Abstract
Tomato brown rugose fruit virus (ToBRFV) has become a major threat to global tomato production, causing significant economic losses. Due to the limited efficacy of conventional control methods, alternative strategies are urgently needed. This study evaluated the effects of biotic and abiotic elicitors—including bacterial polysaccharides from Pseudomonas fluorescens 1442 and Burkholderia gladioli G15, salicylic acid (SA), and mechanical wounding—on resistance induction in tomato plants infected with ToBRFV. The experiment was conducted in a greenhouse under controlled environmental conditions, using a completely randomized design with seven treatments and five replicates. Bacterial elicitors were applied via foliar spray in two applications at one-week intervals; a 0.5 mM SA solution was similarly applied, and mechanical wounding was induced using a sterile punch. Disease severity was visually assessed at pre-flowering, flowering, and fruiting stages. Defense responses were evaluated by measuring the activities of catalase (CAT), peroxidase (POD), and phenylalanine ammonia-lyase (PAL), as well as the expression of defense-related genes, Pathogenesis-Related Protein 1b (PR1b), Coronatine-Insensitive Protein 1 (COI1), and Nonexpressor of PR Genes 1 (NPR1), using qRT-PCR. The results indicated that the combined P. fluorescens and B. gladioli (P + B) treatment significantly enhanced defense enzyme activities, upregulated gene expression, and reduced symptom severity more effectively than individual treatments. Additionally, the combined elicitor treatment improved plant growth parameters, suggesting a synergistic effect that offers a promising strategy for ToBRFV management.
Similar content being viewed by others
Introduction
Global crop yield losses due to plant diseases are estimated to exceed 30% annually, resulting in economic damages valued at hundreds of billions of dollars1. Among these pathogens, tomato brown rugose fruit virus (ToBRFV), a species of the genus Tobamovirus within the family Virgaviridae, has emerged as a formidable challenge to tomato (Solanum lycopersicum) production since its initial detection in the Middle East2,3. In Iran, ToBRFV was first documented in 2021 in greenhouses located near Tehran and Isfahan provinces4,5, causing fruit yellowing, deformation, and necrotic lesions, with yield losses ranging from 10 to 55%6. Its mechanical transmission through contact, agricultural tools, and possibly contaminated seeds facilitates the virus’s rapid dissemination, particularly in controlled environments such as greenhouses7,8. This swift global spread underscores the virus’s economic impact, exacerbating losses in a crop of major importance for both fresh consumption and processing9.
Traditional chemical control methods against viral diseases are often ineffective and contribute to environmental degradation and the emergence of resistant pathogen strains, prompting the search for sustainable biological alternatives such as elicitors10,11. Elicitors, compounds that trigger plant defense responses, typically categorized as biotic or abiotic agents and are gaining recognition as eco-friendly tools for integrated disease management12. Biotic elicitors, particularly plant growth-promoting rhizobacteria (PGPR), stimulate induced systemic resistance (ISR) through mechanisms such as antibiotic production, siderophore secretion, and emission of volatile organic compounds13. Pseudomonas fluorescens, a well-known soil bacterium, exemplifies PGPR by producing siderophores and fluorescein that suppress plant pathogens14. For instance, Simões et al. (2021)15 showed that flagellin from P. fluorescens reduces the severity of bacterial leaf spots in tomatoes by triggering ISR. Similarly, Rudrappa et al. (2010)16 demonstrated that volatile compounds from Bacillus subtilis protect Arabidopsis against Pseudomonas syringae by inducing ISR, a mechanism further supported by Pieterse et al. (2014)17, who emphasized the pivotal role of PGPR in priming plant defenses against diverse biotic threats. These findings underscore the potential of microbial elicitors to enhance plant immunity.
In addition to microbial agents, chemical and physical elicitors have been explored to bolster plant defense mechanisms. Salicylic acid (SA), a key phytohormone, initiates systemic acquired resistance (SAR) by upregulating pathogenesis-related (PR) genes and promoting the synthesis of antimicrobial proteins18. In tobacco, SA enhances resistance to tobacco mosaic virus (TMV) via NPR1-mediated signaling pathways19. Similarly, Jayaraj et al. (2004)20 found that exogenous application of SA and jasmonic acid (JA) in wheat reduced Stagonospora nodorum infection by 56%, correlating with elevated PR protein levels. Physical wounding, an abiotic elicitor, mimics herbivore damage and activates plant defenses by inducing reactive oxygen species (ROS) production and modulating gene expression21. For example, Sokea et al. (2019)22 reported that the protein elicitor Hrip1 from Alternaria tenuissima induces SAR in tomatoes against tomato yellow leaf curl virus (TYLCV), independent of direct antimicrobial activity. Moreover, Qi et al. (2022)23 showed that the synthetic elicitor LY5-24-2 enhances Arabidopsis resistance by reinforcing cell walls, suggesting the broad applicability of elicitor-based strategies. These findings highlight the multifaceted nature of plant defense activation, suggesting unexplored synergies among different elicitor types.
