Abstract
Tripterygium polyglycosides (TG), Triptolide (TP), and Celastrol (CEL), which are extracts from Tripterygium wilfordii Hook F (TwHF), are renowned for their anti-inflammatory and immunomodulatory properties in the treatment of rheumatoid arthritis (RA). However, a comprehensive systematic review and meta-analysis of preclinical evidence supporting the use of Tripterygium wilfordii extracts for RA has not yet been conducted. This study aimed to conduct a systematic review and meta-analysis to evaluate the therapeutic effects and potential underlying mechanisms of Tripterygium wilfordii extracts in CIA model of RA. Six electronic databases, including PubMed, Embase, Web of Science, Cochrane Library, Scopus, and Ovid, were searched from their inception to June 2024. The methodological quality was assessed via SYRCLE’s risk of bias tool. Statistical analyses were performed using Stata 18.0 software and Review Manager 5.4.1 software. This study included 32 articles involving 568 animals. The results revealed that Tripterygium wilfordii extracts significantly reduced joint manifestations (arthritis index, paw swelling degree, and paw thickness); histopathological changes in joints (histopathological score, cartilage damage, bone destruction, synovial hyperplasia, pannus formation, and inflammatory cell infiltration); levels of related cytokines (TNF-α, IL-1β, IL-6, IL-23, IFN-γ, and IL-17); anti-type II collagen antibodies; and T-cell subsets (CD4+ T cells, Th1 cells, and Th17 cells). Moreover, Tripterygium wilfordii extracts significantly increased the levels of relevant cytokines (IL-10, IL-4, and TGF-β) and the number of Treg cells compared with those in the control group. However, animal species and sex, extract category and dosage, and treatment duration were identified as important factors influencing the above results. Tripterygium wilfordii extracts (TG, TP and CEL) exhibit promising therapeutic effects and mechanisms in the treatment of RA. But the effect characteristics and mechanisms vary among different Tripterygium wilfordii extracts. These specific effects and mechanisms also vary depending on the treatment duration and dosage.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is one of the most common immune-mediated inflammatory diseases, often accompanied by symmetrical synovial joint pain, swelling, and stiffness, as well as extra-articular organ damage, and has a high rate of disability and deformity1. It is more prevalent in women than in men, and the global age-standardized prevalence rate of RA demonstrates a significantly increasing trend2,3. The pathogenesis of RA is highly complex and closely related to genetic predisposition and environmental risk factors. The recent significant progress in understanding its pathogenesis has led to improved treatment and quality of life4.
The conventional drugs for RA include non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs), and disease-modifying antirheumatic drugs (DMARDs)3,5,6. Currently, DMARDs remain the mainstream for clinical treatment in RA. Although they have improved clinical outcomes, a portion of patients still do not respond adequately; even long-term use may lead to adverse reactions, such as cardiovascular and gastrointestinal damage, osteoporosis, and other potential health issues, as well as significant costs of treatment. Identifying inexpensive but effective medications is crucial for reducing the global health burden of RA.
Traditional Chinese medicine (TCM) has the advantage of good safety and few adverse reactions, and can be used for long periods. Tripterygium wilfordii Hook F (TwHF), a member of the Celastraceae herbal family, whose extracts are widely recommended and utilized in clinical practice guidelines for the treatment of RA in China, is considered a DMARD derived from TCM7. The commonly used extracts from TwHF involve Tripterygium polyglycosides (TG), Triptolide (TP), and Celastrol (CEL), all renowned for their anti-inflammatory and immunomodulatory properties8,9,10. The 2023 International Consensus Guidance reported that there was no obvious difference between TwHF monotherapy and Conventional Synthetic DMARD methotrexate (MTX) monotherapy on a American College of Rheumatology 20 response (ACR20), ACR50, and ACR70 in the treatment of active RA. Additionally, no obvious difference was observed between the two groups on critical safety outcomes. Based on these findings, the guideline development group suggested that either TwHF monotherapy or MTX monotherapy was an available option for people with active RA as a first line treatment11. The results of Luo et al. indicated that TwHF plus MTX treatment revealed a higher effective rate in RA treatment compared with MTX alone12. Our team’s previous research has also demonstrated that the combination of TG is superior to conventional DMARD monotherapy in improving RA conditions with a good safety profile13.
Synovial hyperplasia and bone destruction are the landmark pathological changes at the joint site of RA, and the infiltration of various inflammatory cells at the bone-cartilage junction has been proven to be critical in mediating these pathological changes14. Although previous studies have confirmed its good clinical efficacy with considerably low costs in RA treatment15,16,17, the effects and mechanisms of Tripterygium wilfordii extracts on these pathological changes, especially on various infiltrating inflammatory cells, remain poorly studied and uncertain, due to the lack of clinical widespread application of the related detections, coupled with the inherent complexity of the detection procedures. This constrains their broader clinical utilization and deserves further exploration.
Systematic reviews and meta-analyses of preclinical animal studies can not only provide valuable information on the efficacy of treatments targeting various pathological changes but also can make unique contributions to the exploration of mechanisms18,19. So far, no systematic review and meta-analysis of preclinical studies has been published on the therapeutic effects and mechanisms of Tripterygium wilfordii extracts in multiple aspects of these pathological changes and the immunological dysfunction in the treatment of RA. Moreover, there is a lack of quality evaluation for animal studies on the therapeutic effects of Tripterygium wilfordii extracts in RA. Therefore, our study conduct a preclinical systematic review and meta-analysis, aiming to explore and elucidate the effects and mechanisms of Tripterygium wilfordii extracts on these pathological changes and the immunological dysfunction, on the basis of systematically evaluating the quality of relevant research. This is imperative for understanding their potential therapeutic effects in RA therapy. As Collagen-induced arthritis (CIA) is the most commonly used animal model of RA, sharing similar immunological and pathological features with human RA, and has been widely used to identify potential pathogenic mechanisms of autoimmunity20,21, this study mainly focuses on the research with CIA as the model. Our study aims to provide valuable preclinical insights that can serve as a cornerstone for future clinical applications of Tripterygium wilfordii extracts.
Methods
Search strategy
The study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines22 and registered on PROSPERO (CRD42024575394).
We searched PubMed, Embase, Web of Science, Cochrane Library, Scopus, and Ovid from inception to June 2024. These databases were searched respectively by using the Medical Subject Heading (MeSH) terms and free-text terms, including the following three primary components: (‘Tripterygium’ OR ‘Tripterygium Glycosides’ OR ‘Triptolide’ OR ‘Celastrol’) AND (‘rheumatoid arthritis’) AND (‘Rat’ OR ‘mice’ OR ‘Experiment’ OR ‘in vivo’ OR ‘animal model’). The detailed search strategy is shown in Table S1.
Inclusion criteria
-
1.
The experimental animal model for RA was the CIA model, and the animal species were rats or mice, with no restrictions on age, weight, or sex.
-
2.
CIA modeling was performed on both the treatment group and the control group. The treatment group was administered with one of the three Tripterygium wilfordii extracts (TG or TP or CEL), while the control group received no intervention, or was given an equal volume of nonfunctional fluids (saline, distilled water, or vehicle) through oral gavage.
-
3.
Primary outcome measures included joint manifestations and histopathological changes in joints:
-
(a)
Joint manifestations encompassed the arthritis index (AI), paw swelling degree, and paw thickness.
-
(b)
Histopathological changes in joints included the histopathological score, cartilage damage, bone destruction, synovial hyperplasia, pannus formation, and inflammatory cell infiltration.
-
(a)
-
4.
Secondary outcome measures included the levels of various cytokines and immune markers, such as tumor necrosis factor-alpha (TNF-α), transforming growth factor beta (TGF-β), interferon-gamma (IFN-γ), interleukin (IL)-1β, IL-6, IL-23, IL-10, IL-2, IL-4, IL-17, anti-type II collagen antibodies, the percentage of CD4+ and CD8+ T cells, and the percentage of CD4+ T cells subsets (Th1, Th2, Th17, and Treg cells).
Exclusion criteria
(1) Other animal models of RA; (2) The treatment group received other pharmaceutical components; (3) Other administration route (intraperitoneal injection); (4) Clinical studies, reviews, conference abstracts, case reports, and in vitro studies; (5) No appropriate data and inadequate information in the literature; (6) Studies without full text; (7) Duplicate publications; (8) Non-SCI indexed journals; (9) Languages other than English.
Data extraction
Two authors (K.C. and X.Y.C.) used NoteExpress 4.0.0.9855 software (http://www.inoteexpress.com) to screen the literature, and extracted data independently, and the third author (S.H.) was consulted to solve disagreements. The main extracted information included: (1) The first author’s name and year of publication; (2) Animal species, sex, age, weight, sample size, modeling method, and anesthetic method; (3) Intervention measures, including extract category, dosage, and treatment duration; (4) Outcome measures; (5) If the data was shown in the form of graphs, we contacted the corresponding author of the relevant research to obtain the raw data. If this was not possible, we extracted data from the graphs through WebplotDigitizer online tool; (6) Standard error of the mean (SEM) was transformed to standard deviation (SD) when necessary, using SD = SEM * √n (n represents the sample size).
