Abstract
In this study, new kappa-carrageenan-based nanocomposites (CG/TiO2) were synthesized using in situ generated TiO2 inorganic nanofiller, which were utilized for Cu2+ ions adsorption from aqueous media. The physicochemical properties of the nanocomposites were studied using Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), and scanning and transmission electron microscopies (SEM and TEM). Data from TGA experiments indicate that nanocomposites have improved thermal properties. Also, morphological investigations and particle size distribution diagrams show the uniform distribution of nanoparticles within the polymer matrix. The effects of pH, contact time, and initial concentration on the adsorption properties of the synthesized nanocomposites were investigated. CG/TiO2 demonstrated efficient adsorption for Cu2+ cations from aqueous solutions, achieving > 80% removal at optimal conditions and reduced Cu2+ levels from 10 to < 1.7 mg/L (below the WHO limit). Kinetic studies of copper cation adsorption showed pseudo-second-order kinetics, and the equilibrium isothermal models for this nanocomposite agreed with the Langmuir isotherm model.
Similar content being viewed by others
Introduction
Water pollution has become one of the most pressing human concerns in recent years. Generally, it has resulted from the indiscriminate discharge of industrial effluents, some of which cannot be treated biologically. Thus, these persistent pollutants, including toxic heavy metals such as Cu2+ ions, may remain in ecosystems for a long time, negatively affecting humans and the environment. Notably, mining and textile effluents frequently contain Cu2+ concentrations exceeding the World Health Organization’s mandated limit of 2.0 mg/L, necessitating the use of advanced remediation strategies1. To remove pollutants, such as various metal ions and dyes, different methods and materials have been developed, including adsorption and degradation processes using nanocomposite materials2,3,4,5,6,7,8,9.
Composites mainly include three distinct components. The properties and behavior of a composite depend on the characteristics of these parts: the continuous phase or the matrix, the discontinuous phase or the reinforcement, and the interface between these two phases. Nanocomposites are materials that are prepared from the distribution of particles with nanometer dimensions in the matrix phase. Due to the very small size of nanoparticles and their high surface-to-volume ratio, which allows them to be in contact with the matrix phase, the atoms on the external surface of these particles provide sufficient forces for adhesion to the matrix, resulting in improved mechanical and physical properties. These properties include hardness, strength, thermal stability, and electrical and thermal conductivity. Therefore, nanocomposites are used widely in military, medical, industrial, and textile applications10,11,12,13,14,15.
Nanocomposites with the polymeric continuous phase or matrix, especially biopolymers, are of great interest. Carrageenan is a natural polymer and a ubiquitous additive in the food industry. It is present in the cell walls and the intercellular matrix of the red seaweed plant known as Irish moss. Additionally, it can be considered a high-molecular-weight sulfated polysaccharide. This compound is categorized into three types: kappa (κ), iota (ι), and lambda (λ). The kappa and iota types are only soluble in hot water, while the lambda type can be dissolved in cold water. Depending on the number and position of the sulfate groups in the carrageenan structure, different types exhibit distinct characteristics. A higher amount of sulfate reduces the strength of the gel and lowers the solubility temperature16. These sulfate ester salts are utilized in the pharmaceutical industry as substrates for drug delivery, owing to the high efficiency of their nanocomposites for surface adsorption17.
Titanium dioxide nanoparticles, however, possess unique optical, electrical, and catalytic properties, making them highly useful for industrial pigments, paper production, and cancer cell elimination, among other applications18,19,20,21,22. Additionally, these nanoparticles can be used to produce nanocomposites for water purification, gas purification, and filtration, especially for air, as well as for disinfection, deodorization, and color removal23,24,25.
Surface modification and effective mixing techniques are the primary methods to attain a homogeneous dispersion of the filler in the polymer matrix. Three strategies, including solution mixing, in situ preparation, and melt mixing, are usually used to produce these nanocomposites. The first two methods generally yield materials with better nanoparticle dispersion. Conversely, melt mixing is more prevalent in industrial processes. In situ particle synthesis can proceed via two main routes: simultaneous inorganic and organic polymerization and sol-gel generated particles in the presence of preformed polymer. A preformed polymer is preferred for producing particle-containing polymer nanocomposites since the simultaneous production of both species typically occurs, often leading to phase separation26.
