Abstract
Chronic kidney disease (CKD) causes a significant health and economic burden across the world. This study aimed to evaluate the effects of zinc on CKD-mineral bone disorder (CKD-MBD) and autophagy in CKD rats by a diet containing 0.25% adenine and low vitamin K and to explore its mechanism of action on autophagy and calcification in a vascular smooth muscle cell (VSMC) calcification model using 10 mM β-glycerophosphate (β-GP). In vivo experiments showed that zinc supplementation improved blood and urinary biochemistry, corrected abnormalities related to bone metabolism in rats with CKD, promoted autophagy, and reduced aortic calcification. In vitro, zinc reduced the calcification of VSMCs induced by β-GP. During VSMC calcification, zinc further upregulated autophagy levels and the phosphorylated extracellular regulatory kinase1/2 (ERK1/2) levels in a high-phosphorus environment. Pretreatment with 3-methyladenine (3-MA), an autophagy inhibitor, or with U0126, an ERK1/2 pathway inhibitor, decreased autophagy and increased calcification. In conclusion, zinc improved CKD-MBD by inhibiting vascular calcification (VC) and ameliorating bone metabolism disorders. Furthermore, zinc alleviates VC in CKD by activating ERK1/2-mediated autophagy.
Similar content being viewed by others
Introduction
CKD represents a significant challenge in global health, with a prevalence exceeding 10%, affecting approximately 800 million people1; cardiovascular disease (CVD) is known to be significantly affected thereby. When end-stage renal disease is brought on by chronic kidney disease (CKD), 40–50% of adult patients are expected to die from CVD2. It is predicted that CKD will rank as the fifth most common cause of mortality by 20403. CKD-MBD, a common complication of CKD, is associated with the incidence and mortality of CVD. It encompasses abnormalities in biochemical markers, bone metabolism, and VC4. VC, especially medial calcification, is a crucial risk factor for CVD in individuals with CKD, with around 54–100% of patients exhibiting such calcifications5,6. Giachelli et al. examined the diverse mechanisms underlying vascular calcification (VC), highlighting how disruptions in calcium and phosphorus homeostasis can contribute to calcification process through various pathways7. Furthermore, Shanahan et al. underscored the pivotal role of calcium and phosphorus in arterial calcification associated with CKD, elucidating key molecules and regulatory factors involved in the calcification cascade8.
Autophagy, a vital physiological process, plays a significant part in several disorders, including CVD9,10. Autophagy happens in three ways: chaperone-mediated autophagy, microautophagy, and macroautophagy11. The most well-defined form of autophagy, macroautophagy — usually called autophagy — includes the large-scale processing of material found in the cytoplasm by autophagosome-mediated lysosomal delivery12. Autophagy is activated in response to diverse stressors, including oxidative stress, nutrient depletion, and hypoxia, and is often linked to cellular protection and survival13. Recent evidence indicates that high phosphorus levels can induce autophagy in VSMCs, and autophagy may inhibit calcification by reducing the release of extracellular matrix vesicles and suppressing the phenotypic transformation of VSMCs14. Thus, modulating autophagy levels in VSMCs under high phosphorus conditions may offer a novel intervention attenuating the development of VSMCs.
Zinc as an essential trace element in cellular metabolism is involved in various pathophysiological processes15,16. In patients with CKD, the level of serum zinc is frequently reduced and is associated with the risk of CVD17,18. Recently, the protective effects of zinc against VC have garnered considerable attention. Multiple studies proved that zinc inhibits VC19,20,21,22. Additionally, high dietary zinc intake in adults is associated with a lower prevalence of severe abdominal aortic calcification23,24. Furthermore, zinc can stimulate osteoblast activity and bone formation while inhibiting osteoclast activity25. These findings show that zinc may be a practical bioactive component in slowing CKD progression and preventing complications.
The role that zinc plays in controlling autophagy has drawn more attention in recent times. Zinc deficiency impedes the maturation and degradation of autophagosomes, while zinc supplementation can positively regulate autophagy levels26,27. Additionally, several studies show that zinc-induced autophagy regulation requires ERK1/228,29,30, indicating that autophagy may be positively regulated by the ERK1/2 signaling pathway within cells. However, whether zinc supplementation can alleviate VC and improve bone metabolism disorders remains unclear, particularly in VC induced by CKD. The effect of zinc on autophagy levels in this scenario is also not well understood. Therefore, this study aims to explore the effects of zinc on VC and bone metabolism in rats with CKD, as well as the role of zinc in regulating autophagy during the VC process and its mechanism of action.
Material and methods
All methods were performed in accordance with the relevant guidelines and regulations, and the experimental protocol has been approved by the Ethics Committee of China Medical University.
Animal studies
Wistar male rats, aged 8–10 weeks, were obtained from Liaoning Changsheng Biotechnology Co., Ltd (Liaoning, China) and raised following the Animal Research Ethics Committee of China Medical University guidelines, and the study is reported in accordance with ARRIVE guidelines. Four groups of thirty-two rats were randomly assigned: control group (CTR), zinc control group (CTR + Zn), calcification group (CKD), and calcification + zinc group (CKD + Zn), each comprising eight rats. From day 1 to day 90, rats were fed a 0.25% adenine, 0.2 mg/kg vitamin K diet containing 1% calcium, 1% phosphorus, 1 IU/g vitamin D and 6% protein to establish the CKD calcification model31. Concurrently, ZnCl2 gavage was initiated, with ZnCl2 dissolved in physiological saline, and administered daily at 60 mg/kg via gavage based on the body weight of each rat for three months while maintaining their diet. The control group received physiological saline based on the body mass. After three months, the rats were euthanized by an overdose of sodium pentobarbital (150 mg/kg, intraperitoneal injection), and bone tissue and artery samples taken for further testing.
