Abstract
The coronavirus disease 2019 (COVID-19) pandemic has led to the development of numerous prognostic models for patient assessment. However, the potential utility of the predisposition, insult/infection, response, organ dysfunction (PIRO) score in evaluating COVID-19 severity and outcomes remains unexplored, presenting a gap in current research. A retrospective analysis was conducted on a cohort of 374 individuals diagnosed with COVID-19 who were admitted to the emergency department of Beijing Youan Hospital. Demographic data, treatment regimens, and seven prognostic scoring systems, including PIRO, were evaluated. To evaluate the models’ prognostic accuracy for 28-day mortality, area under the receiver operating characteristic (AUROC) analysis was employed. Comparative performance between scoring systems was quantified using the DeLong method for paired ROC curves. Of the 374 patients meeting inclusion criteria, 120 (32.1%) died within 28 day of hospitalization. Significant disparities were observed between survivors and non-survivors regarding age, laboratory parameters, and clinical scores. Analysis of patient distribution and mortality rates across different score ranges revealed a positive correlation between score magnitude and 28-day mortality. The PIRO score demonstrated superior prognostic capability, yielding an AUC of 0.898 (95% CI 0.866–0.929). The quick sequential organ failure assessment (qSOFA) score followed closely (AUC 0.882, 95% CI 0.849–0.914). Both critical illness risk score (COVID-GRAM) and national early warning score 2 (NEWS2) exhibited AUCs exceeding 0.85 (COVID-GRAM 0.854, 95% CI 0.812–0.895; NEWS2: 0.851, 95% CI 0.813–0.889). DeLong test analysis revealed statistically significant differences in AUC between PIRO and confusion, urea, respiration, systolic pressure, age ≥ 65 (CURB-65), pneumonia severity index (PSI), COVID-GRAM, rapid acute physiology score (RAPS), and NEWS2 (all p < 0.05). Analysis revealed the PIRO scoring system as a robust predictor of 28-day mortality among COVID-19 cases presenting to the emergency setting, offering potential refinement of risk stratification and clinical management strategies.
Similar content being viewed by others
Introduction
The novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first identified in late 2019, has precipitated an unprecedented global pandemic, imposing significant strain on healthcare infrastructure and resources across nations1,2,3. Although a substantial proportion of SARS-CoV-2 infections manifest as clinically mild or moderate, with patients experiencing favorable prognoses, a subset of patients, particularly those of advanced age or with comorbidities, may experience severe disease progression, potentially leading to critical illness or mortality4. Timely assessment of disease severity and optimal resource allocation are crucial for improving patient outcomes5,6,7,8,9.
Clinical prediction scores have been proven effective in helping doctors predict patient outcomes10. Clinical prediction scores incorporating diverse parameters such as demographics, clinical manifestations, and laboratory indicators have been developed to enhance risk stratification in COVID-1911. These include established pneumonia-related indices like the PSI and CURB-65, as well as the MuLBSTA score6,12,13,14,15,16,17,18,19,20. Sepsis-related metrics, such as the NEWS and NEWS221,22,23, SOFA and qSOFA24,25, have also been applied. Furthermore, novel COVID-19-specific models have emerged, including the COVID-GRAM26,27, the 4 C Mortality Score28,29, the CALL score30,31, and the quick COVID-19 Severity Index (qCSI)17,18.
Emergency departments play a pivotal role in patient triage and initial management during epidemics, various emergency department-based clinical prediction scores are used to predict the prognosis of critically ill patients32. Various scoring systems are employed to assess COVID-19 severity and determine appropriate treatment levels in this setting22,33. These encompass the aforementioned scores as well as emergency department-specific tools like early warning scores (EWSs), rapid emergency medicine score (REMs), and RAPS22,34,35,36. However, the utility of the PIRO score in predicting COVID-19 outcomes remains largely unexplored37.
This study aims to address this knowledge gap by evaluating and comparing the prognostic value of the PIRO score against six widely used clinical prediction tools (qSOFA, CURB-65, PSI, COVID-GRAM, RAPS, and NEWS2) in COVID-19 patients. Additionally, we will analyze patient distribution across different score ranges, examine 28-day mortality rates, and identify demographic and clinical characteristics distinguishing survivors from non-survivors.
Methodology
Study population and research design
Our investigation comprised a retrospective analysis of 506 individuals diagnosed with COVID-19 who sought medical attention at the Emergency Department of Beijing Youan Hospital, from May 1, 2022, to May 31, 2023. Diagnostic classification was performed in accordance with the guidelines delineated in the ninth iteration of the “COVID-19 Diagnosis and Management Protocol” promulgated by the National Health Commission of the People’s Republic of China4. SARS-CoV-2 infection was verified using polymerase chain reaction (PCR) assays. During our study period, the included patients were all infected with the Omicron variant. Patient severity classification into mild/moderate or severe/critical groups was conducted following the COVID-19 clinical management recommendations set forth by the National Institutes of Health (NIH)38. Patients were treated according to the guidelines, with a fixed team of doctors and nursing staff under the guidance of senior physicians, ensuring that patients received appropriate treatment.
This research endeavored to assess the prognostic efficacy of the PIRO Score upon hospital admission for COVID-19 patients. The primary endpoint was defined as mortality within a 28-day period post-admission. The study protocol received approval from the Ethics Committee of Beijing Youan Hospital (protocol identifier: LL-2023-006-K). Research procedures adhered to the ethical guidelines stipulated in the Declaration of Helsinki. This study is based on a research cohort of COVID-19 patients from the entire hospital. All subjects provided written informed consent prior to enrollment. To ensure participant privacy, data were de-identified and stored securely in accordance with institutional protocols.
