Abstract
Oxidative stress plays a key role in the development of metabolic disorders, such as diabetes. This study investigated the phytoconstituents present in Carica papaya (AECP) using an in silico model, and its anti-hyperglycemic activities on high sucrose-induced oxidative stress in Drosophila melanogaster. In silico molecular docking was performed to analyze the binding potential of AECP’s bioactive compounds to key diabetes targets. Flies were fed a diet containing 30% sucrose to induce oxidative stress, followed by administration of AECP at doses of 50 and 100 mg/kg for five days. Biochemical assays assessed were glucose, total thiols, catalase, glutathione S-transferase (GST), and nitric oxide. In silico analysis revealed that carpaine, myricetin 3-rhamnoside, orientin 7-O-rhamnoside, and quercetin in AECP exhibited strong binding potential to key diabetes targets (alpha-amylase, beta-glucosidase, dipeptidyl peptidase 4, PPARG, and SGLT-2)). In fruit flies, sucrose-diet significantly (p < 0.05) reduced total thiol level, and catalase and GST activities while increasing glucose and nitric oxide levels. The AECP in a dose-dependent manner significantly (p < 0.05) reversed these changes, demonstrating its antioxidant and possible anti-hyperglycemic properties. These findings suggest that AECP may be a potential therapeutic agent for mitigating oxidative stress and supports its potential use in managing diabetes.
Similar content being viewed by others
Introduction
Oxidative stress (OS) is a common pathophysiological condition caused by an excessive production of oxidants relative to antioxidants. Oxidative stress has been implicated in the development of several metabolic disorders, including diabetes. Diabetes mellitus is characterized by persistent hyperglycemia resulting from insulin resistance, inadequate or lack of insulin secretion or both1. Globally, the prevalence of diabetes in adults in 2021 was 537 million, with an estimated increase to 783 million by 2045. Type II diabetes accounts for about 90% of all diabetes cases, making it the most prevalent.
Virtual screening (VS) is a powerful tool in drug discovery, enabling researchers to efficiently identify potential lead compounds for further experimental validation2. Molecular docking is a game-changer in virtual screening, mimicking how pharmaceuticals or therapeutic substances attach to protein targets in the body. This technique was particularly influential during the COVID-19 pandemic, where it helped identify potential treatment candidates like remdesivir and nirmatrelvir3. Molecular docking cuts down the time and expense associated with conventional medication development and allows researchers to filter through vast libraries of possible candidates.
Natural antioxidants, present in various parts of plants, have shown to exhibit different health-promoting effects, by providing affordable management of free radical-causing diseases and avoiding possible toxicities associated with conventional medication4. Several medicinal plants, possessing antioxidant properties, have shown promising therapeutic potential against several diseases, including diabetes mellitus. Carica papaya Linn. is commonly called pawpaw, and it belongs to the family Caricaceae. The ripe pawpaw fruit is juicy and sweet when eaten raw. The plant has been reported to possess antimicrobial, antioxidant, anti-inflammatory, anti-hyperlipidemic, anticancer, anti-obesity, and anti-diabetic properties, and these have been attributed to the presence of several phytochemicals in various parts of the plant5.
Although, the anti-diabetic potential of Carica papaya extracts has been reported, most especially with the fruits. However, there is less information on the anti-hyperglycemic and anti-diabetic potential of the leaf extracts on diet-induced hyperglycemia/diabetes using non-mammalian models. This study therefore, centers on the use of in silico methods to study the interaction of some compounds present in C. papaya leaf with protein targets that are relevant to hyperglycemia and diabetes, highlighting the possible compounds that could be responsible for the pharmacological properties of the plant. In addition, this study supports the use of Drosophila melanogaster in biomedical research and the protective effect of the leaf extract on high sucrose diet-induced hyperglycemia and oxidative stress were also investigated.
Materials and methods
Chemicals
Ellman’s reagent (5’5-dithiobis(2-nitrobezoic acid) (DTNB)), 1-chloro-2,4,-dinitrobenzene (CDNB), trichloroacetic acid (EDTA), hydrogen peroxide, and Greiss Reagents were products of Sigma Aldrich Chemical Co. (St. Loius, MO, USA). Glucose kit was sourced from Randox laboratories limited (Crumin country Antrim, UK). Agar was a product of Readymed (Chaitanya group of industries, India). Every chemical used in this study is of analytical grade.