Several research groups have been working with nanocomposites of carrageenan biopolymer and metal oxides. For example, Jo et al. prepared a cellulose/carrageenan/TiO2 nanocomposite to study the adsorption and photo-degradation of methyl blue27. Duman et al. reported the use of magnetic carbon nanotube/carrageenan/Fe3O4 nanocomposites to remove reactive azo dyes, methylene blue, and triphenylmethane28,29. Salgueiro et al. studied methylene blue adsorption of carrageenan-coated Fe3O4 nanoparticles30. Chavez-Baldovino et al. prepared membranes composed of a mixture of κ and λ carrageenans, TiO2 nanoparticles, and Ni/CeO2, which are used as fuel cell electrodes for ethanol oxidation31. Using a solvent casting method, Duan et al. prepared a nanocomposite film of carrageenan/konjac glucomannan/TiO2. The film containing 7 wt% of TiO2 nanoparticles exhibited effective photocatalytic anti-fungal activity and a favorable effect on strawberry storage32. Mohammadnezhad et al. prepared different magnetite/κ-carrageenan nanocomposites using high surface area Fe3O4 nanoparticles with a flower-like 3D morphology4,33. The nanocomposite was used as a convenient and recyclable sorbent for removing Cu2+ ions from aqueous solutions.
Despite extensive research on carrageenan-based composites, the synergistic effects of incorporating low-cost TiO₂ on thermal stability and adsorption are still of interest. In this research, a rapid, low-cost, and eco-friendly in situ synthesis of TiO₂ nanoparticles within a carrageenan matrix is reported using an ultrasound-assisted method. The structural and functional properties of the resulting nanocomposites were systematically explored at TiO₂ loadings of 5, 10, and 15 wt%. Due to the improved characteristics of the prepared nanocomposite, including enhanced adsorption capacity and stability, it can be utilized as an adsorbent for removing copper cations from aqueous solutions. Unlike conventional synthetic composites, this work presents a cost-effective and sustainable solution by minimizing TiO₂ usage (≤ 15 wt%), ensuring homogeneous nanoparticle dispersion through in situ sonochemical synthesis, and achieving efficient adsorption at low filler concentrations.
Experimental section
Materials
Copper(II) nitrate trihydrate, sodium hydroxide (NaOH), hydrochloric acid (HCl), glacial acetic acid (AcOH), titanium propoxide, and n-propanol (n-PrOH) were purchased from Merck. Kappa-carrageenan was obtained from Merck Chemical Co. All materials used in this research were of laboratory-grade purity. Deionized water was used to prepare all solutions at every stage.
Instrumentation
Infrared spectra were recorded using a Jasco FT-IR 680 Spectrophotometer. The spectra of the samples were obtained by preparing KBr pellets. Thermogravimetric analysis of the nanocomposites was conducted using a NETZSCH STA 449F1 in the temperature range of 20–1000 °C under a nitrogen atmosphere at a heating rate of 5 °C/min. To investigate the surface morphology, dispersion, and size of the nanoparticles, a MIRA3 TESCAN scanning electron microscope (SEM) was used. A thin layer of gold (at the atomic level) was coated on the surfaces of the relevant nanocomposites to make the samples conductive. Transmission electron microscope (TEM) images were acquired using a Philips CM-120 instrument from Pare Tavous Mashhad, Iran. For the preparation of the nanocomposites, an ultrasonic device (ultrasonic liquid processor, MISONIX XL-2000 SERIES) was employed. Ultrasonic waves were applied through the probe tip of the ultrasonic device, which was directly placed in the reaction solution system at a frequency of 2.25 × 104 Hz and the highest power level of the device (100%). Flame atomic absorption spectroscopy was conducted using a PerkinElmer spectrometer equipped with an air-acetylene burner and a hollow cathode to examine the amount of copper cation adsorption.
Preparation of carrageenan/titanium dioxide nanocomposites
A typical synthetic procedure for preparing carrageenan/titanium dioxide 5 wt% nanocomposite (CG/TiO2 5 wt%) is as follows (Scheme 1). First, 2 g of carrageenan was added to 40 mL of n-propanol, and ultrasonic waves were applied for 15 min. In the next step, titanium propoxide, equivalent to 5 wt% of titanium dioxide in the nanocomposite, was added to 5 mL of n-propanol, followed by adding to the carrageenan suspension. The resulting mixture was subjected to ultrasonic waves for another 15 min. Finally, a mixture of water and acetic acid was added for hydrolysis and subjected to ultrasonic waves for 15 min. The resulting gel was refluxed for 20 h in a round-bottom flask with a magnetic stirrer. Then, it was dried at room temperature while the magnetic stirrer was spinning, and the CG/TiO2 5 wt% nanocomposite was prepared. Based on the described method, CG/TiO2 10 wt% and CG/TiO2 15wt.% nanocomposites were prepared.
Copper adsorption from aqueous solution
Adsorption experiments were carried out to determine the optimal amount of adsorbent, pH, metal ion concentration, and contact time. The samples were shaken at a speed of 180 rounds per minute throughout all experiments. All experiments were carried out at 25 °C.