Methodological quality assessment
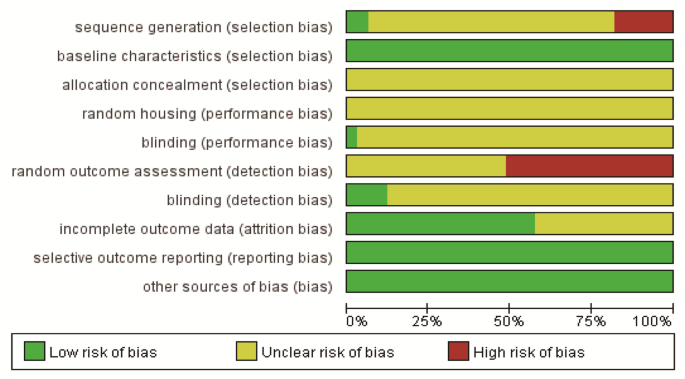
The SYRCLE’s risk of bias (RoB) tool for animal studies23 was independently employed by two authors (K.C. and X.Y.C.) through the evaluation of the following 10 items: (1) sequence generation (selection bias); (2) baseline characteristics (selection bias); (3) allocation concealment (selection bias); (4) random housing (performance bias); (5) blinding (performance bias); (6) random outcome assessment (detection bias); (7) blinding (detection bias); (8) incomplete outcome data (attrition bias); (9) selective outcome reporting (reporting bias); (10) other sources of bias (bias). All disagreements were solved by the author (S.H.). Each item was answered ‘Yes’, ‘Unclear’, or ‘No’, representing low, unclear, or high risk, respectively.
Statistical analysis
This meta-analysis was performed by using Stata 18.0 software (StataCorp, College Station, TX, USA) and Review Manager 5.4.1 software (The Cochrane Collaboration, 2020). For continuous data, the weighted mean difference (WMD) or standardized mean difference (SMD) and 95% confidence interval (CI) were used to express the effect size. Heterogeneity was assessed using the Chi-squared test and I2 statistic. If I2 > 50% or Chi2 test p < 0.1, a random-effects model was used; otherwise, a fixed-effects model was applied.
To further explore the sources of heterogeneity, we conducted meta-regression and subgroup analyses on animal species and sex, extract category and dosage, treatment duration, and sample size to identify potential influencing factors. Meta-regression was performed for outcome measures when the number of included studies reached 10 or more, while subgroup analyses were conducted when the number of studies included in the analysis exceeded three. The dosage classification method was as follows: for different categories of Tripterygium wilfordii extracts in each study, we analyzed the maximum, minimum, and median dosages. Dosages less than the median were defined as low dosages, while those greater than or equal to the median were defined as high dosages. Additionally, sensitivity analysis was implemented to evaluate the stability of the results by sequentially removing each study. Furthermore, when the number of included studies reached 10 or more, funnel plots and Egger’s test were performed to assess the potential publication bias.
Results
Study selection
A total of 3362 articles were retrieved from six online databases. After excluding duplicates, 1746 articles remained. Subsequently, 1326 irrelevant studies were excluded based on the titles and abstracts. Next, following careful examination of the full texts of 420 articles, 388 were excluded. Ultimately, 32 articles were included in the study. The specific flow diagram is shown in Fig. 124,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55.
Characteristics of the included studies
A total of 41 independent experiments are detailed in the 32 studies, with specific information presented in Table 1. The drug used in 2 studies was CEL, 14 studies used TP, and 16 studies used TG. A total of 568 animals were involved, with 282 in the treatment group and 286 in the control group. Sprague–Dawley (SD) rats were used in 11 studies, Wistar rats in 8 studies, DA rats in 1 study, DBA/1 mice in 10 studies, C57BL/6 mice in 1 study, and Kunming mice in 1 study. Male animals were used in 25 studies, female animals in 5 studies, an equal mixture of male and female animals in 1 study, and 1 study did not report the sex of the animals. 22 studies employed bovine type II collagen for modeling, 6 studies utilized chicken type II collagen, and 4 studies used rat type II collagen. Pentobarbital anesthesia was used in 3 studies, chloral hydrate anesthesia in 3 studies, urethane anesthesia in 1 study, isoflurane in 1 study, and anesthesia method was not specified in 24 studies.
Quality assessment
Among the 32 included studies, except for 6 that were not randomly grouped, other studies mentioned random grouping, and 2 of them reported the random sequence generation process. All studies met the evaluation criteria of baseline characteristics between the groups. None of the studies reported allocation concealment and random housing. One study reported blinding of caregivers and experimental researchers. 17 studies did not describe the random methods for outcome assessment, while four studies mentioned blinded assessment of outcomes. 14 studies did not report complete outcome data. No selective outcome reporting was observed. The studies had no other sources of bias. The quality assessment result is shown in Table 2; Fig. 2.
Effectiveness
Primary outcome measures
Joint manifestations
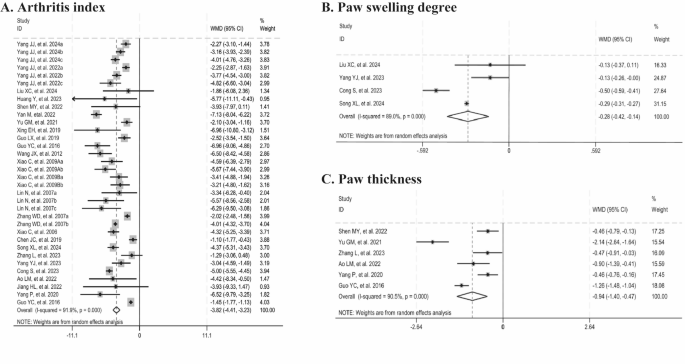
The arthritis index (AI) was used as the outcome measure in 25 studies. A significant reduction in AI was observed in the Tripterygium wilfordii extracts groups (n = 448, WMD = -3.82, 95% CI: -4.41 to -3.23, p = 0.000; I2 = 91.9%, p = 0.000, Fig. 3A). Paw swelling degree was adopted as the outcome measure in four studies. Tripterygium wilfordii extracts also significantly reduced the paw swelling degree compared with the control group (n = 60, WMD = -0.28, 95% CI: -0.42 to -0.14, p = 0.000; I2 = 89.0%, p = 0.0000, Fig. 3B). Besides, six studies assessed paw thickness as an outcome measure. Tripterygium wilfordii extracts groups had a significant reduction on paw thickness (n = 90, WMD = -0.94, 95% CI: -1.40 to -0.47, p = 0.000; I2 = 90.5%, p = 0.000, Fig. 3C). Sensitivity analyses of these three outcomes showed stable results by excluding animal studies one by one.
In addition, we further explored potential sources of heterogeneity through univariate meta-regression and subgroup analyses. The meta-regression analysis of AI identified animal species as the primary source of heterogeneity. Subgroup analysis indicated that, although the difference was not statistically significant, the 95% CI suggested a relatively more pronounced improvement in AI among mice. Moreover, subgroup analysis of paw thickness revealed that animal species and sex could be potential sources of heterogeneity. It was observed that heterogeneity was eliminated in the mice subgroup and in the male subgroup. The application in female animals showed a more pronounced improvement effect, whereas the application of TP did not show a significant improvement effect on paw thickness. However, both applications exhibited high heterogeneity (Tables 3 and 4).