Plant material
Carica papaya leaves were obtained in July, 2023 from Iworoko-Ekiti in Ekiti state, Nigeria, and the plant was authenticated at the Department of Plant Science and Biotechnology, Ekiti State University, Ado-Ekiti, Nigeria, and a voucher number (2024029) was assigned to it. The leaves were separated, air-dried for three weeks, and pulverized using an electric blender. The blended leaves were sieved to obtain a fine powder.
Preparation of aqueous extract of Carica Papaya
Ten grams of the powdered leaf of Carica papaya were soaked in distilled water for 48 h at room temperature and then filtered. The filtrate was evaporated to dryness using a water bath at 45 °C, with a yield of 15%. The resulting extract was stored in an airtight container at −4 °C until further use.
In silico study
Selection of compounds present in Carica papaya Leaves
The compounds within the Carica papaya leaves were examined through searches on different platforms like PubMed, Google Scholar, Science Direct, and ResearchGate. The goal was to compile data on these bioactive compounds, employing diverse range of scholarly sources to ensure a comprehensive and thorough investigation.
Collection and preparation of bioactive compounds in Carica Papaya leaves
The 3D files of bioactive compounds present in C. papaya leaves were retrieved from the PubChem database. The 3D files underwent a comprehensive preparation procedure, such as the removal of water molecule and heteroatoms using Discovery Studio Visualizer software.
Source of diabetes protein targets
The 3D structure of the five diabetes protein targets (alpha amylase, beta glucosidase, dipeptidyl peptidase 4 (DDP-4), peroxisome proliferator-activated receptors (PPARG), sodium-dependent glucose cotransporter (SGLT-2), and sulfonylurea receptor 1 (SUR-1) were retrieved from RCSB protein data bank server. The resolution, dependability, and relevance of the chosen structure to the target enzyme served as guiding principles in the selection process.
Virtual screening workflow
Preparation: Carica papaya leaf’s bioactive compounds were retrieved from the PubChem database and downloaded as 3D structures and then prepared for docking using Discovery Studio software.
Docking: Each compound was docked against the five diabetes protein targets using Python Prescription (PyRx) software with Autodock Vina embedded for binding interactions. Docked poses were ranked based on their predicted binding affinity scores.
Docking and scoring methods
Molecular docking simulations were performed utilizing PyRx with AutoDock vina, during the virtual screening procedure. The scoring functions used in the docking simulations were selected based on their capacity to precisely forecast the binding affinities and interactions between the Carica papaya leaf compounds and the multiple diabetes protein targets.
Absorption, distribution, metabolism, and excretion (ADME) screening and toxicity (T) prediction
ADME prediction
The pharmacokinetic profiles of the selected bioactive compounds were evaluated using the SwissADME web tool (http://www.swissadme.ch/), provided by the Swiss Institute of Bioinformatics. SMILES (Simplified Molecular Input Line Entry System) notations of each compound were submitted to predict key descriptors relevant to ADME. The parameters analyzed included gastrointestinal (GI) absorption, P-glycoprotein (P-gp) substrate identification, lipophilicity (LogKp), water solubility (ESOL model), topological polar surface area (TPSA), blood-brain barrier (BBB) permeability, cytochrome P450 (CYP450) enzyme inhibition, and bioavailability score. These descriptors were used to infer the drug-likeness, oral bioavailability, and pharmacokinetic behavior of the compounds, providing early insights into their suitability for therapeutic application.
In silico toxicity evaluation
An in silico toxicity evaluation was performed for the fourteen compounds using the Greenstone Bio ADMET Prediction Platform (https://admet.ai.greenstonebio.com/), a web-based tool powered by machine learning. The platform predicts a wide range of ADMET properties based on quantitative structure–activity relationship (QSAR) models developed from curated experimental datasets. For each compound, the simplified molecular-input line-entry system (SMILES) notation was input into the platform’s interface. The tool returned predictions for several toxicity-related endpoints, including hERG channel inhibition, clinical toxicity, drug-induced liver injury (DILI), carcinogenicity, acute toxicity (LD50), and skin reaction potential. The output data were compiled and tabulated to facilitate a comparative toxicity analysis across all fourteen compounds. This computational approach provided preliminary insight into the safety profiles of the compounds, offering a rapid, cost-effective alternative to early-stage experimental screening.