Effect of adsorbent dosage
To examine the effect of adsorbent dosage on copper cation adsorption, different amounts of each nanocomposite were placed in several vials, and 20 mL of 10 mg/L of copper cation was added. They were then placed on a shaker for 240 min. Finally, the adsorbents were filtered, and the remaining ion concentration was measured using atomic absorption spectroscopy.
Effect of pH
To determine the effect of pH on copper cation removal efficiency, pH was adjusted in the range of 1–9 using hydrochloric acid (0.1 M) and sodium hydroxide (0.1 M). In this experiment, 20 mL of a 10 mg/L copper cation solution and the optimal amount of adsorbent were added to each vial. The vials were then placed on a shaker for 240 min. Finally, the adsorbents were filtered, and the remaining ion concentration was measured using atomic absorption spectroscopy.
Effect of contact time
Kinetic experiments were conducted using a constant concentration of copper cations (10 mg/L), an optimal amount of adsorbent and pH to determine the optimal adsorption time. Samples were placed on a shaker, and the concentration of the remaining ions was analyzed by atomic absorption spectroscopy every 20 min over 20 to 240 min.
Effect of copper ion concentration
To study the effect of copper ion concentration on the removal efficiency, concentrations ranging from 5 to 50 mg/L were used at optimal amounts of adsorbent, time, and pH in vials containing 20 mL of solution on a shaker. After the optimal time had passed, the remaining copper ion concentration was analyzed using atomic absorption spectroscopy.
Results and discussion
FT-IR spectroscopy
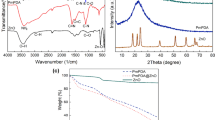
The Fourier transform infrared (FT-IR) spectra of carrageenan, titanium dioxide, and CG/TiO2 5 wt% nanocomposite are shown in Fig. 1. In the spectrum of carrageenan (Fig. 1a), a broad band at 3500 cm− 1 and a weak band at 1639 cm− 1 correspond to the stretching and bending vibrations of hydroxyl groups and absorbed water, respectively. Additionally, the bands observed in 1378, 1427, and 2914 cm− 1 regions correspond to the stretching vibrations of C-H bonds. The bands at 1227, 929 and 847 cm− 1 are attributed to S=O of sulfate ester, C–O stretching within the sugar ring, and C–O–SO3 of the sugar ring, respectively4. In the spectrum of titanium dioxide (Fig. 1b), the bending vibrations of the Ti–OH and the stretching vibrations of the Ti–O appear at 1578 and 1430 cm− 1, respectively18. Furthermore, in the spectrum of CG/TiO2 5 wt%, the decrease in the S=O peak and the presence of bands observed in both titanium dioxide and carrageenan confirm the nanocomposite synthesis.
SEM and TEM
The SEM images of CG/TiO2 5 wt%, CG/TiO2 10 wt%, CG/TiO2 15 wt% nanocomposites are shown in Fig. 2a–c. The uniform observed morphology in these images indicates an excellent and homogeneous dispersion of titanium dioxide nanoparticles within the carrageenan matrix, with the particles exhibiting a spherical shape. The histogram related to the CG/TiO2 5 wt% is presented in Fig. 2d. Based on the obtained data, the average size of the nanoparticles was determined to be 28 nm. The TEM images of CG/TiO2 5 wt% are shown in Fig. 3, and the particle sizes are consistent with the SEM images. In these images, particles with defined crystalline shapes or specified lattice planes were not observed, indicating the amorphous nature of TiO2 in the polymeric matrix.