Inclusion and exclusion criteria
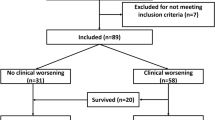
This investigation incorporated subjects who fulfilled the diagnostic criteria delineated in the ninth iteration of the “COVID-19 Diagnosis and Management Protocol,” as promulgated by China’s National Health Commission4. Enrollment was contingent upon obtaining informed consent and voluntary participation. Exclusion parameters encompassed refusal to participate, individuals below 18 years of age, gravid females, mortality within 48 h post-admission, and cases with substantial data deficiencies or inaccessible records. Missing important data refers to the absence of data required for calculating the scores or the absence of information about the primary outcomes. The aforementioned criteria were meticulously applied to ensure a representative cohort for analysis, while adhering to ethical research practices and maintaining data integrity. This rigorous selection process aimed to minimize confounding factors and enhance the validity of subsequent findings.
Data collection
Clinical data were extracted from electronic health records, encompassing demographic characteristics, medical history, baseline parameters, vital signs, arterial blood gas analysis results, laboratory findings, and patient outcomes. Demographic variables included sex and age. The medical history focused on comorbidities such as hypertension, diabetes mellitus, coronary artery disease, cerebrovascular disorders, chronic obstructive pulmonary disease (COPD), hepatic conditions, and malignancies.
Vital sign assessment comprised body temperature (°C), respiratory rate (breaths/min), heart rate (beats/min), and systolic blood pressure (mmHg). Arterial blood gas analysis evaluated pH, partial pressure of carbon dioxide (PaCO2), partial pressure of oxygen (PaO2), peripheral oxygen saturation (SpO2), and the oxygenation index (PaO2/fraction of inspired oxygen [FiO2] ratio).
The laboratory panel included markers of infection (procalcitonin [PCT, ng/mL] and C-reactive protein [CRP, mg/L]), hematological parameters (hemoglobin, total leukocyte count, neutrophil and lymphocyte counts), coagulation indices (international normalized ratio [INR] and D-dimer [mg/L]), and biochemical indicators (alanine aminotransferase [ALT, U/L], aspartate aminotransferase [AST, U/L], albumin [g/L], total and direct bilirubin [µmol/L]).
Composite inflammatory markers were derived from these laboratory parameters, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-CRP ratio (LCR), CRP-to-albumin ratio (CAR), and systemic inflammation index (SII). The SII, calculated as (neutrophil count × platelet count)/lymphocyte count, along with other derived indices, provides critical insights into the severity and nature of the inflammatory response associated with the studied condition, as detailed in Table 1.
Definition of clinical scoring systems
The PIRO score incorporates factors such as comorbidities, advanced age, Infection/Insult, Response, Respiration and lactate (Additional file: Table S1)39.
Organ dysfunction assessment tools, including SOFA, qSOFA, eSOFA, and sSOFA, were utilized to gauge the extent of organ impairment in critically ill patients. These instruments have demonstrated efficacy in predicting in-hospital mortality among adult patients with suspected infection in intensive care unit (ICU) settings (Additional file: Table S2)40,41.
The CURB-65 score, a validated pneumonia severity index, evaluates five parameters: confusion, blood urea nitrogen, respiratory rate, blood pressure, and age ≥ 65 years (Additional file: Table S3)42,43.
The PSI, comprising 20 independent risk factors, stratifies patients into five risk classes based on the cumulative score (Additional file: Table S4)44,45.
The COVID-GRAM model, developed by Chinese researchers in 2020, integrates 10 independent predictors to estimate the probability of progression to severe illness in COVID-19 patients (Additional file: Table S5)26.
The RAPS assesses pulse rate, mean arterial pressure, respiratory rate, and GCS score (Additional file: Table S6)46,47.
Lastly, NEWS2 evaluates respiratory rate, oxygen saturation, supplemental oxygen requirement, heart rate, level of consciousness, and temperature to detect early signs of clinical deterioration (Additional file: Table S7)48,49.
Statistical analysis
Normality of continuous variables was assessed via the Shapiro–Wilk method. Data following normal distribution were summarized as mean ± SD, with between-group differences evaluated by independent t-tests. Non-parametric variables were described using median and IQR, and analyzed with the Mann–Whitney U test. For categorical variables, frequencies and percentages were calculated, and comparisons were conducted using either Pearson’s chi-square or Fisher’s exact test, as appropriate. Multiple group analyses employed the Kruskal–Wallis test. The discriminative ability of prognostic models for 28-day mortality in COVID-19 patients was examined through ROC curve analysis. The selection of the optimal threshold, or Cutoff Values, is based on the Youden Index, where Youden Index = Sensitivity + Specificity − 1. A higher Youden Index indicates better predictive performance, and in this study, the selection of the best threshold uses the Cutoff Values with the highest Youden Index. Clinical utility assessment of these models utilized decision curve analysis (DCA). k-Fold Cross-Validation is used to assess the stability of scoring for patient prognosis prediction. The dataset is randomly divided into 10 equally sized subsets (referred to as ‘folds’), with one fold selected as the validation set in each iteration, and the remaining 10 − 1 folds used as the training set. This training and validation process is repeated 10 times, and the average of the 10 results is taken as a robust estimate of model performance. Calibration plots and Brier scores were further used to evaluate the calibration and accuracy of different scoring systems in predicting the 28-day mortality risk of COVID-19 patients. Calibration plots are utilized to assess the consistency between the model’s predicted probabilities and the actual observed outcomes. For instance, whether the observed mortality rate approximates 30% for patients with a predicted 30% probability of mortality. The Brier score measures the accuracy of predicted probabilities, with lower values indicating better accuracy. A score of 0 represents perfect prediction, while 0.25 is akin to random guessing for binary classification tasks such as life or death. Comparisons of AUC values across different scoring systems were performed using DeLong’s method. A p-value < 0.05 was considered statistically significant. Statistical analyses were conducted using R (version 4.2.1; R Foundation for Statistical Computing) software and SPSS (version 22.0; IBM Corp.). Visualization of data was accomplished using GraphPad Prism 9 (GraphPad Software Inc.).