Predictions of endocrine disruptors properties
The metabolic activity of 15 nuclear receptors was predicted using the Endocrine Disruptome web server (http://endocrinedisruptome.ki.si/, accessed on 09 October 2024). This server simulates the docking of each metabolite with crystal structures of various nuclear receptors, including androgen receptors (AR), oestrogen receptors α and β (ER α/β), glucocorticoid receptor (GR), liver X receptors α and β (LXR α/β), mineralocorticoid receptor (MR), peroxisome proliferator-activated receptors α, β, and γ (PPAR α, PPAR β, and PPAR γ), progesterone receptor (PR), retinoid X receptor α (RXR α), and thyroid receptors α and β (TR α and TR β). The results from the web server are categorized into three levels: red indicates a high binding potential, orange and yellow suggest a moderate binding probability, and green signifies a low likelihood of binding to the receptors.
In vivo study
Drosophila melanogaster stock and culture
Drosophila melanogaster of both genders (Harwich strain, 1–3 days old) was cultured on a cornmeal medium under controlled conditions at the Drosophila Laboratory, Biochemistry Program, College of Sciences, Afe Babalola University Ado-Ekiti, Ekiti State, Nigeria. The flies were fed on basal diet containing a mixture of cornmeal, brewer’s yeast (1% w/v), agar-agar (1% w/v), and methyl paraben (as preservative, 0.08% v/w), and were maintained at temperature of 23 ± 2 ◦C, under 12 h dark/light cycle.
Determination of survival rate
Experimental flies were cultivated on basal diets supplemented with different concentrations (0, 50, 100, 200, and 400 mg/kg diet) of Carica papaya leaf extract (AECP). Survival rates were recorded for 7 days as described by Oboh, et al.6.
Treatments of D. melanogaster with sucrose and AECP
Based on the survival studies, doses of 50 and 100 mg/kg AECP were chosen for further studies. Flies were fed with sucrose and AECP for 5 days, divided into groups as follows: Control (basal diet only), sucrose (30% w/v) diet only, sucrose (30% w/v) + AECP (50 mg/kg diet), sucrose (30% w/v) + AECP (100 mg/kg diet), AECP (100 mg/kg diet) only.
Preparation of flies homogenate for biochemical assays
Flies were anesthetized, weighed, homogenized in 0.1 M potassium phosphate buffer, and centrifuged. The supernatants were used for biochemical assays.
Parameters investigated
Parameters such as glucose level7, total protein level8, level of total thiols9, glutathione-s-transferase (GST) and catalase activities10, and nitric oxide (NO) level11, were carried out in whole flies’ homogenate.
Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA), followed by intergroup multiple comparison using the Tukey post hoc test on the GraphPad Prism (version 8.1). Results were expressed as mean ± standard deviation (SD), n = 5, and values of p < 0.05 were considered statistically significant.
Results and discussion
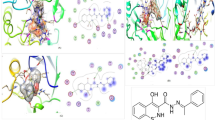
In this study, the in silico model examined 14 bioactive compounds from Carica papaya leaves (Table 1) alongside two reference drugs against five protein targets. Results indicate that carpaine, myricetin 3-rhamnoside, orientin 7-O-rhamnoside, and quercetin demonstrated the strongest binding scores. These compounds, therefore, hold potential as inhibitors of alpha-amylase, beta-glucosidase, DPP-4, PPARG, SGLT-2, and SUR-1 (Table 2), offering diverse pathways for managing diabetes. This aligns with findings by Kong, et al.12 and suggests the potential of Carica papaya-based diabetes treatments. In another research, bioactive compounds in natural compounds, such as quercetin, have been found to inhibit key enzymes involved in carbohydrate digestion, including alpha-amylase and beta-glucosidase13. By inhibiting these enzymes, AECP reduces the rate of carbohydrate breakdown and glucose absorption, thereby lowering blood glucose levels. These findings suggest that developing Carica papaya-based treatments could help manage diabetes.
Molecular investigations were undertaken on the inhibitory activities of the relative compounds in the Carica papaya leaves (Table 2). Two reference medicines (acarbose and metformin) were used to compare the docking score with the compounds in Carica papaya leaves. Four comparable compounds exhibited a good docking score compared to the two reference medicines utilized against various macromolecular targets. The four compounds with good docking scores were carpaine, myricetin 3-rhamnoside, orientin 7-O-rhamnoside and quercetin. These results revealed the following components might be the bioactive compounds contained in Carica papaya leaves that can potentially regulate OS complications like diabetes.