Cell culture
A7R5, purchased from the Cell Bank of a Shanghai Academy of Sciences, was cultured in a 5% CO2 at 37 °C in a humidified incubator using 10% fetal bovine serum (FBS) added to a high-glucose DMEM medium. Upon reaching a confluence of over 80% in the culture dishes, they were split (at random) into the following groups: control group (CTR), calcification group (β-GP), calcification + zinc group (β-GP + Zn), 3-MA + calcification + zinc group (3-MA + β-GP + Zn), and ERK1/2 inhibitor + calcification + zinc group (U0126 + β-GP + Zn). To induce calcification, 10 mmol/L β-GP was added to the DMEM containing 1% FBS for 14 days, while the control group was maintained in DMEM with 1% FBS without β-GP.
Biochemical parameter analysis
Urine and blood samples were collected from the rats at the beginning of the study and every two weeks thereafter. Serum calcium (SCa), serum creatinine (SCr), serum phosphorus (SP), serum zinc (SZn), urinary calcium (Uca), and urinary phosphorus (UP) were analyzed using reagent kits from Jiancheng Bio (Nanjing, China). Levels of serum parathyroid hormone (PTH) were measured using an ELISA kit (Immunoway, USA; Cat# KE1453), while receptor activator of nuclear factor-κB ligand (sRANKL), procollagen type I N-terminal propeptide (PINP), and C-terminal telopeptide of type I collagen (CTX- I) were analyzed using Cusabio ELISA kits (China; Cat# CSB-E05126r, CSB-E12774r, and CSB-E12776r, respectively).
Alizarin red S staining
After deparaffinizing and rehydrating the rat aortic paraffin sections, Alizarin red S staining solution was applied for five minutes; the staining solution was then removed, dried, and mounted with neutral balsam. For 14-day treated cells, they were fixed with neutral formalin for 20 min, and washed three times with distilled water. After adding the Alizarin red S staining solution, the cells were dyed in the dark for thirty minutes at 37 °C. Photographs were taken after removal of the staining solution and washing.
Calcium quantification assay
3 mL of 0.6 mmol/L hydrochloric acid (HCl) was added to the tissue and 14-day treated cells separately; these specimens were then placed in an incubator at 37 °C for 24 h. Thereafter, the supernatant was collected, and using a calcium test kit, the amount of calcium in the tissue and cells was assessed. The calcium content of the vascular tissue (mg/g) was calculated using the formula: the calcium content (mg/g) = [Supernatant calcium concentration (mmol/L) × 2 × 10–3 × 40 mg/L] / Tissue dry mass (g). The calcium concentration of the cells was adjusted based on the protein level using the BCA technique.
Western blot (WB)
To extract protein samples, RIPA buffer containing protease and phosphatase inhibitors was used to lyse cells or blood vessels. The protein extracts were then quantified using the BCA method. Proteins were sorted into equivalent amounts using SDS-PAGE gel electrophoresis and placed onto PVDF membranes. The membranes were then sealed for 1.5 h at room temperature using 10% skimmed milk. After washing with Tris-buffered saline containing 0.1% Tween (TBST), the membranes were left overnight at 4 °C with appropriate primary antibodies: anti-α-SMA (1:1000, Abcam, cat# ab7817), anti-Runx2 (1:1000, Santa, cat# sc-390351), anti-Beclin1 (1:500, CST, cat# 3495), anti-LC3 (1:500, Abcam, cat# ab192890), anti-ERK1/2 (1:500, CST, cat# 4695), anti-Phospho-ERK1/2 (1:500, CST, cat# 4370), and anti-GAPDH (1:1000, CST, cat# 5174). The following day, the membranes were incubated with HRP-conjugated goat anti-rabbit (1:50,000, Abbkine, cat# A21020) and goat anti-mouse (1:50,000, Abbkine, cat# A21010) secondary antibodies for 1.5 h at 37 °C. Then, an ECL reagent was added for image capture using a chemiluminescence imaging system. Finally, the grey-scale values of each band were analyzed and recorded using ImageJ v1.8.0 software (https://imagej.net/ij/).
Immunohistochemistry (IHC)
Following the heat-mediated antigen retrieval treatments, the paraffin sections were incubated individually at 4 °C overnight in a humidified box with primary antibodies that were suitably diluted: anti-α-SMA (1:200, Abcam, cat# ab7817), anti-Runx2 (1:150, Santa, cat# sc-390351), and anti-LC3 (1:200, Abcam, cat# ab192890). After washing, the pieces were blocked for ten minutes at room temperature using 0.3% H2O2. The slices were then exposed to HRP-conjugated goat anti-rabbit (1:500, Abbkine, cat# A21020) and goat anti-mouse (1:500, Abbkine, cat# A21010) secondary antibodies for 15–30 min at 37 °C. The sections were then incubated with DAB solution. Hematoxylin was used to counterstain the nucleus. The slides were ready for microscopy and photography following xylene clearing and neutral gum sealing.