This study investigates the individual and combined effects of P. fluorescens 1442, Burkholderia gladioli G15, salicylic acid (SA), and mechanical wounding on ToBRFV-infected tomato plants by evaluating defense-related gene expression, enzymatic activity, and disease severity. The objectives of this research are to: (1) determine the individual contributions of P. fluorescens 1442, B. gladioli G15, SA, and mechanical wounding in enhancing tomato resistance to ToBRFV; (2) compare the efficacy of the combined bacterial elicitors versus single applications; and (3) quantify their impacts on plant growth, yield, disease severity, enzyme activities (catalase [CAT], peroxidase [POD], and phenylalanine ammonia-lyase [PAL]), and expression of defense-related genes—Coronatine-Insensitive Protein 1 (COI1), Nonexpressor of PR Genes 1 (NPR1), and Pathogenesis-Related Protein 1b (PR1b).
Materials and methods
Sources and Preparation of bacterial and viral isolates
The bacterium P. fluorescens 1442 (P) was obtained from the bacterial collection at the Agricultural Sciences and Natural Resources University of Khuzestan (Mollasani, Iran), and the pathogenic bacterium B. gladioli G15 (B) was originally isolated by the Department of Plant Protection, Shiraz University, and subsequently provided by the Department of Plant Protection, Shahid Chamran University of Ahvaz (Ahvaz, Iran). Additionally, the ToBRFV isolate was provided by the Plant Protection Department of the Agricultural Sciences and Natural Resources University of Khuzestan. To ensure compatibility, the bacterial isolates were tested for antagonistic activity against each other using a modified method described by Jetiyanon and Kloepper (2002)24. Specifically, the diameter of the inhibition zone served as the criterion for antagonism. Subsequently, the bacterial isolates were stored at −80 °C in nutrient broth (NB, Quelab, China) supplemented with 20% glycerol for further analysis.
Preparation of bacterial elicitor
Bacterial isolates were cultured in NB at 25 °C for 36 h. Then, 100 µL of each bacterial suspension was spread onto nutrient agar (NA, Quelab, China) plates and incubated at 25 °C for 48 h. Next, 5 mL of phosphate-buffered saline (0.1 M phosphate buffer, pH 7.0, in 0.85% NaCl) was added to each plate to suspend the bacterial cells. The suspension was autoclaved at 121 °C under 1 atm pressure for 1 h and then centrifuged at 10,000 rpm for 20 min. The pellet was washed with 95% ethanol, and the resulting bacterial polysaccharide pellet was resuspended in distilled water. The solid bacterial elicitors were stored at 4 °C under dark, dry conditions, following a modified protocol25.
Application of elicitors
Tomato (Solanum lycopersicum L.) cultivar ‘Sereen F1’ was selected for this study due to its widespread use in greenhouses and its known susceptibility to ToBRFV, making it an ideal model for resistance studies. Seeds (Syngenta, Switzerland) were supplied by Gol Sam Company (Iran), surface-sterilized with 1% sodium hypochlorite for 1 min, rinsed with sterile distilled water, and sown in 5-L pots filled with a steam-sterilized mixture of peat, perlite, and vermiculite (2:1:1 ratio)26. Plants were grown in a greenhouse under controlled conditions (25 ± 2 °C, 60–70% relative humidity) and irrigated with sterile distilled water (SDW), following standard tomato cultivation practices as described by Jones (2007)26. The bacterial elicitors were resuspended in SDW, adjusted to an optical density (OD600) of 0.6 (600 nm) using a microplate reader (DYNATECH, Spain), and applied as a foliar spray27. A 0.5 mM salicylic acid (SA; Merck Millipore, Germany) solution was prepared by dissolving 0.2 g of SA in SDW and applied as a foliar spray. For the physical treatment (W), uniform mechanical wounds were created on leaves using a sterile puncher28. Treatments were applied to plants at the three-leaf stage and repeated twice at weekly intervals, while control plants were treated with SDW.
Inoculation and symptom evaluation of ToBRFV
To inoculate the plants with ToBRFV, an extract was prepared from infected tomato plants exhibiting characteristic viral symptoms. First, 1 g of young leaves showing rugosity and deformation was harvested and homogenized in 1 mL of phosphate buffer (0.01 M sodium phosphate, pH 7.0) using a mortar and pestle. The homogenate was centrifuged at 3000 rpm for five minutes, and the resulting supernatant was filtered through Whatman No. 1 filter paper. The filtered extract was then injected into both the petiole and leaf blade of tomato plants at the five-leaf stage using a 10-µL syringe (Hamilton, USA). To ensure uniform viral inoculation, two apical leaves per plant were inoculated. Healthy control plants were not treated with elicitors, were sprayed with sterile distilled water (SDW), and were injected with phosphate buffer alone. Virus control (V) plants were inoculated with the ToBRFV extract in phosphate buffer but were not treated with elicitors. All plants were maintained under greenhouse conditions (as described above) for symptom development. Symptom progression was monitored at 7-, 14-, and 21 days post-inoculation (dpi) corresponding to before flowering, during flowering, and after fruiting stages, respectively. Disease severity was assessed using a 0–5 scale: 0 = no symptoms, 1 = mild chlorosis or mosaic, 2 = moderate mosaic and leaf curling, 3 = severe mosaic, curling, and stunting, 4 = severe stunting and leaf deformation, and 5 = plant death. The Disease Severity Index (DSI) and percentage of disease reduction were calculated relative to the control group using the following equation:
Equation 1
In this formula, i represents the symptom severity score (0–5), Yi denotes the number of plants with score i, and N is the total number of plants assessed. This formula quantifies disease severity as a percentage relative to the maximum possible score.
