Abstract
Higher intraoperative mechanical power (MP) is associated with increased postoperative pulmonary complications (PPCs). We hypothesised that periodic alveolar recruitment manoeuvres (PARM) alone, as an open-lung strategy for intraoperative protective ventilation, would reduce MP, thereby potentially mitigating PPCs. Seventy-five non-obese participants were equally allocated to either alveolar recruitment manoeuvres every 30 min alone (PARM group), or medium positive end-expiratory pressure (PEEP) of 6–8 cmH2O alone (PEEP group), or a combination of medium PEEP and PARM (combination group). As a result, the median (interquartile range, IQR) MP in the PARM group was lower than in the other groups (PARM, 4.34 [3.58–5.27]; PEEP, 6.47 [5.83–7.74]; combination, 6.32 [5.16–7.36] J min−1; P < 0.001). The median difference (95% confidence interval, 95% CI) of MP between the PARM and control group (combined PEEP and combination) was 2.05 (1.34–2.74) J min−1, with a significant reduction (32.2%, P < 0.001) in the PARM group. However, no clinical benefit (such as PPCs) was observed despite these physiological improvements. In conclusion, PARM alone as an open-lung strategy for protective ventilation leads to a 32.2% reduction in MP, compared with medium PEEP alone or a combination of PARM and medium PEEP. The association between PARM and PPCs warrant further investigations.
Similar content being viewed by others
Introduction
Postoperative pulmonary complications (PPCs) are associated with prolonged hospital stays and increased mortality1. Pulmonary atelectasis is a common perioperative complication and serves as a significant pathological basis for PPCs2. Rectal neoplasms are increasingly prevalent cancers, and surgical intervention is crucial in their management3,4. Laparoscopic anterior resection has improved the surgical visualization and outcomes, becoming the preferred surgical approach for rectal cancer5. However, it requires the Trendelenburg position and pneumoperitoneum, which can lead to a cranial shift of the diaphragm and increased intrathoracic pressure, resulting in a decrease in functional residual capacity, reduced lung compliance, and increased airway pressure, ultimately promoting the development of atelectasis2,6.
An open-lung strategy, involving the application of positive end-expiratory pressure (PEEP), alveolar recruitment manoeuvres (ARM), or their combination, has been used to reduce atelectasis and is deemed a crucial aspect of protective ventilation7,8. Currently, employing medium PEEP (5 − 10 cmH2O) alone constitutes the predominant strategy in clinical settings7,9. Meanwhile, the combined use of PEEP and periodic ARM (PARM) has shown protective effects in at-risk patients8,10,11. However, studies revealed that PARM, with or without PEEP, improved early postoperative oxygenation, shortened the time for tracheal extubation12and even reduced PPCs13. Besides, using PARM without PEEP led to lower airway pressures and reduced hemodynamic impairment12. Furthermore, a recent large study has shown that sigh ventilation, which is like PARM and also employs periodic deep breathing, may be beneficial in ventilated trauma patients at risk for acute respiratory distress syndrome (ARDS)14. Thus, it seems that PARM alone may also represent a viable open-lung strategy. However, the optimal approach among these strategies remains unclear.
Mechanical power (MP) is a concept that estimates the energy delivered to the respiratory system during mechanical ventilation. It integrates multiple factors of ventilator-associated lung injury (VALI), including plateau pressure (Pplat), peak inspiratory pressure (PIP), PEEP, tidal volume (Vt), and respiratory rate (RR)15. Several studies have indicated that elevated intraoperative MP is associated with increased PPCs15,16,17,18,19. In this context, we hypothesised that intraoperative protective ventilation utilising PARM alone, compared with medium PEEP alone or a combination of medium PEEP and PARM, would reduce intraoperative MP, thereby reducing lung injury and subsequent PPCs. This randomised controlled trial (the role of REcruitment MAneuvers IN intraoperative protective ventilation, study two: REMAIN-2) was conducted in non-obese patients at risk for PPCs undergoing laparoscopic anterior resection. The primary endpoint was MP at the end of surgery.
Methods
Ethical approval
This was a prospective, randomised controlled trial conducted at the Sixth Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China. The trial was approved (2023ZSLYEC-249) by the Ethical Committee of the Sixth Affiliated Hospital, Sun Yat-Sen University (Chairman Professor Lin Yao) on May 11, 2023, and registered at clinicaltrials.gov (reference number NCT05962125, date of registration June 28, 2023). All the study procedures were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from each participant before inclusion.