Micro-CT detection
The right femur was fixed in 70% ethanol for 48 h and then subjected to micro-CT scanning (SkyScan 1276, Bruker, Germany) to obtain imaging of the right distal femur. The micro-CT parameters were set as follows: tube voltage of 70 kV, tube current of 57 μA, and a resolution of 6 μm. The region of interest (ROI) for trabecular bone was defined as a 1.2 mm segment starting 0.78 mm from the growth plate. The ROI for cortical bone was defined as a 1.2 mm segment starting 4.5 mm from the growth plate. After scanning, data analysis and 3-d image reconstruction were performed using CT image analysis software (CtAn v.1.18 and CtVox v3.3.0, https://blue-scientific.com/).
Immunofluorescence staining (IF)
After being fixed for 20 min with a neutral fixative, the cells culturing for 72 h were permeabilized for 20 min with 0.5% Triton X-100, blocked for 30 min at room temperature with 5% BSA, and then incubated overnight with the anti-LC3 (1:200, Abcam, cat# ab192890) at 4 °C. The next day, the cells were cleaned with PBS and then incubated for 2 h at room temperature in the dark with a fluorescent secondary antibody (1:100, Abbkine, cat# A23420). A DAPI solution was used for nuclear staining. Lastly, pictures were taken with a confocal microscope after mounting in glycerol.
Transmission electron microscopy (TEM)
After culturing for 72 h, the VSMCs were treated at 4 °C for 4 h in an electron microscopy fixative. The cells were dehydrated and then soaked in acetone to enclose them. Then, thin sections measuring 70 nm were cut and dyed using a 2% uranyl acetate solution in ethanol under light protection. The sections were stained, cleaned, and allowed to air dry at room temperature for the entire night. Lastly, a TEM was used to examine and record the sections.
Statistical analysis
GraphPad Prism v8.0 (https://www.graphpad.com/) was utilized for the analysis, and the experimental information was represented as mean ± standard deviation. Student’s t-test was used to analyze the variations between the two groups, and ANOVA was used for comparisons involving more than two groups and Tukey’s HSD test was used as a post hoc test if p < 0.05.
Results
Effects of zinc on the body weight, blood biochemistry, and urine biochemistry in CKD rats
Compared to the CTR and CTR + ZH groups, the CKD group showed decreased the body weight, SCa, and UP levels, while levels of SCr, SP, UCa, PTH, sRANKL (an osteoclast activator), PINP (a bone formation marker), and CTX-I (a bone resorption marker) levels increased. Additionally, compared to the CKD group, zinc supplementation elevated the SCa level and reduced the levels of SCr, SP, UCa, PTH, sRANKL, PINP, and CTX-I but had no significant effect on the rat body weight or UP levels. The level of SZn did not differ significantly across any of the aforementioned groups (Table 1).
Effects of zinc on aortic calcification and autophagy in CKD rats
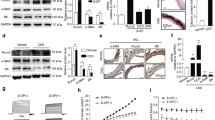
The aortas were subjected to Alizarin Red S staining three months after modelling. The results (Fig. 1A) revealed no significant orange-red calcium deposits or calcified nodules in the aortic walls of the CTR and CTR + ZH groups. In contrast, various degrees of orange-red calcium deposits were observed in the CKD and CKD + Zn groups. Compared to the CKD group, calcium deposition decreased in the CKD + Zn group. These findings were corroborated by the calcium quantification assay (Fig. 1B). Western blots (Fig. 1D–I) showed that compared to the CTR and CTR + ZH groups, the degree of α-SMA protein expression, which is a hallmark of smooth muscle cells, decreased. In contrast, the expressions of RUNX2, the osteogenic gene, and Beclin1 and LC3II/I, the autophagy-associated proteins, were increased in the CKD and CKD + Zn groups. Furthermore, compared to the CKD group, the CKD + Zn group increased α-SMA, Beclin1, and LC3II/I expressions and reduced Runx2 expression. The immunohistochemistry results aligned with the Western Blot findings, indicating that zinc further increased the expression of autophagy proteins in CKD rats (Fig. 1C).
Effects of zinc intake on the calcification and autophagic activity in the aorta. (A) Alizarin red S staining in aortas. Scale bar: 50 μm. (B) Quantitative analysis of the aortic calcium (Ca) content. (C) The immunohistochemistry revealed that the α-SMA activity was lowered in the CKD group but recovered in the CKD + Zn group and showed that Runx2 and LC3 were higher in the CKD group, compared to CTR and CTR + Zn. After zinc supplementation, the Runx2 expression declined, whereas the LC3 expression continued to rise. Scale bar: 50 μm. (D–G) Quantitative analysis of a-SMA, Runx2, Beclin1, LC3 proteins, and GAPDH was a loading control (n = 3). (H, I) Western blots exhibiting α-SMA, Runx2, Beclin1, and LC3 protein levels in aortas. Data are shown as means ± SEM; a P < 0.05 versus CTR, b P < 0.01 versus CKD.