Thermal analyses
The effect of titanium dioxide nanoparticles on the thermal stability of carrageenan was studied using thermogravimetric analysis in a nitrogen atmosphere at a heating rate of 5 °C/min. The TGA curves are shown in Fig. 4, and the data related to these curves, including the temperature of 5 wt% weight loss (T5), the temperature of 10 wt% weight loss (T10), char yield (CY) at 800 and 1000 °C, and the limiting oxygen index (LOI) are provided in Table 1. For the pure polymer, the initial stage of weight loss involves the desorption of solvents and adsorbed water, occurring in the range of 100–170 °C4. The second stage involves chain scission reactions, the removal of hydroxyl side groups and remaining ester groups in the carrageenan structure, occurring in the range of 200–300 °C. The third weight loss stage occurs in the range of 400–750 °C, relating to further degradation of carrageenan and the formation of carbon and hydrocarbons. T5 for the pure polymer is 121 °C, T10 is 202 °C, and its char yield at 800 °C is around 29%. Lower T₅ values for nanocomposites compared to pure carrageenan (121 °C) can be attributed to the disruption of the polymer matrix by TiO2 nanoparticles, since CG has a highly ordered, hydrogen-bonded structure that contributes to its thermal stability. The incorporation of TiO2 nanoparticles (even at 5 wt%) may disrupt hydrogen bonds between polymer chains, weakening the network, and creating defects or voids in the matrix. So, it can host and adsorb more solvent, 5% in the case of T5%, at lower temperature. Notably, the char yield of the nanocomposites increased compared to the pure carrageenan, indicating higher thermal resistance of the nanocomposites than the pure polymer. By adding titanium dioxide nanoparticles to the carrageenan matrix, thermal vibrations of the polymer chains are reduced, and the movement of the chains is restricted. Thus, the nanocomposites consume more thermal energy for degradation, resulting in less mass loss at the same temperature compared to pure carrageenan. Moreover, the inorganic fraction of the nanocomposites has a high melting point and good thermal stability, distributing heat within the nanocomposite, leading to increased thermal resistance. It explains the rise in char yield in the nanocomposites compared to pure carrageenan. The first TGA analysis was measured up to 800 °C, and the obtained char yields did not follow the expected trends (47, 43, and 45% for CG/TiO2 5 wt%, CG/TiO2 10 wt%, and CG/TiO2 15 wt%, respectively). Since higher inorganic content is expected to produce higher char yields, the TGA was remeasured to 1000 °C, and char yields matched well with filler percentage increments (29, 35, 40 for CG/TiO2 5 wt%, CG/TiO2 10 wt%, and CG/TiO2 15 wt%, respectively). Given that the LOI values obtained for carrageenan and the synthesized nanocomposites are above 21, these compounds can be classified as part of self-extinguishing materials. Self-extinguishing materials are those that stop combustion by cutting off the flame source. Therefore, the synthesized nanocomposites are suitable options for applications requiring heat resistance.
Removal of copper cations from aqueous solutions using CG/TiO2 5 wt%
Initially, the optimization of parameters affecting the adsorption process of Cu2+ ions, including the adsorbent dosage, initial concentration of metal ions, contact time between the adsorbent and solution, and pH, was examined. An adsorption test was performed on each nanocomposite (CG/TiO2 5 wt%, 10 wt%, and 15 wt%) under specified conditions: a fixed amount of adsorbent, a solution volume of 20 mL, an initial concentration of 10 mg/L, and a contact time of 240 min. The CG/TiO2 5 wt% nanocomposite was selected as the most efficient adsorbent and was used in subsequent steps.
Effect of adsorbent dosage
Increasing the amount of adsorbent leads to an increase in adsorption capacity because more active sites on the adsorbent surface become available, thus increasing the likelihood of adsorption of the copper cations. However, increasing the amount of adsorbent beyond the optimum level can reduce adsorption effectiveness, as excessive adsorbent can cause agglomeration, decrease the available surface area, and consequently reduce its activity. The removal efficiency of copper cations from aqueous solution using 5, 10, 20, and 30 mg of CG/TiO2 5 wt% nanocomposite were 33.5, 65.7, 78.8, and 77.7%, respectively. Therefore, 20 mg of the nanocomposite was selected as the optimal dosage.
Effect of pH
To determine the optimal pH for the removal of copper cations from aqueous solutions using the CG/TiO2 5 wt%, the percentage of copper cation removal at various pH levels ranging from 2 to 9 was analyzed, maintaining an initial concentration of copper cations at 10 mg/L, as illustrated in Fig. 5. The highest adsorption of copper cations was achieved using the nanocomposite at pH 7.
At low pH levels, there is a competition between hydrogen ions and copper cations for adsorptive sites, and hydrogen ions dominate due to their higher charge density. Even at the lowest pH, some copper cations have been adsorbed, likely due to ion exchange between copper cations and hydrogen ions on the surface of the nanocomposite. As pH increases and the concentration of hydrogen ions decreases, more available sites are freed up, resulting in increased adsorption percentages. As the pH rises from 7, the presence of OH⁻ ions yields a negatively charged adsorbent surface, leading to electrostatic attraction with copper cations and subsequent adsorption. However, as hydroxide precipitation occurs, the overall adsorption capacity diminishes.
Contact time effect
The contact time between the copper cations and the nanocomposite is a key factor affecting the adsorption process. Fig. 6 shows the results obtained over a time range of 20–160 min. Depending on the adsorption kinetics model, as the contact time between the copper cation solution and the nanocomposite increases, the diffusion of copper cations into the inner layers increases. In this regard, three models are examined: pseudo-second-order, Elovich, and intra-particle diffusion models5,6,7,34,35,36,37. The capacity for heavy metal adsorption per unit mass of adsorbent at equilibrium qe (mg/g), the capacity for adsorption at time t, qt (mg/g), and the removal of cations R are calculated according to Eqs. 1–3. C0 (mg/L) is the initial concentration, Ct (mg/L) is the concentration of the metal ions present in the solution at time t, V (L) is the volume of the solution, and M (g) is the mass of the adsorbent used. Initially, the adsorption capacity for copper cations is high due to the presence of unfilled adsorption sites. However, after some time, the adsorption sites become saturated, which leads to a change in the removal of Cu2+ cations. However, the removal percentage remains constant after a specific period, known as the equilibrium time.