The data in Table 3 reveal the pharmacokinetic properties and bioavailability of various metabolites, examining key characteristics including GI absorption potential, ability to cross the BBB, P-gp substrate status, bioavailability score, and skin permeation (Log Kp). Differences in GI absorption values are observed among the metabolites, with myricetin 3-rhamnoside and orientin 7-O-rhamnoside showing low absorption potential, while quercetin, caffeic acid, carpaine, and protocatechuic acid exhibit high GI absorption values, indicating more effective absorption after oral intake. Five of the metabolites, including equisetin and ferulic acid could penetrate the BBB, implying potential activity on the central nervous system. Some metabolites are identified as P-gp substrates, such as equisetin and orientin 7-O-rhamnoside, influencing absorption processes in the body. Skin permeation values suggest that myricetin 3-rhamnoside, prunasin, and orientin 7-O-rhamnoside have poor skin penetration, while carpaine, Hexadecanamide and 9-octadecenamide show better absorption through the skin. Water solubility varies, with compounds like caffeic acid and protocatechuic acid being very soluble, while carpaine is poorly soluble. High TPSA in some compounds may limit permeability. Several compounds inhibit CYP enzymes, notably quercetin, equisetin, and 9-octadecenamide, indicating potential for drug–drug interactions. The bioavailability score indicates that most metabolites possess moderate scores, with compounds including ferulic acid, equisetin, quercetin, caffeic acid, and tenuazonic acid displaying high scores, suggesting their more effective use within the body. Overall, these findings reveal significant differences in the pharmacokinetic profiles of the metabolites, which may have important implications for their potential therapeutic effects, particularly for those with high GI absorption and bioavailability scores that warrant further investigation.
The toxicity prediction result for the fourteen compounds is presented in Table 4. Most compounds demonstrated low potential for hERG channel blocking, with values generally below 0.7, indicating a relatively low risk of cardiac arrhythmia. However, 9-octadecenamide (0.68) and carpaine (0.57) approached higher values, suggesting that their cardiotoxic potential should be monitored. In contrast, compounds like ferulic acid, caffeic acid, tenuazonic acid, and protocatechuic acid had minimal hERG blocking predictions (0.01), indicating a safer cardiac profile. Regarding clinical toxicity, most of the compounds showed low predictions, particularly 2-methoxy-4-vinylphenol (0.0021), and tenuazonic acid and hexadecanamide (0.01), suggesting a favorable safety profile in clinical settings. However, 11-hydroperoxy-12,13-epoxy-9-octadecenoic acid (0.35) was notably higher, implying a need for caution. Predictions for DILI were particularly high for myricetin 3-rhamnoside, quercetin (both 0.93), and orientin 7-O-rhamnoside (0.88), highlighting a potentially significant hepatotoxic risk. This suggests their potential toxicity at high doses. On the other hand, hexadecanamide (0.12) and 9-octadecenamide (0.11) had lower DILI predictions, indicating better liver safety. Most compounds exhibited low carcinogenicity predictions, with quercetin, ferulic acid, and 2-methoxy-4-vinylphenol all at or below 0.03. However, 9-octadecenamide (0.70), hexadecanamide (0.64), and tenuazonic acid (0.43) had elevated values, suggesting a possible carcinogenic concern. In acute toxicity, measured as LD50 in log(1/mol/kg), higher values reflect lower toxicity. Equisetin (3.81), myricetin 3-rhamnoside (3.38), and orientin 7-O-rhamnoside (3.14) exhibited the highest LD50 values, suggesting lower acute toxicity. Lastly, for skin reaction, compounds like 9-octadecenamide (0.92), quercetin (0.89), and 11-hydroperoxy-12,13-epoxy-9-octadecenoic acid (0.84) had high predictions, implying potential for dermal irritation or allergic response.
In this study, the endocrine-disrupting potential of fourteen selected metabolites was evaluated using the Endocrine Disruptome platform, and the results are presented in Figs. 1, 2, 3 and 4. Each compound was assessed for its binding probability to 15 nuclear receptors, including AR, ER α/β, GR, MR, PR, PPAR α/β/γ, TR α/β, RXR α, and LXR α/β. The predicted binding scores were classified based on interaction likelihood as high (red), moderate (orange), or low (green).