Results
Patient selection and baseline characteristics
Of the 506 patients initially admitted to the emergency department, 374 met the inclusion criteria for analysis after excluding 132 individuals (Fig. 1). The cohort comprised 90 (24.1%) mild cases, 117 (31.3%) moderate cases, and 167 (44.6%) severe or critical cases. The 28-day mortality rate was 32.1% (120 patients).
Baseline clinical parameters are summarized in Table 1. The study population was predominantly male (65%, 244/374) with a median age of 72 years. Hypertension was the most prevalent comorbidity (49%, 183/374), followed by diabetes (29%, 110/374) and coronary heart disease (20%, 75/374).
Comparative analysis of survivors and non-survivors
Significant differences were observed between patients who survived and those who died within 28 days (Table 1). Non-survivors were characterized by a higher proportion of males, advanced age, elevated body temperature, increased respiratory and heart rates, and lower oxygenation index at admission (all p < 0.05).
All seven clinical prediction scores (PIRO, qSOFA, CURB-65, PSI, COVID-GRAM, RAPS, NEWS2) demonstrated statistically significant differences between the two groups (all p < 0.05), consistently indicating greater disease severity in non-survivors.
Laboratory findings revealed that non-survivors exhibited significantly higher levels of inflammatory markers (PCT, CRP), hematological parameters (WBC, neutrophil count, lymphocyte count), coagulation indices (INR, D-dimer), and liver function indicators (ALT, AST, DBIL). Conversely, they showed lower levels of albumin and estimated glomerular filtration rate (eGFR) (all p < 0.05). Furthermore, all five blood count-derived inflammatory markers differed significantly between the groups (all p < 0.05).
Analysis of patient distribution based on different clinical scores and the proportion of deaths within 28 days
Figure 2 displays the distribution of patients based on the PIRO score. We can observe that the highest number of patients (83/374, 22.2%) falls into the 4–6 score group, followed by patients with scores of 7–9 (74/374, 19.8%). There is a decreasing trend in the number of patients as the scores increase (Fig. 2A). As depicted in Fig. 2B, it is evident that the proportion of patients who died within 28 days increases continuously with higher PIRO scores. In the 1–3 score group, no patients died within 28 days, while in the group with scores greater than 19, all 16 patients died.
Distribution of 28-day survival and mortality in patients based on the PIRO score. Frequency distribution plot (A), where each group represents a score of 3 and is stacked to visualize the distribution of patients with different scores. Stacked bar chart (B), showing the proportions of 28-day survivors and deceased individuals in different PIRO score groups. PIRO, predisposition, insult/infection, response, organ dysfunction.
We have also presented the distribution of patients based on six other clinical scores, namely qSOFA (A), CURB-65 (B), PSI (C), COVID-GRAM (D), RAPS (E), and NEWS2 (F), in Frequency distribution plots (Fig. 3). Additionally, in Stacked bar charts (Fig. 4A–F), we have depicted the proportions of 28-day survivors and deceased individuals in different score groups based on these six clinical prediction scores.
Frequency distribution plots of 28-day survival and mortality in patients based on six clinical prediction scores. According to qSOFA (A), CURB-65 (B), PSI (C), COVID-GRAM (D), RAPS (E), and NEWS2 (F). The plots illustrate the distribution of patients with different scores. For PSI (C), each group represents a score of 30 and is stacked sequentially. For COVID-GRAM, the first group represents scores from 41 to 80, with each subsequent group incremented by 40 and stacked accordingly. qSOFA, quick sequential organ failure assessment; CURB-65: confusion, urea, respiration, systolic pressure, age ≥ 65; PSI, pneumonia severity index; RAPS: rapid acute physiology score; NEWS2, national early warning score 2.
Stacked bar charts depicting the proportions of 28-day survivors and deceased individuals in different score groups based on six clinical prediction scores. According to qSOFA (A), CURB-65 (B), PSI (C), COVID-GRAM (D), RAPS (E), and NEWS2 (F). The charts provide insights into the distribution of survivors and deceased individuals in different score categories. qSOFA, quick sequential organ failure assessment; CURB-65, confusion, urea, respiration, systolic pressure, age ≥ 65; PSI, pneumonia severity index; RAPS, rapid acute physiology score; NEWS2, national early warning score 2.