Histopathological changes in joints
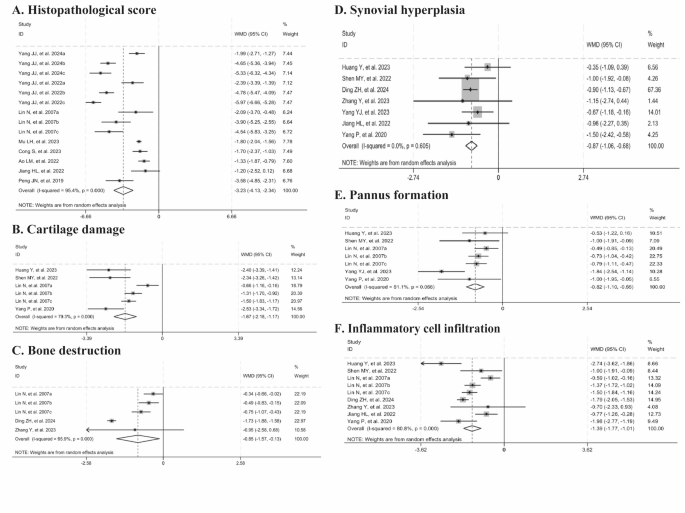
Eight studies showed that histopathological score was significantly reduced with Tripterygium wilfordii extracts (n = 140, WMD = -3.23, 95% CI: -4.13 to -2.34, p = 0.000; I2 = 95.4%, p = 0.000, Fig. 4A). Four studies documented cartilage damage (n = 76, WMD = -1.67, 95% CI: -2.18 to -1.17, p = 0.000; I2 = 79.3%, p = 0.000, Fig. 4B), three reported bone destruction (n = 58, WMD = -0.85, 95% CI: -1.57 to -0.13, p = 0.021; I2 = 95.9%, p = 0.000, Fig. 4C), seven observed synovial hyperplasia (n = 94, WMD = -0.87, 95% CI: -1.06 to -0.68, p = 0.000; I2 = 0%, p = 0.605, Fig. 4D), five noted pannus formation (n = 88, WMD = -0.82, 95% CI: -1.10 to -0.55, p = 0.000; I2 = 51.1%, p = 0.056, Fig. 4E), and seven reported inflammatory cell infiltration (n = 118, WMD = -1.39, 95% CI: -1.77 to -1.01, p = 0.000; I2 = 80.8%, p = 0.000, Fig. 4F). Collectively, these findings suggested that Tripterygium wilfordii extracts significantly ameliorate these pathological indices. Sensitivity analyses showed that the results were consistently stable when animal studies were excluded on a case-by-case basis, thus ensuring the robustness of the histopathological score, cartilage damage, and inflammatory cell infiltration results. After excluding the study by Ding ZH, et al. (2024), the heterogeneity for bone destruction was significantly reduced (I2 = 14%). Similarly, upon the exclusion of the study by Yang YJ, et al. (2023), no heterogeneity was observed in pannus formation (I2 = 0%). The improved results for these analyses remained consistent upon the exclusion of these studies.
Considering the high heterogeneity of the above five indicators, we further employed meta-regression and subgroup analyses. Meta-regression analysis revealed that animal species and extract category were the primary sources of heterogeneity for histopathological score. 95% CI from subgroup analysis indicated that improvements in histopathological score were more pronounced with the use of mice compared to rats, showing a significant difference (95% CI not overlapping). Additionally, improvements in histopathological score were more pronounced with CEL and TP than with TG, with a significant difference for CEL (95% CI not overlapping). Subgroup analysis of pannus formation revealed that extract category, dosage, and treatment duration were major sources of heterogeneity. The results indicated that heterogeneity was eliminated in groups treated with TP, using high dosages, and with a treatment duration of less than or equal to one month. Meanwhile, the results showed that the improvement effect became insignificant when the treatment duration exceeded one month, exhibiting a high degree of heterogeneity in this subgroup. Subgroup analysis of bone destruction also showed that extract category, dosage, and treatment duration were important sources of heterogeneity. The results indicated that heterogeneity was eliminated in groups where TG was applied, low dosages were selected, and the treatment duration was more than one month. The 95% CI suggested that the improvement effect was significantly stronger in the group applying TG, and with a treatment duration of more than one month compared to the group applying TP, and with a treatment duration of less than or equal to one month (95% CI not overlapping) (Tables 3 and 5).
Secondary outcome measures
Cytokines and antibody changes
-
(1)
Cytokines related to the activation and differentiation of innate immune cells.
These cytokines are mainly secreted by innate immune cells, including pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-23, as well as anti-inflammatory cytokines like IL-1056. The levels of TNF-α were reported in eighteen studies, IL-1β in eleven, IL-6 in twelve, IL-23 in three, and IL-10 in ten studies. The meta-analysis results demonstrated significant reductions in TNF-α (n = 274, SMD = -3.36, 95% CI: -4.17 to -2.56, p = 0.000; I2 = 80.9%, p = 0.000, Fig. 5A), IL-1β (n = 158, SMD = -3.77, 95% CI: -4.84 to -2.69, p = 0.000; I2 = 79.1%, p = 0.000, Fig. 5B), IL-6 (n = 176, SMD = -2.40, 95% CI: -3.24 to -1.55, p = 0.000; I2 = 72.7%, p = 0.000, Fig. 5C), and IL-23 (n = 42, SMD = -1.92, 95% CI: -3.24 to -0.60, p = 0.004; I2 = 58.9%, p = 0.088, Fig. 5D) levels in the groups treated with Tripterygium wilfordii extracts compared to the control groups. Conversely, upregulated changes in IL-10 levels (n = 164, SMD = 2.89, 95% CI: 1.62 to 4.15, p = 0.000; I2 = 87.3%, p = 0.000, Fig. 5E) induced by Tripterygium wilfordii extracts were observed in ten studies. Due to the observed heterogeneity in these cytokines, sensitivity analyses were performed, and the findings demonstrated that the results remained stable when individual studies were sequentially excluded for TNF-α, IL-1β, and IL-10. Meanwhile, we discovered that excluding the study by Song XL, et al. (2023) reduced IL-6 heterogeneity to 47%, and the significant improvement persisted (p < 0.00001). Similarly, omitting the study by Shen MY, et al. (2022) markedly decreased IL-23 heterogeneity to 0%, with the significant improvement also remaining (p < 0.0001).
To further explore the sources of heterogeneity, meta-regression and subgroup analyses were conducted. For IL-1β, meta-regression analysis revealed that animal species and extract category may be the primary sources of heterogeneity. The results indicated that the improvements in IL-1β levels were more pronounced when using mice and applying CEL, showing a significant difference (95% CI not overlapping). Meta-regression analysis for TNF-α also identified that extract category may be the main source of heterogeneity. Improvements in TNF-α levels were more pronounced when CEL or TP were applied compared to TG, with a significant difference for CEL (95% CI not overlapping). For IL-10, meta-regression and subgroup analyses found that extract dosage and treatment duration could be the primary sources of heterogeneity. The results revealed that improvements in IL-10 levels were more pronounced with low dosages and treatment durations exceeding one month, showing a significant difference (95% CI not overlapping). In contrast, high dosages did not significantly improve IL-10 levels (Tables 6 and 7).
-
(2)
Cytokines related to the activation and differentiation of adaptive immune T cells.
These cytokines are mainly secreted by adaptive immune T cells. IL-2 is a cytokine related to the activation of CD4 and CD8 cells56. Changes in IL-2 levels were assessed in four studies. The meta-analysis indicated that the effect of Tripterygium wilfordii extracts on IL-2 levels was not statistically significant (n = 56, SMD = -2.11, 95% CI: -4.72 to 0.49, p = 0.112; I2 = 90.9%, p = 0.000, Fig. 5F). IFN-γ is a cytokine associated with Th1 differentiation. Four studies evaluated changes in IFN-γ levels. The meta-analysis results demonstrated that Tripterygium wilfordii extracts significantly downregulated the expression levels of IFN-γ (n = 68, SMD = -1.86, 95% CI: -2.98 to -0.74, p = 0.001; I2 = 67.4%, p = 0.015, Fig. 5G). IL-4 is a cytokine associated with Th2 differentiation. Changes in IL-4 levels were assessed in four studies. The meta-analysis indicated that IL-4 levels (n = 62, SMD = 1.15, 95% CI: 0.54 to 1.76, p = 0.000; I2 = 0%, p = 0.691, Fig. 5H) were significantly upregulated in the groups treated with Tripterygium wilfordii extracts. IL-17 is a cytokine associated with Th17 differentiation. IL-17 levels were reported in five studies and the meta-analysis results demonstrated significant reductions in IL-17 levels (n = 74, SMD = -2.29, 95% CI: -3.03 to -1.56, p = 0.000; I2 = 46.6%, p = 0.112, Fig. 5I) in the groups treated with Tripterygium wilfordii extracts. TGF-β is a cytokine associated with Treg differentiation. Upregulated changes in TGF-β levels (n = 70, SMD = 1.98, 95% CI: 0.83 to 3.14, p = 0.001; I2 = 77.0%, p = 0.002, Fig. 5J) induced by Tripterygium wilfordii extracts were observed and reported in four studies. Due to the observed heterogeneity in the above indicators, sensitivity analyses were conducted. The findings demonstrated that when the study by Mu LH, et al. (2023) was excluded, the heterogeneity of IFN-γ was reduced to 33%, and the results continued to show a significant decrease (p < 0.0001). Additionally, when each study was sequentially excluded from the analysis, the results of the remaining indicators remained stable.
Meanwhile, considering the heterogeneity observed in multiple indicators mentioned above, we further performed subgroup analyses. The subgroup analyses of IFN-γ levels indicated that extract category and dosage might be the main sources of its heterogeneity. The results found that the application of TP, low dosage had a significant effect, while the application of TG, high dosage did not improve significantly (p > 0.05). Subgroup analyses of IL-17 levels suggested that animal species, extract category, and dosage may be the main sources of heterogeneity. Heterogeneity was eliminated in groups treated with TG, using low dosages, and in mice. Although the 95% CI indicated that administration of TG, selection of the low dosage, and application to mice had a slightly better effect compared to administration of TP, selection of the high dosage, and application to rats, this difference was not statistically significant. Additionally, subgroup analyses indicated that animal species may be the main source of TGF-β heterogeneity. The 95% CI showed a significant difference, with more pronounced improvement observed in rats compared to mice (95% CI not overlapping) (Table 8).
