduction were 8%, 25%, 30% with one study reporting completed resolution of T2D16,54,55,56. OAGB outperformed LVSB in achieving diabetes remission, a study reported 84% success rate for OAGB compared to 60.9% for LSG48. OAGB had similar impact on T2D remission compared to RYGB48,57. It should be noted that data available comparing the efficacy of each procedure against others is limited, not from RCTs and may involve very different limb lengths, duration of follow up and even possibly definitions of diabetes remission. Apart from the choice of procedure, multiple factors can estimate T2D remission after MBS including age of the patients, prolonged history of T2D preoperatively, high baseline HbA1c and use of insulin preoperatively according to DIAREM criteria58. This could explain the variability in published rates of HbA1c reduction with subsequent T2D remission following OAGB.
OSA is a common obesity associated complication. The incidence and severity of OSA is positively correlated with BMI48. OSA is a risk factor for cardiovascular disease including hypertension, arrhythmia and heart failure57. Metabolic surgery can resolve/downstage the severity of OSA or the need to use CPAP which could be through both weight loss dependent and independent mechanisms48. RYGB remains superior to intensive lifestyle intervention in OSA treatment48. In our cohort 50% of the patients who used CPAP preoperatively returned their machines postoperatively.
Weight reduction after MBS can help decrease the systemic low-grade inflammatory process associated with obesity. Platelet count is considered a marker for chronic inflammation and tends to decrease following MBS58. Although the mean platelet count in our cohort was within reference range preoperatively, it decreased significantly post operatively43.
The rates of vitamin D, B12 and ferritin deficiencies were higher at baseline than in the postoperative period reflecting good postoperative replacement.
Significantly higher rates of baseline nutritional deficiencies were reported in a study that examined the effect of OAGB on 86 patients55. The development of nutritional deficiencies is a common finding in patients after metabolic surgery who typically consume low quality and calorically dense ultra-processed foods preoperatively56. Vitamin D deficiency is the most common micronutrient deficiency prior to MBS56. Failure to identify and correct nutritional deficiencies preoperatively can increase the risk of developing severe complications postoperatively55.
Multiple other causes for post-surgical nutritional deficiencies have been identified in the literature55,56. Compliance with vitamin supplementation after surgery is vital for preventing deficiencies. The rates of postoperative micronutrient deficiencies in our cohort were low. Low rates were also reported in other studies48,57. However, as the biliopancreatic limb length increases to > 150 cm, the rates of micronutrient deficiencies can increase substantially with a biliopancreatic limb of 280 cm causing the highest rates58. According to another study, there was no significant increase in the incidence of nutritional deficiencies in a small cohort of patients with biliopancreatic length of 230 cm54. It should be noted that nutritional deficiencies following bariatric surgery including OAGB may have detrimental effects to the quality of patient’s life due to several medical complications which may develop.
Hypoalbuminemia and protein malnutrition can be one common nutritional complication of MBS. The recommended dietary protein intake to prevent protein malnutrition is at least 1.1–1.5 g per day43. However, inadequate protein intake, protein intolerance or significant gastrointestinal tract anatomical alteration negatively impact the absorptive capacity of the small intestine, resulting in severe protein malnutrition43. Despite the statistically significant drop in albumin concentration post-op in our cohort, the magnitude of decrease was not clinically significant. Similar results were reported in the literature46,48,53.
In our cohort, there was 15% reported hair loss especially in the first 3 to 6 months postoperatively. Hair loss after MBS is a common finding, although it is not physically harmful, it can have detrimental effects to the mental health and patient quality of life59. It was suggested that hair loss decreases throughout the postoperative period with significantly lower incidence at 24 months postoperatively53. Informing the patient preoperatively about the possibility of hair loss and how to best minimise can mitigate the adverse psychological effects on the patients of this complication.
No patients from our cohort developed anastomotic leaks after surgery. The rates for cholecystectomies, late dumping syndrome and diarrhoea were low and in consensus with the reported rates in other studies46,52,53. This is at variance with what has been reported in YOMEGA trial and likely the outcome of difference in biliopancreatic length59.
Nevertheless, it is important to note that an increasingly reported complication after MBS and particularly RYGB is post-bariatric hypoglycaemia60. It is important that this potentially significant complication of MBS is screened for by clinical teams looking after patients following MBS61,62.