Participants
Eligible patients were aged 60–80 years, scheduled for laparoscopic anterior resection, and had a pulse oxygen saturation (SpO2) of 94% or greater when breathing air, with a grade 2 to 3 risk for PPCs11 (see Supplemental Table S1). Subjects with conditions including an American Society of Anaesthesiologists (ASA) physical status of IV or higher, recent invasive mechanical ventilation, recent pneumonia, severe chronic obstructive pulmonary disease (COPD) or pulmonary bullae, progressive neuromuscular disease, intracranial hypertension, body mass index ≥ 30 kg m−2, or participation in another interventional study were excluded.
Randomisation and masking
A completely randomised design was used. The random allocation sequence was generated using SPSS statistical software, version 17.0 (SPSS Inc., Chicago, Illinois, USA) by an independent statistician. Assigned personnel independent of the research team prepared sequentially numbered, sealed, and opaque envelopes containing the group assignments. The corresponding author screened and enrolled the subjects. Randomisation envelopes were opened by the intraoperative anaesthesiologists immediately before anaesthesia. Patients were equally and randomly assigned to one of three open-lung strategies: PARM alone (PARM group), medium PEEP alone (PEEP group), or a combination of medium PEEP and PARM (combination group). Postoperative outcomes, including PPCs, were evaluated by blinded investigators unaware of treatment allocation. Critically, a comprehensive blinding protocol was implemented: patients, biomarker assay operators, and outcome assessors were all masked to treatment assignments.
Anaesthesia and intervention
No premedication was administered. Before anaesthesia induction, 500–1000 ml of fluid was administered. Propofol (1.5–2.5 ml kg−1), fentanyl (2–4 µg kg−1), and cis-atracurium (0.15–0.25 ml kg−1) were titrated to facilitate tracheal intubation. Anaesthesia was maintained by inhalation (concentration of 1–2% sevoflurane) and intravenous anaesthetics (propofol at a rate of 2–6 mg kg−1 h−1, remifentanil at a rate of 0.05–0.2 µg kg−1 min−1, and intermittently administered cis-atracurium) until the end of surgery. Fluids were infused at a rate of 8–10 ml kg−1 h−1 to ensure hemodynamic stability. Patient-controlled intravenous analgesia was provided for up to three postoperative days. After surgery, patients were routinely transferred to the post-anaesthesia care unit (PACU) or, if necessary, the intensive care unit (ICU).
All patients received volume-controlled ventilation using the Dägger Fabius Tiro system (Dägger, Lübeck, Germany), and had a tidal volume of 7 ml kg−1 of predicted body weight (PBW), an inspiratory to expiratory ratio of 1:1.5, an inspiratory pause of 20%, and an oxygen to air ratio of 1:4 (fraction of inspired oxygen [FiO2] of 35% approximately). The respiratory rate was adjusted to maintain end-tidal carbon dioxide within 30 to 50 mmHg, and a Pplat of 30 cmH2O or less was the target in all the groups. Surgical procedures were conducted in a 30-degree Trendelenburg position with pneumoperitoneum pressure maintained at 12 mmHg.
In the PARM group, PEEP was set at 0 cmH2O, and ARM was initiated within 10 min after tracheal intubation and repeated every 30 min or following any disconnection from the ventilator. For the PEEP group, PEEP was initially set at 6 cmH2O, and adjusted to 8 cmH2O during pneumoperitoneum or in the Trendelenburg position, without ARM. In the combination group, both PEEP and PARM were applied. ARM was under volume-controlled ventilation, referred to previous studies10,20,21 and detailed as follows:
-
1.
PEEP was set at 12 cmH2O, RR at 6 breaths minute−1.
-
2.
Vt was increased in steps of 4 ml kg−1 PBW until a Pplat of 30–35 cmH2O was reached. If the Vt reached the upper limit of the ventilator but the Pplat still did not reach the target value, then PEEP was increased in steps of 4 cmH2O until a targeted Pplat was reached.
-
3.
Three to five breaths were administered under each increased Vt. If the basal Pplat was higher (≥ 20–25 cmH2O), Vt should be increased at least two steps of 5 breaths each.
-
4.
Vt, RR, and PEEP were set back to the settings preceding each ARM.