-
(3)
Antibodies related to the activation of adaptive immune B cells.
Anti-type II collagen antibodies. These antibodies are primarily secreted by adaptive immune B cells. Four studies reduced anti-type II collagen antibodies with Tripterygium wilfordii extracts compared with controls (n = 54, SMD = -4.27, 95% CI: -6.88 to -1.66, p = 0.001; I2 = 89.5%, p = 0.000, Fig. 5K). Sensitivity analyses demonstrated that the results were stable when each study was sequentially excluded from the analysis of anti-type II collagen antibodies. Subgroup analyses revealed that the use of rats and low dosages had significant effects, while the use of mice and high dosages did not show significant improvements (p > 0.05) (Table 9).
Changes in T-cell subsets
-
(1)
Changes in the ratio of CD4+ and CD8+ T cells.
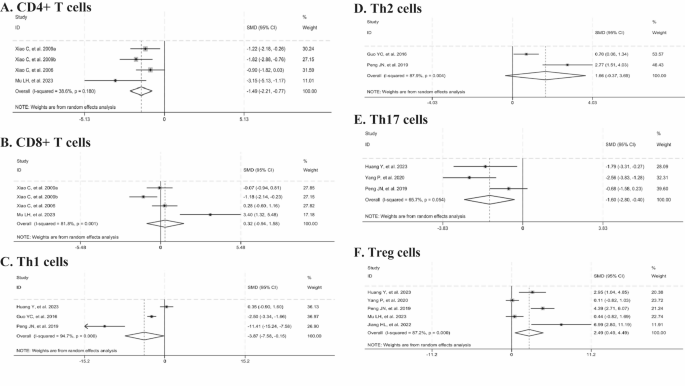
Three studies reported the percentage of CD4+ and CD8+ T cells. The meta-analysis revealed that the intervention with Tripterygium wilfordii extracts significantly reduced the percentage of CD4+ T cells (n = 50, SMD = -1.49, 95% CI: -2.21 to -0.77, p = 0.000; I2 = 38.6%, p = 0.180, Fig. 6A), whereas no statistically significant difference was observed in the percentage of CD8+ T cells (n = 50, SMD = 0.32, 95% CI: -0.94 to 1.58, p = 0.616; I2 = 81.8%, p = 0.001, Fig. 6B), compared to controls.
-
(2)
Changes in the proportions of different subsets of CD4+ T cells.
Three studies documented the percentage of Th1 cells, two reported on Th2 cells, three on Th17 cells, and five on Treg cells. Compared with the control group, Tripterygium wilfordii extracts significantly reduced the levels of Th1 cells (n = 70, SMD = -3.87, 95% CI:-7.58 to -0.15, p = 0.041; I2 = 94.7%, p = 0.000, Fig. 6C) and Th17 cells (n = 48, SMD =-1.60, 95% CI: -2.80 to -0.40, p = 0.009; I2 = 65.7%, p = 0.054, Fig. 6E). In contrast, they significantly elevated the level of Treg cells (n = 66, SMD = 2.49, 95% CI: 0.49 to 4.49, p = 0.015; I2 = 87.2%, p = 0.000, Fig. 6F), whereas no significant difference was observed in Th2 cells (n = 60, SMD = 1.66, 95%CI:-0.37 to 3.69, p = 0.109; I2 = 87.9%, p = 0.004, Fig. 6D). Given the observed heterogeneity, sensitivity analyses were conducted. The results demonstrated that the findings remained stable when individual studies were sequentially excluded from the analyses of Th1 and Treg cells. Upon excluding the study by Peng, JN, et al. (2019), heterogeneity was no longer observed (I2 = 0%), and the statistically significant effect on Th17 cells persisted (p < 0.0001). Subgroup analyses revealed that animal species and treatment duration were the dominant factors contributing to heterogeneity in Treg cells. The results indicated that the use of rats and a treatment duration exceeding one month resulted in significant improvements in Treg levels. In contrast, the use of mice and a treatment duration of less than or equal to one month did not show significant improvements (p > 0.05) (Table 10).
Publication bias
We used the funnel plot and Egger’s test to explore the potential publication bias of AI, histopathological score, TNF-α, IL-6, IL-10, and IL-1β. The funnel plots displayed visual asymmetry, suggesting possible publication bias for these six outcome measures (Fig. S1). Subsequently, Egger’s test indicated evidence of publication bias for TNF-α, IL-6, IL-10, and IL-1β, but not for AI and histopathological score (Fig. S2). The consistency of results before and after the Trim-and-fill analysis demonstrated the stability of the pooled effect sizes for TNF-α, IL-6, IL-10, and IL-1β (Fig. S3 and Table S2).
Discussion
RA is characterized by histopathological changes such as synovial hyperplasia, pannus formation, bone destruction, cartilage erosion, and inflammatory cell infiltration. Notably, synovial hyperplasia and bone destruction are the hallmark pathological alterations at the joint sites of RA. It has also been shown that the infiltration of various inflammatory cells at the bone-cartilage interface plays a pivotal role in mediating these changes14,57. Despite previous studies affirming its significant clinical effectiveness and cost-effectiveness in treating RA, the impacts and underlying mechanisms of Tripterygium wilfordii extracts on these different pathological changes, particularly on the diverse infiltrating inflammatory cells, are not yet fully understood. This uncertainty limits their wider application in clinical practice and warrants further investigation. Therefore, we conducted this preclinical study to explore and clarify the ameliorative effects and mechanisms of Tripterygium wilfordii extracts on these pathological changes, thereby further deepening and expanding its application in the treatment of RA.
In this study, we not only discovered the effect of Tripterygium wilfordii extracts on improving joint swelling in CIA models, but also elucidated their role and characteristics in addressing different pathological stages. Meanwhile, we further explored the mechanisms and features of Tripterygium wilfordii extracts in modulating innate immune-related cytokines, adaptive immune-related cell subsets, and cytokines to ameliorate inflammatory cell infiltration in CIA model. The selection of animals for the CIA model, as well as the extract category and administration regimen, were key factors in observing the effects and studying the mechanisms of Tripterygium wilfordii extracts. These factors were also important sources of heterogeneity in the study. Therefore, in addition to the aforementioned analyses, we conducted meta-regression and subgroup analysis to fully explore the impacts of various factors such as animal species and sex, extract category and dosage, treatment duration, and sample size on the effects and mechanisms of Tripterygium wilfordii extracts.
The improvement effect of Tripterygium wilfordii extracts on joint swelling in the CIA model
Effectiveness evaluation
Joint swelling is the most prominent clinical feature of RA and also a characteristic change in the CIA models. Currently, the main assessment methods for joint swelling in CIA include AI, paw swelling degree, and paw thickness. Our meta-analysis results indicated that Tripterygium wilfordii extracts can significantly ameliorate joint swelling, with notable effects observed in reducing AI and improving paw swelling degree and thickness. Sensitivity analyses of these outcomes showed that the results remained stable even when individual studies were excluded one by one, demonstrating the robustness of this effect. This effect is consistent with the findings in clinical studies11,12,13, further suggesting that the efficacy of Tripterygium wilfordii in improving joint swelling is consistent and stable across species.
Analysis of influencing factors
The heterogeneity analysis revealed that animal species and sex were major factors influencing the improvement of joint swelling in the CIA models treated with Tripterygium wilfordii extracts. The improvement in AI was more pronounced in mice, and the heterogeneity in paw thickness analysis was eliminated. Similarly, when male animals were used, the heterogeneity in paw thickness also disappeared.
These findings suggest that male mice may exhibit a more sensitive and stable response when evaluating the effects of Tripterygium wilfordii extracts on improving joint swelling. This could be related to the better stability and sensitivity of the CIA model constructed using male mice. However, considering that the incidence of RA is much higher in female patients than in males in clinical practice58, it is also necessary to further explore methods for establishing stable RA models using female animals.
The role of T ripterygium wilfordii extracts in improving joint histopathological changes in the CIA model
Effectiveness evaluation
The pathological changes of RA are characterized by inflammatory cell infiltration, synovial proliferation, and bone destruction, along with other features such as pannus formation and cartilage damage. Our meta-analysis results demonstrated that Tripterygium wilfordii extracts can significantly improve the histopathological changes in CIA joints. Not only did they significantly reduce the overall histopathological scores of joint tissues, but they also notably lowered the histopathological scores associated with synovial proliferation, pannus formation, inflammatory cell infiltration, cartilage damage, and bone destruction. A sensitivity analysis further revealed the stability of these effects. Furthermore, the results indicated that among the various histopathological changes in CIA, the scores for synovial proliferation showed the greatest homogeneity, suggesting that the effect of Tripterygium wilfordii extracts in improving synovial proliferation was the most stable. As there are limited pathological data and information available in current clinical studies, these findings provide an important basis for further understanding the pathological mechanisms underlying the clinical improvement of joint swelling observed with Tripterygium wilfordii extracts.