By plotting t/qt versus t, the Pseudo-second order kinetics parameters are obtained. Based on Eq. 4, the slope and intercept provide the equilibrium adsorption capacity (qe) and the rate constant (kad), respectively (Fig. 7)34,35.
By plotting qt versus ln(t), the Elovich kinetic model was evaluated. In Eq. 5, the Elovich rate constant (\(\:\beta\:\)) is obtained using the slope of the linear form of the equation (Fig. 8)36.
By plotting qt versus t1/2, the intra-particle diffusion model is derived. The slope, according to Eq. 6, gives the intra-particle diffusion rate constant kintra (mg g− 1 min− 1/2) (Fig. 9)37. The results of the calculations and parameters obtained from the kinetic equations are presented in Table 2. Considering the graphs from Figs. 7, 8, and 9, the data from Table 2, and the correlation coefficients obtained from the equations, it is concluded that the CG/TiO2 5 wt% follows the pseudo-second-order kinetic model. This model does not fully elucidate the adsorption mechanism, particularly regarding whether intra-particle diffusion or chemisorption is the rate-determining step38. The adsorption mechanism likely involves coordinative, ion exchange, or electrostatic interactions, as schematically illustrated in Scheme 2.
Effect of initial copper concentration on adsorption
By examining the relationship between the equilibrium adsorption capacity and equilibrium concentration of Cu2+ cations using the Langmuir and Freundlich adsorption isotherms, valuable insights are gained into the nature and intensity of interactions between Cu2+ cations and nanocomposites. As the concentration of Cu2+ cations increases, the required energy for mass transfer between the nanocomposites and the copper solution is provided, driven by the concentration difference between the nanocomposite and the solution. Furthermore, as the concentration increases, the frequency of collisions also increases, leading to a faster diffusion process. As shown in Fig. 10, the increase in initial concentrations results in a rise in the adsorption per unit weight of the adsorbent. As observed at the beginning of the process, due to the presence of more vacant sites, the adsorption capacity is high, and the slope is steeper.
To calculate the concentration of Cu2+ cations in each solution, a calibration curve was prepared using solutions with specific concentrations, and their absorption was determined using an atomic absorption spectrometer. In the Langmuir isotherm model, expressed as Eq. 7, the monolayer adsorption assumption can be examined, and the Langmuir isotherm constant and maximum adsorption capacity can be determined by plotting Ce/qe versus Ce and obtaining them from the intercept and slope of the graph, respectively (Fig. 11).
Here, qmax is the maximum possible amount of adsorbed Cu2+ cations (in milligrams of copper cations adsorbed per gram of adsorbent), and KL is a measure of the distribution constant of copper cations between the solid and liquid phases, with units of liters per milligram.
The Freundlich isotherm model, which assumes multilayer adsorption on heterogeneous surfaces, is given by Eq. 8. The model’s constants are determined from the slope and intercept of the graph shown in Fig. 12. Based on the isotherm models and the summarized data in Table 3, and by comparing the correlation coefficients of the models, it is evident that the CG/TiO2 nanocomposite follows the Langmuir adsorption model, characterized by homogeneous adsorption sites and monolayer coverage.
The main characteristic of the Langmuir isotherm model is that it determines whether the adsorption nature is favorable or unfavorable. This characteristic is determined by the dimensionless separation factor RL, which is obtained from Eq. 9 and is expressed by the Langmuir isotherm constant (KL, related to the free energy of adsorption), which shows the tendency of the adsorbent toward the adsorbate5. RL is a positive quantity, and its value predicts the possibility of the adsorption process such that an adsorption reaction can be favorable, unfavorable, linear, or irreversible. Reactions that follow the Langmuir isotherm pattern and are favorable in terms of the separation factor are known as ideal reactions.
If RL = 0, the adsorption is irreversible; if RL is between zero and one, it is favorable; if RL = 1, the adsorption isotherm is linear; and if RL>1, the adsorption is unfavorable. The RL graphs versus Ce are shown in Fig. 13. Given that the obtained RL values fall between 0 and 1, indicating that the adsorption is favorable.
A comparison of the maximum adsorption capacities (qm, mg/g) of some biopolymer/polymer-based adsorbents is presented in Table 4. According to our results, the CG/TiO2 5 wt% nanocomposite had an adsorption capacity of 28.57 mg/g. Even though CG/TiO2 demonstrated moderate qm values, its low cost and ease of preparation make it an attractive candidate for the remediation of toxic heavy metals in aqueous solutions.