ARM administration was postponed if mean arterial pressure (MAP) ≤ 70 mmHg. If ARM led to a MAP ≤ 55 mmHg, it was to be immediately discontinued. In case of relative hypotension (MAP ≥ 70 mmHg) before ARM, we recommended assessing the patient’s condition first, and then using vasopressors and/or rapid fluid resuscitation to ensure hemodynamic stability (with a target MAP of ≥ 75 mmHg for those with a history of hypertension, or ≥ 70 mmHg for others) based on our team’s experience22. During anaesthesia, the hemodynamic management protocol was standardised across groups. Intraoperative hypotension was defined as MAP < 60 mmHg lasting more than 3 min, or MAP ≤ 55 mmHg lasting more than one minute. Bradycardia was defined as Heart rate (HR) ≤ 50 beats per minute (bpm) and the decrease of HR from the basal value ≥ 20% lasting more than 3 min, or HR ≤ 40 bpm. Need for vasopressors was defined as MAP < 60 mmHg and vasopressors used. It was recommended to administer a single dose of dopamine 2 mg intravenously for patients with low blood pressure and bradycardia (HR ≤ 60 bpm), or norepinephrine 5 µg for patients with only low blood pressure. Vasopressors could be administered repeatedly or infused via pump as needed.
In cases of intraoperative hypoxemia (SpO2 ≤ 92%) persisting for more than 3 min, rescue therapy involving a 10 to 20% increase in FiO2 was administered across all groups. If oxygenation did not improve sufficiently when FiO2 reached 100%, PEEP settings equivalent to those in the PEEP group were applied in the PARM group, whereas a single ARM was administered in the PEEP group.
Measurements and follow-up
Intraoperative measurements were taken at three time points. The first point (T1) was 15 to 20 min post-tracheal intubation and before pneumoperitoneum. The second point (T2) was 30 min after pneumoperitoneum. The third point (T3) was at the end of surgery, with the patient repositioned supine without spontaneous breathing. Arterial blood gas analysis was conducted at T1 and T3, and central venous blood gas analysis at T3. Venous blood samples were drawn at T1 and T3. Plasma was immediately extracted and stored at − 80 ℃. Plasma concentrations of lung injury biomarkers, including soluble receptor for advanced glycation end products (sRAGE), Clara Cell Protein 16 (CC16), surfactant protein D (SP-D) and angiopoietin 2 (Ang-2), were measured using validated enzyme-linked immunosorbent assay kits. In the PACU, arterial blood gas analysis and SpO2 measurements were performed once patients were awake and breathing room air. The patients were followed up once daily for the first three postoperative days to collect clinical symptoms and signs of the lungs.
Endpoints
The primary endpoint was MP at T3. MP was calculated as follows14: MP = 0.098 × RR × Vt × (PEEP + ½[Pplat − PEEP] + [Ppeak − Pplat]). Secondary endpoints included: mechanical energy (ME, calculated as the area under the curve of MP and time. ME = MP at T1 × duration of ventilation before pneumoperitoneum + MP at T2 × duration of ventilation under pneumoperitoneum + MP at T3 × duration of ventilation after pneumoperitoneum); MP at T2; PaO2/FiO2 ratio, shunt fraction and alveolar dead space at T3; intraoperative hypoxemia, hypotension or bradycardia; respiratory failure23 at PACU or within three postoperative days; sustained hypoxemia (SpO2 ≤ 92% on room air or a decrease in SpO2 [ΔSpO2] ≥ 5% during two consecutive days) and PPCs grade24 (see Supplemental Table S2) of 2 to 4 within three postoperative days; pneumothorax and pleural effusion23 within seven postoperative days; ratios of plasma concentrations (T3/T1) of lung injury biomarkers; postoperative hospital stays; unplanned admissions to the ICU; and in-hospital mortality. Post-hoc endpoints included time-weighted average MP (MPtwa), calculated as ME divided by the number of minutes of ventilation duration; MP-T3, ME or MPtwa normalised by PBW. Additionally, we defined two variables reflecting intraoperative oxygenation impairment: the PaO2/FiO2 ratio difference (T1 – T3) and the PaO2/FiO2 ratio reduction (T3/T1 < 1).
Sample size calculation
According to a previous study15the estimated MP was 6.6 J min−1 in the PEEP group and the combination group. A quarter reduction of MP in the PARM group was expected according to our clinical experience. With an estimated standard deviation of 1.5 J min−1, assuming a 90% power at a 2-sided α level of 0.05 and a dropout rate of 10%, the sample size was 25 patients in each group. However, this small sample size may be underpowered for detecting differences in clinical outcomes, such as PPCs.
Statistical analysis
Data distribution was assessed using the Shapiro–Wilk test. Normally distributed data were presented as mean ± standard deviation (SD) and compared using one-way analysis of variance (ANOVA) followed by Bonferroni correction. Non-normally distributed data were expressed as medians with interquartile range (IQR) and analyzed using Kruskal-Wallis tests followed by Bonferroni corrections. Categorical variables were described as frequencies (percentages) and analyzed using Fisher’s exact tests. For the primary endpoint and MP at T2, if no significant differences were found between the PEEP and the combination group, these would be merged into a newly defined control group. The Hodges-Lehmann estimator would then be employed to calculate the median differences (95% CIs) between the PARM group and the new control group. All analyses were performed on an intention-to-treat basis. There were no missing data for the endpoints. All statistical tests were two-sided and conducted at an α level of 0.05. Statistical analyses were carried out using SPSS statistical software, version 17.0.