Effect of zinc on the bone metabolism of CKD rats
Three months later, Micro-CT scanning and 3-d imaging (Fig. 2B) were conducted on the femur ROIs in rats from each group (Fig. 2A). The findings (Table 2) indicated that, compared to the CTR group, the bone volume ratio (BV/TV), bone area ratio (BS/TV), trabecular number (Tb.N), and trabecular bone density (Tb.BMD), total cortical bone area (Tt.Ar), cortical bone area (Ct.Ar), cortical area ratio (Ct.Ar/Tt.Ar), and cortical thickness (Ct.Th) were reduced. In contrast, trabecular separation (Tb.Sp) and cortical bone porosity (Ct.Po) were grown in the CKD and CKD + Zn groups. Compared with the CKD group, the CKD + Zn group showed increases in BV/TV, BS/TV, Tb.N, Tb.BMD, Tt.Ar, Ct.Ar, and Ct.Ar/Tt.Ar, and decreases in Tb.Sp and Ct.Po, with no significant differences in Ct.Th. All groups exhibited no notable differences in trabecular thickness (Tb.Th) and cortical bone mineral density (Ct.BMD) levels.
Effect of zinc on β-GP-induced VSMC calcification
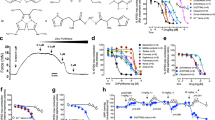
High concentrations of zinc can impair cell viability. To determine a safe concentration for A7R5 cells, ZnCl2 solutions ranging from 10 to 80 µM were added to the culture medium. At 24 and 48 h, the CCK-8 test was used to assess cell viability. The results indicated that zinc at concentrations of 10–40 μM had no effect on the viability of A7R5 cells (Fig. 3A). To trigger calcification, β-GP (10 mmol/l) was applied to VSMCs. Calcium quantification results showed that ZnCl2 concentrations of 10 to 40 µM reduced the calcium content in A7R5 cells under high-phosphate conditions (Fig. 3B). The outcome of the staining with Alizarin Red S was consistent with the calcium quantification results (Fig. 3F). Additionally, ZnCl2-treated cells, at 10 to 40 μM, showed reduced RUNX2 protein expression and elevated α-SMA protein expression in contrast to the β-GP group, according to Western blot assays (Fig. 3C,E). These findings imply that zinc may reduce β-GP-induced VSMC calcification., with the best inhibitory effect observed at 30 µM. Consequently, we used 30 µM zinc as the intervention concentration in subsequent experiments.
Different concentrations of zinc on VSMC calcification. (A) Effects of zinc on the viability of A7R5 cells assessed at 24 h and 48 h. * P < 0.05 versus CTR. (B) Quantitative analysis of the calcium (Ca) content in A7R5 cells with zinc on β-GP-induced calcium deposition for 14 days. (C, E) Western blots showing α-SMA and Runx2 protein levels in A7R5 treated with zinc and β-GP for 14 days. (F) Alizarin red S staining illustrating the effects of zinc on β-GP-induced calcification nodule formation in A7R5 cells for 14 days (200 ×).
Effect of zinc on β-GP-induced VSMC autophagy
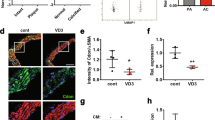
The autophagy-associated proteins were analyzed using a Western blot assay to investigate the possible influence of zinc on VSMC autophagy. As depicted in Fig. 4G, the protein expression levels of LC3II/I and Beclin1 were higher in the β-GP group compared to the CTR group. Additionally, these levels of expression were further elevated in the β-GP + Zn group. Fluorescence microscopy (Fig. 4H) indicated that, following exposure to β-GP, VSMCs showed an increase in the production of fluorescent LC3 puncta and were discovered to have increased even more following treatment with zinc and β-GP. TEM was also employed to assess the autophagy activity. The TEM data (Fig. 4I) disclosed that the β-GP group had more autophagosome accumulation than the CTR group and that accumulation increased even more in the β-GP + Zn group. According to these results, β-GP might enhance VSMC autophagy, and zinc might intensify this autophagic reaction even more.
Zinc promotes autophagy and inhibits VSMC calcification. (A) Quantitative analysis of the calcium (Ca) content in A7R5 cells treated with β-GP, zinc, or 3-MA for 14 days. (B, E) Quantitative analysis of a-SMA, Runx2, Beclin1, LC3 proteins, and GAPDH as a loading control (n = 3). (F) Western blots showing a-SMA and Runx2 protein levels in A7R5 treated with β-GP, zinc, or 3-MA for 14 days. (G) Western blots showing Beclin1 and LC3 protein levels in A7R5 treated with β-GP, zinc, or 3-MA for 72 h. (H) Immunofluorescence staining showing the LC3 expression in A7R5 treated with β-GP, zinc, 3-MA, or U0126 for 72 h (200x). (I) TEM showing autophagosomes (black arrows) in A7R5 treated with β-GP, zinc, 3-MA, or U0126 for 72 h (12,000x). All data are shown as means ± SEM (n = 3); a P < 0.05 versus CTR, b P < 0.01 versus β-GP, c P < 0.01 versus β-GP + Zn. β-GP, β-glycerophosphate; 3-MA, 3-methyladenine; U0126, ERK1/2 pathway inhibitor.