Analysis of influencing factors
-
(1)
Animal selection for CIA model construction.
The heterogeneity analysis revealed that animal species was also an important factor influencing the variability in the effect of Tripterygium wilfordii extracts on improving joint pathology in the CIA model. Similar to the joint score analysis described above, the overall improvement in joint pathology was significantly more prominent when using mice.
-
(2)
Extract categories and selection of administration regimens.
The heterogeneity analysis revealed that extract category, dosage, and treatment duration were also crucial factors influencing the improvement of joint pathology in the CIA model by Tripterygium wilfordii extracts. Different extracts and application regimens not only varied in their action intensity, but also in the characteristics of their ameliorative effects on different pathological aspects.
In terms of overall histopathological changes in joint tissues, the application of CEL and TP was more effective compared to TG, with the difference for CEL being significant. This suggested that CEL and TP, as key active components in TG, may more prominently improve the overall histopathological changes in the joints when other potentially interfering components are eliminated. However, different extracts exhibit varying effects on different pathological aspects. In the case of pannus formation, heterogeneity was eliminated with the application of TP. As for bone destruction, heterogeneity disappeared with the application of TG, and the improvement effect was significantly stronger with TG. These differences in effects suggest that TP may be an important component of TG for improving pannus formation, while other components in TG may play a significant role in improving bone destruction.
Furthermore, different application regimens of various extracts exhibited distinct effects on diverse pathological aspects. Specifically, for pannus formation, heterogeneity was eliminated at high dosages and with treatment duration of one month or less. However, when the treatment duration exceeded one month, the improvement effect became insignificant. In contrast, for bone destruction, heterogeneity was eliminated with low dosages and treatment durations exceeding one month. Additionally, treatment duration longer than one month demonstrated a significantly stronger improvement effect compared to those of one month or less. These differences in effects suggest that varying dosages and treatment durations of Tripterygium wilfordii extracts may exert markedly different impacts on distinct pathological aspects, such as pannus formation and bone destruction. The differences attributed to treatment durations may be related to the fact that pannus formation predominantly occurs during the early stages of RA pathological changes, whereas bone destruction mostly manifests in the later stages59. Given the multi-target characteristics of compounds like TP, differences attributed to dosage may be associated with the specific target actions. This also suggests that we need to choose the appropriate treatment duration and dosages based on the characteristics of pathological changes and drug action properties.
Regulatory effects of T ripterygium wilfordii extracts on abnormal expression of innate immune-related cytokines in the CIA model
Effectiveness evaluation
Inflammatory cell infiltration plays a crucial role in synovial proliferation, pannus formation, bone destruction, and cartilage damage. In the pathological process of RA, inflammatory cell infiltration is primarily associated with dysregulation of innate immune modulation, characterized by increased release of various pro-inflammatory cytokines and decreased release of anti-inflammatory cytokines by synovial fibroblasts, monocyte-macrophages, and other relevant cells. Our meta-analysis results demonstrated that Tripterygium wilfordii extracts significantly inhibited the secretion of pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, and IL-23, while simultaneously increasing the secretion of the anti-inflammatory cytokine IL-10 by innate immune cells in CIA model. Sensitivity analysis further confirmed the robustness of these effects. The results are consistent with those reported in current clinical studies on the effects of Tripterygium wilfordii extracts on innate immune-related pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6. Additionally, this study further elucidated the effects of Tripterygium wilfordii extracts on the innate immune-related pro-inflammatory cytokine IL-23 and the anti-inflammatory cytokine IL-1011,12,13.
Analysis of influencing factors
-
(1)
Animal selection for CIA model construction.
Heterogeneity analysis identified that the animal species was a critical source in the modulatory effects of Tripterygium wilfordii extracts on the innate immune-related pro-inflammatory cytokine IL-1β in CIA model. Similar to the findings for arthritis index and histopathological scores, the improvement in IL-1β levels was significantly more pronounced when using mice.
-
(2)
Extract categories and selection of administration regimens.
Heterogeneity analysis indicated that extract category, dosage, and treatment duration were significant factors influencing the dysregulation of innate immune-related cytokines in CIA model. However, the factors affecting pro-inflammatory and anti-inflammatory cytokines differed, and the characteristics of their effects were distinct.
Firstly, the factors influencing the secretion of pro-inflammatory and anti-inflammatory cytokines in innate immune cells differ. In the regulation of pro-inflammatory cytokines IL-1β and TNF-α, extract category is a significant determinant. Compared to TG, the application of CEL results in more pronounced and statistically significant reductions in IL-1β and TNF-α levels. In contrast, for the modulation of the anti-inflammatory cytokine IL-10, dosage plays a critical role, with low dosages of the extract producing more significant improvements than high dosages. Extract category affect its role in innate immune-related pro-inflammatory cytokines suggesting that CEL may play a more prominent role in inhibiting the secretion of pro-inflammatory cytokines, such as TNF-α and IL-1β, and is likely the major component of TG that exerted this role, and further attention needs to be paid to such major components of TG in such subsequent related studies. Meanwhile, the extract dosage influence its role in modulating innate immune-related anti-inflammatory cytokines, indicating that continuous low-dosage intervention may be the key to the modulation of anti-inflammatory cytokines exerted by Tripterygium wilfordii extracts. Further studies in this area should place greater emphasis on the dosage factor.
Moreover, the impact of treatment duration varies in its characteristics on the secretion of different pro-inflammatory and anti-inflammatory cytokines related to innate immune cells. For the regulation of the anti-inflammatory cytokine IL-10, treatment duration greater than one month showed significantly greater improvements compared to those of less than or equal to one month. Although only a limited number of studies included treatment durations exceeding one month, pro-inflammatory cytokines such as IL-1β and TNF-α displayed a consistent trend, showing significant improvements in treatment durations lasting less than or equal to one month compared to those exceeding one month. The differences in the efficacy among various treatment durations suggest that there may be significant differences in the effects of Tripterygium wilfordii extracts on innate immune-related pro-inflammatory and anti-inflammatory cytokines. These variations might be linked to phenotypic transitions of natural immune cells at different stages of CIA and their associated cytokine secretion patterns. Specifically, pro-inflammatory cytokines such as TNF-α and IL-1β are predominantly secreted during the immune-activation phase, while IL-10 functions primarily as a negative feedback regulator during the immune-enhancing phase60. These findings highlight the importance of considering the characteristics of innate immune cell phenotype transitions and the specific actions of drugs when determining appropriate treatment duration.
Regulatory effects of T ripterygium wilfordii extracts on adaptive immune T-cell subsets and cytokine abnormalities in CIA model
Effectiveness evaluation
-
(1)
Regulatory effects on the imbalance of CD4+ to CD8+ T cell ratios.
The continuous presence and progression of joint pathology in the RA pathological process are closely related to the dysregulation of adaptive immunity. In the adaptive T-cell immunity of RA, there is marked activation of T cells accompanied by increased secretion of IL-2, as well as increased proliferation and differentiation of CD4+ T cells, leading to dysfunctions such as an elevated ratio of CD4+ to CD8+ T cells. The meta-analysis showed that while the regulatory effect on CD8+ T cells was not significant, Tripterygium wilfordii extracts could significantly suppress CD4+ T cells, thereby adjusting the ratio between them. Similarly, the improvement effect on IL-2 levels was not significant. Sensitivity analysis further revealed the stability of these effects. Additionally, the heterogeneity analysis demonstrated that studies on the effects of Tripterygium wilfordii extracts on CD4+ T cells had the best homogeneity. This suggests that Tripterygium wilfordii extracts may exert their effects primarily by inhibiting the proliferation and differentiation of CD4+ T cells, rather than suppressing T cell activation, with this inhibitory effect being both clear and stable. Given the limited clinical data regarding the regulatory effects of Tripterygium wilfordii extracts on the imbalance of CD4+/CD8+ T cell ratio in current clinical studies, these findings provide an important basis for further elucidating the role of Tripterygium wilfordii extracts in modulating CD4+/CD8+ T cell ratio and analyzing their immunological principles for clinical improvement of joint swelling.
-
(2)
Regulatory effects on the imbalance of Th1/Th2 ratio in CD4+ T cell subsets.