Results
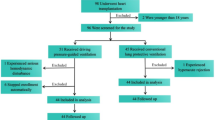
This trial was conducted from August 21, 2023, to November 8, 2023. Seventy-five patients were enrolled in the study, with 25 patients in each group (Fig. 1). No serious protocol violations were noted. No patients were lost to follow-up. Baseline and intraoperative characteristics are shown in Tables 1, 2 and 3. Fewer patients in the combination group experienced longer mechanical ventilation (≥ 3 h) compared to the other groups (PARM, 13; PEEP, 17; combination, 8 patients; P = 0.048).
Primary endpoint
The median (IQR) MP was significantly lower in the PARM group than in the PEEP group and combination group (PARM, 4.34 [3.58–5.27]; PEEP, 6.47 [5.83–7.74]; combination, 6.32 [5.16–7.36] J min−1; P < 0.001) (Fig. 2 A). The median difference (95% CI) between the PARM group and control group was 2.05 (1.34–2.74) J min−1, with a significant reduction (32.2%, P < 0.001) in the PARM group (Fig. 2B).
Secondary endpoints
As shown in Fig. 2 C, no significant difference was observed in ME (P > 0.05). The median (IQR) MP in the PARM group at T2 was significantly lower than in other groups (PARM, 7.72 [5.93–9.78] vs. PEEP, 9.92 [8.07–11.29] vs. combination, 9.78 [8.91–11.68] J min−1; P = 0.001). The PaO2/FiO2 ratio at T3 was significantly lower in the PARM group than in other groups (348 ± 77 vs. 424 ± 78 vs. 446 ± 121 mmHg, P = 0.001). As shown in Table 4, no significant differences were observed in the shunt fraction and alveolar dead space at T3 (P > 0.05). There were no significant differences in the rates of intraoperative hypotension, need for vasopressors, bradycardia, or hypoxemia (P > 0.05). No significant differences (P > 0.05) were observed in the absolute plasma levels (see Supplemental Table S3) and ratios of plasma concentrations (T3/T1) of lung injury biomarkers (Fig. 2E–H).
There were no missing data for all the endpoints. Violin plot A–H: dots represent each individual data point and lines represent medians with IQR; primary endpoint (A), primary endpoint after merging the data of PEEP group and combination group (B), mechanical energy (C), mechanical power (MP) during pneumoperitoneum (D) and the ratios of plasma concentrations of Clara cell protein 16, CC16 (E), soluble advanced glycation end products receptor, sRAGE (F), angiopoietin-2, Ang-2 (G) and surfactant protein D, SP-D (H). Primary endpoint, MP at the end of surgery (T3). As there was no group difference in the primary endpoint between the PEEP group and the combination group, we merged them into one group, i.e. control group. *P < 0.05; # the median difference (95% CI) of the primary endpoint between the PARM group and the combined control group was 2.05 [1.34–2.74] J min−1; P < 0.001), with a significant reduction (32.2%, P < 0.001) in the PARM group. ns, no statistical significance; T1, before surgery; PARM, periodic alveolar recruitment manoeuvre; PEEP, positive end-expiratory pressure.
No significant differences were observed in the PACU respiratory failure, or the respiratory failure, sustained hypoxemia, and pulmonary complications grade ≥ 2 within postoperative three days (P > 0.05). No statistical differences were detected in the extrapulmonary complications, and the length of postoperative hospital stays (P > 0.05). Pneumothorax with a 70% compression of the right lung was found in one patient in the combination group. One patient in the PARM group underwent re-operation and was transferred to the ICU. None of the patients died within 30 days.
Post-hoc analysis
As shown in Supplementary Table S4, MPtwa (PARM, 6.7 ± 1.9; PEEP, 8.6 ± 1.7; combination, 8.6 ± 1.5 J min−1; P < 0.001), MP-T3/PBW (0.08 ± 0.02 vs. 0.12 ± 0.03 vs. 0.10 ± 0.02 J kg−1 min−1; P < 0.001) and MPtwa/PBW (0.11 ± 0.03 vs. 0.15 ± 0.03 vs. 0.15 ± 0.02 J kg−1 min−1; P < 0.001) were significantly lower in the PARM group than in the PEEP group and combination group. As shown in Table 4, no significant differences were observed regarding the PaO2/FiO2 ratio difference and the PaO2/FiO2 ratio reduction among groups.