Growth parameter assessment
At 28 days post-inoculation (dpi), plant height (H) was measured from soil to main stem tip using a ruler. Fresh aerial (WAW) and root (WRW) weights were recorded immediately after harvest using a digital scale; dry weights (DAW, DRW) were measured after oven-drying at 70 °C for 48 h. Fruit number (FN) and weight (FW) were recorded using a digital scale. Five replicates per treatment were assessed26.
Enzyme activity assay
For enzyme analysis, 200 mg of systemic (non-inoculated) upper leaf tissue was ground in liquid nitrogen using a mortar. The resulting powder was homogenized in 1 mL of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA and 1% polyvinylpyrrolidone (PVP) as the extraction buffer, then centrifuged at 15,000 rpm for 15 min at 4 °C. The supernatant was transferred to a new microtube for subsequent assays. Protein concentration in the supernatant was quantified using the Bradford assay29.
Catalase (CAT)
Catalase (CAT, EC 1.11.1.6) activity was measured at 28 days post-inoculation (dpi) according to Chandlee and Scandalios (1984)30. A 100 mg leaf sample was ground in liquid nitrogen, mixed with 1 mL of extraction buffer, and centrifuged at 11,000 rpm for 20 min at 4 °C. The supernatant was used in a reaction mixture consisting of 500 µL of 50 mM potassium phosphate buffer (KH₂PO₄, pH 7.0), 500 µL of H₂O₂, 100 µL of enzyme extract, and 1000 µL of SDW. Absorbance was recorded at 240 nm over 60 s at 25 °C using a Cary 100 UV-Vis spectrophotometer (Agilent, USA), with three replicates per sample. CAT activity was calculated as the amount of enzyme decomposing 1 µmol of H₂O₂ per minute per mg of protein, measured by the decrease in absorbance at 240 nm.
Peroxidase (POD)
Peroxidase (POD, EC 1.11.1.7) activity was assessed at 28 dpi following the method of Thomas et al. (1982)31. The reaction mixture contained 200 µL of enzyme extract, 500 µL of extraction buffer, 1000 µL of H₂O₂, and 1000 µL of guaiacol solution (114 µL guaiacol dissolved in 20 mL of extraction buffer). Absorbance was measured at 470 nm for 60 s at 25 °C, also with three replicates per sample. POD activity was defined as the amount of enzyme oxidizing 1 µmol of guaiacol per minute per mg of protein, measured by the increase in absorbance at 470 nm.
Phenylalanine ammonia-lyase (PAL)
Phenylalanine ammonia-lyase (PAL, EC 4.3.1.24) activity was determined based on the method by Zucker (1965)32. The extraction buffer consisted of 7 mM Na₂HPO₄ (pH 7.0), 2% polyvinylpyrrolidone (PVP), 2 mM EDTA, 18 mM 2-mercaptoethanol, and 0.1% Triton X-100, in a total volume of 50 mL. Leaf tissue was ground in liquid nitrogen, mixed with 800 µL of the extraction buffer, vortexed vigorously, and centrifuged at 15,000 rpm for 15 min at 4 °C. The supernatant was mixed with 1000 µL of reaction buffer (50 mM sodium borate buffer, pH 8.8, containing 5 mM phenylalanine), incubated at 40 °C for 30 min, and absorbance was measured at 290 nm. One unit of PAL activity was defined as the amount of enzyme that produces 1 nanomole of cinnamic acid per minute.
RNA extraction and quality assessment
RNX Plus kit (SinaClon, Iran) was used for RNA isolation from 200 mg of systemic (non-inoculated) upper leaf tissue. The RNA quality was assessed by visualizing 28 S and 18 S ribosomal bands on a 1% agarose gel. The quantity of extracted RNA was measured using a NanoDrop device (Nabi Microdigital, South Korea). Approximately 2000 ng of RNA from each sample was used for cDNA synthesis by a cDNA Synthesis Kit (SinaClon, Iran). The synthesized cDNA was then stored at −20 °C for further experiments.
Detection of ToBRFV by RT-PCR
ToBRFV was detected at 28 days post-inoculation (dpi) by two-step reverse transcription-polymerase chain reaction (RT-PCR) using ToBRFV-F (5′-GAAGTCCCGATGTCTGTAAGG-3′) and ToBRFV-R (5′-GTGCCTACGGATGTGTATGA-3′) primers, which amplify an 840-bp fragment from the viral coat protein33. Approximately 1 µg of total RNA, extracted from 200 mg of systemic (non-inoculated) upper leaf tissue, was reverse transcribed into single-stranded cDNA using a cDNA Synthesis Kit (SinaClon, Iran). The PCR reaction mixture included 100 ng of cDNA, 0.33 µM of each primer, 25 µL of 2X Master Mix, and PCR-grade water to a total volume of 50 µL. The PCR program included an initial denaturation at 95 °C for 5 min, 40 cycles of 95 °C for 30 s, 53 °C for 30 s, and 72 °C for 30 s, and a final extension at 72 °C for 10 min. Agarose gel electrophoresis was performed to visualize the RT-PCR products.