There are several limitations with this study. One is its retrospective nature which resulted in some missing data at different stages of analysis. Another limitation is the limited follow up period to 24 months albeit this is a standard postoperative follow up period in the NHS. A strength of this study is the relatively large sample size, the fact that all procedures were performed by the same surgeon (SEH) ensuring no changes in bypass length. Furthermore, this is one of few studies reporting specifically on the nutritional deficiencies observed following OAGB surgery in a large tertiary institution and provides insight in the management of this emerging surgical procedure. Larger prospective multisite randomized studies are needed to validate the results of our study.
Conclusions
In a single bariatric centre of excellence, OAGB has demonstrated clinically relevant weight reduction and significant improvement of obesity related complications with low rates of surgical complications and nutritional deficiencies.
Data availability
The data relating to this study can be made available to interested parties upon reasonable request towards the corresponding author.
References
Chew, N. W. S. et al. The global burden of metabolic disease: data from 2000 to 2019. Cell. Metab. 35 (3), 414–428e3. https://doi.org/10.1016/j.cmet.2023.02.003 (2023).
Tsigos, V. et al. European guidelines for obesity management in adults. Obes. Facts. 8 (6), 402–424 (2015).
World Health Organization. Obesity and overweight [Online]. World Health Organization. (2020). Available: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight?fbclid=IwAR3FRamKH3FgWsAAFX0GvE4gNvdSx8aU4sU8tmEoXRkTnjWsTmSvOR8y62Q [Accessed 17th April 2021].
GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl. J. Med. 377 (1), 13–27 (2017).
NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 387 (10026), 1377–1396 (2016).
Kelly, T., Yang, W., Chen, C. S., Reynolds, K. & He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. (Lond). 32 (9), 1431–1437 (2008).
Migena Luli, G. et al. The implications of defining obesity as a disease: a report from the Association for the Study of Obesity 2021 annual conference. EClinicalMedicine. 2023 Apr 6:58:101962.https://doi.org/10.1016/j.eclinm.2023.101962
Swinburn, B. A. et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 378 (9793), 804–814 (2011).
Rajan, T. M. & Menon, V. Psychiatric disorders and obesity: A review of association studies. J. Postgrad. Med. 63 (3), 182–190 (2017).
Bianca, M. et al. Laparoscopic adjustable gastric Banding—Should a second chance be given?? Obes. Surg. 30 (8), 2913–2919. https://doi.org/10.1007/s11695-020-04613-1 (2020).
Kehagias Dimitrios, C. et al. Diabetes remission after LRYGBP with and without fundus resection: a randomized clinical trial. Obes. Surg. Vol. 33 (11), 3373–3382. https://doi.org/10.1007/s11695-023-06857-z (2023).
Chaoxing Lin, T. J. J. M. et al. Chin Meng khoo, Nicholas W S chew. Comparison of mechanistic pathways of bariatric surgery in patients with diabetes mellitus: A bayesian network meta-analysis. Obes. (Silver Spring). 30 (7), 1380–1390. https://doi.org/10.1002/oby.23453 (2022).
Georgios, K., Dimitriadis, M. S., Randeva, Alexander, D. & Miras Potential hormone mechanisms of bariatric surgery. Curr. Obes. Rep. 6 (3), 253–265. https://doi.org/10.1007/s13679-017-0276-5 (2017).
Piché, F. L. T. M. E., Marceau, S., Lebel, S., Lafortune, A. & Dimitriadis, G. K. André tchernof, Laurent biertho. Preoperative predictors of type 2 diabetes remission after bilio-pancreatic diversion with duodenal switch. Surg. Obes. Relat. Dis. 20 (6), 507–514. https://doi.org/10.1016/j.soard.2023.11.006 (2024).
Xanthakos, S. A. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr. Clin. North. Am. 56 (5), 1105–1121 (2009).
Sawaya, R. A., Jaffe, J., Friedenberg, L. & Friedenberg, F. K. Vitamin, mineral, and drug absorption following bariatric surgery. Curr. Drug Metab. 13 (9), 1345–1355 (2012).
Matrana, M. R. & Davis, W. E. Vitamin deficiency after gastric bypass surgery: a review. South. Med. J. 102 (10), 1025–1031 (2009).
Lewandowski, H., Breen, T. L. & Huang, E. Y. Kwashiorkor and an acrodermatitis enteropathica-like eruption after a distal gastric bypass surgical procedure. Endocr. Pract. 13 (3), 277–282 (2007).