As shown in Supplementary Table S5, the occurrences of MP >6.7 J min−1 at T3 and MP>9.2 J min−1 at T2 were significantly lower in the PARM group compared with the other two groups. As shown in Supplementary Table S6, the median difference (95% CI) of MP at T2 between the PARM group and control group was 2.27 (1.19–3.28) J min−1, with a significant reduction (23.0%, P < 0.001) in the PARM group. The IQRs of MP overlapped partially (overlapping range: 8.63–9.78 J min−1, 2 (8.0%) in PARM vs. 13 (26.0%) in control) between PARM group and control group at T2. No overlap in the IQRs of MP was found between PARM group and the control group at T3.
Discussion
In this randomised controlled trial involving non-obese patients undergoing laparoscopic anterior resection, we found that utilising PARM alone as an open-lung strategy of protective ventilation resulted in a 32% reduction in MP with no significant changes in shunt fraction, lung injury biomarkers, oxygenation impairment or PPCs, when compared with strategies employing medium PEEP alone or a combination of PARM and medium PEEP.
Previous study has found that most re-expanded atelectatic lung tissue remains inflated for at least 40 min following an ARM in lung-healthy patients during general anaesthesia25. Consequently, the current PARM regimen (ARM/0.5 h) appears to be a reasonable and viable open-lung strategy for short-duration of intraoperative mechanical ventilation in lung-healthy patients. However, to our knowledge, the role of PARM in intraoperative protective ventilation has been rarely investigated. PARM exhibits some similarities with sigh ventilation, but their high-pressure/high-volume ventilation frequency (PARM vs. sigh, 2 vs. 10 times per hour) and per ARM/sigh duration (30–200 vs. 5 s) differ significantly, making them essentially two different treatments. Fixed PEEP and its combination with PARM have been shown to have protective effects in previous studies8,10,26 and are thus included as controls. While individualised PEEP is considered a potentially ideal strategy27the optimal parameters for its individualisation are still under investigation, thus it was not included as a control.
Previous studies have shown that intraoperative elevated MP (e.g. >6.7 or >9.2 J min−1) and ME are correlate with poorer clinical outcomes15,16,17,18,19. MP at the end of surgery encapsulates the cumulative detrimental effects of various factors during intraoperative ventilation, guiding the selection of the primary endpoint. We observed that MPs (MP at three time points, PBW normalised MP and MPtwa) were significantly lower in the PARM group compared to the other groups. Notably, the PARM group had fewer patients with elevated MP during pneumoperitoneum and at the end of surgery, resulting in a 32% reduction in the primary endpoint compared to the control group, with similar findings during pneumoperitoneum. There was no significant difference in ME between the three groups, likely attributed to the shorter ventilation duration of the combination group, as ME is the product of MP and ventilation duration. Above all, our findings underscore the effectiveness of PARM in minimizing intraoperative MP. Contrarily, we found that the open-lung strategy, whether employing PEEP alone or combined with PARM, increased airway pressure, MP and higher MP occurrence. This might provide a reasonable explanation in terms of respiratory mechanics for the unclear relationship between PEEP or individualised PEEP and clinical outcomes20,28,29,30,31,32.
Despite the improved MP with PARM in this study, biomarkers indicative of lung injury33,34,35—including CC16, sRAGE, Ang-2, and SP-D—showed no significant differences among the three groups. Similarly, there were no between-group differences in clinical outcomes such as respiratory failure at the PACU, sustained hypoxemia, PPCs within the first three days, or length of hospital stay. Previous studies have indicated that differences in lung injury biomarkers often emerge after prolonged ventilation durations36 (e.g., > 5 h) or in patients with ARDS34rather than in those with shorter ventilation durations37,38. In addition, high MP is associated with an increased incidence of PPCs only when ventilation duration is extended16. Thus, long ventilation time seems to be a key risk factor for intraoperative VALI. In our study, the ventilation times across all groups were relatively short (3–4 h), which may explain the lack of observable differences in lung injury biomarkers and PPCs. Additionally, the small sample size and the low risk for PPCs in this population may significantly contribute to the null results observed for biomarkers and PPCs. Notably, the length of postoperative hospital stay was marginally longer in the PARM group, potentially linked to a higher incidence of extrapulmonary complications39 in this cohort.