Zinc increases autophagy to reduce β-GP-induced VSMC calcification
To examine whether zinc may mitigate the VSMC calcification by fostering autophagy, the cells were pre-treated with 3-MA (5 mmol/L), an autophagy inhibitor, for half an hour before receiving zinc and β-GP treatments. Calcium quantification revealed that VSMCs that received a 3-MA pre-treatment showed higher levels of calcium deposition than cells that did not receive this therapy (Fig. 4A). Nonetheless, there was a noticeable decline in the fluorescent LC3 puncta seen under the fluorescence microscope (Fig. 4H) and the quantity of autophagosomes seen under the TEM (Fig. 4I). Alizarin Red staining results were consistent with these findings (Fig. 5A). Western blot analysis (Fig. 4B–G) showed that compared to the β-GP + Zn group, α-SMA, Beclin1, and LC3II/I had considerably decreased levels of expression. In contrast, Runx2 protein expression was increased in the 3-MA + β-GP + Zn group. These findings imply that zinc promotes the autophagy activity to reduce β-GP-induced VSMC calcification.
Zinc inhibits the VSMC calcification by activating ERK1/2-mediated autophagy. (A) Alizarin red S staining in A7R5 cells treated with β-GP, zinc, 3-MA, or U0126 for 14 days (200x). (B) Quantitative analysis of the quantitative calcium (Ca) content in A7R5 cells treated with β-GP, zinc, or U0126 for 14 days. (C–G) Quantitative analysis of ERK1/2, Beclin1, LC3, a-SMA, Runx2 proteins, and GAPDH as a loading control (n = 3). (H) Western blots showing a-SMA and Runx2 protein levels in A7R5 treated with β-GP, zinc, or U0126 for 14 days. (I) Western blots illustrating ERK1/2, Beclin1 and LC3 protein levels in A7R5 treated with β-GP, zinc, or U0126 for 72 h. All data are shown as means ± SEM (n = 3); a P < 0.05 versus CTR, b P < 0.01 versus β-GP, c P < 0.01 versus β-GP + U0126. β-GP, β-glycerophosphate; 3-MA, 3-methyladenine; U0126, ERK1/2 pathway inhibitor.
Zinc stimulates the ERK1/2 signaling pathway to increase autophagy.
Before being treated with β-GP and zinc, VSMCs had previously been treated with U0126, the ERK1/2 inhibitor, for 1 h to investigate whether the ERK1/2 signaling pathway contributes to the activation of autophagy by zinc. After pre-treating the VSMCs with U0126, there was a decrease in fluorescent LC3 puncta (Fig. 4H) and the amount of autophagosomes (Fig. 4I). Nevertheless, a notable rise in the amounts of calcium deposition was revealed by the outcomes of Alizarin Red S staining (Fig. 5A) and the calcium quantification test (Fig. 5B). Using Western blot analysis, the β-GP + Zn group was found to have higher levels of protein expression for α-SMA, p-ERK, LC3II/I, and Beclin1 than the β-GP group did; however, Runx2 had lower levels thereof (Fig. 5C–I). The opposite outcome arose when U0126 was used for pre-treatment of the VSMCs (Fig. 5C–I). Therefore, these results suggest that zinc modulates autophagy via the ERK1/2 signaling pathway, which helps reduce calcification in VSMCs under high-phosphate conditions.
Discussion
Zinc is an essential trace element for the human body, participating in protein synthesis and energy metabolism. Recent studies suggest that zinc offers substantial protection against CVD. SZn levels in CKD patients are negatively correlated with coronary artery calcification (CAC), and lower zinc levels are linked to a higher incidence of CVD18. Recent studies found that zinc ameliorates human aortic valve calcification through the GPR39-mediated ERK1 / 2 signaling pathway19. In addition, zinc improved the osteogenic effect of VSMCs under high glucose conditions20. Previous research also found that the zinc deficiency promotes VSMC calcification, a process that is independent of alkaline phosphatase action and partially influenced by Pit1 upregulation21. Additionally, zinc has been demonstrated to provide robust protection against VC induced in Kl/Kl mice, vitamin D3 overload mice, and high-phosphorus diet mice following 5/6 nephrectomy22. This result suggests that zinc supplementation may offer a potential therapeutic strategy to intervene in calcification. Our study, in agreement with it, is the first to confirm that zinc improves blood and urinary biochemistry, slows renal function deterioration, and protects against VC in a CKD rat model. This may be related to its multiple mechanisms, including anti-inflammatory, antioxidant, immune modulation, and improvement of mineral metabolism26. Zinc has been shown to reduce calciprotein formation time32,33,34, which might explain the maintained kidney function: these particles were also shown to induce lysosomal dysfunction35, however, the effects and mechanisms of zinc supplementation still require further investigation. Despite employing more precise methods for serum zinc level assessment as referenced elsewhere36, we observed that zinc supplementation exerted a negligible influence on serum zinc levels. This finding suggests that serum zinc levels may not be a sensitive indicator for evaluating the efficacy of zinc supplementation. Several factors may explain this observation: Firstly, the gavage protocol itself may have contributed to this outcome, as oral administration can lead to transient elevations in zinc levels followed by rapid excretion, potentially masking sustained effects. Additionally, daily gavage represents an unphysiological stressor that might modify zinc’s typical metabolic effects. Secondly, zinc being a trace element, may have a more complex distribution and metabolism in the body than that reflected by serum levels. Thirdly, zinc supplementation might exert its protective effects against VC through other mechanisms, such as enhancing anti-inflammatory or antioxidant actions, which are not directly reflected in changes in serum zinc levels. Therefore, we posit that the potential benefits of zinc supplementation in delaying VC in CKD patients may not be directly related to changes in serum zinc levels. These methodological considerations should be acknowledged as study limitations. Future studies should further investigate other potential effects of zinc supplementation on VC and its mechanisms of action.