Mallory, G. N. & Macgregor, A. M. Folate status following gastric bypass surgery (The great folate Mystery). Obes. Surg. 1 (1), 69–72 (1991).
Booth, H. P. et al. Changing epidemiology of bariatric surgery in the UK: cohort study using primary care electronic health records. Obes. Surg. 26 (8), 1900–1905 (2016).
Welbourn, R. et al. Wendy brown, Lilian KnowBariatric-Metabolic surgery utilisation in patients with and without diabetes: data from the IFSO global registry 2015–2018. Obes. Surg. 31 (6), 2391–2400. https://doi.org/10.1007/s11695-021-05280-6 (2021).
De Luca, M. et al. Mini gastric bypass-One anastomosis gastric bypass (MGB-OAGB)-IFSO position statement. Obes. Surg. 28 (5), 1188–1206 (2018).
British Obesity & Metabolic Surgery Society. Commissioning Guide: Weight Assessment and Management Clinics (tier 3) (UK British Obesity and Metabolic Surgery Society), 2014).
O’Kane, M. et al. British obesity and metabolic surgery society guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery-2020 update. Obes. Rev. 21(11), e13087. (2020).
Charalampos, T. et al. Tailored one anastomosis gastric bypass: 3-Year outcomes of 94 patients. Obes. Surg. 29 (2), 542–551 (2019).
Carbajo, M. A. et al. Evaluation of Weight Loss Indicators and Laparoscopic One-Anastomosis Gastric Bypass Outcomes. Sci Rep. 8(1), 1961. (2018).
Jiménez, J. M. et al. Changes in lipid profile, body weight variables and cardiovascular risk in obese patients undergoing One-Anastomosis gastric bypass. Int. J. Environ. Res. Public. Health. 17 (16), 5858 (2020).
Rutledge, R. & Walsh, T. R. Continued excellent results with the mini-gastric bypass: six-year study in 2,410 patients. Obes. Surg. 15 (9), 1304–1308 (2005).
Boyle, M. & Mahawar, K. One anastomosis gastric bypass performed with a 150-cm biliopancreatic limb delivers weight loss outcomes similar to those with a 200-cm biliopancreatic limb at 18–24 months. Obes. Surg. 30 (4), 1258–1264 (2020).
Musella, M. et al. The laparoscopic mini-gastric bypass: the Italian experience: outcomes from 974 consecutive cases in a multicenter review. Surg. Endosc. 28 (1), 156–163 (2014).
Ruiz-Mar, G., Ruelas-Ayala, A., Ornelas-Oñate, L. A. & Ramirez-Velasquez, J. E. THE ONE ANASTOMOSIS GASTRIC BYPASS TECHNIQUE: RESULTS AFTER ONE YEAR OF FOLLOW-UP. Arq. Bras. Cir. Dig. 32(4), e1476. (2019).
Kermansaravi, M., Pishgahroudsari, M., Kabir, A., Abdolhosseini, M. R. & Pazouki, A. Weight loss after one-anastomosis/mini-gastric bypass - The impact of biliopancreatic limb: A retrospective cohort study. J. Res. Med. Sci. 25, 5 (2020).
Jamal, W. et al. Initial outcomes of one anastomosis gastric bypass at a single institution. Diabetes Metab. Syndr. Obes. 12, 35–41 (2018).
Parmar, C. D. et al. Mini gastric bypass: first report of 125 consecutive cases from united Kingdom. Clin. Obes. 6 (1), 61–67 (2016).
Musella, M. et al. Efficacy of Bariatric Surgery in Type 2 Diabetes Mellitus Remission: the Role of Mini Gastric Bypass/One Anastomosis Gastric Bypass and Sleeve Gastrectomy at 1 Year of Follow-up. A European survey. Obes Surg. ;26(5):933 – 40. (2016). https://doi.org/10.1007/s11695-015-1865-6. PMID: 26341086.
Mahdy, T., Gado, W., Alwahidi, A., Schou, C. & Emile, S. H. Sleeve gastrectomy, One-Anastomosis gastric bypass (OAGB), and single anastomosis sleeve ileal (SASI) bypass in treatment of morbid obesity: a retrospective cohort study. Obes. Surg. 31 (4), 1579–1589. https://doi.org/10.1007/s11695-020-05159-y (2021). Epub 2021 Jan 6. PMID: 33409970.