The PIRO score has a good predictive value for 28-day mortality in COVID-19 patients
ROC curve analysis was performed to evaluate the predictive performance of seven clinical scoring systems for 28-day mortality in COVID-19 patients (Fig. 5A). The PIRO score demonstrated superior prognostic capability with the highest AUC of 0.898 (95% CI 0.866–0.929). The qSOFA score showed comparable performance with an AUC of 0.882 (95% CI 0.849–0.914). Both COVID-GRAM and NEWS2 scores exhibited robust predictive value, with AUCs of 0.854 (95% CI 0.812–0.895) and 0.851 (95% CI 0.813–0.889), respectively.
Prediction of 28-day mortality in COVID-19 patients using seven clinical prediction scores. Receiver Operating Characteristic (ROC) curves (A). The area under the curve (AUC) for qSOFA was 0.882 (95% confidence interval [CI] 0.849–0.914); CURB-65, AUC was 0.843 (95% CI 0.802–0.884); PIRO, AUC was 0.898 (95% CI 0.866–0.929); COVID-GREM, 0.854 (95% CI 0.812–0.895); PSI, AUC was 0.804 (95% CI 0.757–0.854); RAPS, AUC was 0.835 (95% CI 0.790–0.879); NEWS2, AUC was 0.851 (95% CI 0.813–0.889. Decision curve analysis (B). A box plot of the AUC distribution for 10-fold cross-validation of different scoring systems (C). A bar chart of Brier scores for predicting mortality using different scoring systems (D). PIRO, predisposition, insult/infection, response, organ dysfunction; qSOFA, quick sequential organ failure assessment; CURB-65, confusion, urea, respiration, systolic pressure, age ≥ 65; PSI, pneumonia severity index; RAPS, rapid acute physiology score; NEWS2, national early warning score 2.
To evaluate the practical clinical value of the prognostic models, DCA was implemented (Fig. 5B). The results corroborated the superior performance of the PIRO score, followed closely by the qSOFA score. The results of 10-fold cross-validation further demonstrate that the PIRO score has the best predictive performance for patient 28-day mortality, with a median of 0.914 and a range of 0.827–0.955 across 10 tests (Additional file: Table S8 and Fig. 5C). Calibration plots reveal that, except for the RAPS score, the consistency between predicted probabilities and actual observed probabilities for all other scores showed no statistically significant differences (p > 0.05) (Additional file: Fig. S1). Brier score results indicate that the Brier scores for all scoring systems are below 0.2, suggesting good predictive performance for patient prognosis across all scores (Fig. 5D).
Pairwise comparisons of AUCs using DeLong’s test (Table 2) revealed statistically significant differences between PIRO and several other scoring systems, including CURB-65, PSI, COVID-GRAM, RAPS, and NEWS2 (all p < 0.05). Table 3 presents the predictive parameters for 28-day mortality, including optimal cutoff values, positive and negative predictive values, and Youden indices for each scoring system.
Discussion
This study evaluated the predictive efficacy of the PIRO score and six established clinical prediction systems for 28-day mortality in COVID-19 patients. Our findings indicate that while all examined scoring systems effectively predict short-term mortality in SARS-CoV-2 infections, the PIRO score demonstrated superior predictive performance. To our knowledge, this is the first comparative analysis of PIRO against qSOFA, CURB-65, PSI, COVID-GRAM, RAPS, and NEWS2 in prognosticating outcomes for emergency department patients with SARS-CoV-2 infection.
Our cohort comprised 374 COVID-19 patients admitted to the emergency department, with a 28-day mortality rate of 32.1% (120 patients). Significant disparities in age, laboratory parameters, and clinical prediction scores were observed between survivors and non-survivors. Notably, escalating clinical scores correlated with increased 28-day mortality rates. ROC curve analysis revealed the PIRO score’s superior prognostic capability, followed by qSOFA, COVID-GRAM, and NEWS2. This hierarchy was corroborated by DCA. Pairwise comparisons of AUC using DeLong’s test demonstrated statistically significant differences between PIRO and CURB-65, PSI, COVID-GRAM, RAPS, and NEWS2.
The PIRO score has previously demonstrated superior predictive capability for 28-day mortality in community-acquired pneumonia patients in intensive care settings, outperforming the Acute Physiology and Chronic Health Evaluation II (APACHE II) score50,51,52. Its efficacy extends to mortality prediction in sepsis patients, incorporating factors such as comorbidities, sepsis source, and physiological status. Notably, PIRO has shown better performance than the SOFA score in severe sepsis and septic shock39,53. Sepsis is a clinical syndrome, a dysregulated response of the body to infection54. As our understanding of viruses deepens, we are becoming more aware of sepsis caused by viruses55,56. COVID-19, as a viral disease, can progress to sepsis in severe cases, as reported in related articles57. Given the aforementioned description, the PIRO score demonstrates good applicability in sepsis, thus the PIRO score may have a positive effect on predicting the prognosis of COVID-19 patients. Our study corroborates and extends these findings to COVID-19 patients. The PIRO score exhibited the highest predictive performance among the evaluated scoring systems, with an optimal cut-off value of 10.5. It demonstrated a sensitivity of 0.78, specificity of 0.86, and an AUC of 0.898 (95% CI 0.866–0.929). Importantly, PIRO outperformed other widely used clinical prediction scores including CURB-65, PSI, COVID-GRAM, RAPS, and NEWS2. To date, literature on PIRO’s application in COVID-19 prognosis is limited. However, our findings align with the single study we identified on this topic. Kumar et al., in their prospective observational study of 240 intensive care unit patients, reported that the COVID-PIRO score achieved a sensitivity of 95%, specificity of 84%, and an AUC of 0.946 (95% CI 0.918–0.973) for mortality prediction37. This study included patients admitted to a tertiary ICU in central rural India, while our study included patients from a large tertiary hospital emergency department. In our study, a portion of the patients were also admitted to the emergency ICU. Currently, there is limited research on the application of the PIRO score in COVID-19, especially in different settings, which requires further investigation for clarity. Regarding differences in patient populations, disease severity, and various clinical scenarios, the selection of scores can vary. Choosing the appropriate score based on the specific circumstances can better benefit patients. In our study, we applied the PIRO score in the emergency department to predict the prognosis of COVID-19 patients and found it to have good predictive value. This expands the clinical applicability of the PIRO score and provides guidance for emergency department physicians in utilizing the PIRO score.