To recover the adsorbent, a desorption process was performed using 0.05 M hydrochloric acid, followed by drying in an oven at 60 °C. CG/TiO2 5 wt%, the adsorbent, was used in three cycles, with removal efficiencies decreasing from 83.24 to 50.61% and 20.39% after the first and second recycling, respectively. Consequently, the composite exhibits limited recyclability but can serve as an efficient, biodegradable, single-use adsorbent.
Conclusion
In this study, carrageenan-based nanocomposites were synthesized using titanium dioxide as an inorganic nanofiller for the adsorption of Cu2+ ions from aqueous solutions. The physical and chemical properties of these nanocomposites were investigated through FT-IR, SEM, and TGA analyses. The data from thermal gravimetric analysis indicate that the thermal properties of the nanocomposites have improved compared to the pure polymer due to the presence of nanoparticles. Moreover, morphological studies and the particle size distribution diagram exhibit a uniform distribution of the nanoparticles within the polymer matrix. Adsorption studies were performed on the nanocomposite to investigate the effects of various parameters, including pH, contact time, and initial concentration, on the adsorption of Cu2+ ions. Three kinetic models, including pseudo-second-order, Elovich, and particle diffusion models, were evaluated in kinetic studies. Two equilibrium isotherm models, Langmuir and Freundlich, were used to investigate the adsorption mechanism. The data obtained from the adsorption process showed a high affinity of the CG/TiO2 5 wt% nanocomposite for the removal of copper cations. CG/TiO2 5 wt% demonstrated efficient adsorption for Cu2+ cations from aqueous solutions, achieving > 80% removal at optimal conditions. This is particularly significant given the strict international limits on Cu2+ discharge (2 mg/L). CG/TiO2 5 wt% reduced Cu2+ levels from 10 to < 1.7 mg/L (below the WHO limit), confirming its potential for real-world remediation. Kinetic studies revealed that the adsorption of copper cations followed pseudo-second-order kinetics, and the equilibrium adsorption patterns were well-fitted to the Langmuir isotherm model. The main advantages of this nanocomposite include cost-effectiveness due to its low TiO2 loading, well-dispersed particles resulting from in situ synthesis, and eco-friendly conditions. This nanocomposite was tested only for Cu2+ removal, and its long-term stability needs to be checked and improved by carrageenan crosslinking.
Data availability
All data generated or analyzed during this study are included in the published article and its supplementary information files.
References
World Health Organization. Guidelines for Drinking-Water Quality 5th ed. (2022).
Mohammadnezhad, G. & Keikavousi Behbahan, A. Polymer matrix nanocomposites for heavy metal adsorption: A review. J. Iran. Chem. Soc. 17:1259–1281 (2020).
Rostami, M. S. & Khodaei, M. M. Recent advances in chitosan-based nanocomposites for adsorption and removal of heavy metal ions. Int. J. Biol. Macromol. 15:132386 (2024).
Mohammadnezhad, G., Ariaeinezhad, F. & Steiniger, F. S. Magnetite/κ-carrageenan nanocomposites: a convenient recyclable tool for Cu (II) ions adsorption from aqueous solution. Inorg. Chem. Res. 3:129–144 (2019).
Loganathan, M., Raj, A. S., Murugesan, A. & Kumar, P. S. Effective adsorption of crystal violet onto aromatic polyimides: Kinetics and isotherm studies. Chemosphere 304:135332 (2022).
Munireddy, R., Murugesan, L., Arukkani, M., Ponnusamy, S. K. & Gayathri, R. Studies on effective photo-catalytic degradation of rhodamine-B using metal-doped oxidized-activated carbon: Kinetics, isotherm models and degradation mechanism. Korean J. Chem. Eng. 41(7):2183–2200 (2024).
Şenol, Z. M., Kaya, S., Şimşek, S., Katin., K. P., Özer, A. & Marzouki, R. Synthesis and characterization of chitosan-vermiculite-lignin ternary composite as an adsorbent for effective removal of uranyl ions from aqueous solution: Experimental and theoretical analyses. Int. J. Biol. Macromol. 209:1234–1247 (2022).
Mohammadnezhad, G., Soltani, R., Abad, S. & Dinari, M. J. A novel porous nanocomposite of aminated silica MCM-41 and nylon-6: Isotherm, kinetic, and thermodynamic studies on adsorption of Cu (II) and Cd (II). Appl. Polym. Sci. 134(40):45383 (2017).
Mohammadnezhad, G., Dinari, M. & Soltani, R. The preparation of modified boehmite/PMMA nanocomposites by in situ polymerization and the assessment of their capability for Cu2+ ion removal. New. J. Chem. 40:3612–3621 (2016).
Kumar, A. P., Depan, D., Tomer, N. S. & Singh, R. P. Nanoscale particles for polymer degradation and stabilization—trends and future perspectives. Prog. Polym. Sci. 34:479–515 (2009).