Zinc is essential for the normal development and maintenance of bone homeostasis. It is involved in synthesizing, mineralizing, and remodeling bone tissue. Zinc stimulates osteoblast differentiation, inhibits osteoclast formation, and facilitates bone remodeling by regulating the RANKL/RANK/OPG pathway25. In rats given an inadequate zinc diet, the level of serum osteocalcin decreased, serum calcium levels fell, and the level of PTH increased, resulting in osteoporosis37. In our study, zinc was observed to lower PTH, sRANKL, PINP, and CTX-I levels, suggesting that zinc supplementation may ameliorate secondary hyperparathyroidism and improve bone metabolism in CKD by simultaneously suppressing bone resorption and promoting bone formation. Park et al.38 found that zinc increased the RUNX2 expression in human bone marrow-derived mesenchymal stem cells (hBMSCs) via the cAMP-PKA-CREB signaling pathway, promoting osteogenic differentiation in a dose-dependent manner. In contrast, our study discovered that zinc decreases RUNX2 protein expression in VSMCs. The variability in osteogenic responses to zinc may be attributed to differences in cell types. A meta-analysis of 40 studies indicated that serum zinc levels are significantly lower in osteoporosis patients compared to control groups. Supplementing with zinc increased the bone density in the hip and lower back. Additionally, zinc supplementation reduces osteocalcin levels, which is advantageous for bone formation and remodeling39. BV/TV is the most significant among trabecular parameters as it can be directly measured and resembles the bone density and bone mass measurements were made from CT scans. Our study found that zinc supplementation can enhance the bone volume and bone area in CKD rats, improve the bone density, and decrease the number of osteoclasts. These findings suggest that zinc may ameliorate bone metabolic disorders in CKD rats.
Autophagy is an intracellular degradation process that removes damaged organelles and proteins to sustain cellular homeostasis12. Dai et al.14 observed that high phosphorus levels could induce increased matrix vesicle release and increased the expression of autophagy proteins. Pre-treatment with 3-MA reduced autophagy levels, which, in turn, further increased matrix vesicle release and aggravated the VSMC calcification. These findings suggest that, in a calcifying environment, VSMCs can mitigate calcification by reducing vesicle release through autophagy. Recent studies have indicated that autophagy levels increase in cells exposed to high zinc concentrations, zinc ion carriers, or combinations of pro-oxidant agents/conditions with zinc (e.g., alcohol, tamoxifen, ischemia/reperfusion). Conversely, chelation-induced zinc depletion causes different cell lines to experience basal and induced autophagy inactivity. This finding suggested that zinc is necessary for autophagy to function normally40. In rat models of renal ischemia–reperfusion injury26, mouse models of spinal cord injury27, and mouse models of acute alcohol intoxication41, zinc has been observed to confer protective effects by further increasing levels of autophagy. Consistent with previous research, the present research demonstrated that autophagy activation occurs in a CKD model induced by a 0.25% adenine and low vitamin K diet. Furthermore, zinc further enhanced autophagy, whereas zinc supplementation did not significantly affect levels of autophagy in normal rats.
The mechanism through which zinc regulates autophagy remains unclear. Three studies (tamoxifen-treated breast cancer cells, alcohol-treated human liver cancer cells, and hypoxia-reoxygenation-treated human cardiomyocytes) have highlighted the crucial role of ERK1/2 in zinc-mediated regulation of autophagy28,29,30. Zinc as an effective regulator of ERK1/2 is involved in various physiological functions. In rats given an diet deficient in zinc, reduced phosphorylation levels of ERK1/2 in the aorta have been observed, which led to increased apoptosis and accelerated progression of atherosclerosis42. The role of ERK1/2 in phosphate-induced VC remains controversial. One study has shown that phosphates are transported into VSMCs via PIT1 and PIT2. The downstream signaling pathway of PIT1 involves the activation of ERK1/2, which leads to the upregulation of the Runx2 expression in VSMCs. Silencing PIT1 can inhibit phosphate-induced bone differentiation and calcification in VSMCs43. Study two indicated that culturing VSMCs in a calcification medium upregulates the expression of fibroblast growth factor 23 (FGF-23). Treatment with FGF-23 increases the levels of phosphorylation of ERK1/2. However, when cells were treated with FGF-23 and an ERK1/2 inhibitor, the calcification of VSMCs was exacerbated44.
Additionally, research has indicated that, in high-phosphate-induced human arterial smooth muscle cells (hASMCs) under inflammatory conditions, the application of ERK1/2 inhibitors does not affect hASMC calcification45. According to recent research, zinc deficiency may partially affect the calcification of VSMCs by upregulating PIT121. This finding further underscores the significance of PIT1 in VC and reveals the potential dual role of the ERK1/2 signaling pathway in this process. In summary, the role of ERK1/2 in VC may vary depending on experimental conditions and cell types. Further investigation is required to elucidate the specific mechanisms of action, and influencing factors, thereof.