In the pathological process of RA, the Th1 subset of CD4+ T cells plays a pivotal role in inflammatory activities. The proliferation and differentiation of CD4+ T cells are often dominated by the Th1 subset, resulting in an imbalance characterized by an elevated Th1/Th2 ratio. This is accompanied by increased secretion of the Th1 cytokine IFN-γ and decreased secretion of the Th2 cytokine IL-4. Our meta-analysis revealed that, although the regulatory effects of Tripterygium wilfordii extracts on Th2 cell differentiation were not significant, these extracts could significantly inhibit Th1 cell differentiation, thereby modulating the Th1/Th2 ratio. Specifically, these extracts significantly downregulated IFN-γ levels while upregulating IL-4 levels. Sensitivity analysis further confirmed the stability of these effects. Furthermore, the heterogeneity analysis showed that studies on the effects of Tripterygium wilfordii extracts on IL-4 levels exhibited the best homogeneity. This suggests that Tripterygium wilfordii extracts may primarily act by inhibiting Th1 differentiation rather than promoting Th2 differentiation. However, these extracts exhibit a dual effect of suppressing IFN-γ secretion while simultaneously promoting IL-4 secretion. As clinical evidence regarding Tripterygium wilfordii extracts’ modulation of Th1/Th2 imbalance remains scarce, these findings provide an important basis for further understanding the regulation of Th1/Th2 ratios underlying the clinical improvement of joint swelling with Tripterygium wilfordii extracts.
-
(3)
Regulatory effects on the imbalance of Th17/Treg ratio in CD4+ T cell subsets.
In the pathological process of RA, the Th17 subset of CD4+ T cells plays a crucial role in the persistence of inflammation. The proliferation and differentiation of CD4+ T cells are predominantly characterized by the dominance of the Th17 subset, leading to dysregulatory changes such as an elevated Th17/Treg cell ratio. These changes are accompanied by increased secretion of the Th17 cytokine IL-17 and decreased secretion of the Treg cytokine TGF-β. Our meta-analysis showed that Tripterygium wilfordii extracts significantly inhibited Th17 cells and increased Treg cells, thereby regulating the ratio between them. These extracts also significantly downregulated IL-17 levels while upregulating TGF-β levels. Heterogeneity analysis indicated that studies on the effects of Tripterygium wilfordii extracts on IL-17 levels exhibited the best homogeneity. This suggests that Tripterygium wilfordii extracts may simultaneously inhibit Th17 differentiation and promote Treg differentiation, with a more stable effect on inhibiting Th17 differentiation. As there is limited data and information on the regulatory effects of Tripterygium wilfordii extracts on the imbalance of Th17/Treg ratios in current clinical studies, these findings provide an important basis for further understanding the regulation of Th17/Treg ratios underlying the clinical improvement of joint swelling with Tripterygium wilfordii extracts.
Analysis of influencing factors
-
(1)
Animal selection for CIA model construction.
Heterogeneity analysis revealed that animal species was an important factor influencing the immunomodulatory effects of Tripterygium wilfordii extracts on adaptive immune T cells in the CIA model. However, different species exhibited varying sensitivities in their T cell subset responses. Regarding the secretion of the Th17 differentiation cytokine IL-17, heterogeneity was eliminated when using mice, and the improvement effect was more pronounced in mice compared to rats. Conversely, for Treg cell differentiation and related cytokine TGF-β secretion, the improvement was more evident in rats than in mice, with significant differences observed. Due to these species differences, CD4+ T cell subsets from different species may differ in their response during the pathological process of CIA and after intervention. These results suggest that mice may be more sensitive to the differentiation and intervention response of the pro-inflammatory T cell subset Th17, whereas rats may show greater sensitivity to the anti-inflammatory T cell subset Treg. Therefore, the choice of appropriate animal species should be guided by the specific type of T cell immunity under investigation.
-
(2)
Extract categories and selection of administration regimens.
Heterogeneity analysis revealed that extract category and dosage were important factors influencing the immunoregulatory effects of Tripterygium wilfordii extracts on adaptive immune T cells in the CIA model. However, the influencing factors and effect characteristics differed among different T cell subsets.
Firstly, different extracts exert varying effects on distinct T cell subsets. Regarding the secretion of the Th1-related cytokine IFN-γ, the application of TP significantly improved the effect, while the application of TG did not. In contrast, for the secretion of the Th17-related cytokine IL-17, heterogeneity was eliminated with TG treatment, and its improvement was relatively stronger compared to TP. Since TG contains components other than TP, these differences in effects may be attributed to the presence of additional components in TG that either interfere with TP’s ability to improve Th1 differentiation and function or enhance TP’s effect on Th17 differentiation and function. This suggests that we need to select the appropriate extract category according to the specific T cell subset under investigation and the drug action characteristics.
Secondly, different extract application regimens showed variations in their effects on distinct T cell subsets. For the secretion of the Th1-related cytokine IFN-γ and the Th17-related cytokine IL-17, extract dosage was an important factor, with both cytokines demonstrating greater sensitivity and stability at low dosages. At low dosages, heterogeneity was eliminated, and the improvement effect was more pronounced. This may be related to the dose-response characteristics of TP and other active components in specific targets. For Treg cell differentiation, treatment duration was a crucial factor, and the improvements in Treg cells were more apparent when the treatment duration exceeded one month, whereas no significant improvement was observed with treatment duration less than or equal to one month. This is similar to the characteristics of the anti-inflammatory cytokine IL-10 produced by innate immune cells, playing an important role as the negative feedback factors to pro-inflammatory cytokines during the delayed phase in disease progression. This may be the reason why the difference is more pronounced mainly after three months. These findings indicate that selecting an appropriate extract application regimen should be based on the specific T cell subset of interest, as well as the characteristics of dosage and treatment duration.
Regulatory effects of T ripterygium wilfordii extracts on autoantibody secretion by adaptive immune B cells in CIA model
Effectiveness evaluation
Joint pathological changes during the progression of RA are closely associated with the secretion of autoantibodies by adaptive immune B cells. The results of meta-analysis showed that Tripterygium wilfordii extracts significantly reduced the secretion of anti-type II collagen antibodies by B cells. Sensitivity analysis further revealed the stability of this effect. These findings suggest that Tripterygium wilfordii extracts possess the ability to inhibit the secretion of autoantibodies by adaptive immune B cells. As current clinical studies offer limited data on how Tripterygium wilfordii extracts regulate autoantibody secretion by adaptive immune B cells, this result provides an important basis for further understanding the mechanism behind the clinical improvement in joint swelling induced by these extracts.
Analysis of influencing factors
Heterogeneity analysis revealed that animal species and extract dosage were important factors influencing the regulatory effect of Tripterygium wilfordii extracts on the secretion of autoantibodies from B cells in the CIA model. Application in rats demonstrated significant improvement, whereas application in mice did not show such an effect, suggesting that rats may be more sensitive in terms of B cell immune responses. Therefore, it is crucial to select the appropriate animal species based on the type of cellular immunity under investigation. Low dosages of the extracts were associated with significant improvements, while high dosages did not show significant effects. This dose-response characteristic is similar to the T-cell immunomodulatory profile described above. These findings highlight the necessity of selecting an appropriate application regimen based on the type of cellular immunity of interest and the specific dosage.
Highlights
-
(1)
We found in this study that Tripterygium wilfordii extracts significantly ameliorate pathological changes in CIA joints by reducing synovial proliferation, pannus formation, inflammatory cell infiltration, cartilage damage, and bone destruction, thereby alleviating joint swelling in CIA models.
-
(2)
This study shows that the above effects may be partly attributed to the significant inhibitory action of Tripterygium wilfordii extracts on innate immune-related pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-23, as well as their significant promotion of the anti-inflammatory cytokine IL-10. It is also associated with their significant inhibitory effect on the differentiation of Th1 and Th17 cells and related cytokines IFN-γ and IL-17 among CD4+ T cells in adaptive immunity, as well as their promotional effect on the differentiation of Treg cells and their related cytokines TGF-β and Th2-associated cytokine IL-4.
-
(3)
This study shows that there are some differences in the effects and mechanisms among various Tripterygium wilfordii extracts, which suggesting that the above effects and mechanisms are combination from various extracts, and different extracts can be selectively applied in clinical practice according to different pathological and immune states. Regarding the inhibition of the secretion of pro-inflammatory cytokines related to innate immunity, such as TNF-α and IL-1β, CEL exhibits more pronounced effects. TP shows more significant efficacy in inhibiting the secretion of the Th1-related pro-inflammatory cytokine IFN-γ, and more stable and reliable responses in mitigating pannus formation in joint tissues. In contrast, TG demonstrates stronger effects in inhibiting the secretion of the Th17-related pro-inflammatory cytokine IL-17, and demonstrates superior efficacy in reducing bone destruction in joint tissues.