SOFA and qSOFA scores have demonstrated significant utility in screening for sepsis and critically ill patients24,58,59,60. Recent studies have extended their application to predicting outcomes in COVID-19 patients25,33,61,62. One notable study reported an AUC of 0.742 (95% CI 0.657–0.816) for qSOFA and 0.890 (95% CI 0.826–0.955) for SOFA in predicting COVID-19 mortality25. In resource-constrained settings, current UK guidelines recommend using qSOFA in conjunction with NEWS2 for risk stratification and hospital admission screening of COVID-19 patients. Our findings corroborate the prognostic value of qSOFA in COVID-19, revealing an AUC of 0.882 (95% CI 0.849–0.914). This performance exceeds that reported in several previous studies36,48,62,63. The enhanced predictive accuracy observed in our study may be attributed to the characteristics of our patient cohort, which included a higher proportion of critically ill patients. This likely contributed to the high specificity (0.97) of qSOFA in our analysis. However, further research is needed to investigate this in more detail. In urgent scenarios, using qSOFA is an option. However, after patient admission, it is advisable to utilize the PIRO score when feasible, as it incorporates more parameters, enabling a more effective evaluation of the patient’s condition. The PIRO score is also a commonly used score in the emergency department, and emergency physicians are more familiar with its application.
The NEWS2 has been extensively evaluated for its prognostic utility in COVID-19. Literature reports varying degrees of predictive accuracy, with one study demonstrating an impressive AUC of 0.963 (95% CI 0.940–0.979) for disease severity prediction47. Another investigation reported an AUC of 0.882 (95% CI 0.868–0.895) for the composite outcome of mortality or intensive care unit admission using NEWS or NEWS221. Conversely, some studies have reported more modest performance, with AUCs of 0.705 (95% CI 0.638–0.773) for severity prediction and 0.696 (95% CI 0.608–0.784) for mortality23. Our analysis yielded an AUC of 0.851 (95% CI 0.813–0.889) for mortality prediction in hospitalized COVID-19 patients, aligning with the more favorable end of the reported range.
The CURB-65 and PSI, traditionally employed for pneumonia severity assessment, have been naturally extended to COVID-19 prognostication12,15,48,64. Previous research has reported varied performance for these scores. For CURB-65, AUCs ranging from 0.81 (95% CI 0.71–0.91)16 to 0.907 (95% CI 0.866–0.948)23 have been observed for 28-day mortality prediction. A comparative study of SARS-CoV-2 and non-SARS-CoV-2 community-acquired pneumonia (CAP) found both scoring systems to be effective, with PSI slightly outperforming CURB-65. For SARS-CoV-2 CAP, PSI achieved an AUC of 0.82 (95% CI 0.78–0.86) compared to CURB-65’s 0.79 (95% CI 0.75–0.84)42. In our study, CURB-65 had an AUC of 0.843 (0.802–0.884) for predicting 28-day mortality in COVID-19 patients, higher than PSI’s AUC of 0.804 (0.757–0.852).
In response to the COVID-19 pandemic, researchers in mainland China developed the COVID-GRAM scoring system based on data from 1590 patients across 575 hospitals in 31 provincial-level administrative regions. This system, designed to predict the development of critical illness, identified 10 independent risk factors. External validation demonstrated robust performance, with AUC values of 0.88 (95% CI 0.84–0.93) overall, 0.87 (95% CI 0.83–0.91) within Hubei province, and 0.82 (95% CI 0.73–0.90) in other regions, indicating some geographic variability26. Our investigation yielded an AUC of 0.854 (95% CI 0.812–0.895) for COVID-GRAM in predicting 28-day mortality, aligning with findings from several domestic and international studies65,66. This consistency across diverse populations underscores the potential utility of COVID-GRAM as a prognostic tool in COVID-19 management.
Our investigation is subject to several inherent limitations that warrant careful interpretation of the results. The retrospective, single-institution design introduces potential biases and constraints on external validity. This approach, while valuable for initial insights, may not fully capture the diversity of COVID-19 manifestations and outcomes across varied healthcare settings, potentially limiting the broader applicability of our findings. Moreover, the relatively modest cohort size precluded comprehensive external validation procedures. This limitation impedes our ability to assess the robustness and reproducibility of our observations across diverse patient populations. To address these methodological challenges and enhance the validity of our findings, we advocate for the implementation of large-scale, multicenter prospective studies. Such investigations would not only serve to corroborate our results but also significantly augment their generalizability across a broader spectrum of clinical environments and patient demographics. Lastly, our study lacked information on the vaccination status of patients, which could potentially introduce confounding biases.