The present research did not encompass experiments involving the silencing of GPR39 to elucidate its influence on ERK phosphorylation and autophagy triggered by zinc, constituting a notable constraint on our investigation. Subsequent studies should incorporate such experiments to enhance the understanding of the mechanisms of action of zinc. Furthermore, our investigation did not explore other plausible signaling pathways, such as PI3K/AKT/mTOR, which could be implicated in autophagy induced by zinc. Future research should explore these pathways to provide a more comprehensive view.
Conclusions
Zinc supplementation can improve CKD-MBD. Zinc enhances autophagy by activating the ERK1/2 pathway and inhibits VC induced by CKD (Fig. 6). These findings offer novel insights into treating VC in CKD and highlight the significant roles of zinc and the ERK1/2 pathway in maintaining vascular health. Future research should further investigate the role of zinc and the ERK1/2 pathway in in-vivo models and additional studies on the potential of zinc to inhibit VC and reduce cardiovascular disease risk in CKD patients are warranted. These efforts may aid advances clinical treatment strategies.
Data availability
Data will be made available from the corresponding author upon reasonable request.
References
Kovesdy, C. P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2011(12), 7–11 (2022).
Modi, Z. J. et al. Risk of cardiovascular disease and mortality in young adults with end-stage renal disease: An analysis of the US renal data system. JAMA Cardiol. 4, 353–362 (2019).
Kalantar-Zadeh, K. et al. Caring for patients with advanced chronic kidney disease: Dietary options and conservative care instead of maintenance dialysis. J. Ren. Nutr. 33, 508–519 (2023).
Hou, Y.-C., Lu, C.-L. & Lu, K.-C. Mineral bone disorders in chronic kidney disease. Nephrology (Carlton) 23(Suppl 4), 88–94 (2018).
Disthabanchong, S. & Boongird, S. Role of different imaging modalities of vascular calcification in predicting outcomes in chronic kidney disease. World J. Nephrol. 6, 100–110 (2017).
Bansal, N. Evolution of cardiovascular disease during the transition to end-stage renal disease. SSemin Nephrol. 37, 120–131 (2017).
Giachelli, C. M. Vascular calcification mechanisms. J. Am. Soc. Nephrol. 15, 2959–2964 (2004).
Shanahan, C. M., Crouthamel, M. H., Kapustin, A. & Giachelli, C. M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 109, 697–711 (2011).
Abdellatif, M., Sedej, S., Carmona-Gutierrez, D., Madeo, F. & Kroemer, G. Autophagy in cardiovascular aging. Circ. Res. 123, 803–824 (2018).
Bravo-San Pedro, J. M., Kroemer, G. & Galluzzi, L. Autophagy and mitophagy in cardiovascular disease. Circ. Res. 120, 1812–1824 (2017).
Kroemer, G. & Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 9, 1004–1010 (2008).
Levine, B. & Kroemer, G. Biological functions of autophagy genes: A disease perspective. Cell 176, 11–42 (2019).
Mizushima, N. & Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 147, 728–741 (2011).
Dai, X.-Y. et al. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney Int. 83, 1042–1051 (2013).
Chasapis, C. T., Ntoupa, P.-S.A., Spiliopoulou, C. A. & Stefanidou, M. E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 94, 1443–1460 (2020).
Ozyildirim, S. & Baltaci, S. B. Cardiovascular diseases and zinc. Biol. Trace. Elem. Res. 201, 1615–1626 (2023).
Nakatani, S., Mori, K., Shoji, T. & Emoto, M. Association of zinc deficiency with development of CVD events in patients with CKD. Nutrients 13, 1680 (2021).
Zhang, D. et al. Associations of whole blood zinc levels with coronary artery calcification and future cardiovascular events in CKD patients. Biol. Trace Elem. Res. 202, 46–55 (2024).
Chen, Z. et al. Zinc ameliorates human aortic valve calcification through GPR39 mediated ERK1/2 signalling pathway. Cardiovasc. Res. 117, 820–835 (2021).
Henze, L. A. et al. Zinc ameliorates the osteogenic effects of high glucose in vascular smooth muscle cells. Cells 10, 3083 (2021).
Alcantara, E. H., Kwon, J.-H., Kang, M.-K., Cho, Y.-E. & Kwun, I.-S. Zinc deficiency promotes calcification in vascular smooth muscle cells independent of alkaline phosphatase action and partly impacted by Pit1 upregulation. Nutrients 16, 291 (2024).
Voelkl, J. et al. Zinc inhibits phosphate-induced vascular calcification through TNFAIP3-mediated suppression of NF-κB. J. Am. Soc. Nephrol. 29, 1636–1648 (2018).
Chen, W. et al. Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol Dial Transplant 35, 1171–1178 (2020).
Kong, X. & Wang, W. Associations between the composite dietary antioxidant index and abdominal aortic calcification among United States adults: A cross-sectional study. JPEN J. Parenter Enteral. Nutr. 48, 571–579 (2024).
Molenda, M. & Kolmas, J. The role of zinc in bone tissue health and regeneration: A review. Biol. Trace. Elem. Res. 201, 5640–5651 (2023).