Among all tested compounds, quercetin (Fig. 4L) demonstrated the highest binding potential across multiple receptors, particularly AR, GR, MR, PPAR γ, and TR α, indicating a broad spectrum of endocrine-disrupting capacity. Similarly, carpaine (Fig. 2E) exhibited high-affinity binding specifically to the PR receptor, warranting consideration as a potential endocrine-active compound. Orientin 7-O-rhamnoside (Fig. 3İ) showed a moderate binding probability to the PR receptor, suggesting selective interaction potential.
Other metabolites such as ferulic acid (Fig. 2G), protocatechuic acid (Fig. 3J), prunasin (Fig. 3K), and tenuazonic acid (Fig. 4M) demonstrated moderate binding only to the AR α receptor, while exhibiting low affinity to other targets, indicating limited endocrine-disrupting potential. In contrast, compounds including 2-methoxy-4-vinylphenol (Fig. 1A), 9-octadecenamide (Fig. 1B), 11-hydroperoxy-12,13-epoxy-9-octadecenoic acid (Fig. 1C), hexadecanamide (Fig. 2H), equisetin (Fig. 2F), and myricetin 3’-rhamnoside (Fig. 3I) displayed low binding across all nuclear receptors, suggesting minimal endocrine interference.
Overall, AR α and PR emerged as the most frequently affected receptors, with several metabolites showing moderate to high affinity toward them. These findings highlight receptor-specific binding tendencies of phytochemical metabolites and suggest that a subset of these compounds may exhibit endocrine-disrupting behavior, especially through interaction with androgenic and progestogenic pathways.
Oxidative stress results from an excess of ROS, thereby, causing harmful oxidation and inflammation. These processes have been linked to various health conditions, including diabetes, Alzheimer’s disease, rheumatoid arthritis, cancers, cardiovascular diseases, cataracts, and even cosmetic issues like wrinkles12.
The fruit fly, Drosophila melanogaster, is a valuable model for investigating human health due to the significant homology between its genome and that of humans. Its short lifespan, ease of maintenance, and well-understood genetics make it a powerful tool for studying diseases like cancer and developmental studies14. One of the major parameters used to assess the effect of plants and plant products in D. melanogaster is the survival rate15. It was noted that low doses of the AECP (50 and 100 mg/kg diet) improved the survival rate of the flies, which suggests that caution should be taken with the consumption of AECP at high doses (Fig. 5). The observed safe doses (50 and 100 mg/kg diet) were, therefore, used to investigate the protective effect against high-sucrose-induced hyperglycemia and oxidative stress in fruit flies.
Previous studies indicate that sucrose consumption elevates glucose levels, and excess sucrose consumption can trigger OS and its complications16,17. The present study explores the potential of AECP as a countermeasure. A significant (p < 0.05) increase in the level of glucose was noted in flies fed with sucrose alone and in sucrose-fed flies treated with 50 and 100 mg/kg AECP when compared with the control (Fig. 6). Furthermore, a significant (p < 0.05) reduction in glucose level was observed in the sucrose-fed diet fortified with 50 mg/kg AECP compared to the sucrose-only treated group. Results indicate that AECP reversed the glucose elevation caused by a high sucrose diet in the flies. This finding supports the result of the in silico analysis which suggest that the hit compounds (bioactive compounds) in the Carica papaya may interact with the diabetic protein targets to reduce the excess glucose level.
Effect of aqueous extract of Carica papaya leaf on glucose level in sucrose-induced oxidative stress in flies. Data are presented as Mean ± SD of 40 flies/vial (n = 5). ap < 0.05 when compared with the control, bp < 0.05 when compared with sucrose only. AECP: Aqueous extract of Carica papaya; Suc: Sucrose.
The antioxidant potential of Carica papaya has been documented in various studies. The high antioxidant activity of Carica papaya extracts can be attributed to bioactive compounds such as alkaloids, flavonoids, saponins, glycosides, phytosterol, flavonoids, tannins terpenoids, and vitamins5. These compounds neutralize free radicals and boost the body’s antioxidant defense system. The role of catalase and GST in maintaining cellular health shows the importance of these enzymes in mitigating the effects of OS. Catalase, by breaking down hydrogen peroxide, prevents the formation of hydroxyl radicals, one of the most reactive and damaging types of free radicals18. Glutathione-s-transferase, through its detoxification role, helps eliminate reactive intermediates that could otherwise contribute to cellular damage and dysfunction as seen in this study.