Conclusion
The PIRO scoring system has demonstrated considerable efficacy in prognosticating 28-day mortality among COVID-19 patients presenting to emergency departments. This model offers valuable insights for clinical risk assessment. To further establish its validity and generalizability, we anticipate future research involving comprehensive, multi-institutional investigations to rigorously evaluate this prognostic tool across diverse healthcare settings and patient populations.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PIRO:
-
Predisposition, insult/infection, response, organ dysfunction
- COVID-19:
-
Coronavirus disease 2019
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- qSOFA:
-
Quick sequential organ failure assessment
- COVID-GRAM:
-
Critical illness risk score
- NEWS2:
-
National early warning score 2
- CURB-65:
-
Confusion, urea, respiration, systolic pressure, age ≥ 65
- PSI:
-
Pneumonia severity index
- RAPS:
-
Rapid acute physiology score
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- EWSs:
-
Early warning scores
- REMs:
-
Rapid emergency medicine score
- PCR:
-
Polymerase chain reaction
- COPD:
-
Chronic obstructive pulmonary disease
- RR:
-
Respiratory rate
- HR:
-
Heart rate
- SBP:
-
Systolic blood pressure
- PaCO2 :
-
Arterial carbon dioxide tension
- PaO2 :
-
Oxygen tension
- SpO2 :
-
Peripheral oxygen saturation
- FiO2 :
-
Fraction of inspired oxygen
- PCT:
-
Procalcitonin
- CRP:
-
C-reactive protein
- HGB:
-
Hemoglobin
- WBC:
-
White blood cell
- INR:
-
International normalized ratio
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- TBIL:
-
Total bilirubin
- DBIL:
-
Direct bilirubin
- CAR:
-
C-reactive protein-to-albumin ratio
- BCDIMs:
-
Blood count-derived inflammatory markers
- NLR:
-
Neutrophil-to-lymphocyte ratio
- PLR:
-
Platelet-to-lymphocyte ratio
- LCR:
-
Lymphocyte-to-c-reactive protein ratio
- SII:
-
Systemic inflammation index
- DCA:
-
Decision curve analysis
- ICU:
-
Intensive care unit
- APACHE II:
-
Acute physiology and chronic health evaluation II
- CAP:
-
Community-acquired pneumonia
- SII:
-
(neutrophil count × platelet count)/lymphocyte count.
References
Verelst, F., Kuylen, E. & Beutels, P. Indications for healthcare surge capacity in European countries facing an exponential increase in coronavirus disease (COVID-19) cases, March 2020. Euro. Surveill. 25(13). (2020).
Szliszka, E. et al. Ethanolic extract of propolis (EEP) enhances the apoptosis- inducing potential of TRAIL in cancer cells. Molecules. 14 (2), 738–754 (2009).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet. 395 (10223), 497–506 (2020).
Diagnosis and Treatment protocol for COVID-19 patients (tentative 9 version). https://www.gov.cn/zhengce/zhengceku/2022-03/15/content_5679257.htm (accessed 20 Mar 2022).
Tjendra, Y. et al. Predicting disease severity and outcome in COVID-19 patients: A review of multiple Biomarkers. Arch. Pathol. Lab. Med. 144 (12), 1465–1474 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in wuhan, china: a retrospective cohort study. Lancet. 395 (10229), 1054–1062 (2020).
Duan, J. et al. Predicting SARS-CoV-2 infection among Hemodialysis patients using multimodal data. Front. Nephrol. 3, 1179342 (2023).
Wu, Z. et al. Presepsin as a prognostic biomarker in COVID-19 patients: combining clinical scoring systems and laboratory inflammatory markers for outcome prediction. Virol. J. 21 (1), 96 (2024).
Geng, N. et al. sTREM-1 as a predictive biomarker for disease severity and prognosis in COVID-19 Patients. J. Inflamm. Res. 17, 3879–3891 (2024).
Rahmatinejad, Z. et al. Comparing in-hospital mortality prediction by senior emergency resident’s judgment and prognostic models in the emergency department. Biomed. Res. Int. 2023, 6042762. (2023).
Cai, Y. Q. et al. Advances in clinical prediction scores for prognosis of coronavirus disease-2019. Zhonghua Jie He He Hu Xi Za Zhi. 45 (7), 706–711 (2022).
Han, R. et al. Prognostic value of immune-inflammatory index in PSI IV-V patients with COVID-19. Biomed. Res. Int. 2021, 9987931 (2021).
Wu, C. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern. Med. 180 (7), 934–943 (2020).
Natanov, D. et al. Predicting COVID-19 prognosis in hospitalized patients based on early status. mBio 14 (5), e0150823 (2023).
Liu, A. et al. Low CRB-65 scores effectively rule out adverse clinical outcomes in COVID-19 irrespective of chest radiographic Abnormalities. Biomedicines. 11(9). (2023).
Guo, J. et al. CURB-65 May serve as a useful prognostic marker in COVID-19 patients within wuhan, china: a retrospective cohort study. Epidemiol. Infect. 148, e241 (2020).
Ak, R., Kurt, E. & Bahadirli, S. Comparison of 2 risk prediction models specific for COVID-19: the Brescia-COVID respiratory severity scale versus the quick COVID-19 severity Index. Disaster Med. Public. Health Prep. 15 (4), e46–e50 (2021).
Haimovich, A. D. et al. Development and validation of the quick COVID-19 severity index: A prognostic tool for early clinical Decompensation. Ann. Emerg. Med. 76 (4), 442–453 (2020).