Quantification of defense gene expression by qRT-PCR
qRT-PCR was performed at 28 days post-inoculation (dpi) to quantify expression of defense-related genes using a StepOnePlus System (ABI) with the SYBR Green 1.25 mL High Rox Kit (Amplicon, Denmark). Real-time PCR reactions used 100 ng of cDNA, synthesized from 2000 ng of RNA extracted from systemic (non-inoculated) upper leaf tissue, 0.33 µM of each primer, 25.6 µL of 2X Master Mix, and 25.2 µL of PCR-grade water. Each gene analysis included two biological and two technical replicates, with thermal cycling conditions of 95 °C for 10 min, followed by 40 cycles of 95 °C for 20 s, 57 °C for 20 s, and 72 °C for 20 s, ending at 60 °C for 1 min. Primers for COI1, PR1b, NPR1, and the reference gene LOC544055 were used (Table 1)34.
Statistical analysis of gene expression, disease severity, and growth parameters
Gene expression changes were calculated using the ΔΔCT method, normalized to LOC544055, and compared to controls35. Briefly, ΔCt was measured for each sample by subtracting the cycle threshold (Ct) value of the reference gene (LOC544055) from the Ct value of the target genes (COI1, PR1b, NPR1). Then, data normalization was accomplished by calculating ΔΔCt by subtracting the mean ΔCt of the reference gene from the ΔCt of each target gene. The fold change in gene expression was measured by the formula 2 − ΔΔCt35. Growth parameters (plant height, fresh and dry weights, fruit number, and weight) and gene expression data were analyzed in a completely randomized design with five and three replicates, respectively, using SAS software ver. 9.3 (IBM, USA) via ANOVA (Analysis of Variance). Duncan’s multiple range test was applied at P = 0.01 and P = 0.05 to identify significant differences among treatments. Normal distribution of data from the disease severity assay was not observed; therefore, a non-parametric statistical approach was applied. The Kruskal-Wallis H test was used in SPSS software ver. 22 (IBM, USA). qRT-PCR efficiency was evaluated using REST (Relative Expression Software Tool) software36.
Result
Effect of elicitors on growth parameters in ToBRFV-Infected tomato
Growth parameters were assessed at 28 dpi. Typical viral symptoms—including mottling, mosaic, yellowing, and leaf deformation—were observed on tomato plants mechanically inoculated with ToBRFV (Figure S1). The results showed that the highest tomato plant height (230 cm) was recorded in the simultaneous treatment of the bacterial elicitors (P + B), which was significantly higher (at the 5% significance level) than both virus-infected non-treated (V) (190 cm) and healthy (C) plants (212 cm) (Table 2). Other treatments, including P and B, SA, and W, also significantly increased plant height compared to virus-infected non-treated plants, but did not differ significantly from healthy plants.
Regarding root biomass, the highest fresh root weights were observed in the SA (110 g) and W (115 g) treatments, which were comparable to healthy controls (112 g) (Table 2). The P + B treatment also significantly enhanced both fresh and dry root weights relative to the V treatment. Virus infection alone led to a general reduction in plant growth parameters, including root biomass. However, the application of bio-elicitors derived from plant growth-promoting and pathogenic bacteria stimulated the plant’s defense responses, mitigating virus-induced damage.
Measurements of aerial biomass revealed that only the P + B treatment significantly increased fresh shoot weight (297.1 g) compared to the C (252 g) treatment, while all treatments showed improvements over the V treatment (Table 2). These findings emphasize the beneficial role of bacterial elicitors in promoting plant growth and alleviating the negative effects of ToBRFV infection.
Impact of elicitors on disease severity in tomato
In this study, a visual assessment method was employed to evaluate symptoms caused by ToBRFV in tomato plants, serving as an effective preliminary tool for differentiating levels of disease severity37. The Kruskal-Wallis H test revealed highly significant differences in disease severity among treatments at all growth stages. Given this overall significance, post-hoc pairwise comparisons were performed using Dunn’s test with Bonferroni adjustment, (p < 0.05). The results revealed that the highest disease severity index occurred in plants treated with the Pseudomonas elicitor alone, with values of 0%, 16%, and 44% recorded before flowering, during flowering, and after fruiting, respectively (Table 3). However, this treatment did not significantly suppress viral symptoms compared to virus-infected non-treated plants.
In contrast, the lowest disease severity was observed in plants treated simultaneously with Pseudomonas and Burkholderia elicitors, showing disease severity indices of 0%, 8%, and 6% at the same respective stages. Control (healthy) plants exhibited no visible symptoms, with a consistent disease severity index of 0% throughout the experiment, indicating the absence of infection (Table 3).
PCR-based detection of ToBRFV
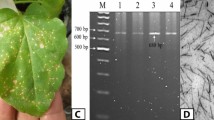
To confirm primer specificity for ToBRFV and determine the optimal annealing temperature, a temperature gradient PCR was performed at 28 dpi. The results indicated that 53 °C was the most effective temperature for primer annealing. PCR using cDNA as the template verified the successful and specific amplification of the target gene, confirming both the quality of the synthesized cDNA and the precision of the primers. Agarose gel electrophoresis revealed a distinct PCR product of approximately 840 bp in samples from treated tomato plants (Fig. 1). These findings suggest that while bacterial elicitors mitigated the virus-induced growth inhibition, they were not fully effective in preventing viral replication in infected tissues, as evidenced by the continued presence of ToBRFV in PCR assays.