While pulmonary atelectasis increases shunt fraction40our findings indicated no significant differences in shunt fraction between the groups, implying that the three open-lung strategies tested may be similarly effective in preventing atelectasis formation. Interestingly, the PaO2/FiO2 ratios at both the beginning and end of surgery were lower in the PARM group than in the other two groups. This result may imply that the open-lung strategies incorporating PEEP are better at enhancing oxygenation. However, previous studies20,30,31 have demonstrated that improvements in intraoperative oxygenation (e.g., higher SpO2 or PaO2/FiO2 ratios, or reduced hypoxemia occurrence) do not necessarily correlate with a reduction in postoperative complications (PPCs) in at-risk patients undergoing abdominal surgery. Similarly, in ARDS patients, a liberal oxygenation strategy targeting SpO2 levels of 96% or higher showed no advantage in terms of new organ dysfunction, ICU admission, or 90-day mortality compared to a conservative strategy targeting SpO2 levels of 88–92%41. This dissociation between oxygenation improvement and clinical benefits may be related to the potential risks of oxygen toxicity42. In our study, we further found no between-group differences in PaO2/FiO2 ratio reduction, PaO2/FiO2 ratio difference, or incidence of intraoperative hypoxemia. This suggests that none of the tested open-lung strategies exacerbated oxygenation impairment. Above all, the higher PaO2/FiO2 ratios observed in the groups utilising PEEP may not be clinically significant.
Although ARM theoretically increase the risk of pneumothorax, previous large trials11,20,30 have not reported a heightened incidence. In this study, pneumothorax occurred in one patient of the combination group, while no such events were observed in the PARM or PEEP group. Despite normal intraoperative airway pressures and oxygenation, the patient developed wheezing and hypoxemia on the third postoperative day. The pneumothorax was diagnosed on the fifth day and treated with closed thoracic drainage. Finally, the patient was discharged on the eleventh day. This incident underscores that the potential adverse effects of high intraoperative airway pressures, possibly linked to ARM, cannot be entirely dismissed.
Contrary to findings in previous studies10,20,30no significant differences were observed in the rates of intraoperative hypotension or the need for vasopressors among the groups, potentially due to proactive hypotension management and liberal fluid administration. Specifically, we administered 500–1000 ml of fluid prior to anaesthesia and suggested vasopressor administration preemptively if blood pressure was low, as per our earlier findings22. Furthermore, the use of PARM did not lead to an increase in intraoperative bradycardia. These results suggest that ARM’s potential hemodynamic effects can be mitigated with vigilant monitoring and preventive strategies.
The study presented several limitations. First, the primary endpoint, i.e. MP, is an intermediate measure that, while supported by numerous observational studies, has not been validated in randomised trials. However, given that this was a small sample size study with innovative elements, a clinical outcome such as PPCs, which would require a larger sample size, was deemed unsuitable for a primary endpoint. Consequently, the use of an intermediate measure was a practical compromise. Second, driving pressure, a possible predictor of PPCs43 was suboptimal in the PARM group. However, driving pressure contains fewer factors contributing to VALI, whereas MP encompasses more injury factors including driving pressure itself, enhancing its relevance as an outcome measure. Third, although small, the sample size was scientifically calculated, and the results regarding the primary endpoint validated its appropriateness. Fourth, the study population was homogenous, which, while potentially limiting the generalizability of the findings, ensured balanced subject characteristics and reliable results for a study of this scale. Fifth, while the baseline characteristics, such as the duration of mechanical ventilation, were not perfectly balanced, the strict randomisation process and the nature of the primary endpoint minimise concerns about the effect of this imbalance. Sixth, the study did not utilise advanced imaging techniques like computed tomography, lung ultrasonography, or electrical impedance tomography to evaluate atelectasis; future studies should address this gap. Seventh, we conducted PARM using a stepwise increase in tidal volume, and it remains uncertain whether other ARM techniques would yield similar results. However, existing literature suggests comparable efficacy across various ARM methods44,45,46. Eighth, given the high incidence of atelectasis after anaesthesia induction, baseline measurements of MP without PEEP and ARM were not considered in the study design. Ninth, the biomarkers presented may lack sufficient sensitivity to detect low-grade injuries in short-duration surgeries. However, there are currently no identified perioperative biomarkers with greater specificity and sensitivity for lung injury, indicating the need for further investigation.
In conclusion, in non-obese patients undergoing laparoscopic anterior resection, employing PARM alone as an open-lung strategy for protective ventilation led to a significant reduction in MP without significant changes in shunt fraction, lung injury biomarkers, oxygenation impairment or PPCs, compared with medium PEEP alone or a combination of PARM and medium PEEP. Thus, PARM alone may represent a viable open-lung strategy for short-duration intraoperative ventilation in non-obese patients. The implications of PARM for PPCs merit further explorations, which are currently in progress.
Data availability
Due to ethical restrictions, the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fernandez-Bustamante, A. et al. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA Surg. 152, 157–166 (2017).