Ma, B. et al. Applicability of mulbsta scoring system as diagnostic and prognostic role in early warning of severe COVID-19. Microb. Pathog. 150, 104706 (2021).
Preetam, M. & Anurag, A. MuLBSTA score in COVID-19 pneumonia and prediction of 14-day mortality risk: A study in an Indian cohort. J. Family Med. Prim. Care. 10 (1), 223–227 (2021).
Kostakis, I. et al. The performance of the National early warning score and National early warning score 2 in hospitalised patients infected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Resuscitation. 159, 150–157 (2021).
Rahmatinejad, Z. et al. Comparison of six scoring systems for predicting In-hospital mortality among patients with SARS-COV2 presenting to the emergency Department. Indian J. Crit. Care Med. 27 (6), 416–425 (2023).
De Santos Castro, P. A. et al. Head-to-head comparison of six warning scores to predict mortality and clinical impairment in COVID-19 patients in emergency department. Intern. Emerg. Med. 18 (8), 2385–2395 (2023).
Matono, T. et al. Diagnostic accuracy of quick SOFA score and inflammatory biomarkers for predicting community-onset bacteremia. Sci. Rep. 12 (1), 11121 (2022).
Liu, S. et al. Predictive performance of SOFA and qSOFA for in-hospital mortality in severe novel coronavirus disease. Am. J. Emerg. Med. 38 (10), 2074–2080 (2020).
Liang, W. et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern. Med. 180 (8), 1081–1089 (2020).
Gong, J. et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): A multicenter study using the risk nomogram in Wuhan and guangdong, China. Clin. Infect. Dis. 71 (15), 833–840 (2020).
Doganay, F. & Ak, R. Performance of the CURB-65, ISARIC-4 C and COVID-GRAM scores in terms of severity for COVID-19 patients. Int. J. Clin. Pract. 75 (10), e14759 (2021).
Knight, S. R. et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: development and validation of the 4 C mortality Score. BMJ. 370, m3339 (2020).
Erturk Sengel, B. et al. Application of CALL score for prediction of progression risk in patients with COVID-19 at university hospital in Turkey. Int. J. Clin. Pract. 75 (10), e14642 (2021).
Ji, D. et al. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL Score. Clin. Infect. Dis. 71 (6), 1393–1399 (2020).
Rahmatinejad, Z. et al. Internal validation of the predictive performance of models based on three ED and ICU scoring systems to predict inhospital mortality for intensive care patients referred from the emergency department. Biomed. Res. Int. 2022, 3964063 (2022).
Holten, A. R. et al. Predicting severe COVID-19 in the emergency department. Resusc. Plus. 4, 100042 (2020).
Ozdemir, S. et al. Effectiveness of the rapid emergency medicine score and the rapid acute physiology score in prognosticating mortality in patients presenting to the emergency department with COVID-19 symptoms. Am. J. Emerg. Med. 49, 259–264 (2021).
Martin-Rodriguez, F. et al. Early warning scores in patients with suspected COVID-19 infection in emergency Departments. J. Pers. Med., 11(3). (2021).
Ruangsomboon, O. et al. The utility of the rapid emergency medicine score (REMS) compared with three other early warning scores in predicting in-hospital mortality among COVID-19 patients in the emergency department: a multicenter validation study. BMC Emerg. Med. 23 (1), 45 (2023).
Kumar, S. et al. COVID-PIRO (Predisposition, insult, response, organ Dysfunction) score: A reliable predictor of outcomes in COVID-19 patients admitted in intensive care Unit. Cureus. 13 (10), e18960 (2021).
Diagnosis and Treatment protocol for COVID-19. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/(Accessed (accessed 20 Mar 2022.).[EB/OL].
Rubulotta, F. et al. Predisposition, insult/infection, response, and organ dysfunction: A new model for staging severe sepsis. Crit. Care Med. 37 (4), 1329–1335 (2009).
Asai, N. et al. Efficacy and accuracy of qSOFA and SOFA scores as prognostic tools for community-acquired and healthcare-associated pneumonia. Int. J. Infect. Dis. 84, 89–96 (2019).
Gulec, T. et al. Can we recognize severe community-acquired pneumonia without pneumonia severity index? Use of modified qSOFA with procalcitonin. Heliyon. 9 (9), e19937 (2023).
Bradley, J. et al. Pneumonia severity index and CURB-65 score are good predictors of mortality in hospitalized patients with SARS-CoV-2 community-acquired pneumonia. Chest 161 (4), 927–936 (2022).
Lim, W. S. et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 58 (5), 377–382 (2003).
Sligl, W. I. & Marrie, T. J. Severe community-acquired pneumonia. Crit. Care Clin. 29 (3), 563–601 (2013).
Berastegui-Cabrera, J. et al. Prepandemic viral community-acquired pneumonia: diagnostic sensitivity and specificity of nasopharyngeal swabs and performance of clinical severity scores. J. Med. Virol. 95 (1), e28317 (2023).
Ozdemir, S. et al. Predictive ability of the MEWS, REMS, and RAPS in geriatric patients with SARS-CoV-2 infection in the emergency department. Disaster Med. Public. Health Prep. 17, e174 (2022).
Li, C. et al. Prehospital physiological parameters related illness severity scores can accurately discriminate the severe/critical state in adult patients with COVID-19. Ann. Med. 55 (2), 2239829 (2023).