The results of gel electrophoresis of PCR products obtained from tomato brown rugose fruit virus detection assay showing the amplification of an 840 bp fragment from the virus genome. M: DNA ladder (1 kb), C+: positive control (virus-infected plant), C−: negative control (virus free plant), P: Pseudomonas fluorescens 1442 elicitor, B: Burkholderia gladioli G15, P + B: plant simultaneously treated with the bacterial elicitors, SA: Salicylic acid-treated plant, W: wound plant, V: virus-inoculated plant. The primer details were presented in Table 1.
Enhancement of antioxidant enzymes
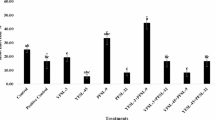
Enzyme activities (CAT, EC 1.11.1.6; POD, EC 1.11.1.7; PAL, EC 4.3.1.24) were measured at 28 dpi in systemic (non-inoculated) upper leaf tissue. Peroxidase (POD) activity in tomato plants was highest in the P + B treatment (0.316 mg/min), while the lowest activity was recorded in the V treatment (0.048 mg/min) (Fig. 2). This combined treatment significantly enhanced POD levels compared to all other treatments, whereas virus-infected non-treated plants exhibited significantly lower activity.
Enzymatic activity of catalase (CAT), peroxidase (POD), and phenylalanine ammonia-lyase (PAL) in plants treated with abiotic and biotic elicitors. -: negative control (virus free plant), P: Pseudomonas fluorescens 1442 elicitor, B: Burkholderia gladioli G15, P + B: plant simultaneously treated with the bacterial elicitors, SA: Salicylic acid-treated plant, W: wound plant, V: virus-inoculated plant. The letters on each bar represent the results of Duncan’s multiple range test based on which the values with the same letters do not show any significant difference at a 5% probability level. The error value was shown on each bar.
Similarly, catalase (CAT) activity peaked (0.306 mg/min) in the P + B treatment and lowered to its lowest level (0.102 mg/min) in the virus-infected non-treated group (Fig. 2). The P + B treatment markedly boosted CAT activity compared to other treatments.
For phenylalanine ammonia-lyase (PAL), the highest enzyme level (0.63 mg) was observed under P + B treatment, while the lowest PAL activity (0.31 mg) occurred in the virus-infected non-treated plants (Fig. 2). PAL activity was significantly increased by the P + B treatment compared to all other treatments. Notably, PAL levels in virus-infected non-treated plants were not significantly different from those in the control (healthy) plants, suggesting virus infection alone did not substantially suppress PAL expression.
Defense-related gene expression
At 28 days post-inoculation (dpi), qRT-PCR was performed to quantify expression of defense-related genes in response to ToBRFV infection. The quality of the extracted RNA was confirmed by the clear visualization of 28 S and 18 S ribosomal bands on a 1% agarose gel, indicating intact RNA (data not shown). RNA purity was verified using spectrophotometric measurements at 230, 260, and 280 nm, and the calculated A260/A280 and A260/A230 ratios confirmed the absence of contaminants (data not shown). The synthesized cDNA also showed a high A260 value, indicating efficient reverse transcription (data not shown). Melting curve analysis in the qRT-PCR assay revealed single peaks for all genes, confirming primer specificity and the absence of non-specific amplification or primer-dimer formation (Fig. 3).
In response to ToBRFV infection, the COI1 gene expression was significantly upregulated (p < 0.01), with the highest expression observed in the P + B treatment—an 1800-fold increase compared to the virus-infected non-treated control. No significant induction of COI1 was observed in P and B treatments (Fig. 4).
The effect of different treatments on the relative transcription levels of PR1b, NPR1, and COI1 genes, -: negative control (virus free plant), P: Pseudomonas fluorescens 1442 elicitor, B: Burkholderia gladioli G15, P + B: plant simultaneously treated with the bacterial elicitors, SA: Salicylic acid-treated plant, W: wound plant, V: virus-inoculated plant. The letters on each bar represent the results of Duncan’s multiple range test based on which the values with the same letters do not show any significant difference at a 5% probability level. The error value was shown on each bar.
NPR1 gene expression also showed a significant increase (p < 0.01) in the P + B treatment, exhibiting a 600-fold elevation over virus-infected non-treated plants. In contrast, P, B, and W treatments did not result in significant changes (Fig. 4).
Likewise, PR1b expression was significantly enhanced (p < 0.01) by the P + B treatment, with a 240-fold increase compared to virus-infected non-treated plants. Notably, significant upregulation of PR1b was also observed in plants treated with SA and by W (Fig. 4). The quality of the extracted RNA was confirmed by the clear visualization of 28 S and 18 S ribosomal bands on a 1% agarose gel, indicating intact RNA (data not shown). RNA purity was verified using spectrophotometric measurements at 230, 260, and 280 nm, and the calculated A260/A280 and A260/A230 ratios confirmed the absence of contaminants (data not shown). The synthesized cDNA also showed a high A260 value, indicating efficient reverse transcription (data not shown). Melting curve analysis in the qRT-PCR assay revealed single peaks for all genes, confirming primer specificity and the absence of non-specific amplification or primer-dimer formation (Fig. 3).
In response to ToBRFV infection, the COI1 gene expression was significantly upregulated (p < 0.01), with the highest expression observed in the P + B treatment—an 1800-fold increase compared to the virus-infected non-treated control. No significant induction of COI1 was observed in P and B treatments (Fig. 4).