Magouliotis, D. E. et al. One-Anastomosis Gastric Bypass Versus Sleeve Gastrectomy for Morbid Obesity: a Systematic Review and Meta-analysis. Obes Surg. ;27(9):2479–2487. (2017). https://doi.org/10.1007/s11695-017-2807-2. PMID: 28681256.
Jia, D., Tan, H., Faramand, A. & Fang, F. One Anastomosis Gastric Bypass Versus Roux-en-Y Gastric Bypass for Obesity: a Systematic Review and Meta-Analysis of Randomized Clinical Trials. Obes Surg. ;30(4):1211–1218. (2020). https://doi.org/10.1007/s11695-019-04288-3. PMID: 31749109.
Kessler, Y. et al. Nutritional status following one anastomosis gastric bypass. Clin. Nutr. 39 (2), 599–605 (2020).
Klop, B., Elte, J. W. & Cabezas, M. C. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 5 (4), 1218–1240. https://doi.org/10.3390/nu5041218 (2013). PMID: 23584084; PMCID: PMC3705344.
Heffron, S. P. et al. Changes in lipid profile of obese patients following contemporary bariatric surgery: A Meta-Analysis. Am. J. Med. 129 (9), 952–959 (2016).
Bettini, S. et al. Improvement of lipid profile after One-Anastomosis gastric bypass compared to sleeve gastrectomy. Nutrients 13 (8), 2770. https://doi.org/10.3390/nu13082770 (2021). PMID: 34444930; PMCID: PMC8401377.
Magouliotis, D. E., Tasiopoulou, V. S. & Tzovaras, G. One anastomosis gastric bypass versus Roux-en-Y gastric bypass for morbid obesity: a meta-analysis. Clin. Obes. 8 (3), 159–169. https://doi.org/10.1111/cob.12246 (2018). Epub 2018 Mar 24. PMID: 29573175.
Jurowich, C. et al. Improvement of type 2 diabetes mellitus (T2DM) after bariatric surgery–who fails in the early postoperative course? Obes Surg. ;22(10):1521-6. (2012). https://doi.org/10.1007/s11695-012-0676-2. PMID: 22588846.
Malhotra, A. & White, D. P. Obstructive sleep apnoea. Lancet. ;360(9328):237 – 45. (2002). https://doi.org/10.1016/S0140-6736(02)09464-3. PMID: 12133673.
Stansbury, R. C. & Strollo, P. J. Clinical manifestations of sleep apnea. J. Thorac. Dis. 7 (9), E298–310. https://doi.org/10.3978/j.issn.2072-1439.2015.09.13 (2015). PMID: 26543619; PMCID: PMC4598518.
Sarkhosh, K. et al. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg. ;23(3):414 – 23. (2013). https://doi.org/10.1007/s11695-012-0862-2. PMID: 23299507.
Fredheim, J. M. et al. Obstructive sleep apnea after weight loss: a clinical trial comparing gastric bypass and intensive lifestyle intervention. J. Clin. Sleep. Med. 9 (5), 427–432. https://doi.org/10.5664/jcsm.2656 (2013). PMID: 23674932; PMCID: PMC3629315.
Dallal, R. M., Leighton, J. & Trang, A. Analysis of leukopenia and anemia after gastric bypass surgery. Surg Obes relat dis. ;8(2):164–168. https://doi.org/10.1016/j.soard.2011.02.006. (2012). Mar-Apr Epub 2011 Feb 24. PMID: 21459685.
Brooks, G. C., Blaha, M. J. & Blumenthal, R. S. Relation of C-reactive protein to abdominal adiposity. Am. J. Cardiol. 106 (1), 56–61. https://doi.org/10.1016/j.amjcard.2010.02.017 (2010). Epub 2010 May 13. PMID: 20609648.
Wolf, E. et al. Preoperative micronutrient status in morbidly obese patients before undergoing bariatric surgery: results of a cross-sectional study. Surg. Obes. Relat. Dis. 11 (5), 1157–1156 (2015 Sep-Oct).
Guan, B., Yang, J., Chen, Y., Yang, W. & Wang, C. Nutritional Deficiencies in Chinese Patients Undergoing Gastric Bypass and Sleeve Gastrectomy: Prevalence and Predictors. Obes Surg. ;28(9):2727–2736. (2018). https://doi.org/10.1007/s11695-018-3225-9. PMID: 29754386.