-
(4)
This study implies that the therapeutic effects and mechanisms of Tripterygium wilfordii extracts vary depending on the treatment durations at the same time, which suggesting that different treatment durations can be selectively applied in clinical practice according to different pathological and immune states. For mitigating pannus formation in joint tissues, a treatment duration of less than or equal to one month shows more stable and reliable outcomes. Conversely, for addressing bone destruction in joint tissues, treatment durations exceeding one month yields more significant results. For the inhibition of the secretion of pro-inflammatory cytokines such as TNF-α and IL-1β, treatment duration of less than or equal to one month exhibits a more pronounced effect. However, in terms of promoting the secretion of the anti-inflammatory cytokine IL-10, as well as enhancing the differentiation of Treg cells, treatment durations exceeding one month demonstrates more stable and reliable effects.
-
(5)
Additionally, this study implies that the therapeutic effects and mechanisms of Tripterygium wilfordii extracts vary with treatment dosage, which suggesting that different treatment dosages can be selectively applied in clinical practice according to different pathological and immune states. In promoting the secretion of the innate immune-related anti-inflammatory cytokine IL-10, inhibiting the secretion of T-cell pro-inflammatory cytokines such as Th1-associated IFN-γ and Th17-associated IL-17, suppressing B-cell anti-type II collagen antibody production, and ameliorating bone destruction, low-dosage extracts exhibit more stable and reliable improvements. Conversely, high-dosage extracts show relatively more reliable efficacy in mitigating pannus formation.
-
(6)
Furthermore, this study also implies that the choice of animal species in establishing the CIA model can lead to certain differences in effect evaluation, which suggesting that different animal species can be selectively applied in studies according to the different focus of research for pathological and immune states. Specifically, mice exhibit more sensitive and stable responses in assessing the effects on CIA-related joint swelling, improvement in joint pathology, and modulation of innate immunity and T-cell pro-inflammatory cytokines such as IL-1β and IL-17. In contrast, rats show more sensitive and stable responses in evaluating the modulation of T-cell anti-inflammatory subsets, including Treg cells and the cytokine TGF-β, as well as B-cell antibody secretion.
Limitations
At present, there is a lack of systematic and in-depth comparative studies on the differences in the effects and mechanisms of various Tripterygium wilfordii extracts, dosages, and treatment durations. A consensus on the optimal extract, dosage, and treatment duration for specific effects and mechanisms has not yet been formed. Due to the limited number of relevant studies, these factors that this study focuses on are relatively dispersed in subgroups of some indicators, with only a few studies conducted within each subgroup. This limitation makes it challenging to perform meta-regression and subgroup analyses. Furthermore, the heterogeneity analysis in this study represents an initial exploration that provides important clues for subsequent studies, but the effects of the corresponding factors still require further investigation to be confirmed.
In addition, this meta-analysis has the following limitations: (1) To ensure study quality, only English articles published in SCI-indexed journals were included, while studies in other languages or non-SCI journals were excluded, which may introduce selection bias. (2) The methodological quality of the included studies did not fully meet expectations, potentially affecting the reliability of the meta-analysis results. (3) Some of the included studies had relatively small sample sizes, and most were conducted in China, which may limit the generalizability of the findings. (4) Although meta-regression and subgroup analyses were performed for primary outcomes, some outcome measures still exhibited publication bias, potentially attributable to experimental design, extract categories, animal species, or variations in operational techniques among animal experimenters. (5) While multiple animal models of RA exist, this study exclusively analyzed the CIA model, which may introduce certain biases in fully replicating the pathological progression of human RA.
Conclusion
Tripterygium wilfordii extracts have demonstrated significant therapeutic effects on various pathological changes in CIA joints, including reducing synovial proliferation, pannus formation, inflammatory cell infiltration, cartilage damage, and bone destruction. These effects may be partly attributed to the significant inhibitory action of Tripterygium wilfordii extracts on innate immune-related pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-23, as well as their significant enhancement of the anti-inflammatory cytokine IL-10. It is also associated with their significant inhibitory effects on the differentiation of Th1 and Th17 cells and related cytokines IFN-γ and IL-17 among CD4+ T cells in adaptive immunity, as well as their promotional effects on the differentiation of Treg cells and the related cytokines TGF-β and the Th2-associated cytokine IL-4. This is of great significance for understanding the therapeutic effects and mechanisms of the extracts and for guiding their further application in clinical practice. At the same time, there are some differences in the effects and mechanisms among various Tripterygium wilfordii extracts, and also vary depending on the treatment durations and dosages, which suggests that different extracts, treatment durations, and dosages can be selectively applied in clinical practice according to different pathological and immune states.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Brown, P., Pratt, A. G. & Hyrich, K. L. Therapeutic advances in rheumatoid arthritis. BMJ-BRIT Med. J. 384, e70856 (2024).
Cao, F. et al. Temporal trends in the prevalence of autoimmune diseases from 1990 to 2019. Autoimmun. Rev. 22 (8), 103359 (2023).
Finckh, A. et al. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 18 (10), 591–602 (2022).
Di Matteo, A., Bathon, J. M. & Emery, P. Rheumatoid arthritis. Lancet 402 (10416), 2019–2033 (2023).
Xu, Y. et al. Applications and recent advances in transdermal drug delivery systems for the treatment of rheumatoid arthritis. Acta Pharm. Sin B. 13 (11), 4417–4441 (2023).
Prasad, P., Verma, S., Surbhi, Ganguly, N. K., Chaturvedi, V. & Mittal, S. A. Rheumatoid arthritis: advances in treatment strategies. Mol. Cell. Biochem. 478 (1), 69–88 (2023).
Chinese Rheumatology Association. 2018 Chinese guideline for the diagnosis and treatment of rheumatoid arthritis. Chin. J. Intern. Med. 57 (4), 242–251 (2018).
Fan, D. et al. The effect of triptolide in rheumatoid arthritis: from basic research towards clinical translation. Int. J. Mol. Sci. 19, 2 (2018).
Zhang, X. et al. Efficacy and safety of tripterygium wilfordii Hook F plus TNF inhibitor for active rheumatoid arthritis: A multicentre, randomized, double-blind, triple-dummy controlled trial. Clin. Immunol. 255, 109749 (2023).
Zeng, L. et al. Exploring the mechanism of Celastrol in the treatment of rheumatoid arthritis based on systems Pharmacology and multi-omics. Sci. REP-UK. 14 (1), 1604 (2024).
Zhang, X. et al. 2023 international consensus guidance for the use of tripterygium wilfordii Hook F in the treatment of active rheumatoid arthritis. J. Autoimmun. 142, 103148 (2024).
Luo, Y. et al. Tripterygium wilfordii Hook F combination therapy with methotrexate for rheumatoid arthritis: an updated meta-analysis. J. Ethnopharmacol. 307, 116211 (2023).
Feng, Z. et al. Efficacy of tripterygium glycosides (TG) in rheumatoid arthritis as a disease-modifying anti-rheumatic drug (DMARD) in combination with conventional dmards: a systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 184, 106405 (2022).
Scherer, H. U., Häupl, T. & Burmester, G. R. The etiology of rheumatoid arthritis. J. Autoimmun. 110, 102400 (2020).
Zhou, Y. Z. et al. Comparison of the impact of tripterygium wilfordii Hook F and methotrexate treatment on radiological progression in active rheumatoid arthritis: 2-year follow up of a randomized, non-blinded, controlled study. Arthritis Res. Ther. 20 (1), 70 (2018).
Wang, X. et al. Treatment of rheumatoid arthritis with combination of methotrexate and tripterygium wilfordii: A meta-analysis. Life Sci. 171, 45–50 (2017).
Lv, Q. W. et al. Comparison of tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): a randomised, controlled clinical trial. Ann. Rheum. Dis. 74 (6), 1078–1086 (2015).
Ritskes-Hoitinga, M. & Pound, P. The role of systematic reviews in identifying the limitations of preclinical animal research, 2000–2022: part 1. J. Roy Soc. Med. 115 (5), 186–192 (2022).
de Vries, R. B. et al. The usefulness of systematic reviews of animal experiments for the design of preclinical and clinical studies. Ilar J. 55 (3), 427–437 (2014).
Kong, J. S., Jeong, G. H. & Yoo, S. A. The use of animal models in rheumatoid arthritis research. J. Yeungnam Med. Sci. 40 (1), 23–29 (2023).
Wang, S. et al. Advances in experimental models of rheumatoid arthritis. Eur. J. Immunol. 53 (1), e2249962 (2023).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ-Brit Med. J. 372, n71 (2021).
Hooijmans, C. R. et al. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 14, 43 (2014).
Ao, L. et al. Matrine inhibits synovial angiogenesis in collagen-induced arthritis rats by regulating HIF-VEGF-Ang and inhibiting the PI3K/Akt signaling pathway. Mol. Immunol. 141, 13–20 (2022).
Chen, J. et al. Effects of Huatan Tongluo Decoction on vascular endothelial growth factor receptor 2 expression in synovial tissues of rats with collagen-induced arthritis. J. Tradit Chin. Med. 39 (2), 191–198 (2019).