NPR1 gene expression also showed a significant increase (p < 0.01) in the P + B treatment, exhibiting a 600-fold elevation over virus-infected non-treated plants. In contrast, P, B, and W treatments did not result in significant changes (Fig. 4).
Likewise, PR1b expression was significantly enhanced (p < 0.01) by the P + B treatment, with a 240-fold increase compared to virus-infected non-treated plants. Notably, significant upregulation of PR1b was also observed in plants treated with SA and by W (Fig. 4).
Discussion
This study demonstrates that the application of abiotic and biotic elicitors, particularly bacterial elicitors from P. fluorescens 1442 and B. gladioli G15, significantly improved plant growth and reduced the severity of ToBRFV infection in tomato plants. These findings align with de Almeida Halfeld-Vieira et al. (2023)38, who reported that Xanthomonas crude lipopolysaccharide (LPS) enhanced systemic resistance and reduced disease severity in tomatoes against bacterial spot. Similarly, the commercial product Virus Stop® reduced TMV severity39, and systemic resistance was induced by Bacillus spp. and Rhodopseudomonas palustris GJ-22 against TMV40,41. Additionally, commercial elicitors like Virablock® and Silicant® have been shown to improve growth and yield in ToBRFV-infected tomatoes42,43. Our results are also consistent with Sofla et al. (2023)34, who reported that bacterial elicitors significantly enhanced growth parameters and reduced disease symptoms in virus-infected tomato plants.
The increased activity of PAL in response to Pseudomonas and Burkholderia elicitors is consistent with previous findings, emphasizing PAL’s key role in plant defense. PAL is involved in the biosynthesis of phenolic compounds and lignin, which contribute to structural barriers and antimicrobial activity. Similar increases in PAL activity have been reported by Huang et al. (2010)44 and Gerasimova et al. (2005)45. Our findings further demonstrate that the P + B application produced a stronger defense response than P or B treatment, consistent with reports of synergistic effects from chitosan and yeast elicitors in tobacco46. This suggests that P. fluorescens and B. gladioli polysaccharides effectively boost plant immunity via MAMP-triggered responses, as supported by de Almeida Halfeld-Vieira et al. (2023)38.
The elevation of POD activity in the P + B treatments confirms its vital role in plant defense. As shown by Durner et al. (1997)47, POD contributes to cell wall reinforcement and the conversion of phenolics to antimicrobial quinones, limiting pathogen invasion and replication. These results align with studies demonstrating that bacterial polysaccharides enhance systemic resistance in tomatoes38.
Similarly, CAT activity, which aids in scavenging reactive oxygen species by converting hydrogen peroxide into water and oxygen, was significantly enhanced by the P + B treatment. This agrees with Sharma and Ahmad (2014)48 and the findings by Ghanem (2022)49, where B. subtilis treatments increased CAT and POD activities, improving resistance to TYLCV. Collectively, these enzymatic changes highlight the effectiveness of bacterial elicitors in enhancing antioxidant defenses and systemic resistance.
Regarding gene expression, the COI1 gene—central to the jasmonic acid (JA) signaling pathway—was significantly upregulated following P + B treatment, suggesting activation of JA-mediated defenses. This is consistent with findings that COI1 is involved in defense against Ralstonia solanacearum and nematodes50. Similarly, NPR1, a regulatory protein in the SA signaling pathway, was significantly induced only by the P + B treatment, supporting the hypothesis of synergistic activation of SA- and JA-dependent pathways. NPR1’s role in modulating PR gene expression17,51 reinforces its importance in the plant immune network.
The significant upregulation of PR1b—a pathogenesis-related gene—by the P + B treatment and SA application suggests an enhanced defense response, as also reported by Shah (2003)52. The strong expression of PR1b indicates activation of downstream defense mechanisms that limit pathogen spread. These results collectively support the conclusion that simultaneous bacterial elicitor treatment triggers a robust and coordinated defense response involving both enzymatic and genetic pathways.
The persistence of ToBRFV replication in elicitor-treated plants, as detected by RT-PCR, likely reflects the nature of viral infections, where elicitors enhance systemic resistance but do not directly inhibit viral replication within infected cells. Bacterial elicitors, such as those from P. fluorescens and B. gladioli, primarily induce defense pathways (e.g., SA and JA signaling) that limit symptom expression and viral spread by strengthening cell walls, producing antimicrobial compounds, and enhancing antioxidant defenses17,53. However, once ToBRFV establishes infection, its rapid replication and mechanical transmission may outpace complete suppression by induced resistance.
The coordinated upregulation of both enzymatic (POD, CAT, PAL) and genetic (NPR1, COI1, PR1b) defense components in P + B-treated plants suggests a two-tiered resistance mechanism: (1) rapid ROS scavenging through enhanced CAT/POD activities mitigates oxidative damage during early infection, while (2) sustained PAL activity and PR gene induction establish long-term systemic resistance. This temporal synergy explains the significant symptom reduction despite viral persistence, as the immediate antioxidant response buffers cellular damage while SA/JA-mediated signaling orchestrates durable defense gene activation17,48.