In this study, the level of total thiols was significantly (p < 0.05) reduced in flies fed with a high-sucrose diet only and groups treated with a sucrose diet fortified with 50 and 100 mg/kg AECP when compared to the control group (Fig. 7). However, a significant (p < 0.05) increase in the level of total thiols in flies treated with 100 mg/kg AECP-only was observed when compared with sucrose-only fed flies. Also, a significant (p < 0.05) decrease in catalase activity was observed in sucrose-only treated flies when compared to the control (Fig. 8). The catalase activity was significantly (p < 0.05) increased in flies treated with both doses of AECP when compared to the group treated with sucrose only. The GST activity was significantly (p < 0.05) decreased in the group treated with sucrose only when compared to the control group and the group treated with 50 mg/kg diet AECP (Fig. 9). The GST activity was also significantly increased (p < 0.05) in the groups treated with AECP (100 mg/kg) when compared to the group treated with sucrose only. This could be associated with the antioxidant activity of the AECP. In addition, the binding score of carpaine as a potential compound, could enhance catalase activity as observed in the in silico analysis.
It has been reported that fruit extract of papaya increased the activities of antioxidant enzymes in biological systems, and ameliorated lipid peroxidation5supporting the notion that Carica papaya can enhance the body’s ability to manage OS. This aligns with the findings of this study, where the antioxidant effects of AECP restored enzyme activities and potentially improved overall cellular health and resilience against OS. This study adds to the growing body of evidence supporting the health benefits of Carica papaya, particularly its role in enhancing antioxidant defenses. The restoration of total thiols level, catalase and GST activities in the treatment groups showed the potential of Carica papaya as a natural remedy for managing OS and its associated complications. These findings are particularly relevant in dietary management and the potential use of Carica papaya in functional foods and nutraceuticals to reduce OS and improve overall health.
Effect of aqueous extract of Carica papaya leaf on the level of total thiol in sucrose-induced oxidative stress in flies. Data are presented as Mean ± SD of 40 flies/vial (n = 5). ap < 0.05 when compared with the control, bp < 0.05 when compared with sucrose only. AECP: Aqueous Extract of Carica papaya; Suc: Sucrose.
Effect of aqueous extract of Carica papaya leaf on the activity of catalase in sucrose-induced oxidative stress in flies. Data are presented as Mean ± SD of 40 flies/vial (n = 5). ap < 0.05 when compared with the control, bp < 0.05 when compared with sucrose only. AECP: Aqueous extract of Carica papaya; Suc: Sucrose.
Effect of aqueous extract of Carica papaya on the glutathione-s-transferase activity in sucrose-induced oxidative stress in flies. Data are presented as Mean ± SD of 40 flies/vial (n = 5). ap < 0.05 when compared with the control, bp < 0.05 when compared with sucrose only. AECP: Aqueous Extract of Carica papaya; Suc: Sucrose.
Elevated level of glucose (hyperglycemia) is associated with elevated production of NO through elevated expression of inducible NO synthase (iNOS) and endothelial NO synthase (eNOS) gene and protein levels19. In this study, a significant (p < 0.05) increase in the level of NO was noted in flies exposed to the sucrose diet only when compared to the control (Fig. 10). However, a significant (p < 0.05) reduction was noted in the sucrose diet-fed flies treated with both doses of AECP compared to the sucrose diet only. This suggests the ability of AECP to inhibit the production of NO.
Effect of aqueous extract of Carica papaya leaf on the level of nitric oxide in sucrose-induced oxidative stress in flies. Data are presented as Mean ± SD of 40 flies/vial (n = 5). ap < 0.05 when compared with the control, bp < 0.05 when compared with sucrose only. AECP: Aqueous extract of Carica papaya; Suc: Sucrose.
Conclusion
Carica papaya exhibits promising potential in preventing or delaying the onset of oxidative stress (OS)-related diabetes and its associated complications. The anti-hyperglycemic and antioxidant activities of Carica papaya extracts (AECP) leaves (most especially at low doses) highlight its therapeutic relevance in the management of metabolic disorders. Molecular docking studies further support this potential by demonstrating strong interactions between key metabolites—particularly carpaine, quercetin and caffeic acid—and several diabetes-related targets and nuclear receptors, suggesting possible molecular mechanisms for their bioactivity. The BBB permeability of some compounds, including equisetin and ferulic acid, could suggest the action of the leaf extract of C. papaya against the complications of diabetes, most especially neuropathy. Further studies are recommended to confirm this. Taken together, these integrated in silico evaluations provide a strong rationale for further experimental and clinical investigations into the therapeutic applications of Carica papaya and its bioactive compounds, particularly those with high GI absorption and docking affinity. Based on this findings, it could be suggested that AECP has the ability to improve insulin secretion and sensitivity, as well as glucose metabolism. Although, more studies are warranted to elucidate the underlying molecular mechanisms and optimize their potential use in managing OS-associated diseases such as diabetes.