Bradley, P. et al. Utility of established prognostic scores in COVID-19 hospital admissions: multicentre prospective evaluation of CURB-65, NEWS2 and qSOFA. BMJ Open. Respir. Res., 7(1). (2020).
De Socio, G. V. et al. National early warning score 2 (NEWS2) better predicts critical coronavirus disease 2019 (COVID-19) illness than COVID-GRAM, a multi-centre study. Infection. 49 (5), 1033–1038 (2021).
Rello, J. et al. PIRO score for community-acquired pneumonia: a new prediction rule for assessment of severity in intensive care unit patients with community-acquired pneumonia. Crit. Care Med. 37 (2), 456–462 (2009).
Lisboa, T. et al. The ventilator-associated pneumonia PIRO score: a tool for predicting ICU mortality and health-care resources use in ventilator-associated pneumonia. Chest. 134 (6), 1208–1216 (2008).
Sivayoham, N. et al. An observational cohort study of the performance of the REDS score compared to the SIRS criteria, NEWS2, CURB65, SOFA, MEDS and PIRO scores to risk-stratify emergency department suspected sepsis. Ann. Med. 53 (1), 1863–1874 (2021).
Moreno, R. P. et al. Sepsis mortality prediction based on predisposition, infection and response. Intensive Care Med. 34 (3), 496–504 (2008).
Meyer, N. J. & Prescott, H. C. Sepsis and septic Shock. N. Engl. J. Med. 391 (22), 2133–2146 (2024).
Xu, J. Q. et al. Viral sepsis: diagnosis, clinical features, pathogenesis, and clinical considerations. Mil Med. Res. 11 (1), 78 (2024).
Lin, G. L. et al. Epidemiology and immune pathogenesis of viral Sepsis. Front. Immunol. 9, 2147 (2018).
Li, H. et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 395 (10235), 1517–1520 (2020).
Schoe, A. et al. Mortality prediction by SOFA score in ICU-patients after cardiac surgery; comparison with traditional prognostic-models. BMC Anesthesiol. 20 (1), 65 (2020).
Ferreira, F. L. et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 286 (14), 1754–1758 (2001).
Rahmatinejad, Z. et al. Predictive performance of the SOFA and mSOFA scoring systems for predicting in-hospital mortality in the emergency department. Am. J. Emerg. Med. 37 (7), 1237–1241 (2019).
Gershengorn, H. B. et al. Predictive value of sequential organ failure assessment score across patients with and without COVID-19 Infection. Ann. Am. Thorac. Soc. 19 (5), 790–798 (2022).
Richter, T. et al. Validation of the qSOFA and CRB-65 in SARS-CoV-2-infected community-acquired pneumonia. ERJ Open. Res. 9(3). (2023).
Brajkovic, M. et al. The predictive value of risk factors and prognostic scores in hospitalized COVID-19 Patients. Diagnostics (Basel). 13(16). (2023).
Liu, K. et al. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J. Infect. 80 (6), e14–e18 (2020).
Shi, Y. et al. Validation of pneumonia prognostic scores in a statewide cohort of hospitalised patients with COVID-19. Int. J. Clin. Pract. 75 (3), e13926 (2021).
Arminanzas, C. et al. Usefulness of the COVID-GRAM and CURB-65 scores for predicting severity in patients with COVID-19. Int. J. Infect. Dis. 108, 282–288 (2021).
Acknowledgements
We would like to express our gratitude to Beijing You’an Hospital for granting access to the clinical data of the patients.
Funding
This work was supported by National Key Research and Development Program of China [Grant No. 2022YFC2305002], COVID-19 Special Project of Beijing You’an Hospital [Grant No. 2023-6], Middle-aged and Young Talent Incubation Programs (Clinical Research) of Beijing You’an Hospital [Grant No. BJYAYY-YN2022-12, BJYAYY-YN2022-13], Beijing research center for respiratory infectious diseases project [Grant No. BJRID2024-001], Beijing Natural Science Foundation [Grant No. M22030] and Beijing Natural Science Foundation [Grant No. 7232079].
Author information
Authors and Affiliations
Contributions
N.G.: Conceptualization, writing—original draft, validation, supervision, software, project administration, methodology, investigation, data curation. Z.W.: Conceptualization, writing—original draft, validation, supervision, software, project administration, methodology, investigation, formal analysis. C.L.: Data curation, validation, supervision, software, project administration. Y.C.: Data curation, validation, supervision, software, project administration. Y.M.: Data curation, validation, supervision, software, project administration. M.L.: Data curation, validation, supervision, software, project administration. B.W.: Validation, supervision, software, methodology, investigation. H.S.: Manuscript revising, validation, supervision, acquisition of funding. Y.M.: Conceptualization, methodology, validation, supervision, software, project administration, manuscript revising, acquisition of funding. B.L.: Conceptualization, methodology, validation, supervision, software, project administration, manuscript revising, acquisition of funding. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethical Committee of Beijing Youan Hospital (Approval No. LL-2023-006-K). All participating patients provided informed consent, and the data used in the study were anonymized.
Consent for publication
All authors approved the publication of this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Geng, N., Wu, Z., Lai, C. et al. Evaluating the predictive performance of PIRO score against six clinical prediction scores for COVID-19 outcomes in the emergency department. Sci Rep 15, 27657 (2025). https://doi.org/10.1038/s41598-025-13131-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13131-y