In conclusion, this study reveals that the simultaneous application of bacterial elicitors from P. fluorescens 1442 and B. gladioli G15 markedly enhances resistance in tomato plants against ToBRFV. This treatment significantly elevated the expression of key defense-related genes—NPR1, COI1, and PR1b—and increased the activity of critical defense enzymes—PAL, POD, and CAT. In contrast, individual treatments with these elicitors or with SA showed limited effects, indicating a synergistic interaction when both elicitors are applied together. Wounding induced some resistance but was notably less effective than bio-elicitor treatments.
In addition to bolstering plant immunity, the combined elicitor treatment improved agronomic traits such as stem height and fruit weight, highlighting its dual Brole in disease management and growth promotion. Therefore, the application of bacterial elicitors represents a promising, eco-friendly, and sustainable strategy for controlling viral infections like ToBRFV in tomato cultivation, while simultaneously improving crop performance.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Savary, S. et al. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 3, 430–439 (2019).
Salem, N., Mansour, A., Ciuffo, M. & Falk, B. W. Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol. 161, 503–506 (2016).
Luria, N. et al. A new Israeli tobamovirus isolate infects tomato plants harboring Tm-22 resistance genes. PLoS ONE. 12, e0170429 (2017).
Esmaeilzadeh, F. & Koolivand, D. First report of tomato brown rugose fruit virus infecting bell pepper in Iran. J. Plant. Pathol. 104, 893 (2022).
Ghorbani, A., Rostami, M., Seifi, S. & Izadpanah, K. First report of tomato brown rugose fruit virus in greenhouse tomato in Iran. New. Dis. Rep. 44, e12040 (2021).
Avni, B. et al. Tomato genetic resistance to tobamoviruses is compromised. Acta Hortic. 1316, 89–98 (2019).
Panno, S. et al. Spread of tomato brown rugose fruit virus in Sicily and evaluation of the Spatiotemporal dispersion in experimental conditions. Agronomy 10, 834 (2020).
Zhang, S., Griffiths, J. S., Marchand, G., Bernards, M. A. & Wang, A. Tomato brown rugose fruit virus: an emerging and rapidly spreading plant RNA virus that threatens tomato production worldwide. Mol. Plant. Pathol. 23, 1262–1277 (2022).
FAO. FAO Year Book (FAO, 2021).
Enebe, M. C. & Babalola, O. O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: a survival strategy. Appl. Microbiol. Biotechnol. 102, 7821–7835 (2018).
Garrido, J. & Luque-Romero, J. Integrated Pest Management in Mediterranean Greenhouses (European Crop Protection, 2014).
Moreno-Escamilla, J. O. et al. Effect of elicitors in the nutritional and sensorial quality of fruits and vegetables. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality (eds Paliyath, G. Academic Press, 71–91 (2018).
Jha, C. K. & Saraf, M. Plant growth promoting rhizobacteria (PGPR): a review. J. Agric. Res. Dev. 5, 108–119 (2015).
Weller, D. M. Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology 97, 250–256 (2007).
Simões, C. T., Carvalho, V. N. & Halfeld-Vieira, B. D. A. Prospecting of pathogen-derived elicitors for the control of tomato bacterial spot. J. Plant. Prot. Res. 61, 192–199 (2021).
Rudrappa, T. et al. The rhizobacterial elicitor acetoin induces systemic resistance in Arabidopsis Thaliana. Commun. Integr. Biol. 3, 130–138 (2010).
Pieterse, C. M. et al. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52, 347–375 (2014).
Malamy, J., Carr, J. P., Klessig, D. F. & Raskin, I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250, 1002–1004 (1990).
Ding, P. & Ding, Y. Stories of Salicylic acid: a plant defense hormone. Trends Plant. Sci. 25, 549–565 (2020).
Jayaraj, J., Muthukrishnan, S., Liang, G. H. & Velazhahan, R. Jasmonic acid and Salicylic acid induce accumulation of β-1, 3-glucanase and thaumatin-like proteins in wheat and enhance resistance against stagonospora nodorum. Biol. Plant. 48, 425–430 (2004).
Muthamilarasan, M. & Prasad, M. Plant innate immunity: an updated insight into defense mechanism. J. Biosci. 38, 433–449 (2013).
Sokea, T. et al. Micro-pathogen elicitor Hrip1 protein isolated from Alternaria tenuissima induced disease resistance against tomato yellow leaf curl virus (TYLCV) in tomato (Solanum lycopersicum). J. Appl. Microb. Res. 2, 8–16 (2019).
Qi, X. et al. Plant defense responses to a novel plant elicitor candidate LY5-24-2. Int. J. Mol. Sci. 23, 5348 (2022).
Jetiyanon, K. & Kloepper, J. W. Mixtures of plant growth-promoting rhizobacteria for induction of systemic resistance against multiple plant diseases. Biol. Control. 24, 285–291 (2002).
Luiz, C., Neto, A. R. & Di Piero, R. M. Resistance to Xanthomonas gardneri in tomato leaves induced by polysaccharides from plant or microbial origin. J. Plant. Pathol. 97, 119–127 (2015).
Jones, J. B. Jr Tomato plant culture: in the field, greenhouse, and home garden, Second Edition (2nd ed.). CRC press, (2007).
Bonaterra, A. et al. Bacteria as biological control agents of plant diseases. Microorganisms 31, 1759 (2022).