Lagier, D., Zeng, C. & Fernandez-Bustamante, A. Vidal melo, M. F. Perioperative pulmonary atelectasis: part II. clinical implications. Anesthesiology 136, 206–236 (2022).
Dekker, E., Tanis, P. J., Vleugels, J. L. A., Kasi, P. M. & Wallace, M. B. Colorectal cancer. Lancet 394, 1467–1480 (2019).
Siegel, R. L., Wagle, N. S., Cercek, A., Smith, R. A. & Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 73, 233–254 (2023).
Jayne, D. et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA 318, 1569–1580 (2017).
Shono, A. et al. Positive end-expiratory pressure and distribution of ventilation in Pneumoperitoneum combined with steep Trendelenburg position. Anesthesiology 132, 476–490 (2020).
Young, C. C. et al. Lung-protective ventilation for the surgical patient: international expert panel-based consensus recommendations. Br. J. Anaesth. 123, 898–913 (2019).
Odor, P. M., Bampoe, S., Gilhooly, D., Creagh-Brown, B. & Moonesinghe, S. R. Perioperative interventions for prevention of postoperative pulmonary complications: systematic review and meta-analysis. BMJ 368, m540 (2020).
Chiumello, D., Coppola, S., Fratti, I., Leone, M. & Pastene, B. Ventilation strategy during urological and gynaecological robotic-assisted surgery: a narrative review. Br. J. Anaesth. 131, 764–774 (2023).
Li, H. et al. Intra-operative open-lung ventilatory strategy reduces postoperative complications after laparoscopic colorectal cancer resection: A randomised controlled trial. Eur. J. Anaesthesiol. 38, 1042–1051 (2021).
Futier, E. et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl. J. Med. 369, 428–437 (2013).
Wei, K., Min, S., Cao, J., Hao, X. & Deng, J. Repeated alveolar recruitment maneuvers with and without positive end-expiratory pressure during bariatric surgery: a randomized trial. Minerva Anestesiol. 84, 463–472 (2018).
Pei, S. et al. Recruitment maneuver to reduce postoperative pulmonary complications after laparoscopic abdominal surgery: a systematic review and meta-analysis. J. Clin. Med. 11, 5841 (2022).
Albert, R. K. et al. Sigh ventilation in patients with trauma: the SiVent randomized clinical trial. JAMA 330, 1982–1990 (2023).
Santer, P. et al. Mechanical power during general anesthesia and postoperative respiratory failure: a multicenter retrospective cohort study. Anesthesiology 137, 41–54 (2022).
Yoon, S. et al. Association of mechanical energy and power with postoperative pulmonary complications in lung resection surgery: a post hoc analysis of randomized clinical trial data. Anesthesiology 140, 920–934 (2024).
Tartler, T. M. et al. High mechanical power and driving pressures are associated with postoperative respiratory failure independent from patients’ respiratory system mechanics. Crit. Care Med. 52, 68–79 (2024).
Elefterion, B. et al. Intraoperative mechanical power and postoperative pulmonary complications in noncardiothoracic elective surgery patients: a 10-year retrospective cohort study. Anesthesiology 140, 399–408 (2024).
van Meenen, D. M. P. et al. Effect of mechanical power on mortality in invasively ventilated ICU patients without the acute respiratory distress syndrome: an analysis of three randomised clinical trials. Eur. J. Anaesthesiol. 40, 21–28 (2023).
Bluth, T. et al. Effect of intraoperative high positive end-expiratory pressure (PEEP) with recruitment maneuvers vs low PEEP on postoperative pulmonary complications in obese patients: a randomized clinical trial. JAMA 321, 2292–2305 (2019).
Zhang, N. R. et al. Intraoperative protective ventilation with or without periodic lung recruitment manoeuvres on pulmonary complications after major abdominal surgery (REMAIN-1): protocol for a randomised controlled trial. BMJ. Open 15, e093360 (2025).
Zhang, N. R., Zheng, Z. N., Wang, K. & Li, H. Incidence, characteristics and risk factors for alveolar recruitment maneuver-related hypotension in patients undergoing laparoscopic colorectal cancer resection. World J. Gastrointest. Surg. 15, 1454–1464 (2023).
Jammer, I. et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European perioperative clinical outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur. J. Anaesthesiol. 32, 88–105 (2015).
Fernandez-Bustamante, A. et al. An anesthesia-centered bundle to reduce postoperative pulmonary complications: the PRIME-AIR study protocol. Plos One 18, e0283748(2023).
Rothen, H. U., Sporre, B., Engberg, G., Wegenius, G. & Hedenstierna, G. Reexpansion of atelectasis during general anaesthesia May have a prolonged effect. Acta Anaesthesiol. Scand. 39, 118–125 (1995).