Wen, X., Lin, Y., Han, C., Zhang, K., Ran, X. & Li, Y. Thermomechanical and optical properties of biodegradable poly(L-lactide)/silica nanocomposites by melt compounding. J. Appl. Polym. Sci. 114:3379–3388 (2009).
Wang, X., Wu, L. & Li, J. Influence of nanozirconia on the thermal stability of poly(methyl methacrylate) prepared by in situ bulk polymerization. J. Appl. Polym. Sci. 117:163–170 (2010).
Sabzi, M., Mirabedini, S., Zohuriaan-Mehr, J. & Atai, M. Surface modification of TiO2 nano-particles with silane coupling agent and investigation of its effect on the properties of polyurethane composite coating. Prog. Org. Coat. 65:222–228 (2009).
Carraher, C. E. Jr., Roner, M. R., Lambert, R. E., Arroyo, L. & Miller, L. C. Synthesis of organotin polyamine ethers containing thiamine (vitamin B1) and preliminary ability to inhibit select cancer cell lines. J. Inorg. Organomet. Polym. Mater. 25:1414–1424 (2015).
Leopold, N., Cîntă-Pînzaru, S., Baia, M., Antonescu, E., Cozar, O. & Kiefer, W. Raman and surface-enhanced Raman study of thiamine at different pH values. Vib. Spectrosc. 39:169–176 (2005).
Klein, L. C. Sol–Gel Technology for Thin Films, Fibers, Preforms, and Electronics and Speciality (Shapes Noyes Publication, 1988).
Das, M., Lalsangi, S., Santra, S. & Banerjee, R. Nanocellulose as a carrier for improved drug delivery: Progresses and innovation. J. Drug Deliv. Technol. 9:105743 (2024).
Ariaeinezhad, F., Mohammadnezhad, G., Zare, M., Akintola, O. & Plass, W. Controllable and facile one-pot synthesis of high surface area amorphous, crystalline, and triphasic TiO2: catalytic and photocatalytic applications. J. Mater. Chem. A 12:6488–6506 (2024).
Damoom, M. M., Saeed, A., Alshammari, E. M., Alhawsawi, A. M., Yassin, A. Y., Abdulwahed, J. M. & Al-Muntaser, A. A. The role of TiO2 nanoparticles in enhancing the structural, optical, and electrical properties of PVA/PVP/CMC ternary polymer blend: nanocomposites for capacitive energy storage. J. Sol–Gel Sci. Technol. 108:742–755 (2023).
Yin, Z. F., Wu, L., Yang, H. G. & Su, Y. H. Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 15:4844–4858 (2013).
Pfaff, G. High stability, high solid content, low viscosity titanium dioxide dispersion. Phys. Sci. Rev. 6:679–696 (2021).
Lee, H. S., Im, S. J., Kim, J. H., Kim, H. J., Kim, J. P. & Min, B. R. Polyamide thin-film nanofiltration membranes containing TiO2 nanoparticles. Desalination 219:48–56 (2008).
Kong, Y., Du, H., Yang, J., Shi, D., Wang, Y., Zhang, Y. & Xin, W. Study on polyimide/TiO2 nanocomposite membranes for gas separation. Desalination 146:49–55 (2002).
Nejad, S. A. T., Soleimani-Gorgani, A. & Pishvaei, M. Multifunctional screen-printed films using polymer nanocomposite based on PPy/TiO2: conductive, photocatalytic, self-cleaning and antibacterial functionalities. Iran. Polym. J. 32:647–659 (2023).
Gholamrezapor, E. & Eslami, A. Modification of magnetic TiO2 nanocomposite with CoIII, ZnII and NiII porphyrins for photodegradation of methylene blue under the blue Vis-LED light irradiation. Inorg. Chem. Res. 5:19–36 (2021).
Kickelbick, G. Nanoparticles and composites. Sol-Gel Handb. 14, 225–244 (2015).
Jo, S., Oh, Y., Park, S., Kan, E. & Lee, S. H. Cellulose/carrageenan/TiO2 nanocomposite for adsorption and photodegradation of cationic dye. Biotechnol. Bioprocess. Eng. 22:734–738 (2017).
Duman, O., Tunç, S., Bozoğlan, B. K. & Polat, T. G. Removal of triphenylmethane and reactive azo dyes from aqueous solution by magnetic carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite. J. Alloys Compd. 687:370–383 (2016).
Duman, O., Tunç, S., Polat, T. G. & Bozoğlan, B. K. Synthesis of magnetic oxidized multiwalled carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic methylene blue dye adsorption. Carbohydr. Polym. 147:79–88 (2016).