Hadj Abdallah, N. et al. Zinc mitigates renal ischemia-reperfusion injury in rats by modulating oxidative stress, endoplasmic reticulum stress, and autophagy. J. Cell Physiol. 233, 8677–8690 (2018).
Lin, J.-Q. et al. Zinc provides neuroprotection by regulating NLRP3 inflammasome through autophagy and ubiquitination in a spinal contusion injury model. CNS Neurosci. Ther. 27, 413–425 (2021).
Hwang, J. J. et al. Zinc(II) ion mediates tamoxifen-induced autophagy and cell death in MCF-7 breast cancer cell line. Biometals 23, 997–1013 (2010).
Liuzzi, J. P. & Yoo, C. Role of zinc in the regulation of autophagy during ethanol exposure in human hepatoma cells. Biol. Trace Elem. Res. 156, 350–356 (2013).
Bian, X. et al. Zinc prevents mitochondrial superoxide generation by inducing mitophagy in the setting of hypoxia/reoxygenation in cardiac cells. Free Radic. Res. 52, 80–91 (2018).
Neven, E. et al. Disturbances in bone largely predict aortic calcification in an alternative rat model developed to study both vascular and bone pathology in chronic kidney disease. J. Bone Miner Res. 30, 2313–2324 (2015).
Nakatani, S. et al. Association between serum zinc and calcification propensity (T50) in Patients with Type 2 diabetes mellitus and in vitro effect of exogenous zinc on T50. Biomedicines 8, 337 (2020).
Sohail, A. et al. Association of serum zinc with mineral stress in chronic kidney disease. Clin. Kidney J. 17, sfae258 (2024).
Geroldinger-Simic, M. et al. Accelerated calciprotein crystallization time (T50) is correlated with impaired lung diffusion capacity in systemic sclerosis. Front. Immunol. 15, 1425885 (2024).
Kunishige, R. et al. Calciprotein particle-induced cytotoxicity via lysosomal dysfunction and altered cholesterol distribution in renal epithelial HK-2 cells. Sci. Rep. 10, 20125 (2020).
Hall, A. G., King, J. C. & McDonald, C. M. Comparison of serum, plasma, and liver zinc measurements by AAS, ICP-OES, and ICP-MS in diverse laboratory settings. Biol. Trace Elem. Res. 200, 2606–2613 (2022).
Suzuki, T., Kajita, Y., Katsumata, S., Matsuzaki, H. & Suzuki, K. Zinc deficiency increases serum concentrations of parathyroid hormone through a decrease in serum calcium and induces bone fragility in rats. J. Nutr. Sci. Vitaminol. 61, 382–390 (2015).
Park, K. H. et al. Zinc promotes osteoblast differentiation in human mesenchymal stem cells via activation of the cAMP-PKA-CREB signaling pathway. Stem Cells Dev. 27, 1125–1135 (2018).
Ceylan, M. N., Akdas, S. & Yazihan, N. Is zinc an important trace element on bone-related diseases and complications? A meta-analysis and systematic review from serum level, dietary intake, and supplementation aspects. Biol. Trace Elem. Res. 199, 535–549 (2021).
Liuzzi, J. P. & Pazos, R. Interplay between autophagy and zinc. J. Trace Elem. Med Biol. 62, 126636 (2020).
Liuzzi, J. P., Narayanan, V., Doan, H. & Yoo, C. Effect of zinc intake on hepatic autophagy during acute alcohol intoxication. Biometals 31, 217–232 (2018).
Allen-Redpath, K. et al. Marginal dietary zinc deficiency in vivo induces vascular smooth muscle cell apoptosis in large arteries. Cardiovasc. Res. 99, 525–534 (2013).
Chavkin, N. W., Chia, J. J., Crouthamel, M. H. & Giachelli, C. M. Phosphate uptake-independent signaling functions of the type III sodium-dependent phosphate transporter, PiT-1, in vascular smooth muscle cells. Exp. Cell Res. 333, 39–48 (2015).
Zhu, D., Mackenzie, N. C. W., Millan, J. L., Farquharson, C. & MacRae, V. E. A protective role for FGF-23 in local defence against disrupted arterial wall integrity?. Mol. Cell Endocrinol. 372, 1–11 (2013).
Toita, R. et al. Protein kinase A (PKA) inhibition reduces human aortic smooth muscle cell calcification stimulated by inflammatory response and inorganic phosphate. Life Sci. 209, 466–471 (2018).
Acknowledgements
This project was funded by grants from the National Natural Science Foundation of China (Grant Nos 82270779 and U21A20349) and the Liaoning Province Science and Technology Fund (Grant No. 2023-MSLH-398).
Author information
Authors and Affiliations
Contributions
GG, XF, and LY designed the experiments; GG performed the majority of the experiments; JL carried out the immunofluorescence experiment; TX conducted the TEM; XL and DS performed all statistical analysis; GG, JL, TX, and LY wrote the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval
The Animal Research Ethics Committee of China Medical University authorized the present research in accordance with the tenets of The Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, G., Long, J., Xu, T. et al. Zinc alleviates vascular calcification by activating ERK1/2-mediated autophagy. Sci Rep 15, 34039 (2025). https://doi.org/10.1038/s41598-025-13352-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-13352-1