Data availability
All data generated or analyzed during this study are included in this article.
References
Ayyoub, S. et al. Biosynthesis of gold nanoparticles using leaf extract of dittrichia viscosa and in vivo assessment of its anti-diabetic efficacy. Drug Delivery Translational Res. 12, 2993–2999 (2022).
Lin, X., Li, X. & Lin, X. A. Review on applications of computational methods in drug screening and design. Molecules 25, 1375 (2020).
Jang, W. D., Jeon, S., Kim, S. & Lee, S. Y. Drugs repurposed for COVID-19 by virtual screening of 6,218 drugs and cell-based assay. Proc. Natl. Acad. Sci. U S A 118, e2024302118 (2021).
Rahaman, M. M. et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: an update. Food Sci. Nutr. 11, 1657–1670 (2023).
Ugbogu, E. A. et al. Ethnomedicinal uses, nutritional composition, phytochemistry and potential health benefits of carica Papaya. Pharmacol. Res. - Mod. Chin. Med. 7, 100266 (2023).
Oboh, G., Atoki, A. V., Ademiluyi, A. O. & Ogunsuyi, O. B. African Jointfir (Gnetum africanum) and Editan (Lasianthera africana) leaf alkaloid extracts exert antioxidant and anticholinesterase activities in fruit fly (Drosophila melanogaster). Food Sci. Nutr. 11, 2708–2718 (2023).
Barham, D. & Trinder, P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 97, 142–145 (1972).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Ellman, G. L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82, 70–77 (1959).
Sinha, A. K. Colorimetric assay of catalase. Anal. Biochem. 47, 389–394 (1972).
Green, L. C. et al. Analysis of nitrate, nitrite, and [15 N]nitrate in biological fluids. Anal. Biochem. 126, 131–138 (1982).
Kong, Y. R. et al. Beneficial role of carica papaya extracts and phytochemicals on oxidative stress and related diseases: A mini review. Biology 10, 287 (2021).
Loukili, E. H. et al. Chemical analysis, antihyperglycemic properties and enzyme inhibition of Opuntia dillenii (Ker Gawl.) Haw: A detailed analysis of pulp and peel extracts. J. Pharm. Anal. https://doi.org/10.1016/j.jpha.2025.101320 (2025).
Mirzoyan, Z. et al. Drosophila melanogaster: A model organism to study cancer. Front. Genet. 10, 51 (2019).
Lopez-Ortiz, C. et al. Drosophila melanogaster as a translational model system to explore the impact of phytochemicals on human health. Int. J. Mol. Sci. 24, 13365 (2023).
Adesanoye, O. A. et al. Beneficial actions of esculentin-2CHa(GA30) on high sucrose-induced oxidative stress in Drosophila melanogaster. Food Chem. Toxicol. 157, 112620 (2021).
Yang, C., Yu, Y. & An, J. Effect of High-Sucrose diet on the occurrence and progression of diabetic retinopathy and dietary modification strategies. Nutrients 16, 1393 (2024).
Nandi, A., Yan, L. J., Jana, C. K. & Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid. Med. Cell. Longev. 2019, 9613090 (2019).
Adela, R. et al. Hyperglycaemia enhances nitric oxide production in diabetes: A study from South Indian patients. Plos One. 10, e0125270 (2015).
Author information
Authors and Affiliations
Contributions
O.I.O. and O.B.Ad. conceived and conceptualized the study. O.I.O. S.O.A., O.T.O. and O.B.Ad. supervised the work. S.J.I., O.S.O. and E.C.A. performed formal analysis and wrote the original draft. I.A.A. and O.B.Ak. performed formal analysis, data processing, and contributed to writing, review, and editing. All authors contributed to reviewing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Oloyede, O.I., Ibrahim, S.J., Anadozie, S.O. et al. In Silico evaluation of phytoconstituents from Carica Papaya and its anti-hyperglycemic activities on high sucrose-induced oxidative stress in Drosophila melanogaster. Sci Rep 15, 29834 (2025). https://doi.org/10.1038/s41598-025-13246-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13246-2