Salgueiro, A. M., Daniel-da-Silva, A. L., Girão, A. V., Pinheiro, P. C. & Trindade, T. Unusual dye adsorption behavior of κ-carrageenan coated superparamagnetic nanoparticles. Chem. Eng. J. 229:276–284. (2013).
Chavez-Baldovino, E., Malca-Reyes, C. A., Masso, R., Feng, P., Camacho, A., Sarmiento, J., Borrero Negrón, J. I., Pagán-Torres, Y. J., Díaz-Vázquez, L. M. Borrero Negrón, J. I. & Díaz-Vázquez, L. M., Optimizing sustainable energy generation in ethanol fuel cells: An exploration of carrageenan with TiO2 nanoparticles and Ni/CeO2 composites. ACS Omega 8:20642–20653 (2023).
Duan, N., Li, Q., Meng, X., Wang, Z. & Wu, S. Preparation and characterization of k-carrageenan/konjac glucomannan/TiO2 nanocomposite film with efficient anti-fungal activity and its application in strawberry preservation. Food Chem. 364:130441 (2021).
Mohammadnezhad, G. & Ariaeinezhad, F. Synthesis of mesoporous flower-like iron oxide nanostructures from iron alkoxide precursor and their application in the catalytic reduction of 4-nitrophenol. J. Porous Mater. 28:791–801 (2021).
Azizian, S. J. Kinetic models of sorption: A theoretical analysis. Colloid Interface Sci. 276:47–52 (2004).
Ho, Y. S., Wase, D. A. J. & Forster, C. F. Kinetic studies of competitive heavy metal adsorption by sphagnum moss peat. Environ. Technol. 17:71–77 (1996).
Ho, Y. S. Review of second-order models for adsorption systems. J. Hazard. Mater. 136:681–689 (2006).
Namasivayam, C. & Ranganathan, K. Removal of Cd (II) from wastewater by adsorption on “waste” Fe (III) Cr (III) hydroxide. Water Res. 29 1744 (1995).
Bullen, J. C., Saleesongsom, S., Gallagher, K., & Weiss, D. J. A revised pseudo-second-order kinetic model for adsorption, sensitive to changes in adsorbate and adsorbent concentrations. Langmuir 37(10):3189–3201 (2021).
Salehi, E., Madaeni, S. S., Rajabi, L., Vatanpour, V., Derakhshan, A. A., Zinadini, S., Ghorabi, S. & Monfared, H. A. Novel chitosan/poly (vinyl) alcohol thin adsorptive membranes modified with amino functionalized multi-walled carbon nanotubes for Cu (II) removal from water: preparation, characterization, adsorption kinetics and thermodynamics. Sep. Purif. Technol. 89:309–319 (2012).
Liang, X., Duan, J., Xu, Q., Wei, X., Lu, A. & Zhang, L. Ampholytic microspheres constructed from chitosan and carrageenan in alkali/urea aqueous solution for purification of various wastewater. Chem. Eng. J. 317:766–776 (2017).
Su, C., Berekute, A. K. & Yu, K. P. Chitosan@TiO2 composites for the adsorption of copper (II) and antibacterial applications. Sustain. Environ. Res. 32(1):27 (2022).
Mohammadnezhad, G., Moshiri, P., Dinari, M. & Steiniger, F. In situ synthesis of nanocomposite materials based on modified-mesoporous silica MCM-41 and methyl methacrylate for copper (II) adsorption from aqueous solution. J. Iran. Chem. Soc. 16:1491–1500 (2019).
Abba, M. U., Man, H. C., Azis, R. A. S., Isma Idris, A., Hazwan Hamzah, M., Yunos, K. F. & Katibi, K. K. Novel PVDF-PVP hollow fiber membrane augmented with TiO2 nanoparticles: preparation, characterization and application for copper removal from leachate. Nanomaterials 11(2):399 (2021).
Acknowledgements
The authors express their gratitude for the support provided to this work by the Research Affairs Division of the Isfahan University of Technology (IUT). We thank Mrs. Wermann for the measurement of the thermogravimetric data.
Funding
This study was funded by Isfahan University of Technology (IUT) and Friedrich Schiller University Jena.
Author information
Authors and Affiliations
Contributions
G.M.: Conceptualization, Methodology, Validation, Resources, Funding acquisition, Writing-original draft, Writing-review & editing, Supervision. P.M.: Investigation, Formal analysis, Methodology, Data curation. M.D.: Conceptualization, Methodology, Validation, Resources, Writing-review & editing W.P.: Resources, Funding acquisition, Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mohammadnezhad, G., Moshiri, P., Dinari, M. et al. In situ synthesis of TiO2 nanofiller in preparation of carrageenan-based nanocomposites and its application in the adsorption of copper(ІI) from aqueous solution. Sci Rep 15, 32294 (2025). https://doi.org/10.1038/s41598-025-13482-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13482-6