Okroj, F. L. T. D. et al. Georgios K dimitriadis. Medication and supplement Pharmacokinetic changes following bariatric surgery: A systematic review and meta-analysis. Obes. Rev. 25 (8), e13759. https://doi.org/10.1111/obr.13759 (2024).
Zamaninour, N., Pazouki, A., Kermansaravi, M., Seifollahi, A. & Kabir, A. Changes in body composition and biochemical parameters following laparoscopic one anastomosis gastric bypass: 1-Year Follow-Up. Obes. Surg. 31 (1), 232–238 (2021).
Jedamzik, J. et al. Impact of limb length on nutritional status in one-anastomosis gastric bypass: 3-year results. Surg. Obes. Relat. Dis. 16 (4), 476–484 (2020).
Ahuja, A. et al. MGB-OAGB: Effect of Biliopancreatic Limb Length on Nutritional Deficiency, Weight Loss, and Comorbidity Resolution. Obes Surg. ;28(11):3439–3445. (2018). https://doi.org/10.1007/s11695-018-3405-7. PMID: 30032419.
Schielein, M. C. et al. Stigmatization caused by hair loss - a systematic literature review. J Dtsch Dermatol Ges. ;18(12):1357–1368. (2020). https://doi.org/10.1111/ddg.14234. Epub 2020 Oct 4. PMID: 33015951.
Ledoux, S. et al. What Are the Micronutrient Deficiencies Responsible for the Most Common Nutritional Symptoms After Bariatric Surgery? Obes Surg. ;30(5):1891–1897. (2020). https://doi.org/10.1007/s11695-020-04412-8. PMID: 31960214.
Robert, M. et al. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): a multicentre, randomised, open-label, non-inferiority trial. Lancet. ;393(10178):1299–1309. (2019). https://doi.org/10.1016/S0140-6736(19)30475-1. Epub 2019 Mar 6. Erratum in: Lancet. 2019;393(10178):1298. PMID: 30851879.
Kehagias Dimitrios, C., Lampropoulos, S. S., Vamvakas, E., Kehagia, N. & Georgopoulos, I. K. Post-Bariatric hypoglycemia in individuals with obesity and type 2 diabetes after laparoscopic Roux-en-Y gastric bypass: A prospective cohort study. Biomedicines 12,8 1671. 26 Jul. https://doi.org/10.3390/biomedicines12081671 (2024).
David, C. et al. The efficacy of GLP-1RAs for the management of postprandial hypoglycemia following bariatric surgery: a systematic review. Obes. (Silver Spring). 31 (1), 20–30. https://doi.org/10.1002/oby.23600 (2023).
Jonathan Hazlehurst, B. et al. Society for endocrinology guidelines for the diagnosis and management of post-bariatric hypoglycaemia. Endocr. Connect. 13 (5), e230285. https://doi.org/10.1530/EC-23-0285 (2024).
Acknowledgements
We would like to acknowledge the support received by Miss Ioanna Papandreou in data collection for this project.
Funding
This study has received no funding.
Author information
Authors and Affiliations
Contributions
WAM collected data, analysed samples, interpreted results, and wrote the manuscript. RR analysed samples, interpreted results and reviewed the final version of the paper. YHC reviewed the final version of the manuscript. HSJC reviewed the final version of the manuscript. TM analysed samples, interpreted results and reviewed the final version of the manuscript. NH analysed sampled and reviewed the final version of the manuscript. FLT reviewed the final version of the manuscript. PR reviewed the final version of the manuscript. SG treated patients and reviewed the final version of the manuscript. AL treated patients and reviewed the final version of the manuscripts. RA performed statistical analyses, interpreted results and reviewed the final version of the manuscript. NWSC reviewed the final version of the manuscripts. RPV supervised the project, interpreted results and reviewed the final version of the manuscript. SHE operated on the patients and reviewed the final version of the manuscript. GKD treated patients, conceived the idea of this work and designed the study, supervised the project, interpreted results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Al-Hasani, W., Ranasinghe, R., Chin, Y.H. et al. One anastomosis gastric bypass produces considerable weight reduction and resolution of medical complications with an acceptable rate of nutritional deficiencies. Sci Rep 15, 30106 (2025). https://doi.org/10.1038/s41598-025-12997-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12997-2