Cong, S. et al. Saussurea involucrata oral liquid regulates gut microbiota and serum metabolism during alleviation of collagen-induced arthritis in rats. Phytother Res. 37 (4), 1242–1259 (2023).
Guo, Y., Xing, E., Liang, X., Song, H. & Dong, W. Effects of total saponins from rhizoma dioscoreae nipponicae on expression of vascular endothelial growth factor and angiopoietin-2 and Tie-2 receptors in the synovium of rats with rheumatoid arthritis. J. Chin. Med. Assoc. 79 (5), 264–271 (2016).
Guo, Y. et al. Therapeutic effect of Dioscin on collagen-induced arthritis through reduction of Th1/Th2. Int. Immunopharmacol. 39, 79–83 (2016).
Guo, L. X. et al. Saponins from clematis Mandshurica rupr. Regulates gut microbiota and its metabolites during alleviation of collagen-induced arthritis in rats. Pharmacol. Res. 149, 104459 (2019).
Huang, Y. et al. Triptolide alleviates collagen-induced arthritis in mice by modulating Treg/Th17 imbalance through the JAK/PTEN-STAT3 pathway. Basic. Clin. Pharmacol. 133 (1), 43–58 (2023).
Jiang, H. et al. Saponins from Nigella glandulifera seeds attenuate collagen-induced rheumatoid arthritis in rats via the OPG/RANKL/NF-κB and Ang/Tie-2 pathways. J. Ethnopharmacol. 283, 114714 (2022).
Li, J. et al. The mechanism of action of paeoniae radix rubra-angelicae sinensis radix drug pair in the treatment of rheumatoid arthritis through PI3K/AKT/NF-κB signaling pathway. Front. Pharmacol. 14, 1113810 (2023).
Lin, N. et al. Triptolide, a diterpenoid triepoxide, suppresses inflammation and cartilage destruction in collagen-induced arthritis mice. Biochem. Pharmacol. 73 (1), 136–146 (2007).
Liu, X. et al. Quantitative proteomic analysis of Circulating exosomes reveals the mechanism by which triptolide protects against collagen-induced arthritis. Immun. Inflamm. Dis. 12 (6), e1322 (2024).
Mu, L. et al. Chemical compositions of Souliea vaginata (Maxim) Franch rhizome and their potential therapeutic effects on collagen-induced arthritis in rats. J. Ethnopharmacol. 310, 116416 (2023).
Peng, J. et al. Dynamic alterations in the gut microbiota of Collagen-Induced arthritis rats following the prolonged administration of total glucosides of Paeony. Front. Cell. Infect. Mi. 9, 204 (2019).
Song, X. et al. Study of the mechanism underlying the anti-inflammatory effect of Miao medicine comprising Raw and processed radix wikstroemia indica using the sweat soaking method. J. Ethnopharmacol. 324, 117770 (2024).
Wang, J. et al. Effect of triptolide on T-cell receptor beta variable gene mRNA expression in rats with collagen-induced arthritis. Anat. Rec. 295 (6), 922–927 (2012).
Xiao, C. et al. The effects of triptolide on enteric mucosal immune responses of DBA/1 mice with collagen-induced arthritis. Planta Med. 72 (14), 1268–1272 (2006).
Xiao, C. et al. Effects of triptolide from radix tripterygium wilfordii (Leigongteng) on cartilage cytokines and transcription factor NF-kappaB: a study on induced arthritis in rats. Chin. MED-UK. 4, 13 (2009).
Xiao, C. et al. The effect of triptolide on CD4 + and CD8 + cells in the peyer’s patch of DA rats with collagen induced arthritis. Nat. Prod. Res. 23 (18), 1699–1706 (2009).
Xing, E. et al. Effects of Dioscin on T helper 17 and regulatory T-cell subsets in chicken collagen type II-induced arthritis mice. J. Chin. Med. Assoc. 82 (3), 202–208 (2019).
Yan, M. et al. The effect of triptolide combined with Crocin on arthritis in mice: from side effect Attenuation to therapy. Front. Pharmacol. 13, 908227 (2022).
Yang, P. et al. Zishen Tongluo formula ameliorates collagen-induced arthritis in mice by modulation of Th17/Treg balance. J. Ethnopharmacol. 250, 112428 (2020).
Yang, J. et al. Celastrol inhibits rheumatoid arthritis by inducing autophagy via Inhibition of the PI3K/AKT/mTOR signaling pathway. Int. Immunopharmacol. 112, 109241 (2022).
Yang, J. et al. Celastrol Regulates the Hsp90-NLRP3 Interaction to Alleviate Rheumatoid Arthritis. Inflammation. (2024).
Yu, G. M., Zhou, L. F., Zeng, B. X., Huang, J. J. & She, X. J. The antioxidant effect of triptolide contributes to the therapy in a collagen-induced arthritis rat model. Redox Rep. 26 (1), 197–202 (2021).
Zhang, W. et al. Therapeutic effect of combined triptolide and glycyrrhizin treatment on rats with collagen induced arthritis. Planta Med. 73 (4), 336–340 (2007).
Zhu, Y. et al. Tripterygium wilfordii glycosides ameliorates collagen-induced arthritis and aberrant lipid metabolism in rats. Front. Pharmacol. 13, 938849 (2022).
Ding, Z. et al. Altered Iron-Mediated Metabolic Homeostasis Governs the Efficacy and Toxicity of Tripterygium Glycosides Tablets Against Rheumatoid Arthritis. Engineering. (2024).
Yang, Y. J. et al. Tubson-2 Decoction ameliorates rheumatoid arthritis complicated with osteoporosis in CIA rats involving isochlorogenic acid A regulating IL-17/MAPK pathway. Phytomedicine 116, 154875 (2023).
Zhang, L. et al. Effect of salvia miltiorrhiza bunge extracts on improving the efficacy and reducing the toxicity of tripterygium wilfordii polyglycosides in the treatment of rheumatoid arthritis. J. Ethnopharmacol. 317, 116782 (2023).
Zhang, Y., Wang, X., Ding, Z., Lin, N. & Zhang, Y. Enhanced efficacy with reduced toxicity of tripterygium glycoside tablet by compatibility with total glucosides of Paeony for rheumatoid arthritis therapy. Biomed. Pharmacother. 166, 115417 (2023).
Shen, M. Y. et al. Triptolide inhibits Th17 differentiation via controlling PKM2-mediated Glycolysis in rheumatoid arthritis. Immunopharm Immunot. 44 (6), 838–849 (2022).
He, Y. C., Yao, Y. M., Xue, Q. W., Fang, X. & Liang, S. Anti-rheumatoid arthritis potential of diterpenoid fraction derived from rhododendron Molle fruits. Chin. J. Nat. Med. 19 (3), 181–187 (2021).
Wang, X., Ni, T., Miao, J., Huang, X. & Feng, Z. The role and mechanism of triptolide, a potential new DMARD, in the treatment of rheumatoid arthritis. Ageing Res. Rev. 104, 102643 (2025).
Guo, Q. et al. Rheumatoid arthritis: pathological mechanisms and modern Pharmacologic therapies. Bone Res. 6, 15 (2018).
Jin, W., Smith, A., Global, R. A. & Burden Consortium. Rheumatoid arthritis: Spatiotemporal distributions and regional disparities in 953 global to local locations, 1980–2040, with deep learning-empowered forecasts and evaluation of interventional policies’ benefits. Ann. Rheum. Dis. 84 (6), 1104–1116 (2025).
Chen, W. S., Xu, B. L., Lin, C. Y., Tian, J. & Zhang, X. L. Bone changes at different time after the occurrence of collagen-induced arthritis in rats. Chin. J. Gerontol. 40 (24), 5250–5254 (2020).
Shi, L. Q., Dong, T., Dian, Z. J. & Ouyang, H. M. Study on the expression of interleukins and tumor necrosis factor in serum of patients with rheumatoid arthritis. Int. J. Lab. Med. 36 (23), 3416–3417 (2015).
Funding
This study was supported by the National Natural Science Foundation of China (82374187) and the Natural Science Foundation of Jiangsu Province (BK20211299). This study was also supported by the Major Basic Research Project of the Natural Science Foundation of the Jiangsu Higher Education Institutions (24KJA360003).
Author information
Authors and Affiliations
Contributions
K.C. and Z.F. conceptualized the study. K.C. and X.Y.C. wrote the original draft and completed database searching. K.C., X.Y.C. and S.H. completed literature screening, data extraction, and quality assessment. L.L.Z., X.P.Z. and Y.L. provided senior advice and supervision. Z.F. provided funding acquisition and methodology advice.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, K., Chen, X., Huang, S. et al. Therapeutic effects and mechanisms of Tripterygium wilfordii extracts in rheumatoid arthritis: a systematic review and meta-analysis of preclinical studies. Sci Rep 15, 27960 (2025). https://doi.org/10.1038/s41598-025-13241-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13241-7