León, J., Rojo, E. & Sánchez-Serrano, J. J. Wound signalling in plants. J. Exp. Bot. 52, 1–9 (2001).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 (1–2), 248–254 (1976).
Chandlee, J. M. & Scandalios, J. G. Analysis of variants affecting the catalase developmental program in maize scutellum. Theor. Appl. Genet. 69, 71–77 (1984).
Thomas, R. L., Jen, J. J. & Morr, C. V. Changes in soluble and bound peroxidase—IAA oxidase during tomato fruit development. J. Food Sci. 47, 158–161 (1982).
Zucker, M. Induction of phenylalanine deaminase by light and its relation to chlorogenic acid synthesis in potato tuber tissue. Plant. Physiol. 40, 779–784 (1965).
Ling, K. S., Tian, T., Gurung, S., Salati, R. & Gilliard, A. First report of tomato brown rugose fruit virus infecting greenhouse tomato in the united States. Plant. Dis. 103, 1439 (2019).
Sofla, A. S. S. A., Taheri, H., Parizipour, M. H. G. & Soleymani, F. Molecular and phenotypic responses of rhizobacteria-treated tomato plants to tomato mosaic virus under greenhouse conditions. Iran. J. Biotechnol. 21, e3220 (2023).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆CT method. Methods 25, 402–408 (2001).
Pfaffl, M. W., Horgan, G. W. & Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30, e36 (2002).
Martinelli, F. et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 35, 1–25 (2015).
de Halfeld-Vieira, A., Simões, B., Carvalho, V. N. & C. T., & Efficacy of Xanthomonas crude lipopolysaccharide on the control of the tomato bacterial spot. Physiol. Mol. Plant. Pathol. 124, 101959 (2023).
Hernández-Santiago, R., Vargas-Hernández, M. & Zamora-Macorra, E. J. Evaluation of TMV resistance inducers in tomato. Rev. Mex Cienc. Agríc. 11, 377–390 (2020).
Wang, S. et al. Molecular mechanism of plant growth promotion and induced systemic resistance to tobacco mosaic virus by Bacillus spp. J. Microbiol. Biotechnol. 19, 1250–1258 (2009).
Su, P. et al. Photosynthetic bacterium Rhodopseudomonas palustris GJ-22 induces systemic resistance against viruses. Microb. Biotechnol. 10, 612–624 (2017).
Ortiz-Martínez, L. E. & Ochoa-Martínez, D. L. Elicitors and biostimulants in the production of tomato infected with tomato brown rugose fruit virus. J. Plant. Dis. Prot. 130, 351–360 (2023).
Cham, A. K., Ojeda Zacarías, M. D. C., Lozoya Saldaña, H., Saenz, E. O. & Alvarado Gomez, O. G. Effects of elicitors on the growth, productivity and health of tomato (Solanum lycopersicum L.) under greenhouse conditions. J. Agric. Sci. Technol. 24, 1129–1142 (2022).
Huang, J. et al. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant. Physiol. 153, 1526–1538 (2010).
Gerasimova, N. G., Pridvorova, S. M. & Ozeretskovskaya, O. L. Role of L-phenylalanine ammonia lyase in the induced resistance and susceptibility of potato plants. Appl. Biochem. Microbiol. 41, 103–105 (2005).
Gómez-Vásquez, R. et al. Phenylpropanoids, phenylalanine ammonia lyase and peroxidases in elicitor-challenged cassava (Manihot esculenta) suspension cells and leaves. Ann. Bot. 94, 87–97 (2004).
Durner, J., Shah, J. & Klessig, D. F. Salicylic acid and disease resistance in plants. Trends Plant. Sci. 2, 266–274 (1997).
Sharma, I. & Ahmad, P. Catalase: a versatile antioxidant in plants. In Oxidative Damage To Plants (ed (ed Ahmad, P.) 131–148 (Academic, (2014).
Ghanem, H. M. Induction systemic resistance in tomato plants against tomato yellow leaf curl virus in protected cultivation using a local bacterial isolate Bacillus subtilis. J. Plant. Prot. Res. 62, 1–10 (2022).
Bhattarai, K. K. et al. Tomato susceptibility to root-knot nematodes requires an intact jasmonic acid signaling pathway. Mol. Plant-Microbe Interact. 21, 1205–1214 (2008).
Dong, X. Genetic dissection of systemic acquired resistance. Curr. Opin. Plant. Biol. 4, 309–314 (2001).
Shah, J. The Salicylic acid loop in plant defense. Curr. Opin. Plant. Biol. 6, 365–371 (2003).
del Carmen Orozco-Mosqueda. Bacterial elicitors of the plant immune system: an overview and the way forward. Plant. Stress. 7, 100138 (2023).
Acknowledgements
The authors thank Shahid Chamran University of Ahvaz for supporting this research (Grant Number: SCU.AP1403.33951).
Author information
Authors and Affiliations
Contributions
R.Z. conducted the experiment with assistance from M.A. and M.H.G.P. M.A. conceived and designed the study with input from M.H.G.P. All authors reviewed and revised the manuscript, with M.A. serving as the corresponding author.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zohoursoleimani, R., Aeini, M. & Ghodoum Parizipour, M.H. Effects of abiotic and biotic elicitors on tomato resistance to tomato brown rugose fruit virus. Sci Rep 15, 27216 (2025). https://doi.org/10.1038/s41598-025-13578-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13578-z