Severgnini, P. et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 118, 1307–1321 (2013).
Ma, X. J. et al. Individualised positive end-expiratory pressure titrated intra-operatively by electrical impedance tomography optimises pulmonary mechanics and reduces postoperative atelectasis: a randomised controlled trial. Eur. J. Anaesthesiol. 40, 805–816 (2023).
Barbosa, F. T., Castro, A. A. & de Sousa-Rodrigues, C. F. Positive end-expiratory pressure (PEEP) during anaesthesia for prevention of mortality and postoperative pulmonary complications. Cochrane Database Syst. Rev. 6, CD007922 (2014).
Kim, Y. J. et al. Effect of driving pressure-guided positive end-expiratory pressure on postoperative pulmonary complications in patients undergoing laparoscopic or robotic surgery: a randomised controlled trial. Br. J. Anaesth. 131, 955–965 (2023).
Hemmes, S. N., de Abreu, G., Pelosi, M., Schultz, M. J. & P. & High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 384, 495–503 (2014).
Ferrando, C. et al. Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): a randomised controlled trial. Lancet Respir Med. 6, 193–203 (2018).
Park, M. et al. Driving pressure-guided ventilation and postoperative pulmonary complications in thoracic surgery: a multicentre randomised clinical trial. Br. J. Anaesth. 130, E106–E118 (2023).
Serpa Neto, A. et al. Kinetics of plasma biomarkers of inflammation and lung injury in surgical patients with or without postoperative pulmonary complications. Eur. J. Anaesthesiol. 34, 229–238 (2017).
Blondonnet, R., Constantin, J. M., Sapin, V. & Jabaudon, M. A pathophysiologic approach to biomarkers in acute respiratory distress syndrome. Dis. Markers. 3501373 (2016). (2016).
Yadav, H. et al. Evolution of validated biomarkers and intraoperative parameters in the development of postoperative ARDS. Respir Care. 63, 1331–1340 (2018).
Jabaudon, M. et al. Association between intraoperative ventilator settings and plasma levels of soluble receptor for advanced glycation end-products in patients without pre-existing lung injury. Respirology 20, 1131–1138 (2015).
van der Woude, M. C. et al. Pulmonary levels of biomarkers for inflammation and lung injury in protective versus conventional one-lung ventilation for oesophagectomy: A randomised clinical trial. Eur. J. Anaesthesiol. 37, 1040–1049 (2020).
Determann, R. M. et al. Lung epithelial injury markers are not influenced by use of lower tidal volumes during elective surgery in patients without preexisting lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L344–L350 (2008).
Li, H. et al. Factors associated with prolonged postoperative length of hospital stay after laparoscopic colorectal cancer resection: a secondary analysis of a randomized controlled trial. BMC Surg. 22, 438 (2022).
Zeng, C., Lagier, D. & Lee, J. W. Vidal melo, M. F. Perioperative pulmonary atelectasis: part I. biology and mechanisms. Anesthesiology 136, 181–205 (2022).
Panwar, R. et al. Conservative versus Liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. Am. J. Respir Crit. Care Med. 193, 43–51 (2016).
Chu, D. K. et al. Mortality and morbidity in acutely ill adults treated with Liberal versus Conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet 391, 1693–1705 (2018).
Neto, A. S. et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med. 4, 272–280 (2016).
Hartland, B. L., Newell, T. J. & Damico, N. Alveolar recruitment maneuvers under general anesthesia: a systematic review of the literature. Respir Care. 60, 609–620 (2015).
Güldner, A. et al. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications. Anesthesiology 123, 692–713 (2015).
Kheir, J. N. et al. Comparison of 2 lung recruitment strategies in children with acute lung injury. Respir Care. 58, 1280–1290 (2013).
Acknowledgements
We acknowledge Li-Shuo Shi from the Clinical Research Center, The Sixth Affiliated Hospital, Sun Yat-sen University, for his valuable advice regarding the statistical analyses.
Funding
This research was supported by the 1010 Program of the Sixth Affiliated Hospital of Sun Yat-sen University (No. 1010PY[2023]−29).
Author information
Authors and Affiliations
Contributions
H.L. and N.R.Z. helped design, write and revise the study. H.L. helped the patient recruitment. J.G., C.L.L., L.Z.Z., J.L., X.K.G. and T.S.W collected, analysed data and wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, J., Liu, CL., Zhang, LZ. et al. Open-lung strategies and mechanical power during protective ventilation for laparoscopic anterior resection: a randomised controlled trial. Sci Rep 15, 27727 (2025). https://doi.org/10.1038/s41598-025-13213-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-13213-x